Biosynthesis of Silver Nanoparticles Using Phytochemicals Extracted from Aqueous Clerodendrum glabrum for Anti-Diabetes and Anti-Inflammatory Activity: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Plant Material
2.2.2. Biosynthesis of Silver Nanoparticles
2.2.3. Stability Testing of CG-Ag Nanoparticles
2.2.4. Characterisation of Silver Nanoparticles
2.2.5. Phytochemical Analyses
Analysing for Flavonoids
Analysing for Phenols
Analysing for Tannins
Analysing for Alkaloids
Analysing for Terpenoids
Analysing for Anthraquinones
Analysing for Saponin
Analysing for Glycosides
Analysing for Steroids
2.2.6. Cytotoxicity of the Extract
2.2.7. Investigation of Anti-Diabetic Activity
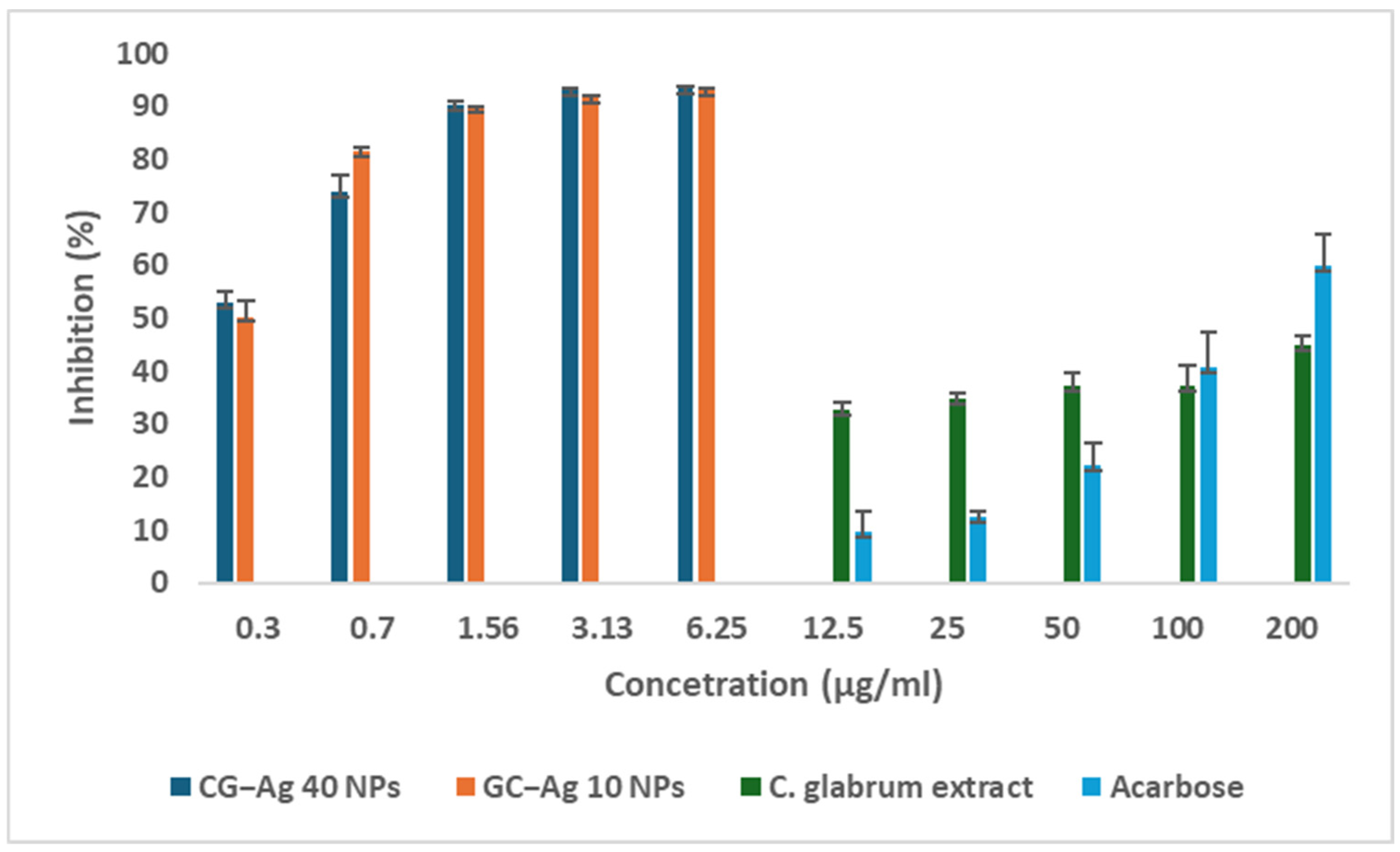
α-Glucosidase Activity Inhibition Assay
α-Amylase Activity Inhibition Assay
2.2.8. Investigating Anti-Inflammatory Activity
Hyaluronidase Activity Inhibition Assay
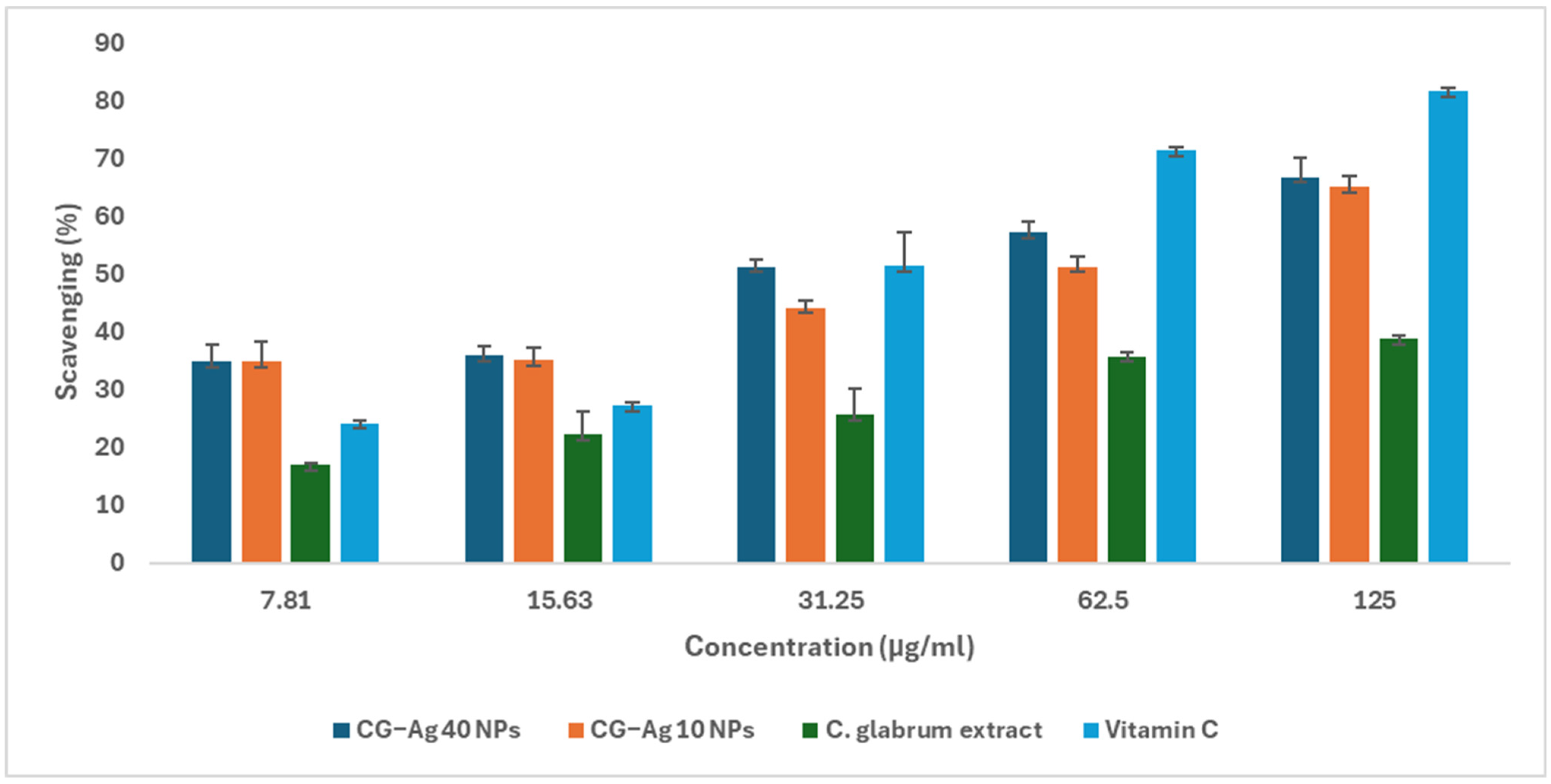
Nitric Oxide (NO) Scavenging Assay
3. Results
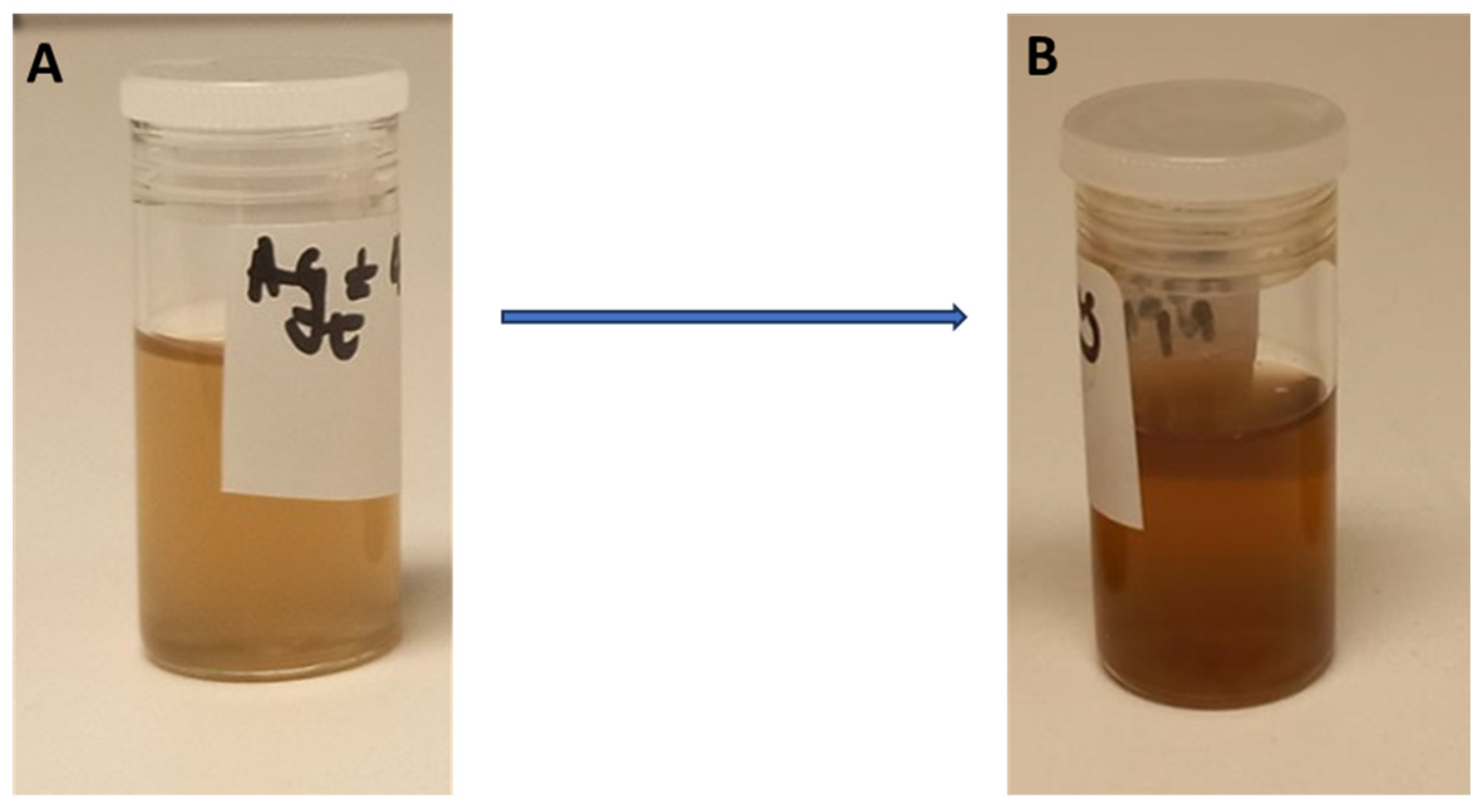
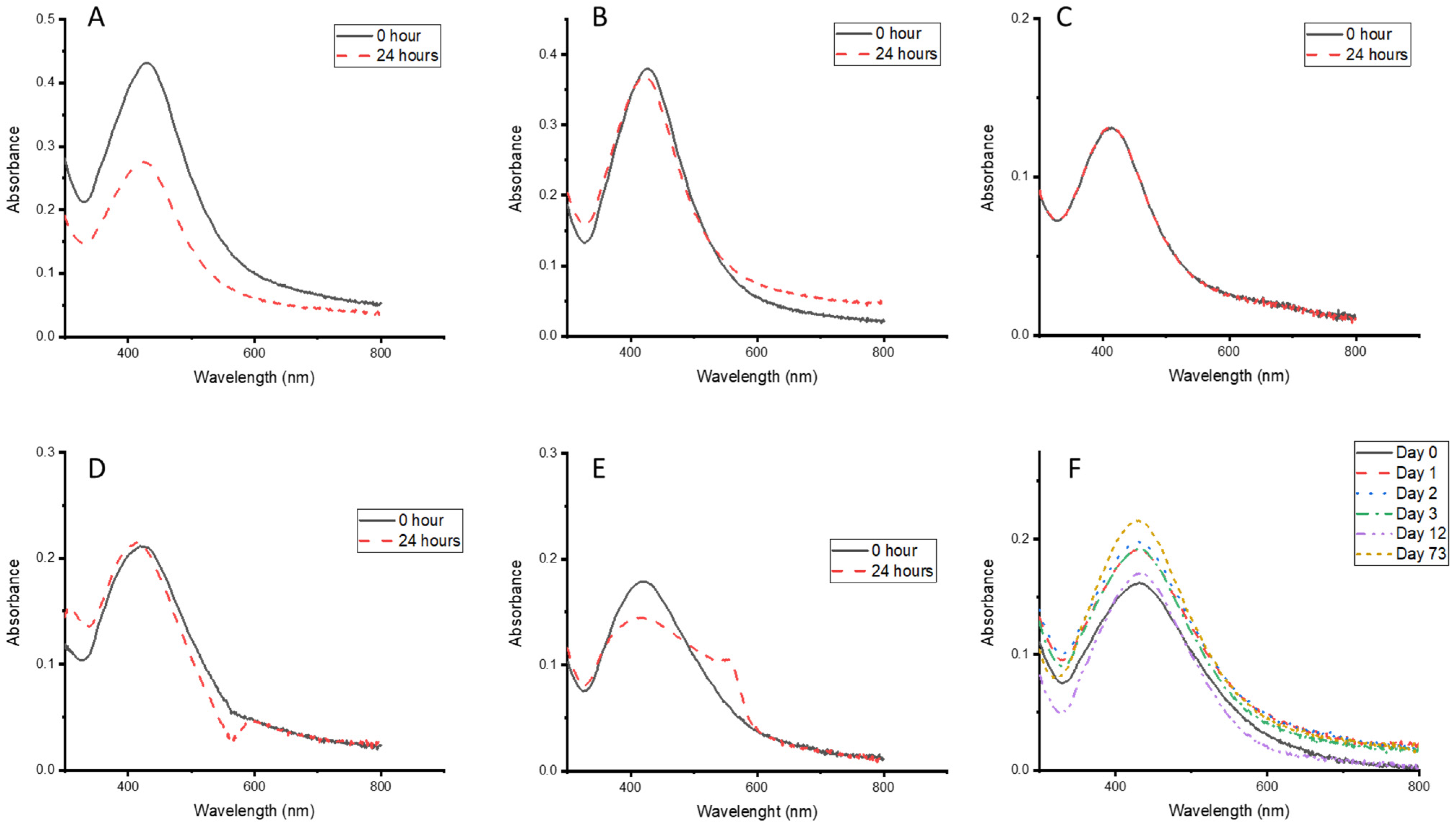
3.1. Synthesis, Optimisation, and Characterisation of the Synthesised CG-Ag NPs
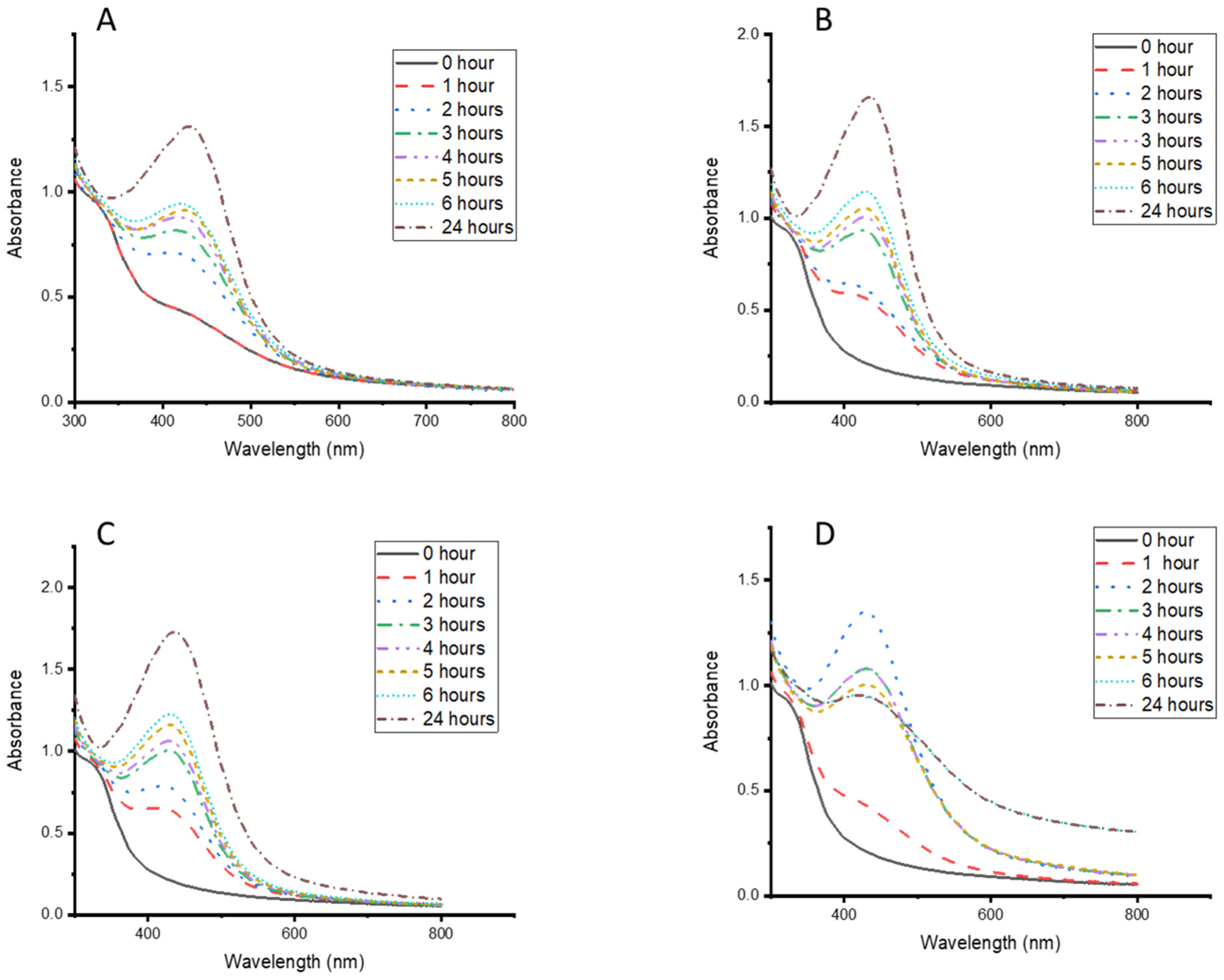
3.2. Effects of Temperature and Time on the Synthesis of CG-Ag NPs
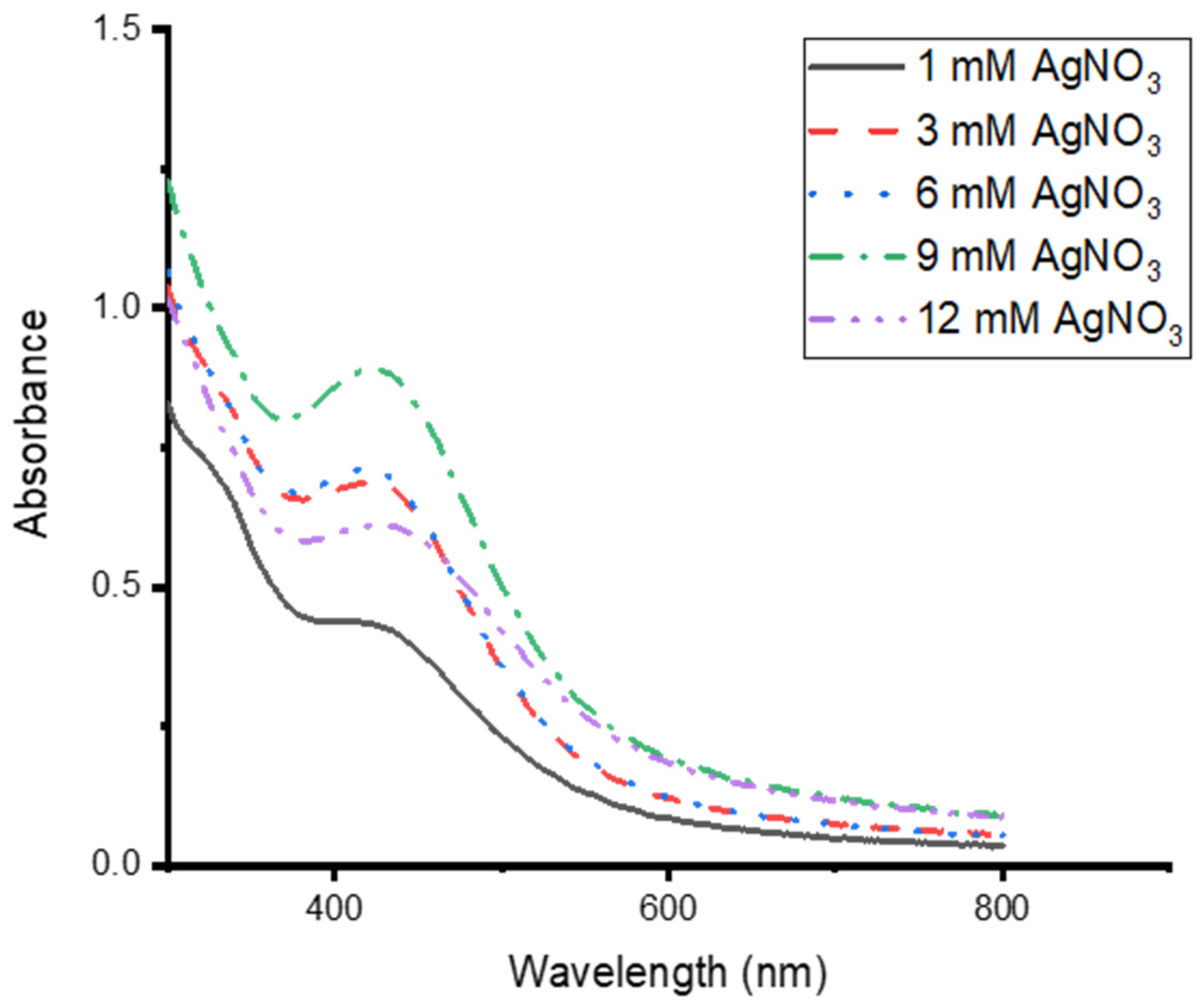
3.3. Effects of AgNO3 Concentration on the Synthesis of CG-Ag NPs
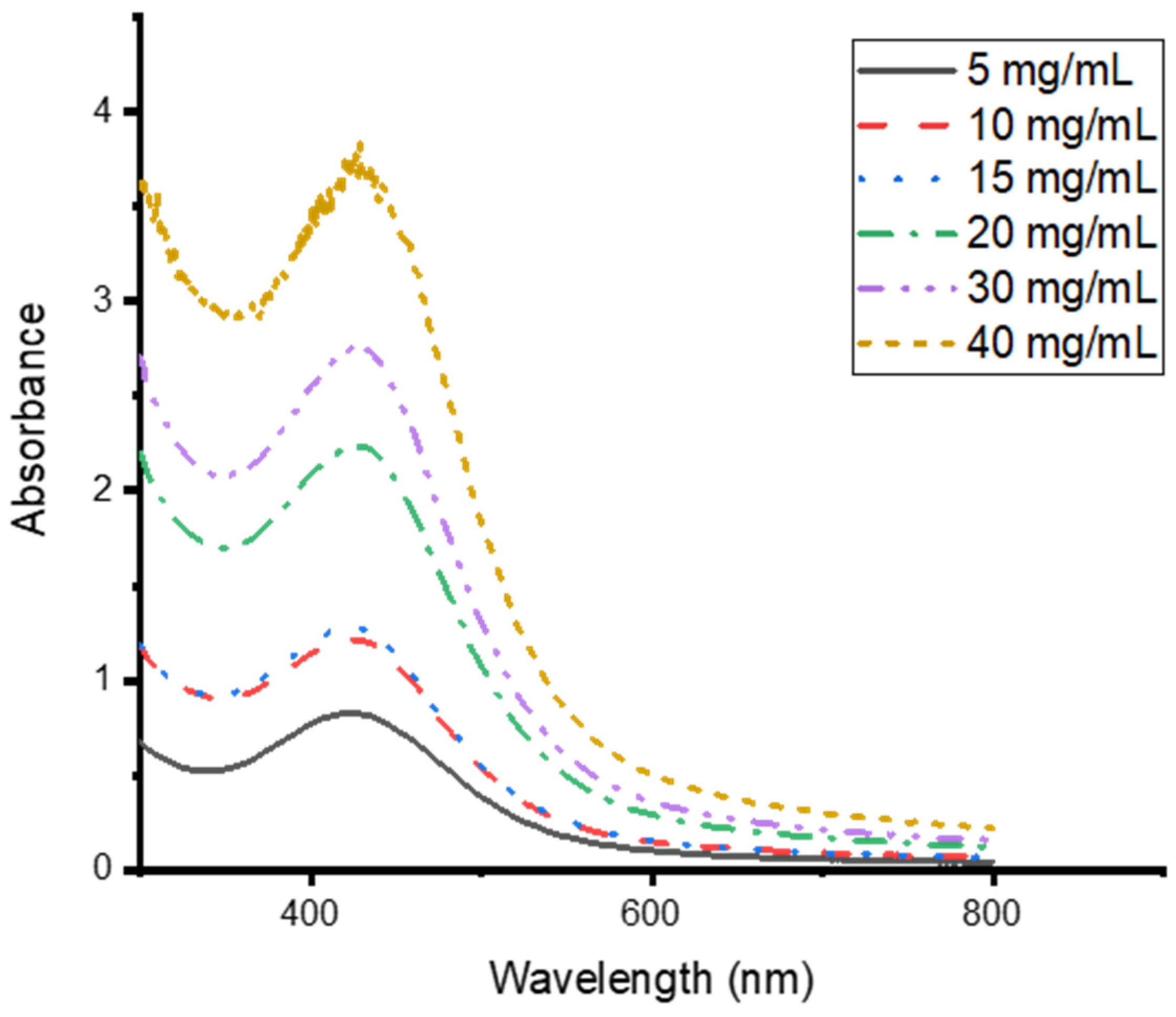
3.4. Effects of CG Extract Concentration on the Synthesis of CG-Ag NPs
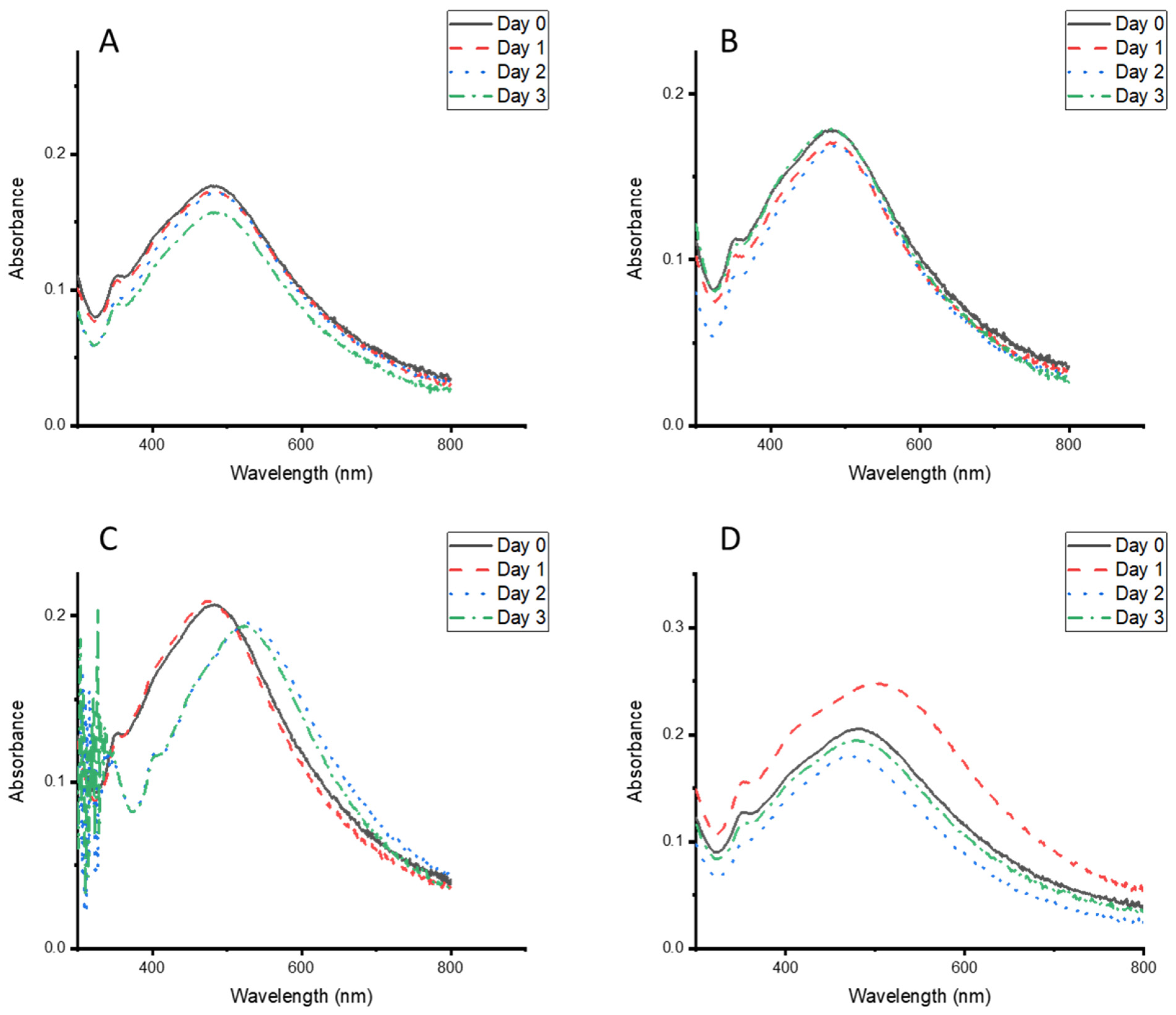
3.5. Stability of the CG-Ag NPs Under Different Conditions
3.6. Phytochemical Analysis
3.7. Dynamic Light Scattering Analysis
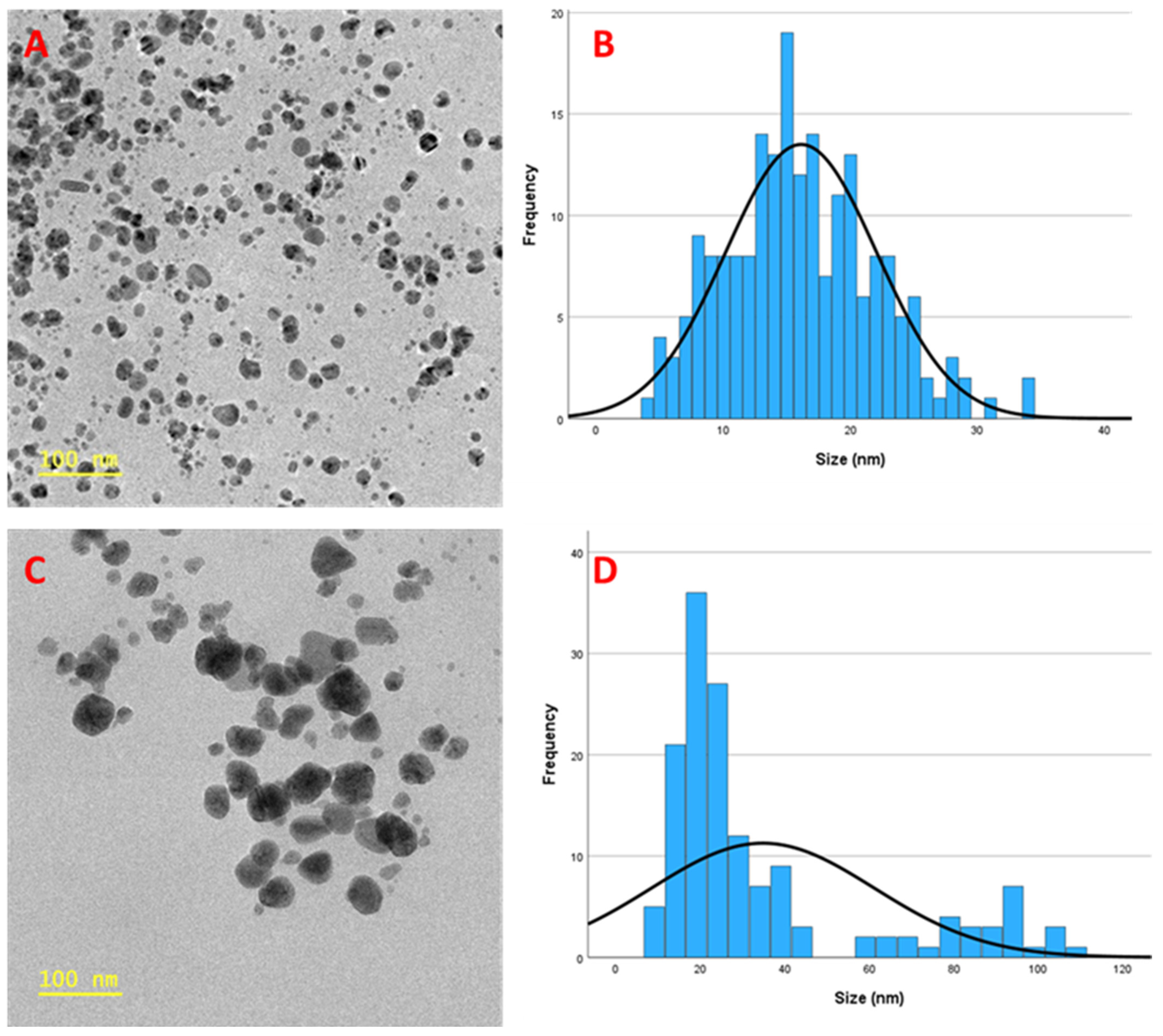
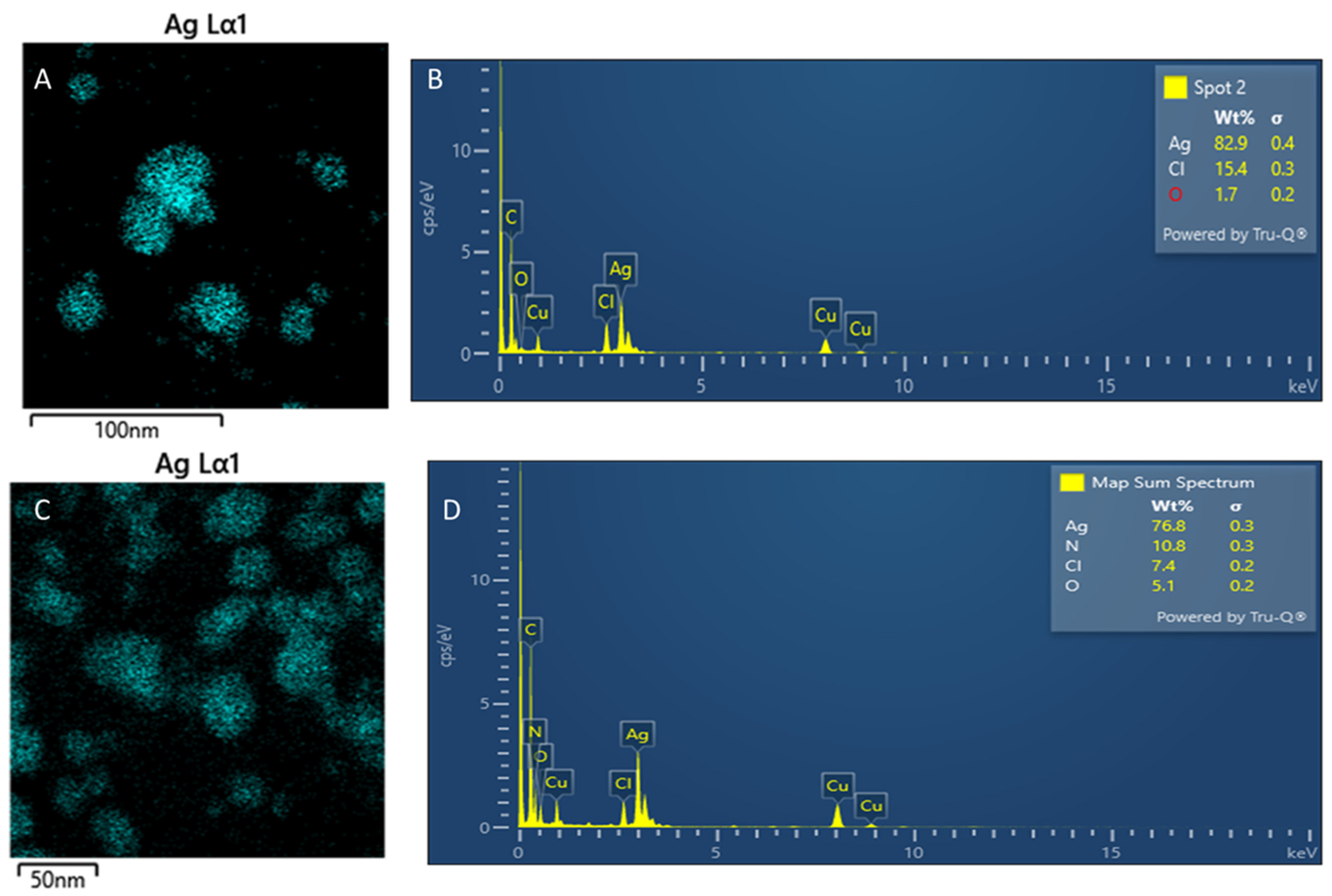
3.8. High-Resolution Transmission Electron Microscopy (HR-TEM) Analysis
3.9. Cytotoxicity
3.10. Investigation of Anti-Diabetic Activity
