Hair-Template Confinement Assembly of Nanomaterials Enables a Robust Single-Hair Surface-Enhanced Raman Spectrocopy Platform for Trace Analysis
Abstract
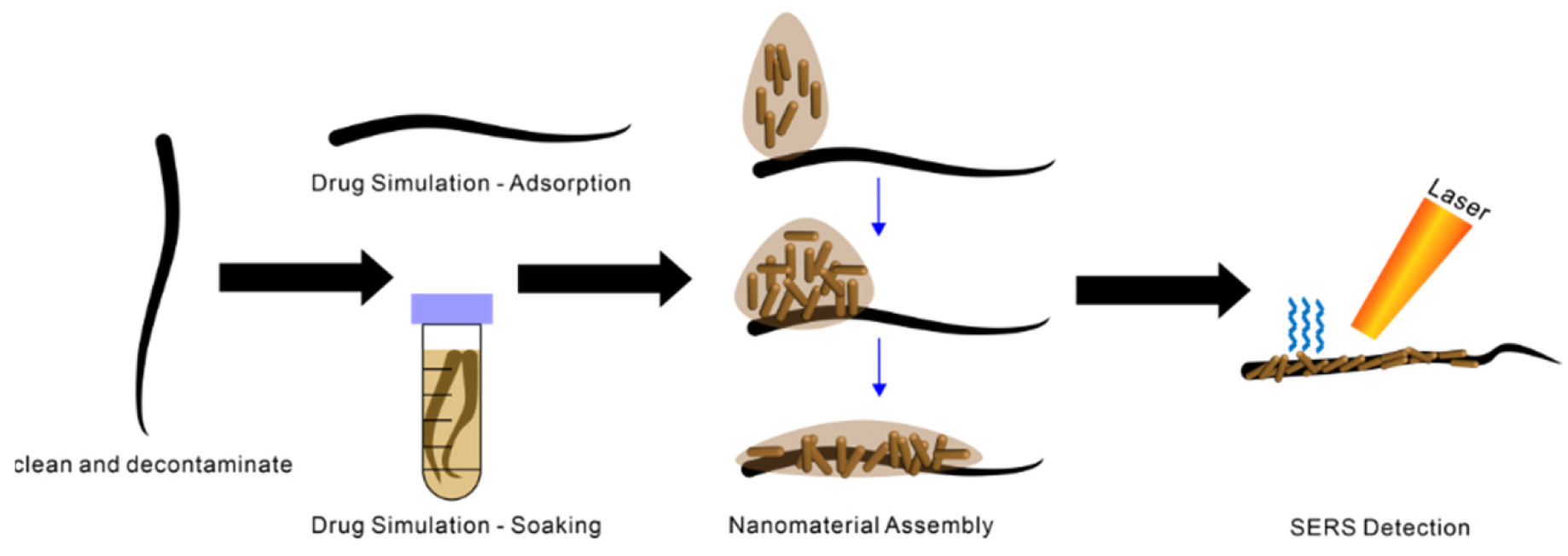
1. Introduction
2. Materials and Methods
2.1. Preparation of Noble Metal Nanomaterials
- Preparation of gold nanoparticle seeds: 103 μL of 1% HAuCl4 solution was mixed with 0.1 M CTAB under magnetic stirring at 29 °C until homogeneous. Then, 60 μL of freshly prepared 10 mM NaBH4 solution was rapidly added. Stirring was stopped after 2 min, and the solution was allowed to stand.
- Growth process: 206 μL of 1 w% HAuCl4 was mixed with 10 mL of 0.1 M CTAB, followed by sequential addition of 125 μL of 0.008 M AgNO3, 100 μL of 2 M HNO3, and 60 μL of 0.1 M AA at 29 °C. Finally, 12 μL of the seed solution from step (1) was added.
- Synthesis of CTAB-stabilized gold nanorods (CTAB-AuNR): The solution from step (2) was mixed thoroughly and left undisturbed in a 28 °C water bath for 12 h to obtain a gold nanorod colloid.
