Assessing the Chronic Environmental Risk of Graphene Oxide Using a Multimarker Approach Across Three Trophic Levels of the Aquatic Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Pristine GO Nanoparticles
2.2. Preparation of GO Stock Suspensions for the Ecotoxicity Assays
2.3. Ecotoxicity Testing
2.3.1. Aliivibrio Fischeri Cultures and Test Protocol
2.3.2. Algae and Cyanobacteria Cultures and Test Protocol
2.3.3. Daphnia Magna Cultures
2.3.4. Daphnia Magna Reproduction Test
2.3.5. Daphnia Magna Heart Rate
2.3.6. Daphnia Magna Feeding Activity
2.3.7. Daphnia Magna Body Length
2.4. Data Evaluation and Statistical Analysis
3. Results and Discussion
3.1. Results of the A. fischeri Bioluminescence Inhibition Test
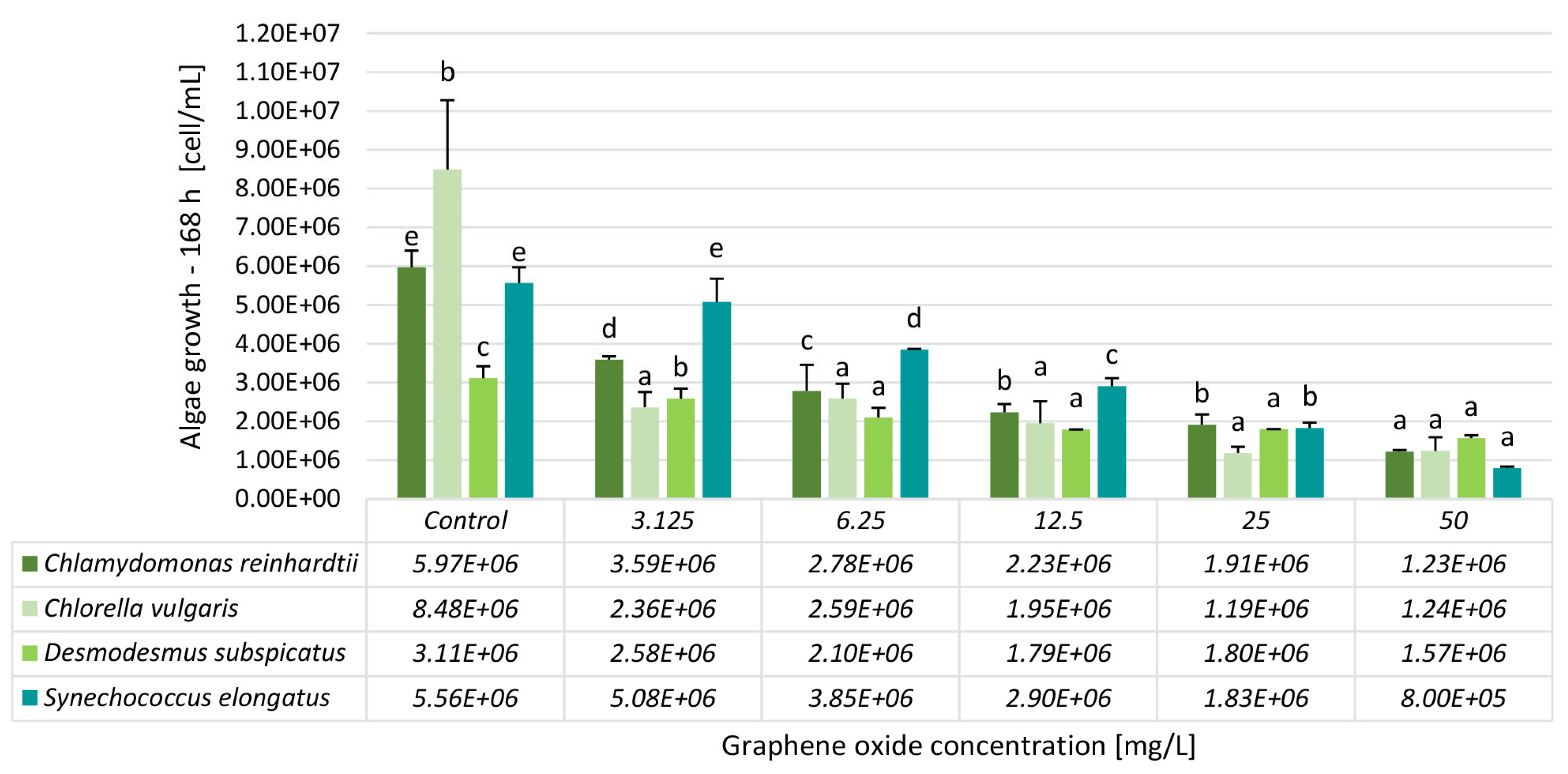
3.2. Results of the Algae and Cyanobacteria Growth Inhibition Tests
3.3. Results of the D. magna Heart Rate Test
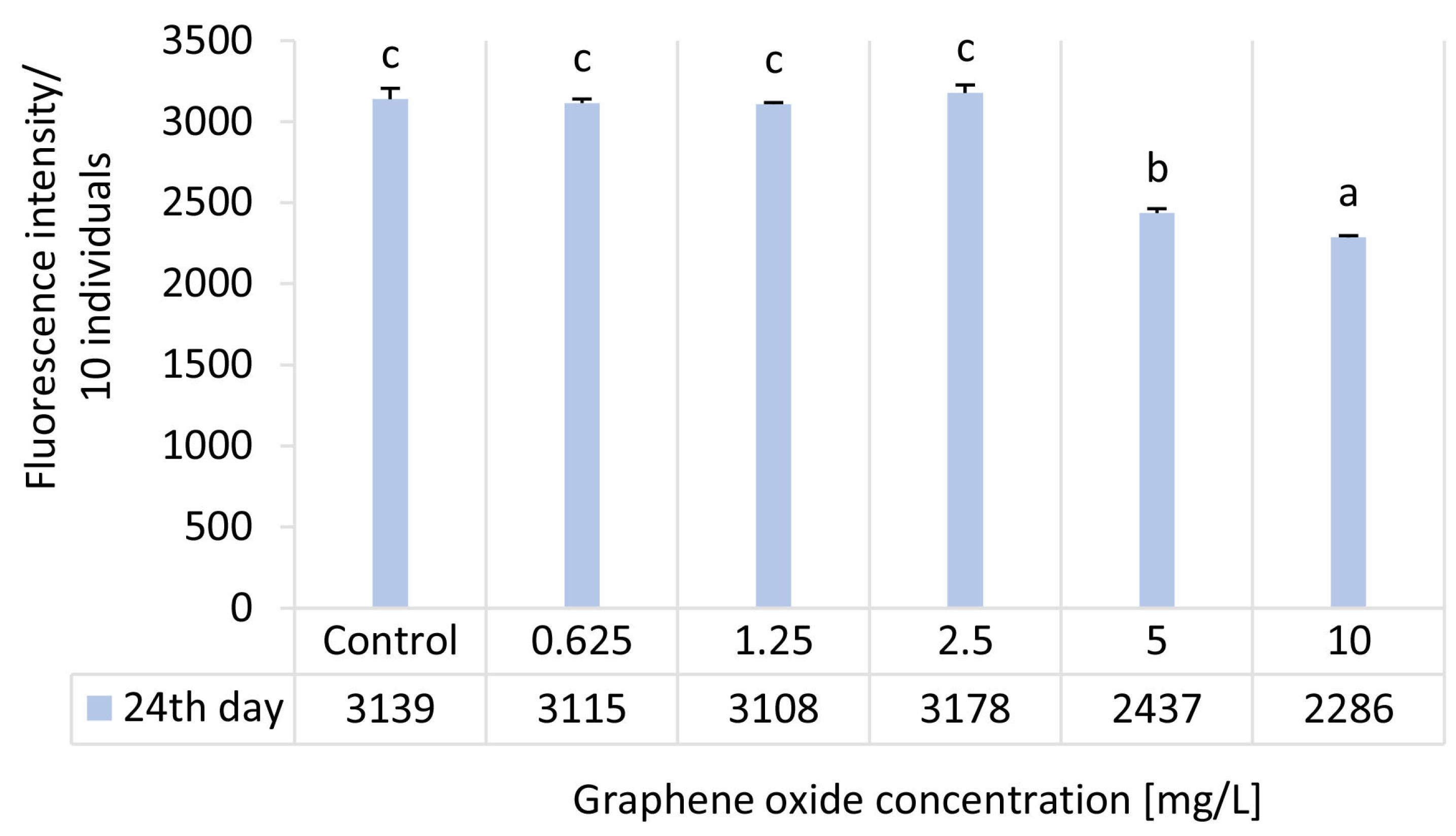
3.4. Results of the D. magna Feeding Activity Test
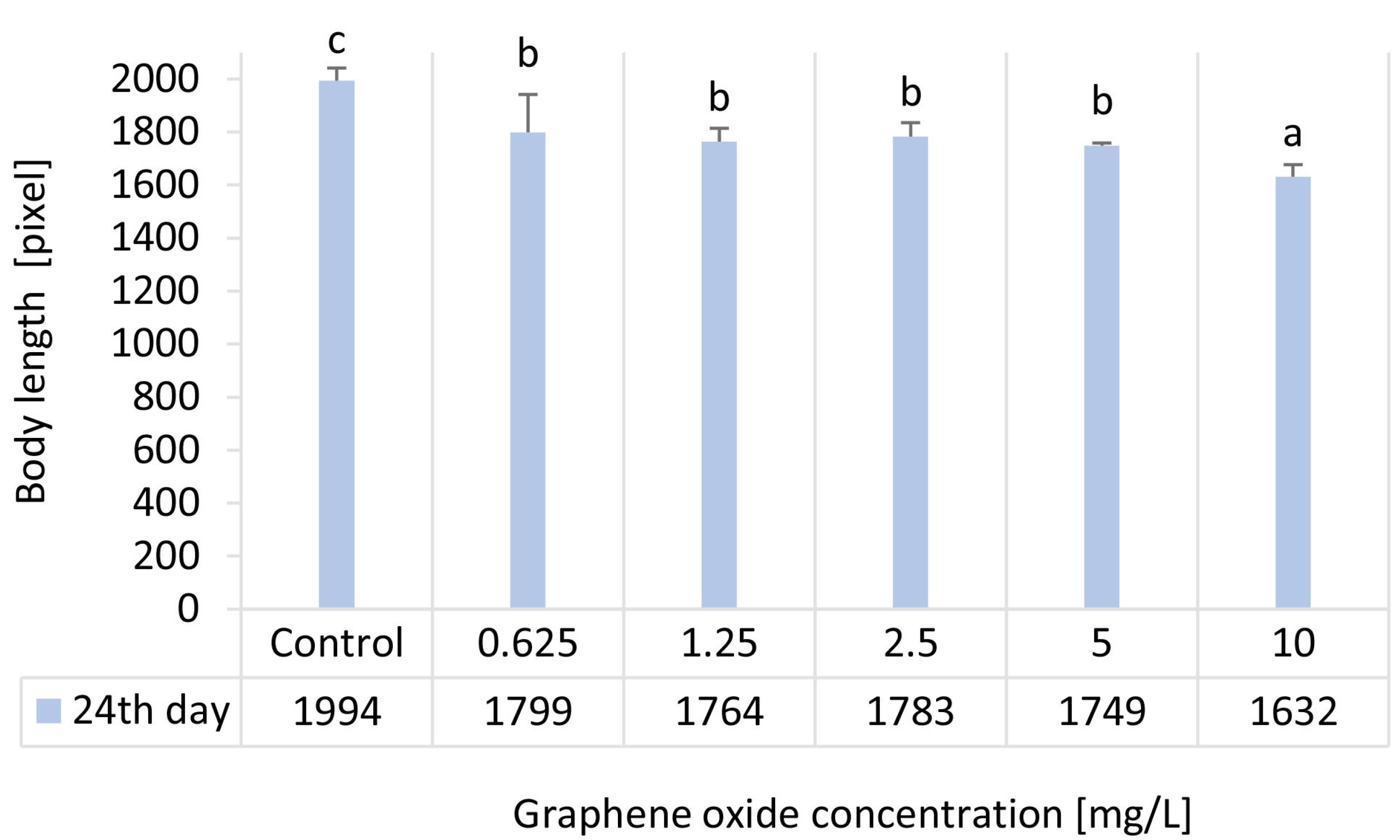
3.5. Results of Body Length Determination of D. magna
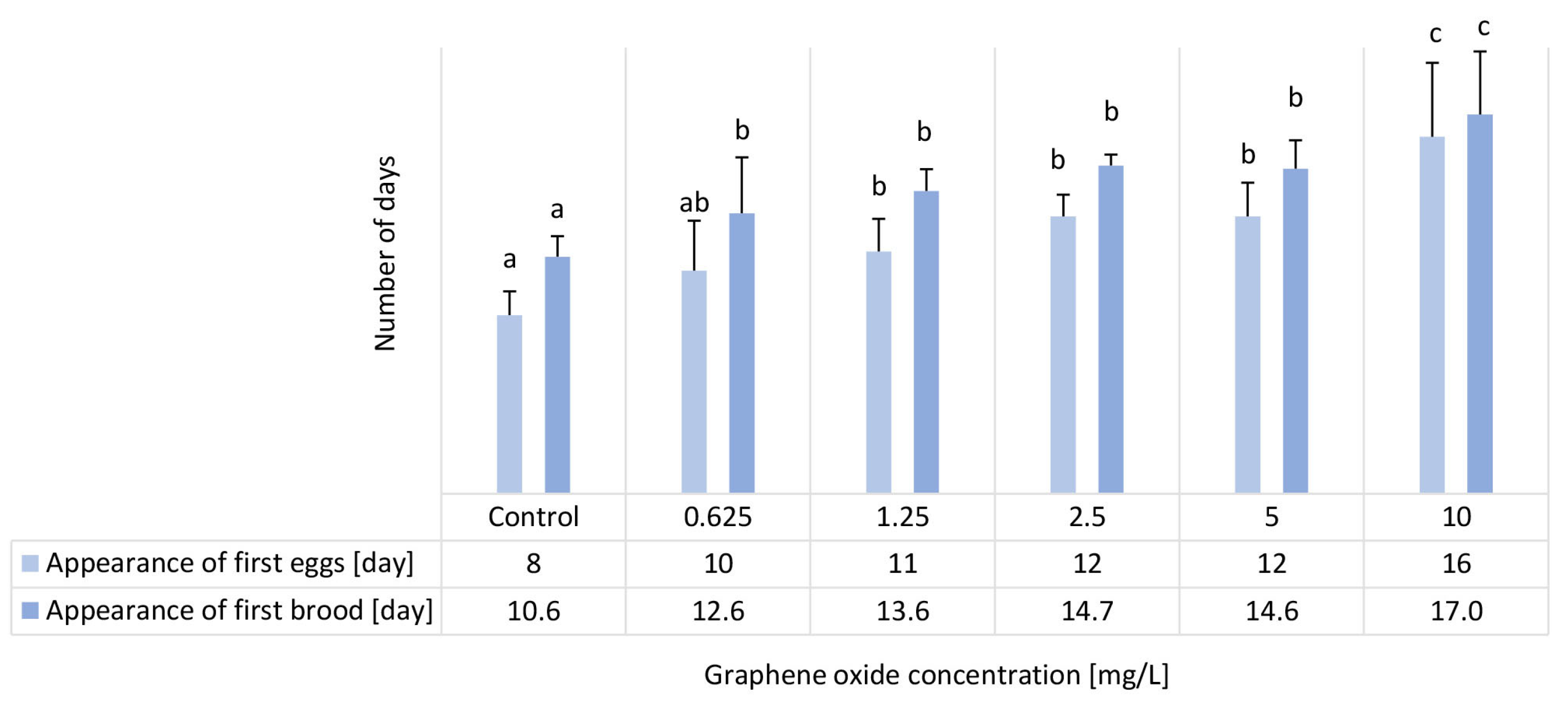
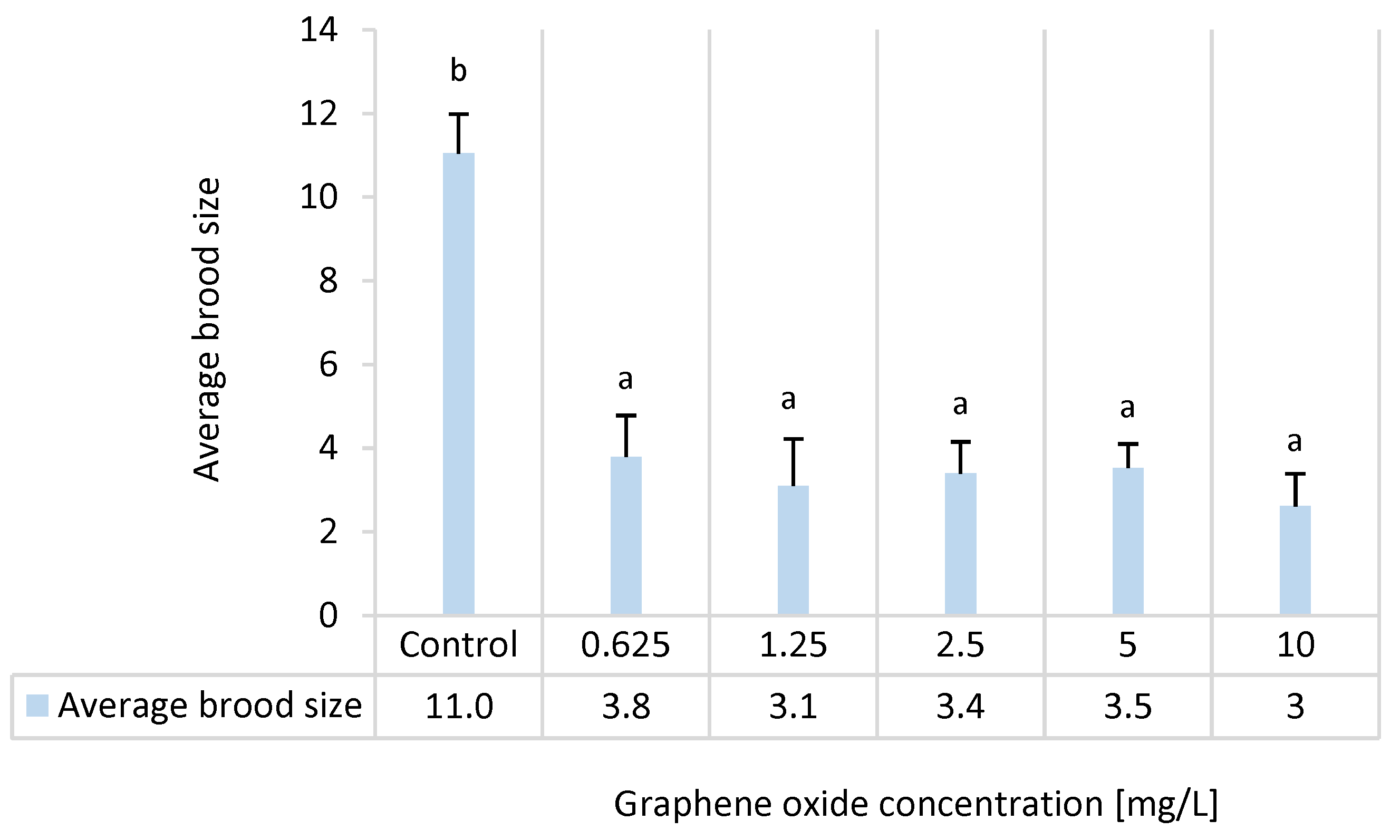
3.6. Results of the D. magna Reproduction Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | Effective Concentration |
| GO | Graphene Oxide |
| LOEC | Lowest Observed Effect Concentration |
| NOEC | No Observed Effect Concentration |
| PEC | Predicted Environmental Concentration |
| PNEC | Predicted No Effect Concentration |
| RCR | Risk Characterisation Ratio |
References
- Novoselov, K.S.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Malhotra, N.; Villaflores, O.B.; Audira, G.; Siregar, P.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. Toxicity studies on graphene-based nanomaterials in aquatic organisms: Current understanding. Molecules 2020, 25, 3618. [Google Scholar] [CrossRef]
- Németh, I.; Vaszita, E.; Molnár, M. Influence of nZnO on Enzyme-Mediated PAH-Removal from Contaminated Soil. Period. Polytech. Chem. Eng. 2025, 69, 361–375. [Google Scholar] [CrossRef]