3.11. Hyaluronic Degradation Inhibition Assay
3.12. Nitric Oxide Scavenging
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CG | C. glabrum |
| NP | Nanoparticle |
| Ag | Silver |
| Ag NPs | Silver nanoparticles |
| CG-Ag NPs | Clerodendrum glabrum–silver nanoparticles |
| NO | Nitric oxide |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| DNS | 3,5-Dinitrosalicylic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| DMSO | Dimethyl sulfoxide |
| RPMI | Roswell Park Memorial Institute 1640 medium |
| DMEM | Dulbecco’s Modified Eagle Medium |
| PBS | Phosphate-buffered saline |
| DLS | Dynamic light scattering |
| HR-TEM | High-resolution transmission electron microscopy |
| UV–Vis | Ultraviolet–visible spectroscopy |
| SEM | Standard error of the mean |
| SD | Standard deviation |
References
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon Orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Palanisamy, S.; Goutham, G.; Koodalingam, A.; Prabhu, N.M.; Kannapiran, E.; Basu, M.J.; Arulvasu, C.; et al. Biosynthesis of Silver Nanoparticles Using Aqueous Extract of Phyllanthus acidus L. Fruits and Characterization of Its Anti-Inflammatory Effect against H2O2 Exposed Rat Peritoneal Macrophages. Process Biochem. 2017, 55, 172–181. [Google Scholar] [CrossRef]
- Al Baloushi, K.S.Y.; Senthilkumar, A.; Kandhan, K.; Subramanian, R.; Kizhakkayil, J.; Ramachandran, T.; Shehab, S.; Kurup, S.S.; Alyafei, M.A.M.; Al Dhaheri, A.S.; et al. Green Synthesis and Characterization of Silver Nanoparticles Using Moringa Peregrina and Their Toxicity on MCF-7 and Caco-2 Human Cancer Cells. Int. J. Nanomed. 2024, 19, 3891–3905. [Google Scholar] [CrossRef] [PubMed]