2.2. Instruments and Operation
2.3. Construction of the Visualized Single D-SERS Detection Platform
3. Results and Discussion
3.1. Morphology and Optical Properties of Nanomaterials on Single Hair Surface
3.1.1. UV-Vis Spectral Analysis and SEM Characterization
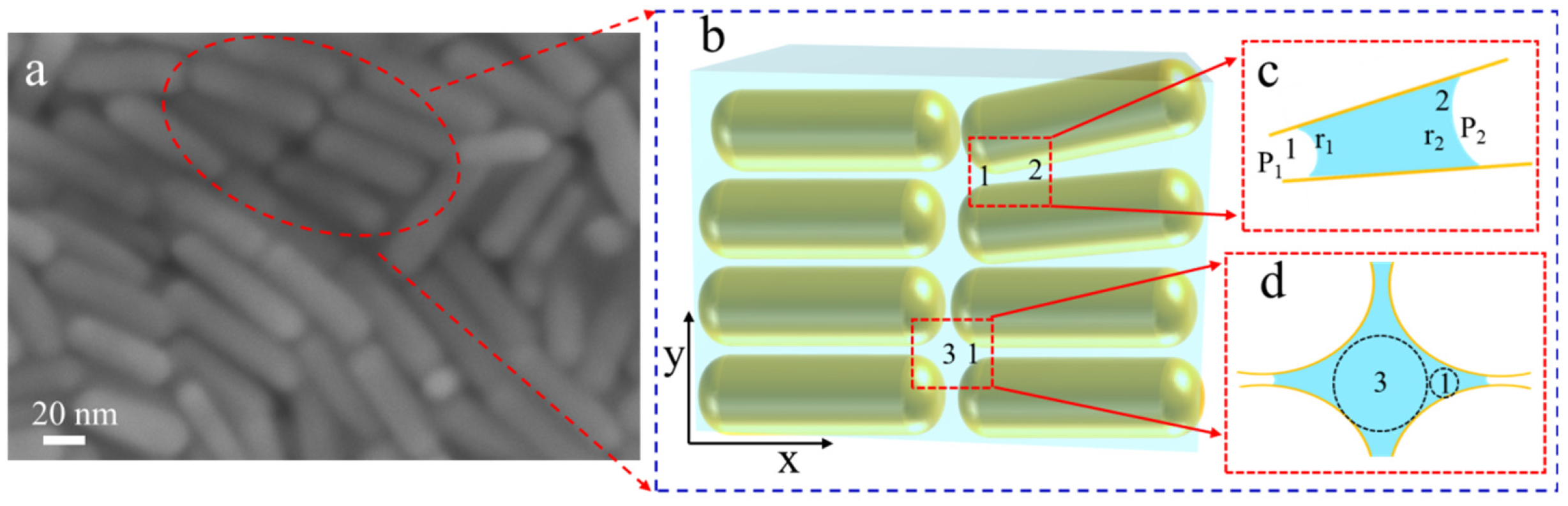
3.1.2. Mechanistic Investigation of the Confined Assembly of CTAB-AuNR on Single Hair Surface
3.2. Mechanistic Analysis of Active Entry of Target Molecules into Nanoparticle Gaps
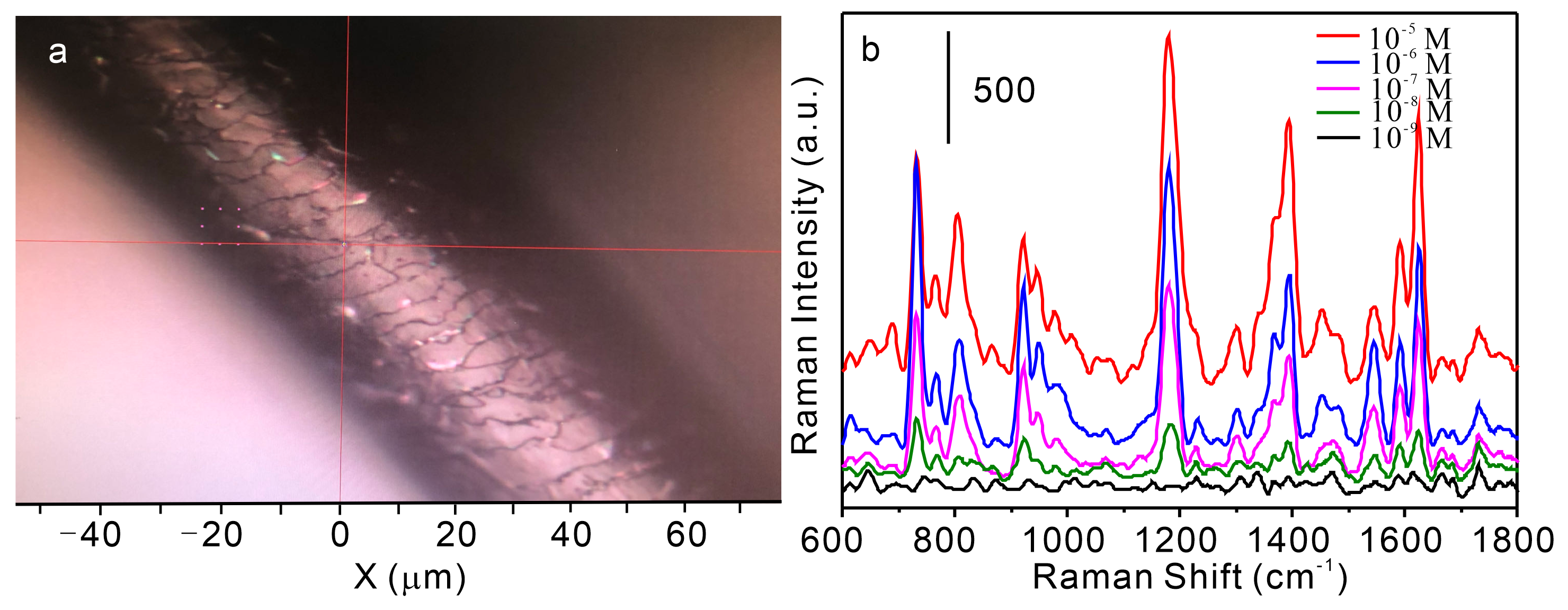
3.3. SERS Performance Characterization of the Single D-SERS Detection Platform
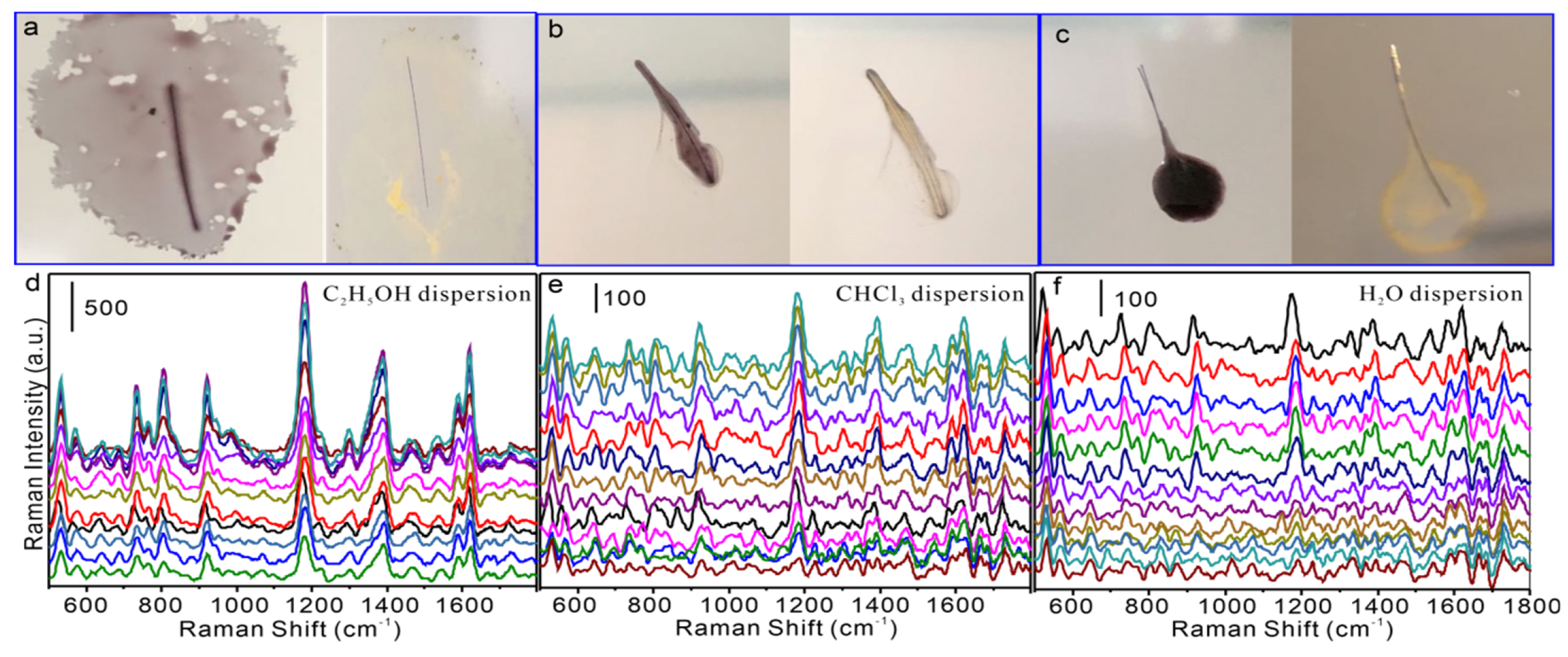
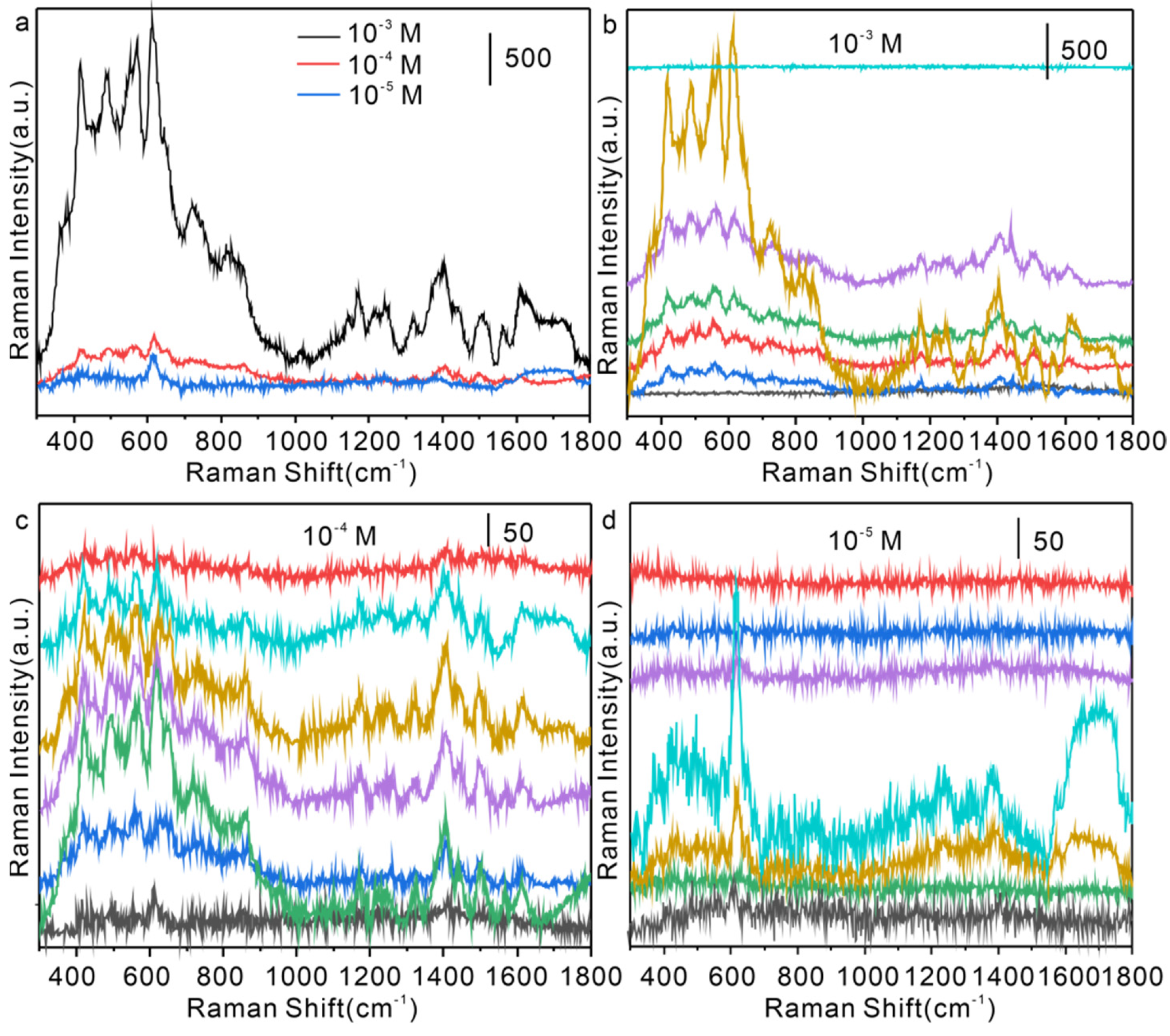
3.4. Application of Nanomaterials on Single Hair Surface for Real Sample Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NaBH4 | Sodium borohydride |
| NaOH | Sodium hydroxide |
| AgNO3 | Silver nitrate |
| HNO3 | Nitric acid |
| PVP | Polyvinylpyrrolidone |
| H2O2 | Hydrogen peroxide |
| AA | Ascorbic acid |
| PPD | p-Phenylenediamine |
| SERS | Surface-enhanced raman spectroscopy |
| D-SERS | Dynamic-SERS |
| LSPR | Localized surface plasmon resonance |
| UV-Vis | Ultraviolet-visible |
| SEM | Scanning electron microscopy |
| RSD | relative standard deviation |
References