- Fekete-Kertész, I.; László, K.; Molnár, M. Towards understanding the factors behind the limited integration of multispecies ecotoxicity assessment in environmental risk characterisation of graphene-family materials—A bibliometric review. C 2023, 9, 90. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Olszyna, A.R. The ecotoxicity of graphene family materials: Current status, knowledge gaps and future needs. J. Nanopart. Res. 2015, 17, 40. [Google Scholar] [CrossRef]
- Montagner, A.; Bosi, S.; Tenori, E.; Bidussi, M.; Alshatwi, A.A.; Tretiach, M.; Prato, M.; Syrgiannis, Z. Ecotoxicological effects of graphene-based materials. 2D Mater. 2016, 4, 12001. [Google Scholar] [CrossRef]
- Freixa, A.; Acuña, V.; Sanchís, J.; Farré, M.; Barceló, D.; Sabater, S. Ecotoxicological effects of carbon based nanomaterials in aquatic organisms. Sci. Total Environ. 2018, 619–620, 328–337. [Google Scholar] [CrossRef]
- Pretti, C.; Oliva, M.; Di Pietro, R.; Monni, G.; Cevasco, G.; Chiellini, F.; Pomelli, C.; Chiappe, C. Ecotoxicity of pristine graphene to marine organisms. Ecotoxicol. Environ. Saf. 2014, 101, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Domi, B.; Rumbo, C.; García-Tojal, J.; Elena Sima, L.; Negroiu, G.; Tamayo-Ramos, J.A. Interaction analysis of commercial graphene oxide nanoparticles with unicellular systems and biomolecules. Int. J. Mol. Sci. 2020, 21, 205. [Google Scholar] [CrossRef]
- Bergua, J.F.; Álvarez-Diduk, R.; Hu, L.; Hassan, A.H.; Merkoçi, A. Improved Aliivibrio fischeri based-toxicity assay: Graphene-oxide as a sensitivity booster with a mobile-phone application. J. Hazard. Mater. 2021, 406, 124434. [Google Scholar] [CrossRef]