- Majoumouo, M.S.; Sibuyi, N.R.S.; Tincho, M.B.; Mbekou, M.; Boyom, F.F.; Meyer, M. Enhanced Anti-Bacterial Activity Of Biogenic Silver Nanoparticles Synthesized From Terminalia Mantaly Extracts. Int. J. Nanomed. 2019, 14, 9031–9046. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Dias, H.V.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The Greener Synthesis of Nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Singh, P.; Ahn, S.; Kang, J.-P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; El-Agamy Farh, M.; Aceituno, V.C.; Kim, Y.J.; et al. In Vitro Anti-Inflammatory Activity of Spherical Silver Nanoparticles and Monodisperse Hexagonal Gold Nanoparticles by Fruit Extract of Prunus serrulata: A Green Synthetic Approach. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2022–2032. [Google Scholar] [CrossRef]
- Kanwar, R.; Rathee, J.; Salunke, D.B.; Mehta, S.K. Green Nanotechnology-Driven Drug Delivery Assemblies. ACS Omega 2019, 4, 8804–8815. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Duan, S.-S.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial Effect of Silver Nanoparticles on Staphylococcus Aureus. BioMetals 2011, 24, 135–141. [Google Scholar] [CrossRef]
- Singh, S.; Arya, H.; Sahu, W.; Reddy, K.S.; Nimesh, S.; Alotaibi, B.S.; Hakami, M.A.; Almasoudi, H.H.; Hessien, K.B.G.; Hasan, M.R.; et al. Green Synthesized Silver Nanoparticles of Terminalia Bellirica Leaves Extract: Synthesis, Characterization, in-Silico Studies, and Antimalarial Activity. Artif. Cells Nanomed. Biotechnol. 2024, 52, 238–249. [Google Scholar] [CrossRef]
- Younas, M.; Rasool, M.H.; Khurshid, M.; Khan, A.; Nawaz, M.Z.; Ahmad, I.; Lakhan, M.N. Moringa oleifera Leaf Extract Mediated Green Synthesis of Silver Nanoparticles and Their Antibacterial Effect against Selected Gram-Negative Strains. Biochem. Syst. Ecol. 2023, 107, 104605. [Google Scholar] [CrossRef]