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Huang, Z.; Peng, J.; Xu, L.; Liu, P. Development and Application of Surface-Enhanced Raman Scattering (SERS). Nanomaterials 2024, 14, 1417. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-Based Plasmon-Enhanced Raman Spectroscopy for Surface Analysis of Materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhao, Z.; Lian, K.; Yin, L.; Wang, J.; Man, S.; Liu, G.; Ma, L. SERS-Based CRISPR/Cas Assay on Microfluidic Paper Analytical Devices for Supersensitive Detection of Pathogenic Bacteria in Foods. Biosens. Bioelectron. 2022, 207, 114167. [Google Scholar] [CrossRef]
- Zhao, Q.; Hilal, H.; Kim, J.; Park, W.; Haddadnezhad, M.; Lee, J.; Park, W.; Lee, J.-W.; Lee, S.; Jung, I.; et al. All-Hot-Spot Bulk Surface-Enhanced Raman Scattering (SERS) Substrates: Attomolar Detection of Adsorbates with Designer Plasmonic Nanoparticles. J. Am. Chem. Soc. 2022, 144, 13285–13293. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Ji, C.; Tan, J.; Du, B.; Zhao, X.; Yu, J.; Man, B.; Xu, K.; Zhang, C.; Li, Z. Ferroelectrically Modulate the Fermi Level of Graphene Oxide to Enhance SERS Response. Opto-Electron. Adv. 2023, 6, 230094. [Google Scholar] [CrossRef]
- Kim, W.H.; Lee, J.U.; Jeon, M.J.; Park, K.H.; Sim, S.J. Three-Dimensional Hierarchical Plasmonic Nano-Architecture Based Label-Free Surface-Enhanced Raman Spectroscopy Detection of Urinary Exosomal miRNA for Clinical Diagnosis of Prostate Cancer. Biosens. Bioelectron. 2022, 205, 114116. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, H.; Xiang, Z.; Wu, L.; Jin, S. Biologically Inspired Superwetting Surface Enhanced Raman Scattering (SERS) Substrates. ACS Appl. Nano Mater. 2024, 7, 23337–23367. [Google Scholar] [CrossRef]