- Németh, I.; László, K.; Bulátkó, A.; Vaszita, E.; Molnár, M. Ecotoxicity Assessment of Graphene Oxides Using Test Organisms from Three Hierarchical Trophic Levels to Evaluate Their Potential Environmental Risk. Nanomaterials 2023, 13, 2858. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, X.; Wang, Z.; Dai, Y.; Xing, B. Mechanistic understanding toward the toxicity of graphene-family materials to freshwater algae. Water Res. 2017, 111, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Singh Raman, A.P.; Meena, H.; Goswami, A.G.; Bhawna; Kumar, V.; Jain, P.; Kumar, G.; Sagar, M.; Rana, D.K.; et al. An Update on Graphene Oxide: Applications and Toxicity. ACS Omega 2022, 7, 34869–34888. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, L.; Cheng, S.; Zhu, L.; Liu, L.; Wen, P.; Zhou, L.; Xue, W.; Lu, S.; Zhang, W.; et al. An Overview of Light-Mediated Impact of Graphene Oxide on Algae: Photo-Transform, Toxicity and Mechanism. Water 2022, 14, 2997. [Google Scholar] [CrossRef]
- Huang, Y.; Yao, H.; Li, X.; Li, F.; Wang, X.; Fu, Z.; Li, N.; Chen, J. Differences of functionalized graphene materials on inducing chronic aquatic toxicity through the regulation of DNA damage, metabolism and oxidative stress in Daphnia magna. Sci. Total Environ. 2023, 876, 162735. [Google Scholar] [CrossRef] [PubMed]
- Gyarmati, B.; Farah, S.; Farkas, A.; Sáfrán, G.; László, K. Long-Term Aging of Concentrated Aqueous Graphene Oxide Suspension Seen by Rheology and Raman Spectroscopy. Nanomaterials 2022, 12, 916. [Google Scholar] [CrossRef]
- Bold, H.C. The morphology of Chlamydomonas chlamydogama, sp. nov. Bull. Torrey Bot. Club 1949, 76, 101–108. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.; Herdman, M.; Stanier, R. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- OECD. OECD Guideline for Testing Chemicals 201. Freshwater Alga and Cyanobacterial, Growth Inhibition Test; OECD Publisher: Paris, France, 2011. [Google Scholar]
- OECD. OECD Guideline for Testing Chemicals 202. Daphnia magna Acute Immobilization Test; OECD Publisher: Paris, France, 2004. [Google Scholar]