- Amabeoku, G.J.; Green, I.; Kabatende, J. Anticonvulsant Activity of Cotyledon orbiculata L. (Crassulaceae) Leaf Extract in Mice. J. Ethnopharmacol. 2007, 112, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Elbagory, A.M.; Hussein, A.A.; Meyer, M. The In Vitro Immunomodulatory Effects of Gold Nanoparticles Synthesized from Hypoxis hemerocallidea Aqueous Extract and Hypoxoside on Macrophage and Natural Killer Cells. Int. J. Nanomed. 2019, 14, 9007–9018. [Google Scholar] [CrossRef]
- Verma, A.; Gautam, S.P.; Bansal, K.K.; Prabhakar, N.; Rosenholm, J.M. Green Nanotechnology: Advancement in Phytoformulation Research. Medicines 2019, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Kang, S.G.; Lee, G.B.; Vinayagam, R.; Do, G.S.; Oh, S.Y.; Yang, S.J.; Kwon, J.B.; Singh, M. Anti-Inflammatory, Antioxidative, and Nitric Oxide-Scavenging Activities of a Quercetin Nanosuspension with Polyethylene Glycol in LPS-Induced RAW 264.7 Macrophages. Molecules 2022, 27, 7432. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, G.P.; Van Wyk, B.E.; Feng, Y.; Cock, I.E. A Review of the Traditional Use of Southern African Medicinal Plants for the Treatment of Inflammation and Inflammatory Pain. J. Ethnopharmacol. 2022, 283, 114436. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of Inflammation: A New Therapeutic Frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Abdul Khaliq, H.; Ortiz, S.; Alhouayek, M.; Muccioli, G.G.; Quetin-Leclercq, J. Dereplication and Quantification of Major Compounds of Convolvulus Arvensis L. Extracts and Assessment of Their Effect on LPS-Activated J774 Macrophages. Molecules 2022, 27, 963. [Google Scholar] [CrossRef]
- Wisastra, R.; Dekker, F.J. Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked. Cancers 2014, 6, 1500. [Google Scholar] [CrossRef]
- Adebayo, S.A.; Dzoyem, J.P.; Shai, L.J.; Eloff, J.N. The Anti-Inflammatory and Antioxidant Activity of 25 Plant Species Used Traditionally to Treat Pain in Southern African. BMC Complement. Altern. Med. 2015, 15, 159. [Google Scholar] [CrossRef]
