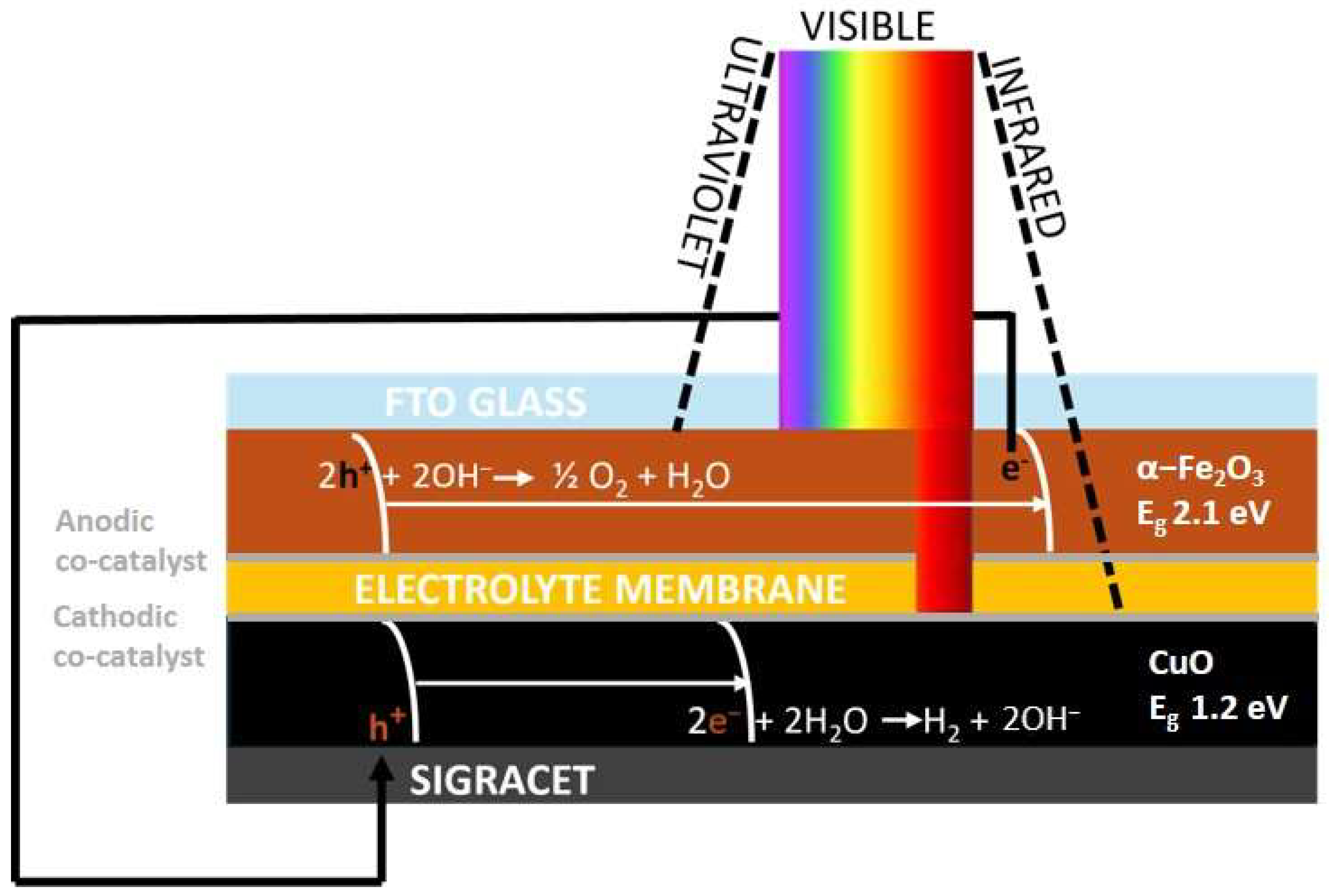
Experimental Investigation of Nickel-Based Co-Catalysts for Photoelectrochemical Water Splitting Using Hematite and Cupric Oxide Nanostructured Electrodes
Abstract
1. Introduction
- Q1: How do crystallographic, morphological, and surface features of selected Ni-based co-catalysts correlate with OER on Fe2O3 and HER on CuO under identical preparation and testing protocols?
- Q2: What relative activity trends emerge when comparing oxide-type (e.g., NiOx, NiFeOx) versus metallic/alloy co-catalysts (e.g., Ni, NiCu, NiMo) on n-type and p-type photoelectrodes?
- Q3: How does co-catalyst loading modulate performance and does it manifest a volcano-type dependence, revealing optimal ranges tied to active-site availability and transport/optical constraints?
2. Materials and Methods
2.1. Ni-Based Co-Catalysts Development
2.1.1. Synthesis of NiOx and Ni
2.1.2. Synthesis of NiFeOx
2.1.3. Synthesis of NiWO4
2.1.4. Synthesis of NiCu
2.1.5. Synthesis of NiMo
2.2. Physico-Chemical Characterization
2.3. Preparation of Semiconductors and Co-Catalysts Deposition
2.4. Half-Cell Characterization
2.5. Electrochemical Surface Area (ECSA) Determination
- Photoanodes: from 0.05 to 0.15 V vs. Ag/AgCl, scan rates 10–100 mV s−1.
- Photocathodes: from −0.15 to −0.05 V vs. Ag/AgCl, scan rates 10–100 mV s−1.