- OECD. OECD Guideline for Testing Chemicals 211. Daphnia magna Reproduction Test; OECD Publisher: Paris, France, 2012. [Google Scholar]
- Fekete-Kertész, I.; László, K.; Terebesi, C.; Gyarmati, B.S.; Farah, S.; Márton, R.; Molnár, M. Ecotoxicity assessment of graphene oxide by Daphnia magna through a multimarker approach from the molecular to the physiological level including behavioral changes. Nanomaterials 2020, 10, 2048. [Google Scholar] [CrossRef]
- Kamaya, M.; Sonamoto, M.; Nagashima, K.; Ginatullina, E.N. A simple bioassay using fluorescent microbeads and Daphnia magna. J. Environ. Sci. Eng. 2011, 5, 1613–1616. [Google Scholar]
- Jia, P.P.; Sun, T.; Junaid, M.; Yang, L.; Ma, Y.B.; Cui, Z.S.; Wei, D.P.; Shi, H.F.; Pei, D.S. Nanotoxicity of Different Sizes of Graphene (G) and Graphene Oxide (GO) In Vitro and In Vivo. Environ. Pollut. 2019, 247, 595–606. [Google Scholar] [CrossRef]
- Yin, J.; Fan, W.; Du, J.; Li, X.; Li, Y.; Qu, Y. The toxicity of graphene oxide affected by algal physiological characteristics: A comparative study in cyanobacteria, green algae, and diatoms. Environ. Pollut. 2020, 260, 113847. [Google Scholar] [CrossRef]
- Malina, T.; Maršálková, E.; Holá, K.; Tuček, J.; Scheibe, M.; Zbořil, R.; Maršálek, B. Toxicity of Graphene Oxide against Algae and Cyanobacteria: Nanoblade-Morphology-Induced Mechanical Injury and Self-Protection Mechanism. Carbon 2019, 155, 386–396. [Google Scholar] [CrossRef]
- Cruces, E.; Barrios, A.C.; Cahue, Y.P.; Januszewski, B.; Gilbertson, L.M.; Perreault, F. Similar toxicity mechanisms between graphene oxide and oxidized multi-walled carbon nanotubes in Microcystis aeruginosa. Chemosphere 2021, 265, 129137. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, T.; Shi, L.; Li, X.; Wang, P. The effects of humic acid on the toxicity of graphene oxide to Scenedesmus obliquus and Daphnia magna. Sci. Total Environ. 2019, 649, 163–171. [Google Scholar] [CrossRef]