- Milliken, S.; Fraser, J.; Poirier, S.; Hulse, J.; Tay, L.-L. Self-Assembled Vertically Aligned Au Nanorod Arrays for Surface-Enhanced Raman Scattering (SERS) Detection of Cannabinol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 196, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.G.; Pant, U.; Lou-Franco, J.; Logan, N.; Cao, C. Directed Assembly of Au Nanostar@Ag Satellite Nanostructures for SERS-Based Sensing of Hg2+ Ions. ACS Appl. Nano Mater. 2023, 6, 10431–10440. [Google Scholar] [CrossRef]
- Xu, L.; Lu, Z.-M.; Zhang, X.-J.; Chai, L.-J.; Wang, S.-T.; Zhang, S.-Y.; Shen, C.-H.; Li, B.; Shi, J.-S.; Xu, Z.-H. Metabolic Activity Profiling of High-Temperature Daqu Microbiota Using Single-Cell Raman Spectroscopy and Deuterium Isotope Probing. Anal. Chem. 2025, 97, 18199–18207. [Google Scholar] [CrossRef]
- Zheng, Z.; Shi, M.; Xu, Y.; Liu, S.; Zhong, H.; Shou, Q.; Huang, J.; Luan, T.; Li, Z.; Xing, X. Light-Induced Dynamic Assembly of Gold Nanoparticles in a Lab-on-Fiber Platform for Surface-Enhanced Raman Scattering Detection. ACS Appl. Nano Mater. 2022, 5, 8005–8011. [Google Scholar] [CrossRef]
- González-Gómez, X.; Cambeiro-Pérez, N.; Martínez-Carballo, E.; Simal-Gándara, J. Screening of Organic Pollutants in Pet Hair Samples and the Significance of Environmental Factors. Sci. Total Environ. 2018, 625, 311–319. [Google Scholar] [CrossRef]