3. Results
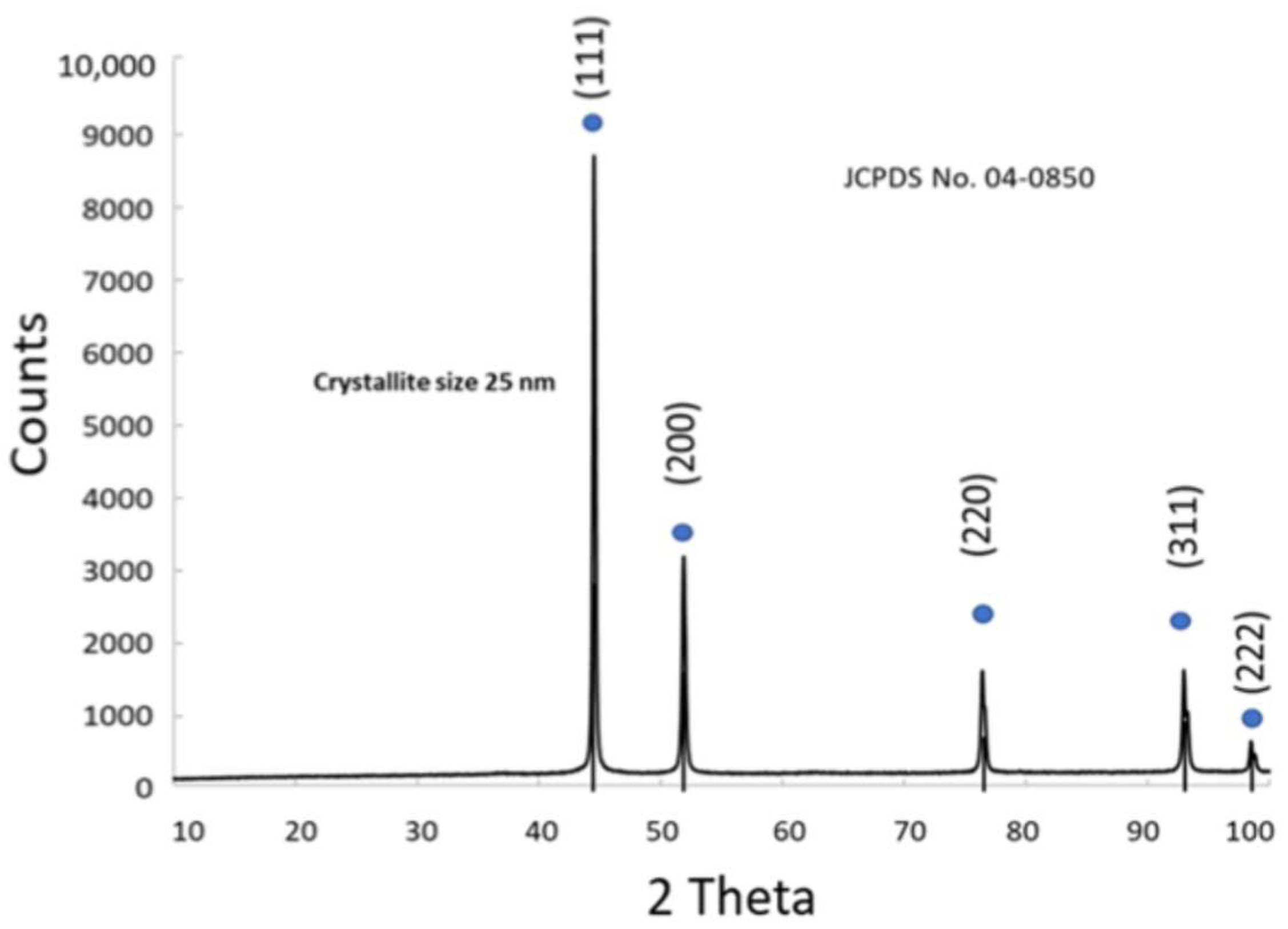
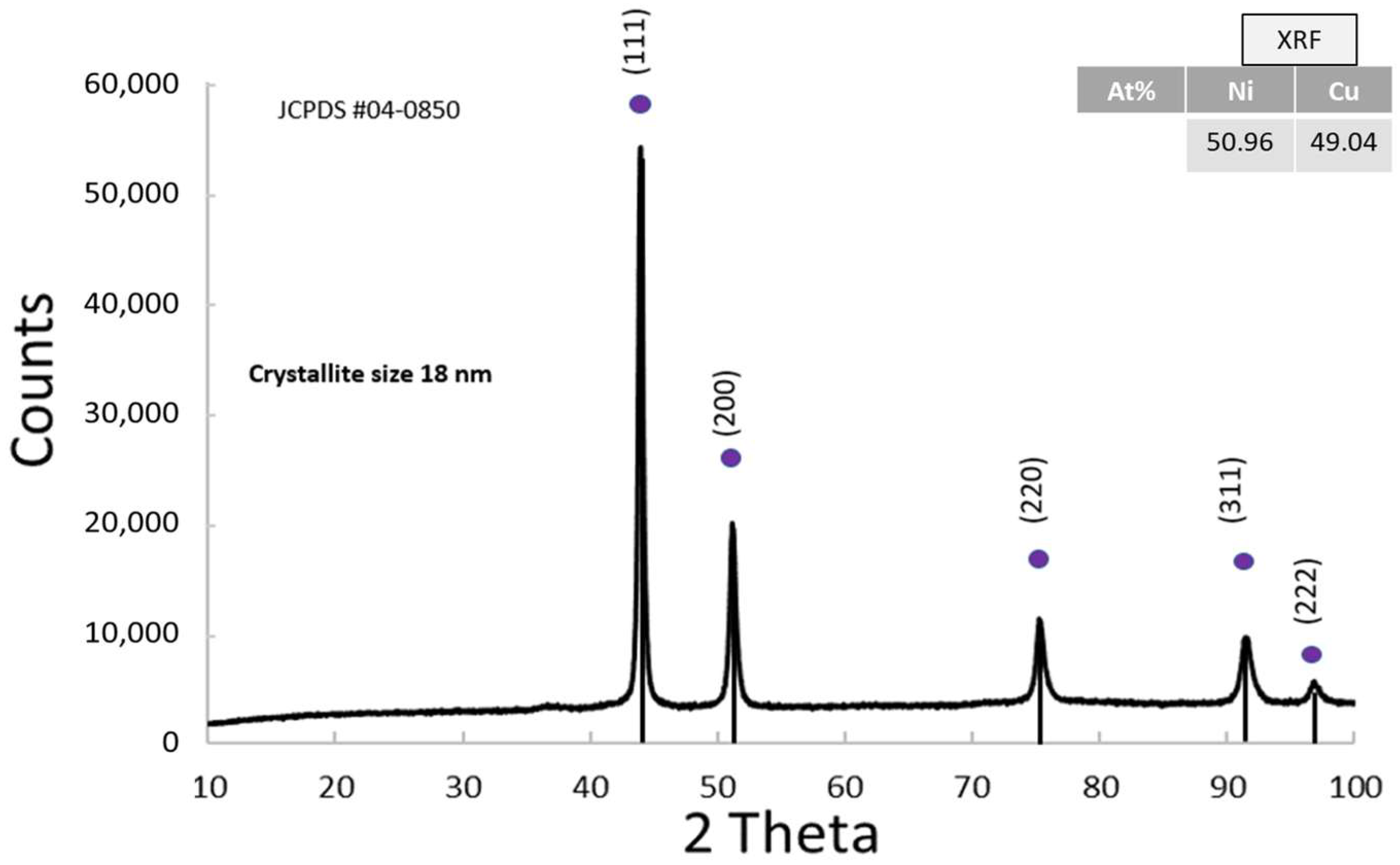
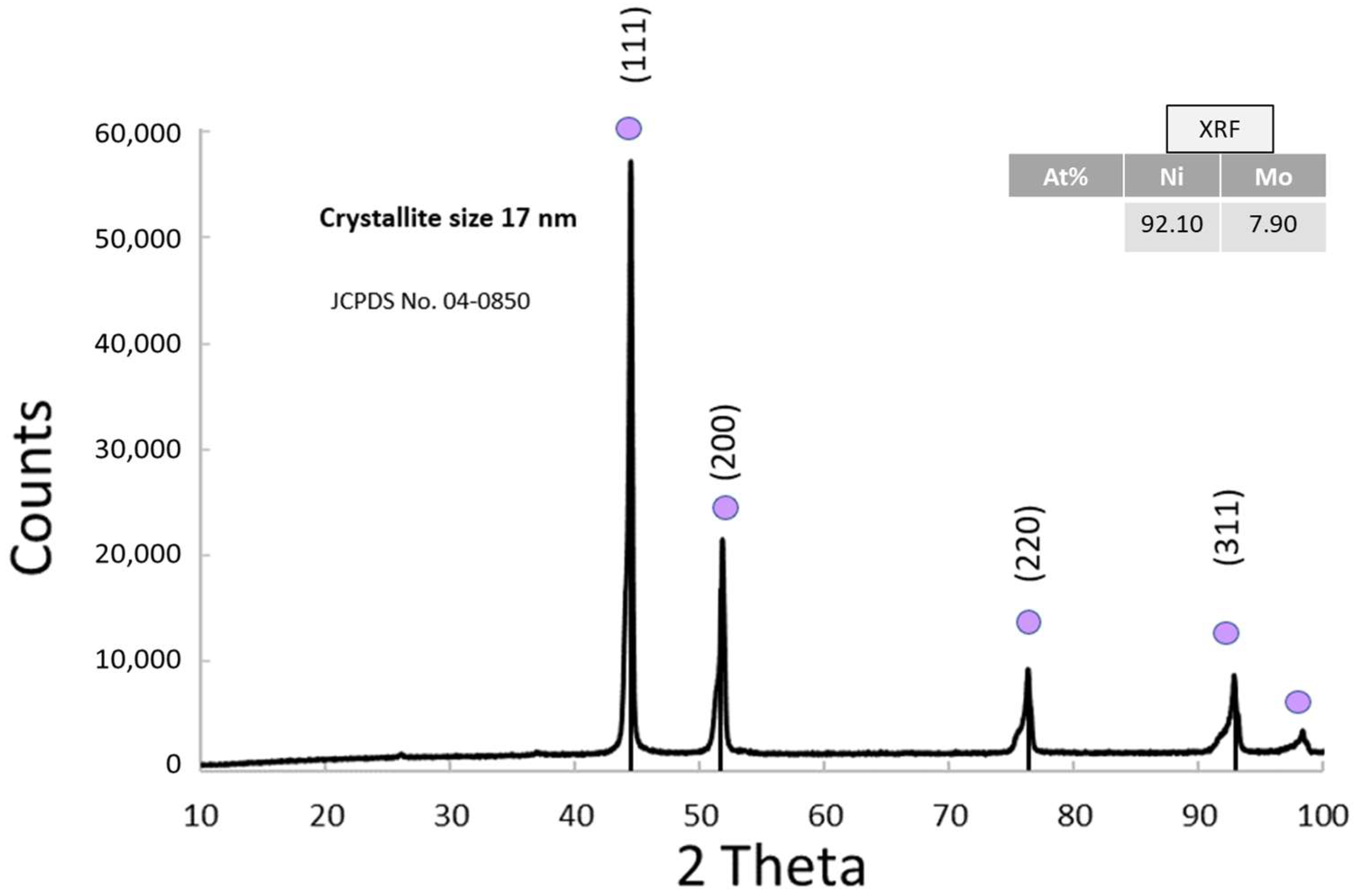
3.1. Physico-Chemical Characterization of Co-Catalysts by XRD and XRF Analysis
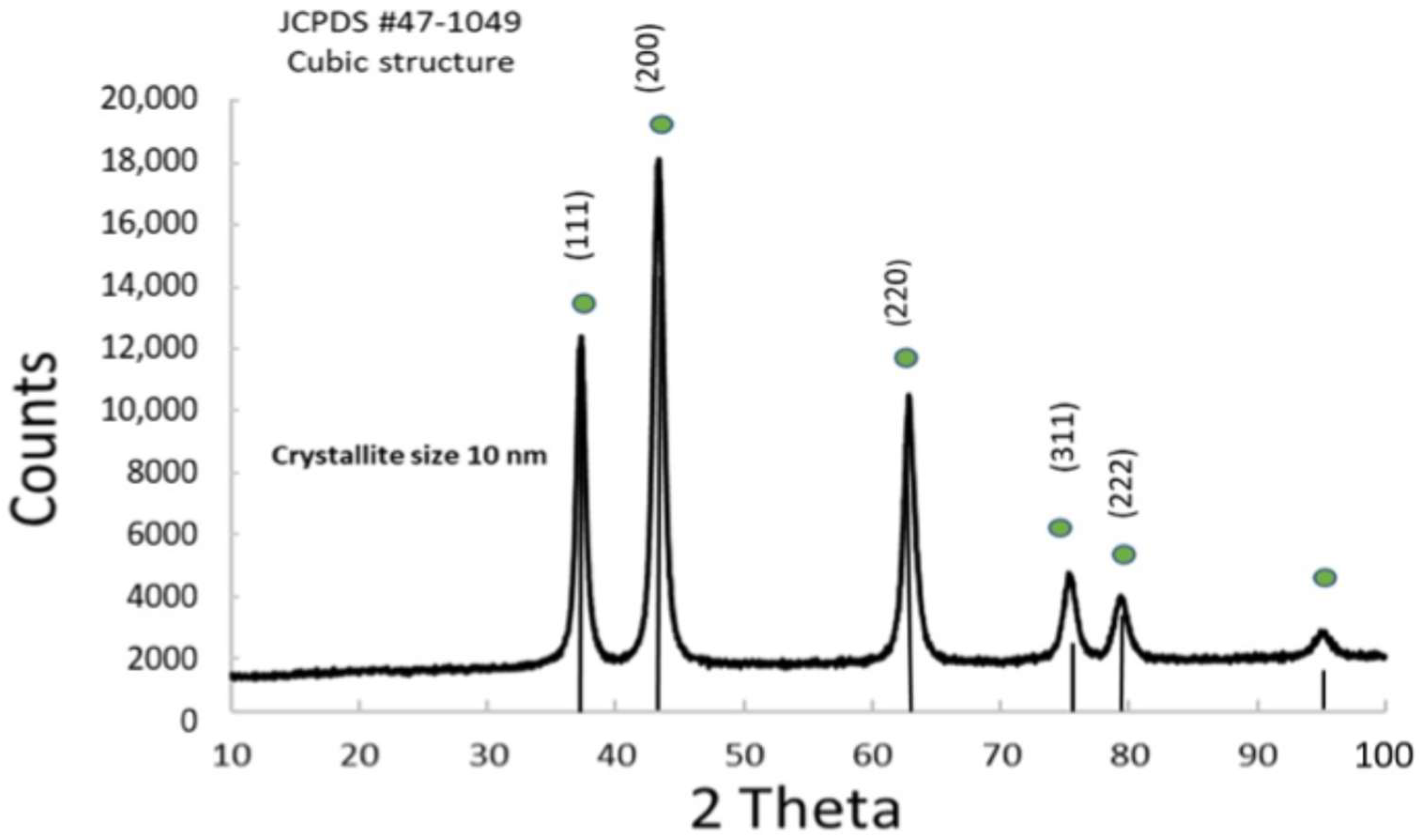
3.1.1. Physico-Chemical Properties of NiOx
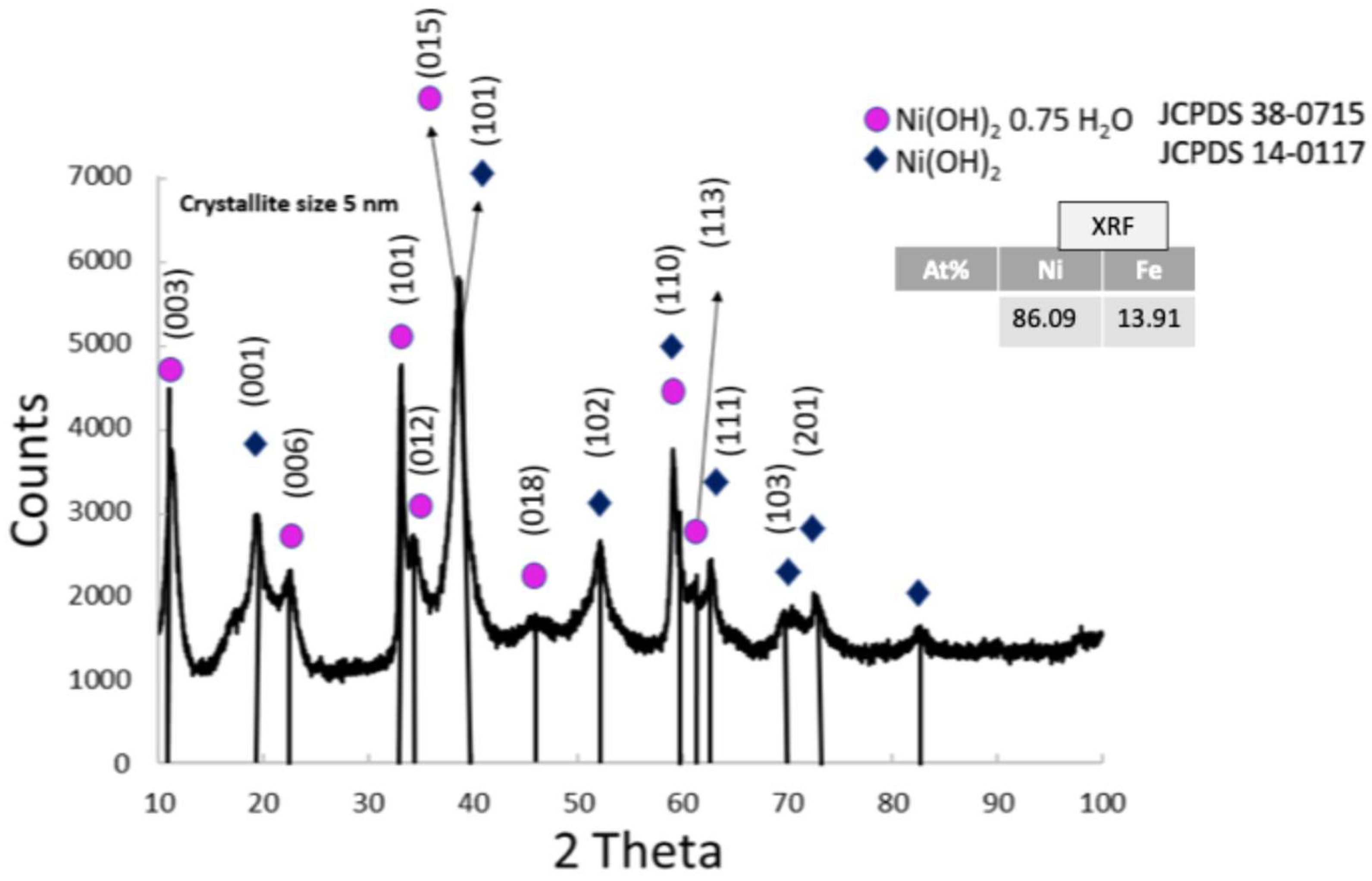
3.1.2. Physico-Chemical Properties of NiFeOx