- Schmuck, J.; Rondan, W.; Reno, U.; Vasquez, J.; Regaldo, L.; Gagneten, A.M.; Champi, A. Lyophilized and sonicated graphene oxide and its nanoecotoxicity applications. Diam. Relat. Mater. 2024, 145, 111145. [Google Scholar] [CrossRef]
- Villegas-Navarro, A.; Rosas-L, E.; Reyes, J.L. The heart of Daphnia magna: Effects of four cardioactive drugs. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 127–134. [Google Scholar] [CrossRef]
- Fekete-Kertész, I.; Kunglné-Nagy, Z.; Molnár, M. Ecological impact of micropollutants on aquatic life determined by an innovative sublethal endpoint, Daphnia magna heartbeat rate. Carpathian J. Earth Environ. Sci. 2016, 11, 345–354. [Google Scholar]
- Bownik, A.; Pawłocik, M.; Sokołowska, N. Effects of neonicotinoid insecticide acetamiprid on swimming velocity, heart rate and thoracic limb movement of Daphnia magna. Pol. J. Nat. Sci. 2017, 32, 481–493. [Google Scholar]
- Tkaczyk, A.; Bownik, A.; Dudka, J.; Kowal, K.; Ślaska, B. Daphnia magna model in the toxicity assessment of pharmaceuticals: A review. Sci. Total Environ. 2021, 763, 143038. [Google Scholar] [CrossRef] [PubMed]
- Saputra, F.; Suryanto, M.E.; Audira, G.; Luong, C.T.; Hung, C.H.; Roldan, M.J.; Vasquez, R.D.; Hsiao, C.D. Using DeepLabCut for markerless cardiac physiology and toxicity estimation in water fleas (Daphnia magna). Aquat. Toxicol. 2023, 263, 106676. [Google Scholar] [CrossRef]
- Chung, W.; Song, J.M.; Lee, J. The evaluation of titanium dioxide nanoparticle effects on cardiac and swimming performance of Daphnia magna. Int. J. Appl. Environ. Sci. 2016, 11, 1375–1385. [Google Scholar]
- Kim, Y.; Samadi, A.; Gwag, E.H.; Park, J.; Kwak, M.; Park, J.; Lee, T.G.; Kim, Y.J. Physiological and behavioral effects of SiO2 nanoparticle ingestion on Daphnia magna. Micromachines 2021, 12, 1105. [Google Scholar] [CrossRef]
- Park, J.; Park, C.; Lee, Y.; Ryu, C.; Park, J.; Kim, Y. Acute adverse effects of metallic nanomaterials on cardiac and behavioral changes in Daphnia magna. Environments 2022, 9, 26. [Google Scholar] [CrossRef]