- Le, N.D.B.; Hou, S.; Tonga, G.Y.; Jerri, H.A.; Elci, S.G.; Mizuhara, T.; Normand, V.; Benczédi, D.; Vachet, R.W.; Rotello, V.M. Nanoparticle Probes for Quantifying Supramolecular Determinants of Biosurface Affinity. Part. Part. Syst. Charact. 2017, 34, 1700100. [Google Scholar] [CrossRef]
- Labarre, L.; Squillace, O.; Liu, Y.; Fryer, P.J.; Kaur, P.; Whitaker, S.; Marsh, J.M.; Zhang, Z.J. Hair Surface Interactions against Different Chemical Functional Groups as a Function of Environment and Hair Condition. Int. J. Cosmet. Sci. 2023, 45, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, L.; Ding, Y.; Liu, C.; Mao, Z.; Jiang, Q.; Chen, J.; Chen, F.; Cao, Y. Fabrication of Flexible Electrospinning Nano-Fiber Membrane for Detection of Respiratory Tract Transmission Virus Based on SERS. Talanta 2024, 266, 125127. [Google Scholar] [CrossRef]
- Esparza, I.; Wang, R.; Kurouski, D. Surface-Enhanced Raman Analysis of Underlaying Colorants on Redyed Hair. Anal. Chem. 2019, 91, 7313–7318. [Google Scholar] [CrossRef] [PubMed]
- Eastman, R.R.; Jursa, T.P.; Benedetti, C.; Lucchini, R.G.; Smith, D.R. Hair as a Biomarker of Environmental Manganese Exposure. Environ. Sci. Technol. 2013, 47, 130117145235002. [Google Scholar] [CrossRef]
- Hsu, Y.-T.; Chen, C.-H.; Hsu, J.-Y.; Chen, H.-W.; Liu, K.-K. Femtosecond Laser-Induced Au Nanostructure-Decorated with Plasmonic Nanomaterials for Sensitive SERS-Based Detection of Fentanyl. Talanta 2025, 284, 127264. [Google Scholar] [CrossRef] [PubMed]