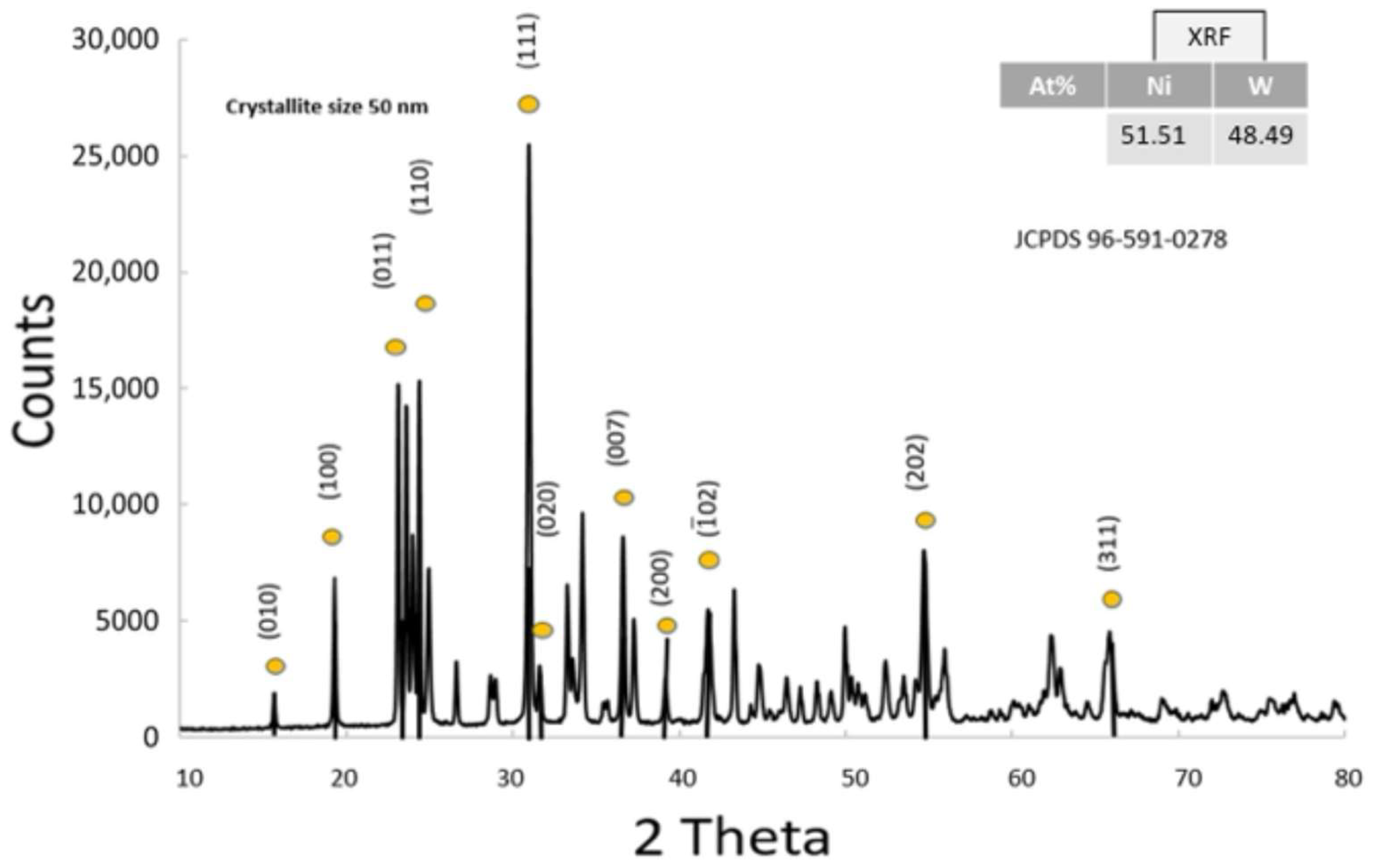
3.1.3. Physico-Chemical Properties of NiWO4
3.1.4. Physico-Chemical Properties of Ni
3.1.5. Physico-Chemical Properties of NiCu
3.1.6. Physico-Chemical Properties of NiMo
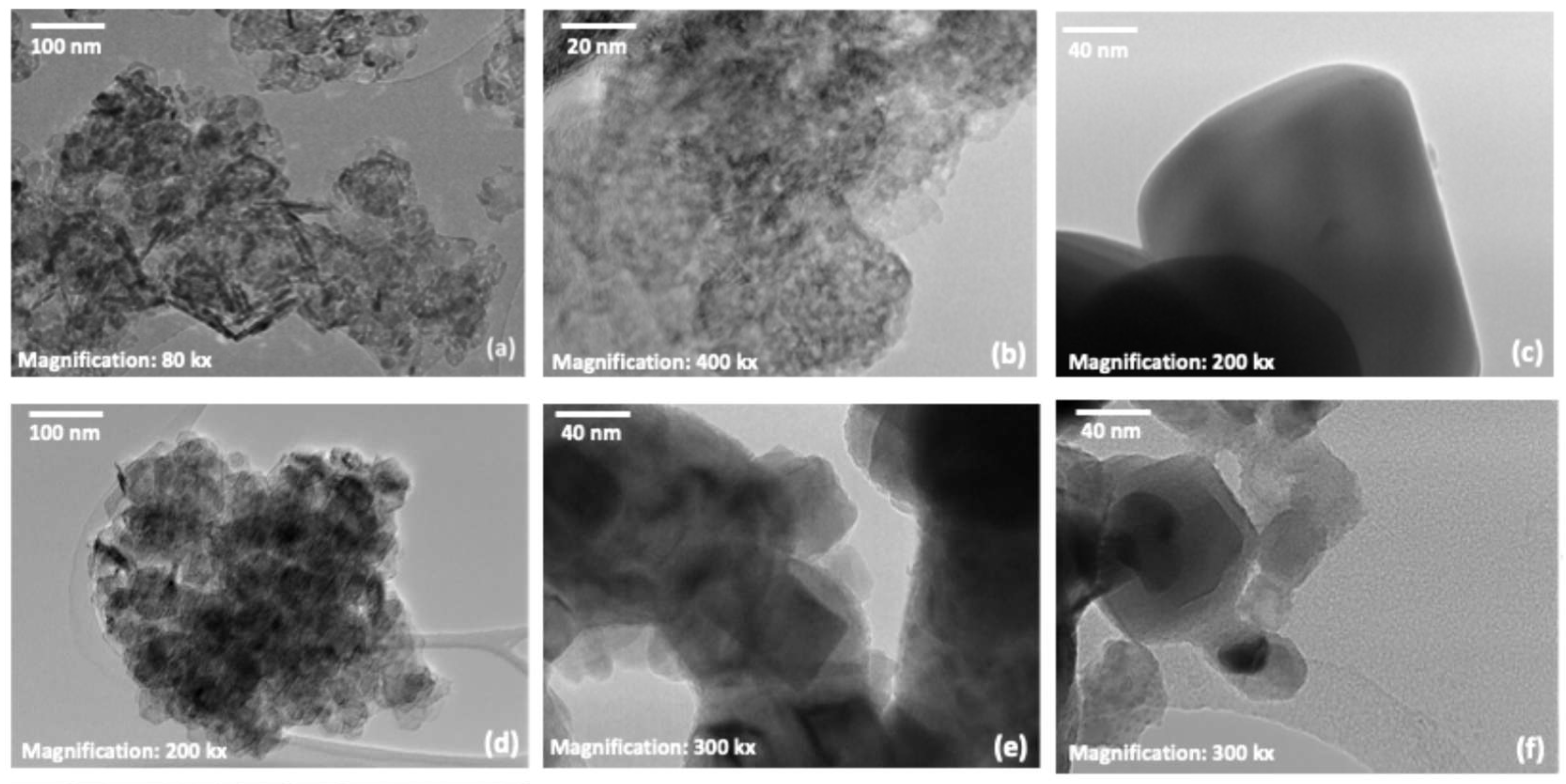
3.2. Physico-Chemical Characterization of Co-Catalysts by TEM-EDX and SEM-EDX Analysis
3.3. Physico-Chemical Characterization of Co-Catalysts by Brunauer–Emmett–Teller (BET) Analysis