- Lovern, S.B.; Strickler, J.R.; Klaper, R. Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C60, and C60HxC70Hx). Environ. Sci. Technol. 2007, 41, 4465–4470. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.H.; Kim, I.Y.; Heo, M.B.; Park, J.W.; Lee, S.W.; Lee, T.G. Real-time heart rate monitoring system for cardiotoxicity assessment of Daphnia magna using high-speed digital holographic microscopy. Sci. Total Environ. 2021, 780, 146405. [Google Scholar] [CrossRef] [PubMed]
- Luo, N. Investigating the acute cardiac restoration for rescuing Daphnia magna stressed from environmental disturbances using antioxidants. J. Glob. Ecol. Environ. 2023, 19, 40–51. [Google Scholar] [CrossRef]
- Guo, X.K.; Dong, S.P.; Petersen, E.J.; Gao, S.X.; Huang, Q.G.; Mao, L. Biological uptake and depuration of radio-labeled graphene by Daphnia magna. Environ. Sci. Technol. 2013, 47, 12524–12531. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, W.; Xu, Z.; Peng, W.; Luo, S. Transgenerational effects of reduced graphene oxide modified by Au, Ag, Pd, Fe3O4, Co3O4 and SnO2 on two generations of Daphnia magna. Carbon 2017, 122, 669–679. [Google Scholar] [CrossRef]
- Liu, Y.; Han, W.; Xu, Z.; Fan, W.; Peng, W.; Luo, S. Comparative toxicity of pristine graphene oxide and its carboxyl, imidazole or polyethylene glycol functionalized products to Daphnia magna: A two generation study. Environ. Pollut. 2018, 237, 218–227. [Google Scholar] [CrossRef]
- Loureiro, S.; Gonçalves, S.F.; Gonçalves, G.; Hortiguela, M.J.; Rebelo, S.; Ferro, M.C.; Vila, M. Eco-friendly profile of pegylated nano-graphene oxide at different levels of an aquatic trophic chain. Ecotoxicol. Environ. Saf. 2018, 162, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yang, Y.; Tao, Y.; Jiang, Y.; Chen, B.; Zhu, X.; Li, B. A mechanism study on toxicity of graphene oxide to Daphnia magna: Direct link between bioaccumulation and oxidative stress. Environ. Pollut. 2018, 234, 953–959. [Google Scholar] [CrossRef]