- Yikna, B.B.; Yehualashet, A.S. Medicinal Plant Extracts Evaluated In Vitro and In Vivo for Antidiabetic Activities in Ethiopia: Bases for Future Clinical Trials and Related Investigations. Evid. Based Complement. Alternat. Med. 2021, 2021, e9108499. [Google Scholar] [CrossRef]
- Cherrada, N.; Elkhalifa Chemsa, A.; Gheraissa, N.; Zaater, A.; Benamor, B.; Ghania, A.; Yassine, B.; Kaddour, A.; Afzaal, M.; Asghar, A.; et al. Antidiabetic Medicinal Plants from the Chenopodiaceae Family: A Comprehensive Overview. Int. J. Food Prop. 2024, 27, 194–213. [Google Scholar] [CrossRef]
- Melesie Taye, G.; Bule, M.; Alemayehu Gadisa, D.; Teka, F.; Abula, T. In Vivo Antidiabetic Activity Evaluation of Aqueous and 80% Methanolic Extracts of Leaves of Thymus schimperi (Lamiaceae) in Alloxan-Induced Diabetic Mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Issa, I.A.; Hussen Bule, M. Hypoglycemic Effect of Aqueous and Methanolic Extract of Artemisia Afra on Alloxan Induced Diabetic Swiss Albino Mice. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 752486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kou, X.; Zhao, H.; Mak, K.-K.; Balijepalli, M.K.; Pichika, M.R. Zingiber Officinale Var. Rubrum: Red Ginger’s Medicinal Uses. Molecules 2022, 27, 775. [Google Scholar] [CrossRef]
- Piazza, S.; Martinelli, G.; Vrhovsek, U.; Masuero, D.; Fumagalli, M.; Magnavacca, A.; Pozzoli, C.; Canilli, L.; Terno, M.; Angarano, M.; et al. Anti-Inflammatory and Anti-Acne Effects of Hamamelis Virginiana Bark in Human Keratinocytes. Antioxidants 2022, 11, 1119. [Google Scholar] [CrossRef]
- Sinatra, R. Causes and Consequences of Inadequate Management of Acute Pain. Pain Med. 2010, 11, 1859–1871. [Google Scholar] [CrossRef]