- Kamata, T.; Shima, N.; Sasaki, K.; Matsuta, S.; Takei, S.; Katagi, M.; Miki, A.; Zaitsu, K.; Nakanishi, T.; Sato, T.; et al. Time-Course Mass Spectrometry Imaging for Depicting Drug Incorporation into Hair. Anal. Chem. 2015, 87, 5476–5481. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.; Fakhrullina, G.; Fakhrullin, R.; Lvov, Y. Self-Assembly of Clay Nanotubes on Hair Surface for Medical and Cosmetic Formulations. Nanoscale 2018, 10, 18205–18216. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Shang, J.; Liu, C.; Zhao, S.; Huang, C.; Bai, Y.; Li, Y. A Universal Replica Molding Strategy Based on Natural Bio-Templates for Fabrication of Robust Superhydrophobic Surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130879. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Cao, X.; Qin, M.; Li, P.; Zhou, B.; Tang, X.; Ge, M.; Yang, L.; Liu, J. Probing Catecholamine Neurotransmitters Based on Iron-Coordination Surface-Enhanced Resonance Raman Spectroscopy Label. Sens. Actuators B Chem. 2018, 268, 350–358. [Google Scholar] [CrossRef]
- Mitarai, N.; Nori, F. Wet Granular Materials. Adv. Phys. 2006, 55, 1–45. [Google Scholar] [CrossRef]
- Qin, M.; Ge, M.; Li, P.; Chen, S.; Huang, G.; Tong, X.; Han, W.; Ren, D.; He, Y.; Lin, D.; et al. Natural <3 Nm Interbedded Gaps to Trap Target Molecules and Provide an Enhanced Raman Spectroscopy Method. Adv. Opt. Mater. 2022, 10, 2200551. [Google Scholar] [CrossRef]