- Cano, A.M.; Maul, J.D.; Saed, M.; Shah, S.A.; Green, M.J.; Cañas-Carrell, J.E. Bioaccumulation, stress, and swimming impairment in Daphnia magna exposed to multiwalled carbon nanotubes, graphene, and graphene oxide. Environ. Toxicol. Chem. 2017, 36, 2199–2204. [Google Scholar] [CrossRef]
- Kirk, K.L. Suspended clay reduces Daphnia feeding rate: Behavioural mechanisms. Freshw. Biol. 1991, 25, 357–365. [Google Scholar] [CrossRef]
- Souza, J.P.; Venturini, F.P.; Santos, F.; Zucolotto, V. Chronic Toxicity in Ceriodaphnia dubia Induced by Graphene Oxide. Chemosphere 2018, 190, 218–224. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances, Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances, Directive 98/8/EC of the European Parliament and of the Council; Publications Office of the European Union: Luxembourg, 2003; Available online: https://op.europa.eu/en/publication-detail/-/publication/9aebb292-39c5-4b9c-b4cb-97fb02d9bea2/language-en (accessed on 31 August 2025).
- Hong, H.; Nowack, B. Form-Specific Prospective Environmental Risk Assessment of Graphene-Based Materials in European Freshwater. Environ. Sci. Technol. 2024, 58, 21750–21759. [Google Scholar] [CrossRef] [PubMed]
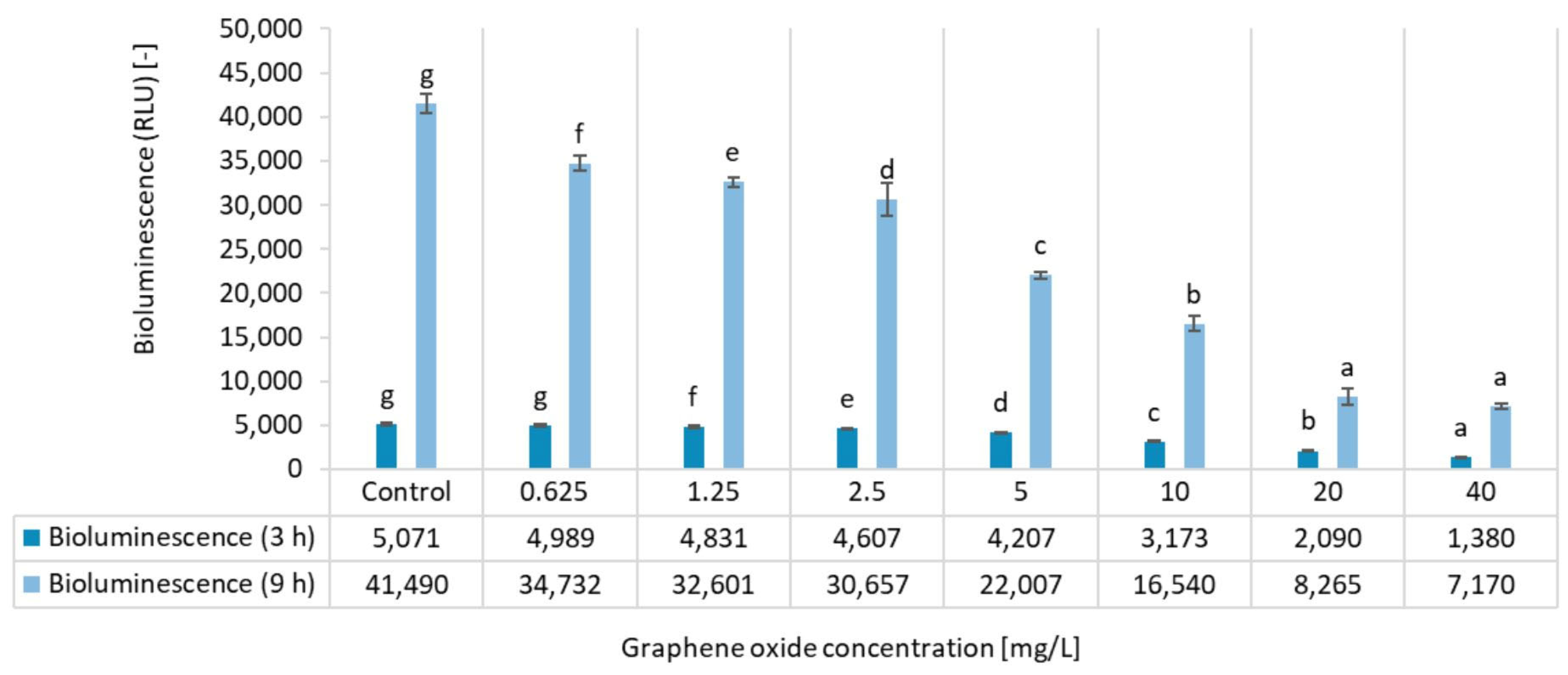


| Inhibition [%] | ||
|---|---|---|
| Concentration [mg/L] | 3 h of Exposure | 9 h of Exposure |
| 0.625 | 2 ± 2 | 16 ± 2 |
| 1.25 | 5 ± 2 | 21 ± 1 |
| 2.5 | 9 ± 2 | 26 ± 4 |
| 5 | 17 ± 1 | 47 ± 1 |
| 10 | 37 ± 2 | 60 ± 2 |
| 20 | 59 ± 1 | 80 ± 2 |
| 40 | 73 ± 2 | 83 ± 1 |
| EC20 | 5.36 ± 0.27 | 1.26 ± 0.06 |
| EC50 | 14.92 ± 0.75 | 6.05 ± 0.30 |
| LOEC | 1.25 | 0.625 |
| Inhibition [%]—168 h | ||||
|---|---|---|---|---|
| Conc. [mg/L] | Chlamydomonas reinhardtii | Chlorella vulgaris | Desmodesmus subspicatus | Synechococcus elongatus |
| 3.125 | 40 ± 1 | 72 ± 5 | 17 ± 2 | 9 ± 1 |
| 6.25 | 53 ± 13 | 70 ± 5 | 33 ± 4 | 31 ± 0 |