- Cock, I.E.; Van Vuuren, S.F. The Traditional Use of Southern African Medicinal Plants for the Treatment of Bacterial Respiratory Diseases: A Review of the Ethnobotany and Scientific Evaluations. J. Ethnopharmacol. 2020, 263, 113204. [Google Scholar] [CrossRef]
- Teclegeorgish, Z.W.; Mokgalaka, N.S.; Vukea, N.; de la Mare, J.-A.; Tembu, V.J. Cytotoxicity of Triterpenoids from Clerodendrum glabrum against Triple Negative Breast Cancer Cells In Vitro. S. Afr. J. Bot. 2020, 133, 144–150. [Google Scholar] [CrossRef]
- Yadav, A.; Verma, P.K.; Chand, T.; Bora, H.R. Ethno-Medicinal Knowledge of Clerodendrum L. among Different Tribes of Nambor Reserve Forest, Assam, India. J. Pharmacogn. Phytochem. 2018, 7, 1567–1570. [Google Scholar]
- Rehman, G.; Umar, M.; Shah, N.; Hamayun, M.; Ali, A.; Khan, W.; Khan, A.; Ahmad, S.; Alrefaei, A.F.; Almutairi, M.H.; et al. Green Synthesis and Characterization of Silver Nanoparticles Using Azadirachta Indica Seeds Extract: In Vitro and In Vivo Evaluation of Anti-Diabetic Activity. Pharmaceuticals 2023, 16, 1677. [Google Scholar] [CrossRef] [PubMed]
- Asam Raza, M.; Farwa, U.; Waseem Mumtaz, M.; Kainat, J.; Sabir, A.; Al-Sehemi, A.G. Green Synthesis of Gold and Silver Nanoparticles as Antidiabetic and Anticancerous Agents. Green Chem. Lett. Rev. 2023, 16, 2275666. [Google Scholar] [CrossRef]
- Nayal, R.; Mejjo, D.; Abajy, M.Y. Anti Inflammatory Properties and Safety of Green Synthesized Metal and Metal Oxide Nanoparticles: A Review Article. Eur. J. Med. Chem. Rep. 2024, 11, 100169. [Google Scholar] [CrossRef]
- Rajkumar, G.; Panambara, P.A.H.R.; Sanmugarajah, V. Comparative Analysis of Qualitative and Quantitative Phytochemical Evaluation of Selected Leaves of Medicinal Plants in Jaffna, Sri Lanka. Borneo J. Pharm. 2022, 5, 93–103. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- AAT Bioquest Inc. IC50 Calculator. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 22 May 2025).