- Scatena, L.F.; Brown, M.G.; Richmond, G.L. Water at Hydrophobic Surfaces: Weak Hydrogen Bonding and Strong Orientation Effects. Science 2001, 292, 908–912. [Google Scholar] [CrossRef]
- Yu, Q.; Kong, X.; Chen, C.; Kang, C.; Meng, M.; Huang, S. Synthesis of Ag NPs Layer and Its Application as SERS Substrate in the Determination of P-Phenylenediamine. J. Solid State Electrochem. 2021, 25, 683–688. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, M.; Chen, S.; Xie, T.; Ma, M.; Wang, C. Hair-Template Confinement Assembly of Nanomaterials Enables a Robust Single-Hair Surface-Enhanced Raman Spectrocopy Platform for Trace Analysis. Nanomaterials 2025, 15, 1557. https://doi.org/10.3390/nano15201557
Qin M, Chen S, Xie T, Ma M, Wang C. Hair-Template Confinement Assembly of Nanomaterials Enables a Robust Single-Hair Surface-Enhanced Raman Spectrocopy Platform for Trace Analysis. Nanomaterials. 2025; 15(20):1557. https://doi.org/10.3390/nano15201557
Chicago/Turabian StyleQin, Miao, Siyu Chen, Tao Xie, Mingwen Ma, and Cong Wang. 2025. "Hair-Template Confinement Assembly of Nanomaterials Enables a Robust Single-Hair Surface-Enhanced Raman Spectrocopy Platform for Trace Analysis" Nanomaterials 15, no. 20: 1557. https://doi.org/10.3390/nano15201557
APA StyleQin, M., Chen, S., Xie, T., Ma, M., & Wang, C. (2025). Hair-Template Confinement Assembly of Nanomaterials Enables a Robust Single-Hair Surface-Enhanced Raman Spectrocopy Platform for Trace Analysis. Nanomaterials, 15(20), 1557. https://doi.org/10.3390/nano15201557