| 12.5 | 63 ± 6 | 77 ± 7 | 42 ± 0 | 48 ± 4 |
| 25 | 68 ± 9 | 86 ± 2 | 42 ± 0 | 67 ± 5 |
| 50 | 79 ± 2 | 85 ± 4 | 50 ± 2 | 86 ± 4 |
| EC20 | 1.38 ± 0.09 | 0.7 ± 0.25 | 3.65 ± 0.06 | 5.34 ± 0.15 |
| EC50 | 5.82 ± 0.36 | 2.52 ± 0.28 | 50 ± 0.8 | 14.79 ± 0.41 |
| Ecotoxicity Test Endpoint | Inhibition [%]—24 d | ||||
|---|---|---|---|---|---|
| Graphene Oxide Concentration [mg/L] | |||||
| 0.625 | 1.25 | 2.5 | 5 | 10 | |
| Heart rate | 21.4 ± 11.2 | 22 ± 5.4 | 21.3 ± 7.3 | 19.4 ± 5.4 | 26.9 ± 3.0 |
| Feeding activity | 0.8 ± 0.8 | 1 ± 0.3 | −1.2 ± 1.6 | 22.4 ± 0.9 | 27.2 ± 0.3 |
| Body length | 12.9 ± 4.2 | 12.1 ± 2.8 | 10.6 ± 2.6 | 12.3 ± 0.5 | 18.2 ± 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekete-Kertész, I.; László, K.; Bulátkó, A.; Gyarmati, B.; Molnár, Z.; Molnár, M. Assessing the Chronic Environmental Risk of Graphene Oxide Using a Multimarker Approach Across Three Trophic Levels of the Aquatic Ecosystem. Nanomaterials 2025, 15, 1553. https://doi.org/10.3390/nano15201553
Fekete-Kertész I, László K, Bulátkó A, Gyarmati B, Molnár Z, Molnár M. Assessing the Chronic Environmental Risk of Graphene Oxide Using a Multimarker Approach Across Three Trophic Levels of the Aquatic Ecosystem. Nanomaterials. 2025; 15(20):1553. https://doi.org/10.3390/nano15201553
Chicago/Turabian StyleFekete-Kertész, Ildikó, Krisztina László, Anna Bulátkó, Benjámin Gyarmati, Zoltán Molnár, and Mónika Molnár. 2025. "Assessing the Chronic Environmental Risk of Graphene Oxide Using a Multimarker Approach Across Three Trophic Levels of the Aquatic Ecosystem" Nanomaterials 15, no. 20: 1553. https://doi.org/10.3390/nano15201553
APA StyleFekete-Kertész, I., László, K., Bulátkó, A., Gyarmati, B., Molnár, Z., & Molnár, M. (2025). Assessing the Chronic Environmental Risk of Graphene Oxide Using a Multimarker Approach Across Three Trophic Levels of the Aquatic Ecosystem. Nanomaterials, 15(20), 1553. https://doi.org/10.3390/nano15201553