- Shayegan, N.; Haghipour, S.; Tanideh, N.; Moazzam, A.; Mojtabavi, S.; Faramarzi, M.A.; Irajie, C.; Parizad, S.; Ansari, S.; Larijani, B.; et al. Synthesis, In Vitro α-Glucosidase Inhibitory Activities, and Molecular Dynamic Simulations of Novel 4-Hydroxyquinolinone-Hydrazones as Potential Antidiabetic Agents. Sci. Rep. 2023, 13, 6304. [Google Scholar] [CrossRef]
- Wickramaratne, M.N.; Punchihewa, J.C.; Wickramaratne, D.B.M. In-Vitro Alpha Amylase Inhibitory Activity of the Leaf Extracts of Adenanthera Pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef]
- Bahta, T.; Karim, A.; Periasamy, G.; Gebremedhin, G.; Ur-Rehman, N.; Bitew, H.; Hagazi, K. Analgesic, Anti-Inflammatory and In-Vitro Hyaluronidase Inhibitory Properties of the Leaf Extract and Solvent Fractions of Otostegia fruticosa (Forssk.) Schweinf. Ex Penzig. Iran. J. Pharm. Res. IJPR 2020, 19, 218–230. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, S. In Vitro α-Amylase Inhibition and Antioxidant Activities of Methanolic Extract of Amaranthus Caudatus Linn. Oman Med. J. 2011, 26, 166–170. [Google Scholar] [CrossRef]
- Campêlo, L.M.L.; de Almeida, A.A.C.; de Freitas, R.L.M.; Cerqueira, G.S.; de Sousa, G.F.; Saldanha, G.B.; Feitosa, C.M.; de Freitas, R.M. Antioxidant and Antinociceptive Effects of Citrus Limon Essential Oil in Mice. J. Biomed. Biotechnol. 2011, 2011, 678673. [Google Scholar] [CrossRef]
- Jaast, S.; Grewal, A. Green Synthesis of Silver Nanoparticles, Characterization and Evaluation of Their Photocatalytic Dye Degradation Activity. Curr. Res. Green Sustain. Chem. 2021, 4, 100195. [Google Scholar] [CrossRef]
- Asefian, S.; Ghavam, M. Green and Environmentally Friendly Synthesis of Silver Nanoparticles with Antibacterial Properties from Some Medicinal Plants. BMC Biotechnol. 2024, 24, 5. [Google Scholar] [CrossRef]
- Khodadadi, B.; Bordbar, M.; Yeganeh-Faal, A.; Nasrollahzadeh, M. Green Synthesis of Ag Nanoparticles/Clinoptilolite Using Vaccinium Macrocarpon Fruit Extract and Its Excellent Catalytic Activity for Reduction of Organic Dyes. J. Alloys Compd. 2017, 719, 82–88. [Google Scholar] [CrossRef]
- Suárez-Cerda, J.; Alonso-Nuñez, G.; Espinoza-Gómez, H.; Flores-López, L.Z. Synthesis, Kinetics and Photocatalytic Study of “Ultra-Small” Ag-NPs Obtained by a Green Chemistry Method Using an Extract of Rosa ‘Andeli’ Double Delight Petals. J. Colloid Interface Sci. 2015, 458, 169–177. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, J.; Wei, J. Effect of Temperature on the Size of Biosynthesized Silver Nanoparticle: Deep Insight into Microscopic Kinetics Analysis. Arab. J. Chem. 2020, 13, 1011–1019. [Google Scholar] [CrossRef]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green Synthesis of Silver Nanoparticles: A Comprehensive Review of Methods, Influencing Factors, and Applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Cupido, C.N.; Meyer, M.; Hussein, A.A. Large Scale Screening of Southern African Plant Extracts for the Green Synthesis of Gold Nanoparticles Using Microtitre-Plate Method. Molecules 2016, 21, 1498. [Google Scholar] [CrossRef]
- Benakashani, F.; Allafchian, A.R.; Jalali, S.A.H. Biosynthesis of Silver Nanoparticles Using Capparis spinosa L. Leaf Extract and Their Antibacterial Activity. Karbala Int. J. Mod. Sci. 2016, 2, 251–258. [Google Scholar] [CrossRef]
- Dam, X.T.; Nguyen, X.T.; Nguyen, T.C.; Nguyen, T.D.; Duong, T.M.; Bui, T.D.; Thai, H. Bio-Synthesis of Silver Nanoparticles Decorated on Hydrotalcite Using Psidium Guajava Leaf Extract and Its Anti-Bacterial Effect. ChemistrySelect 2024, 9, e202303711. [Google Scholar] [CrossRef]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis Prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef]
- Matotoka, M.M.; Mashabela, G.T.; Masoko, P. Phytochemical Content, Antibacterial Activity, and Antioxidant, Anti-Inflammatory, and Cytotoxic Effects of Traditional Medicinal Plants against Respiratory Tract Bacterial Pathogens. Evid.-Based Complement. Altern. Med. ECAM 2023, 2023, 1243438. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, J.; Anjum, M.R.; Sankari, M.; Narasimha, G.; Krishna, S.B.N.; Kishori, B. Green Synthesis, Characterization and Biological Activities of Silver Nanoparticles Synthesized from Neolamarkia cadamba. Admet Dmpk 2023, 11, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, N.; Jahan, N.; Khalil-ur-Rahman; Anwar, T.; Qureshi, H. Green Synthesized Silver Nanoparticles: Optimization, Characterization, Antimicrobial Activity, and Cytotoxicity Study by Hemolysis Assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef]
- Lekkala, V.D.V.V.; Muktinutalapati, A.V.; Lebaka, V.R.; Lomada, D.; Korivi, M.; Li, W.; Reddy, M.C. Green Synthesis and Characterization of Silver Nanoparticles from Tinospora cordifolia Leaf Extract: Evaluation of Their Antioxidant, Anti-Inflammatory, Antibacterial, and Antibiofilm Efficacies. Nanomaterials 2025, 15, 381. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yıldıztekin, M.; Han, D.; Keskin, C.; Baran, A.; Baran, M.F.; Eftekhari, A.; Ava, C.A.; Kandemir, S.İ.; Cebe, D.B.; et al. Biosynthesis, Characterization, and Investigation of Antimicrobial and Cytotoxic Activities of Silver Nanoparticles Using Solanum tuberosum Peel Aqueous Extract. Heliyon 2023, 9, e19061. [Google Scholar] [CrossRef]
- Wasilewska, A.; Basa, A.; Zambrzycka, M.; Swiecicka, I.; Kalska-Szostko, B. Apple Extract as an Effective Silver Reducer in the Synthesis of Ag Nanoparticles. Nanomaterials 2025, 15, 595. [Google Scholar] [CrossRef]
- Ansar, S.; Tabassum, H.; Aladwan, N.S.M.; Naiman Ali, M.; Almaarik, B.; AlMahrouqi, S.; Abudawood, M.; Banu, N.; Alsubki, R. Eco Friendly Silver Nanoparticles Synthesis by Brassica Oleracea and Its Antibacterial, Anticancer and Antioxidant Properties. Sci. Rep. 2020, 10, 18564. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. African Flora Has the Potential to Fight Multidrug Resistance of Cancer. BioMed Res. Int. 2015, 2015, 914813. [Google Scholar] [CrossRef]