3.4. Physico-Chemical Characterization of Co-Catalysts by XPS Survey Analysis
3.5. Electrochemical Characterization of Electrodes
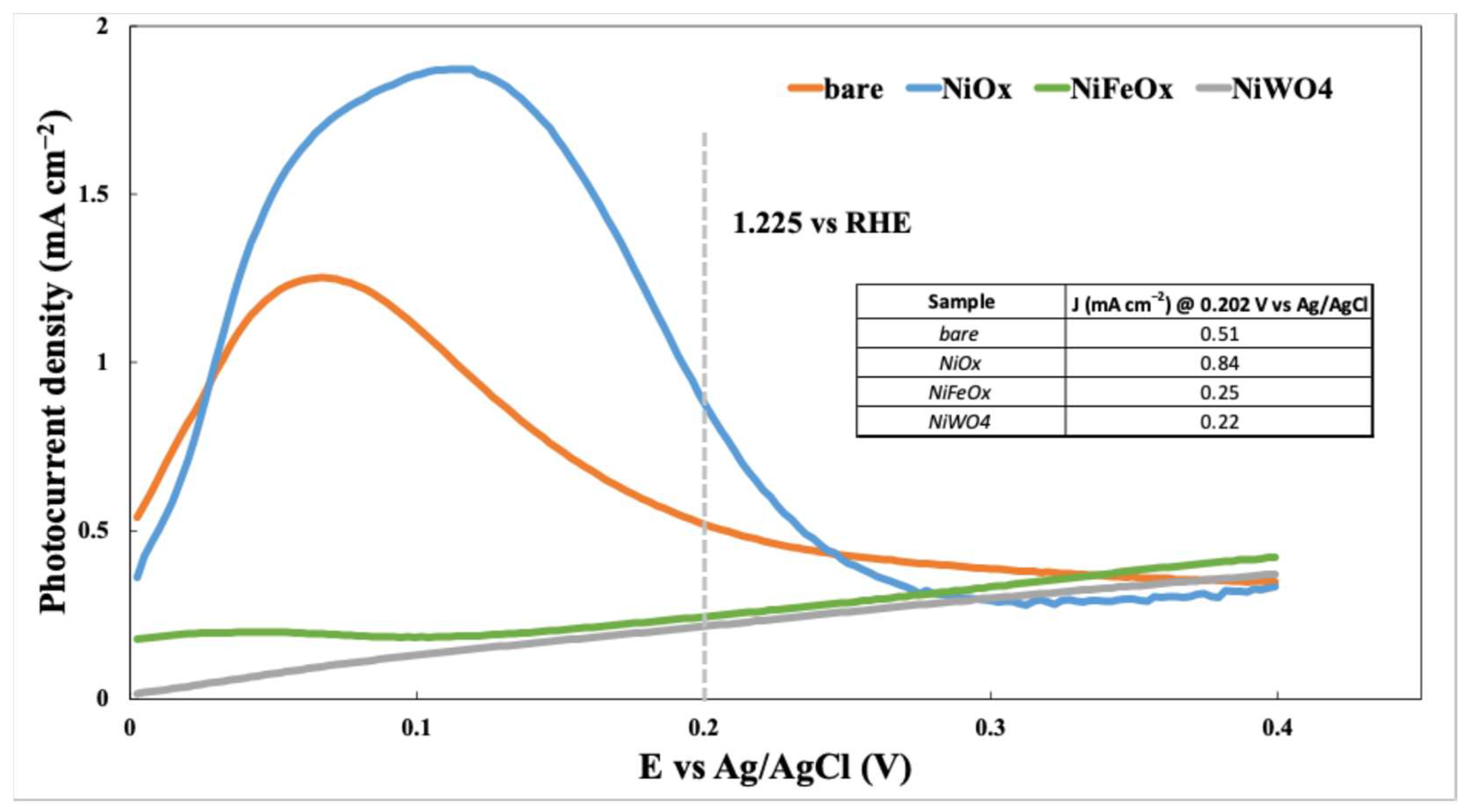
- NiOx: This shows the most significant improvement in photocurrent density, reaching values exceeding 0.8 mA/cm2 at approximately 0.2 V vs. Ag/AgCl. This suggests that NiOx is a highly