- Motene, M.V.; Maepa, C.; Sigidi, M.T. Optimizing the Antimicrobial, Antioxidant, and Cytotoxic Properties of Silver Nanoparticles Synthesized from Elephantorrhiza elephantina (Burch.) Extracts: A Comprehensive Study. Plants 2025, 14, 822. [Google Scholar] [CrossRef]
- Jini, D.; Sharmila, S.; Anitha, A.; Pandian, M.; Rajapaksha, R.M.H. In Vitro and in Silico Studies of Silver Nanoparticles (AgNPs) from Allium Sativum against Diabetes. Sci. Rep. 2022, 12, 22109. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Khan, H.A.; Ghufran, M.; Shams, S.; Jamal, A.; Khan, A.; Abdullah; Awan, Z.A.; Khan, M.I. Green Synthesis of Silver Nanoparticles from Plant Fagonia cretica and Evaluating Its Anti-Diabetic Activity through Indepth In-Vitro and In-Vivo Analysis. Front. Pharmacol. 2023, 14, 1194809. [Google Scholar] [CrossRef]
- Ogundajo, A.L.; Ashafa, A.O.T. Medicinal Properties of Clerodendrum glaburum E May Leaf Extracts: Phytochemical Constituents, Antioxidant, Cytotoxicity, and Carbohydrate-Metabolizing Enzyme Inhibitory Potentials. Comp. Clin. Pathol. 2019, 28, 927–936. [Google Scholar] [CrossRef]
- Abbigeri, M.B.; Thokchom, B.; Singh, S.R.; Bhavi, S.M.; Harini, B.P.; Yarajarla, R.B. Antioxidant and Anti-Diabetic Potential of the Green Synthesized Silver Nanoparticles Using Martynia annua L. Root Extract. Nano TransMed 2025, 4, 100070. [Google Scholar] [CrossRef]
- Anandachockalingam, A.; Shanmugam, R.; Ryntathiang, I.; Dharmalingam Jothinathan, M.K. Green Synthesis of Silver Nanoparticles Using Zingiber Officinale and Ocimum Gratissimum Formulation for Its Anti-Inflammatory and Antidiabetic Activity: An In Vitro Study. Cureus 2024, 16, e58098. [Google Scholar] [CrossRef] [PubMed]
- González-Garibay, A.S.; Sánchez-Hernández, I.M.; Torres-González, O.R.; Hernández-Aviña, A.D.S.; Villarreal-Amézquita, A.A.; Padilla-Camberos, E. Antidiabetic Activity of Silver Nanoparticles Biosynthesized with Stenocereus queretaroensis Flower Extract. Pharmaceuticals 2025, 18, 1310. [Google Scholar] [CrossRef]
- Okumus, E. Green Synthesis of Silver Nanoparticles Using Hebeloma Excedens Mushroom Extract as a New Source: Anti-Lipid Peroxidation, Bioaccessibility and Antidiabetic Properties. J. Food Meas. Charact. 2024, 18, 5157–5169. [Google Scholar] [CrossRef]
- Dilipan, E.; Sivaperumal, P.; Kamala, K.; Ramachandran, M.; Vivekanandhan, P. Green Synthesis of Silver Nanoparticles Using Seagrass Cymodocea serrulata (R.Br.) Asch. & Magnus, Characterization, and Evaluation of Anticancer, Antioxidant, and Antiglycemic Index. Biotechnol. Appl. Biochem. 2023, 70, 1346–1356. [Google Scholar] [CrossRef]
- Patil, S.; Rajiv, P.; Sivaraj, R. An investigation of antioxidant and cytotoxic properties of green synthesized silver nanoparticles. Indo Am. J. Pharm. Sci. 2015, 2, 1453–1459. [Google Scholar]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.-K.; Ravishankar Rai, V.; Ramachandra, Y.L. Characterization, Antibacterial, Antioxidant, Antidiabetic, Anti-Inflammatory and Antityrosinase Activity of Green Synthesized Silver Nanoparticles Using Calophyllum tomentosum Leaves Extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]





| Phytochemical Constituents | C. glabrum Extract | GC-Ag NPs |
|---|---|---|
| Terpenoids | + | + |
| Alkaloids | − | − |
| Steroids | + | + |
| Glycosides | + | − |
| Anthraquinones | − | − |
| Flavonoids | − | − |
| Saponins | + | + |
| Tannins | − | − |
| Phenols | + | − |
| Sample ID | Size (nm) | Zeta Potential (mV) | Polydispersity Index (PI) |
|---|---|---|---|
| CG-Ag 10 NP | 87.12 | −25.38 | 0.3088 |
| CG-Ag 40 NP | 148.7 | −21.22 | 0.2687 |
| C. glabrum Extract | CG-Ag 40 NPs | CG-Ag 10 NPs | Doxorubicin | |
|---|---|---|---|---|
| Estimated IC50 (µg/mL) | 139.85 | 173.34 | >200 | <1.56 |
| Plant Name | Size (nm) | Zeta Potential (mV) | α-Amylase (Concentration) | α-Glucosidase (Concentration) | Reference |
|---|---|---|---|---|---|
| C. glabrum | 148.7 | −21.22 | 93.32 (6.25 µg/mL) | 99.25 (0.95 µg/mL) | In this study |
| Azadirachta indica | ND | ND | 73.85% (100 µg/mL) | ND | [32] |
| Fagonia cretica | ND | ND | 83.53% (1000 μg/mL) | 81.74% (1000 μg/mL) | [64] |
| M. annua | 64 | −21.6 | 78.48 (250 µg/mL) | ND | [66] |
| Z. officinale and O. gratissimum | ND | ND | 78% (50 µg/mL) | 80% (50 µg/mL) | [67] |
| Stenocereus queretaroensis | 99.5 | −32.8 | 70.1% (10 μg/mL) | 99.8% (10 μg/mL) | [68] |
| Hebeloma excedens | 190.40 | −51.57 | 50% (7.08 mg/mL) | 50% (29.20 mg/mL) | [69] |
| Cymodocea serrulata (R.Br.) Asch. & Magnus | ND | ND | 57.31% (125 μg/mL) | 54.5% (125 μg/mL) | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhlongo, K.; Mangoato, I.; Matsabisa, M. Biosynthesis of Silver Nanoparticles Using Phytochemicals Extracted from Aqueous Clerodendrum glabrum for Anti-Diabetes and Anti-Inflammatory Activity: An In Vitro Study. Nanomaterials 2025, 15, 1560. https://doi.org/10.3390/nano15201560
Mhlongo K, Mangoato I, Matsabisa M. Biosynthesis of Silver Nanoparticles Using Phytochemicals Extracted from Aqueous Clerodendrum glabrum for Anti-Diabetes and Anti-Inflammatory Activity: An In Vitro Study. Nanomaterials. 2025; 15(20):1560. https://doi.org/10.3390/nano15201560
Chicago/Turabian StyleMhlongo, Kulani, Innocensia Mangoato, and Motlalepula Matsabisa. 2025. "Biosynthesis of Silver Nanoparticles Using Phytochemicals Extracted from Aqueous Clerodendrum glabrum for Anti-Diabetes and Anti-Inflammatory Activity: An In Vitro Study" Nanomaterials 15, no. 20: 1560. https://doi.org/10.3390/nano15201560
APA StyleMhlongo, K., Mangoato, I., & Matsabisa, M. (2025). Biosynthesis of Silver Nanoparticles Using Phytochemicals Extracted from Aqueous Clerodendrum glabrum for Anti-Diabetes and Anti-Inflammatory Activity: An In Vitro Study. Nanomaterials, 15(20), 1560. https://doi.org/10.3390/nano15201560