effective co-catalyst for boosting the photoanode’s activity.
- NiFeOx and NiWO4: Over the potential range, both deliver photocurrents that are well below those of NiOx and generally below those of the bare photoanode. At 0.20 V vs. Ag/AgCl, they are lower than the bare photoanode, indicating that they do not provide an improvement under our conditions. This likely reflects less favorable surface states and interfacial charge-transfer kinetics compared to NiOx.
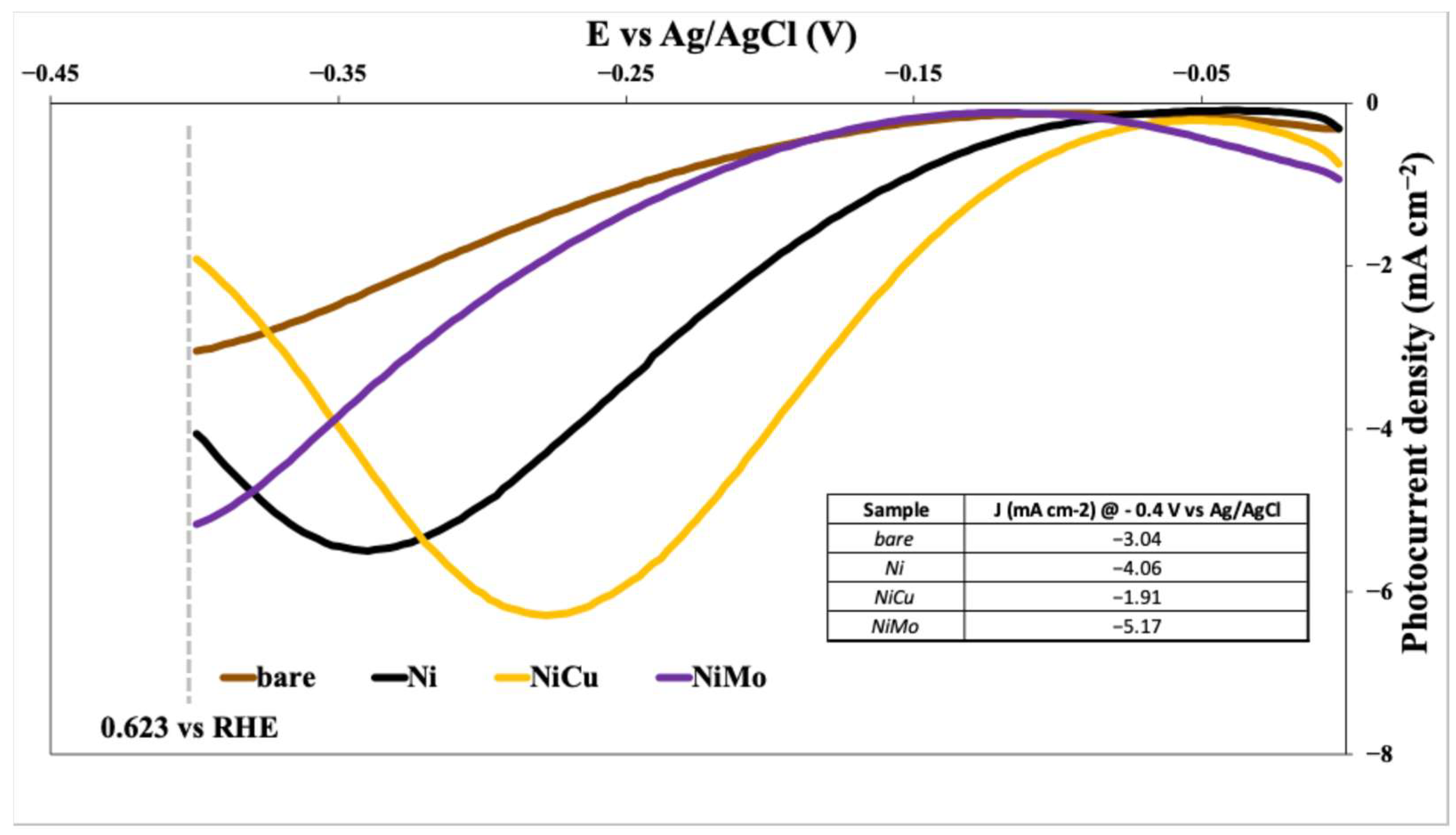
- Significant Enhancement: All three co-catalysts (Ni, NiCu, and NiMo) demonstrably improve the photocurrent density of the CuO photocathode compared to the bare CuO electrode. This indicates that these materials effectively enhance the hydrogen evolution reaction (HER) kinetics.
- NiMo Superiority: Among the tested materials, NiMo provided the highest enhancement in photocurrent density over the entire potential range, confirming the strong synergistic interaction between Ni and Mo in promoting HER activity. This superior performance likely stems from optimized electronic properties and/or enhanced catalytic activity at the co-catalyst-electrolyte interface.
- NiCu Performance: The NiCu co-catalyst also demonstrates a noticeable improvement over the bare CuO and the Ni-only co-catalyst, although it falls short of NiMo’s performance. This indicates that the copper addition positively influences HER activity, but possibly less than the combined effects of nickel and molybdenum.
- Potential-Dependent Behavior: The photocurrent density is strongly potential-dependent for all samples. At more negative potentials, the HER is favored, and thus, photocurrent density increases. The specific shapes of the curves may reveal information about the charge transfer mechanisms and reaction kinetics for each co-catalyst.
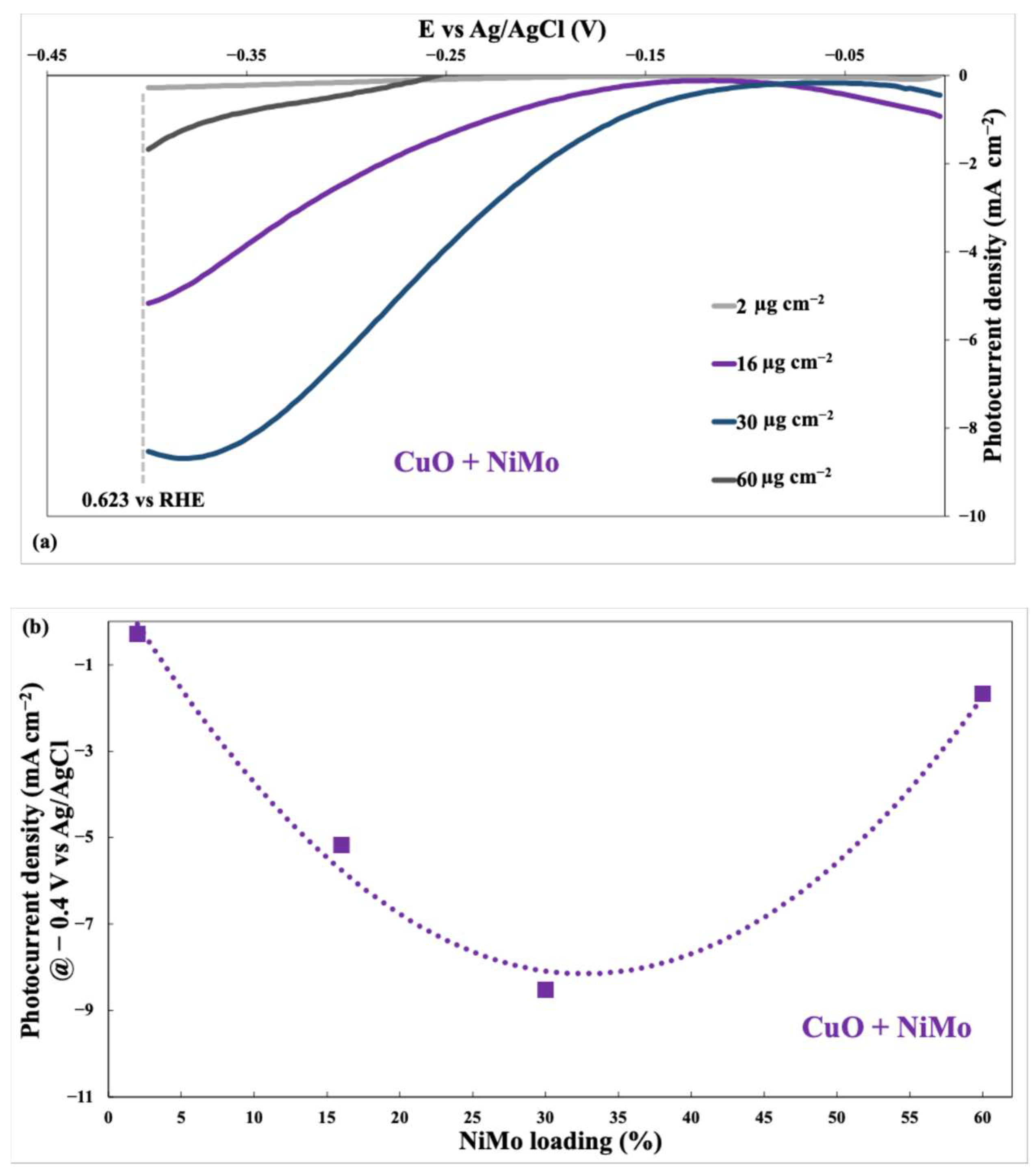
- Blocking of active sites. Excessive NiMo could cover active sites on the CuO surface, reducing the number of sites available for HER.
- Increased charge transfer resistance. A thick layer of NiMo might hinder efficient charge transfer between the CuO and the electrolyte.
- Light scattering/absorption. A high NiMo loading could scatter or absorb light, reducing the amount of light reaching the CuO and decreasing the photocurrent generation.
3.6. ECSA Results
4. Discussion
4.1. Anodic Co-Catalysts: Hematite Photoanode
4.2. Cathodic Co-Catalysts: CuO Photocathode
4.3. Effect of Co-Catalyst Loading
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Yang, Y.; Javanroodi, K.; Nik, V.M. Climate Change and Renewable Energy Generation in Europe—Long-Term Impact Assessment on Solar and Wind Energy Using High-Resolution Future Climate Data and Considering Climate Uncertainties. Energies 2022, 15, 302. [Google Scholar] [CrossRef]
- Giacoppo, G.; Trocino, S.; Lo Vecchio, C.; Baglio, V.; Díez-García, M.I.; Aricò, A.S.; Barbera, O. Numerical 3D Model of a Novel Photoelectrolysis Tandem Cell with Solid Electrolyte for Green Hydrogen Production. Energies 2023, 16, 1953. [Google Scholar] [CrossRef]
- Liu, D.; Kuang, Y. Particle-Based Photoelectrodes for PEC Water Splitting: Concepts and Perspectives. Adv. Mater. 2024, 36, e2311692. [Google Scholar] [CrossRef]
- Juodkazis, K.; Juodkazyte, J.; Jelmakas, E.; Kalinauskas, P.; Valsiunas, I.; Mecinskas, P.; Juodkazis, S. Photoelectrolysis of water: Solar hydrogen—Achievements and perspectives. Opt. Express 2010, 18, A147. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Giacoppo, G.; Barbera, O.; Carbone, A.; Baglio, V.; Aricò, A.S.; Monforte, G.; Trocino, S. Scalability and Investigation of the Geometrical Features and Shapes of a Tandem Photo-Electrolysis Cell Based on Non-Critical Raw Materials. Catalysts 2024, 14, 98. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, Y.; Zhang, X.; Yang, H.; Xie, R.; Zhang, S.; Lv, Y.; Xiong, L. Progress in Promising Semiconductor Materials for Efficient Photoelectrocatalytic Hydrogen Production. Molecules 2024, 29, 289. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.; Li, C. Surface Passivation Engineering for Photoelectrochemical Water Splitting. Catalysts 2023, 13, 217. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Yang, J.; Ye, M.; Zhang, Y.; Jang, Y.J.; Zhang, H. Hematite photoanode for efficient photoelectrochemical water splitting: Recent advances and outlook. Chem. Commun. 2025, 61, 7724–7736. [Google Scholar] [CrossRef] [PubMed]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Bisquert, J.; Hamann, T.W. Electrochemical and photoelectrochemical investigation of water oxidation with hematite electrodes. Energy Environ. Sci. 2012, 5, 7626. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Reddy, J.; Wang, C.; Zhang, J.Z.; Li, Y. The Influence of Oxygen Content on the Thermal Activation of Hematite Nanowires. Angew. Chem. Int. Ed. 2012, 51, 4074–4079. [Google Scholar] [CrossRef]
- Kim, J.Y.; Magesh, G.; Youn, D.H.; Jang, J.-W.; Kubota, J.; Domen, K.; Lee, J.S. Single-crystalline, wormlike hematite photoanodes for efficient solar water splitting. Sci. Rep. 2013, 3, 2681. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting—Superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Lopes, T.; Andrade, L.; Le Formal, F.; Gratzel, M.; Sivula, K.; Mendes, A. Hematite photoelectrodes for water splitting: Evaluation of the role of film thickness by impedance spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 16515. [Google Scholar] [CrossRef]
- Trocino, S.; Lo Vecchio, C.; Campagna Zignani, S.; Carbone, A.; Saccà, A.; Baglio, V.; Gómez, R.; Aricò, A.S. Dry Hydrogen Production in a Tandem Critical Raw Material-Free Water Photoelectrolysis Cell Using a Hydrophobic Gas-Diffusion Backing Layer. Catalysts 2020, 10, 1319. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Muayqil, E.; Al Abass, N.; Alhajji, M.A.; Bubshait, A.A.; Alhazmi, N.E.; Almuqhim, A.A. Surface engineering of CuO-Cu2O heterojunction thin films for improved photoelectrochemical water splitting. Renew. Energy 2024, 235, 121326. [Google Scholar] [CrossRef]
- Baran, T.; Visibile, A.; Busch, M.; He, X.; Wojtyla, S.; Rondinini, S.; Minguzzi, A.; Vertova, A. Copper Oxide-Based Photocatalysts and Photocathodes: Fundamentals and Recent Advances. Molecules 2021, 26, 7271. [Google Scholar] [CrossRef]
- Dubale, A.A.; Pan, C.-J.; Tamirat, A.G.; Chen, H.-M.; Su, W.-N.; Chen, C.-H.; Rick, J.; Ayele, D.W.; Aragaw, B.A.; Lee, J.-F.; et al. Heterostructured Cu2O/CuO decorated with nickel as a highly efficient photocathode for photoelectrochemical water reduction. J. Mater. Chem. A 2015, 3, 12482–12499. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Trocino, S.; Giacoppo, G.; Barbera, O.; Baglio, V.; Díez-García, M.I.; Contreras, M.; Gómez, R.; Aricò, A.S. Water Splitting with Enhanced Efficiency Using a Nickel-Based Co-Catalyst at a Cupric Oxide Photocathode. Catalysts 2021, 11, 1363. [Google Scholar] [CrossRef]
- Angeles-Olvera, Z.; Crespo-Yapur, A.; Rodríguez, O.; Cholula-Díaz, J.; Martínez, L.; Videa, M. Nickel-Based Electrocatalysts for Water Electrolysis. Energies 2022, 15, 1609. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, P.C. Realizing NiO nanocrystals from a simple chemical method. Bull. Mater. Sci. 2010, 33, 653–656. [Google Scholar] [CrossRef]
- Hu, C.; Lv, C.; Zeng, N.; Liu, A.; Liu, Y.; Hu, L.; Li, P.; Yao, Y.; Cai, J.; Tang, T. Recent Advances in Ni-Based Electrocatalysts for Hydrogen Evolution Reaction. Energy Technol. 2023, 11, 2201048. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, Y.; Huang, X.; Shen, W.; Yu, J.; Li, J. A Facile Synthesis of Three Dimensional β-Ni(OH)2 Composed of Ultrathin Nanosheets for High Performance Pseudocapacitor. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2089–2097. [Google Scholar] [CrossRef]
- Patil, D.J.; Malavekar, D.B.; Lokhande, V.C.; Bagwade, P.P.; Khot, S.D.; Ji, T.; Lokhande, C.D. Binder-Free Synthesis of Mesoporous Nickel Tungstate for Aqueous Asymmetric Supercapacitor Applications: Effect of Film Thickness. Energy Technol. 2022, 10, 2200295. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Simeonidis, K.; Vilalta-Clemente, A.; Tuna, F.; Tsiaoussis, I.; Angelakeris, M.; Dendrinou-Samara, C.; Kalogirou, O. Controlling the crystal structure of Ni nanoparticles by the use of alkylamines. J. Magn. Magn. Mater. 2009, 321, 2723–2728. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An Advanced Ni–Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef]
- Mancheva, M.N.; Iordanova, R.S.; Klissurski, D.G.; Tyuliev, G.T.; Kunev, B.N. Direct Mechanochemical Synthesis of Nanocrystalline NiWO4. J. Phys. Chem. C 2007, 111, 1101–1104. [Google Scholar] [CrossRef]
- McKone, J.R.; Sadtler, B.F.; Werlang, C.A.; Lewis, N.S.; Gray, H.B. Ni–Mo Nanopowders for Efficient Electrochemical Hydrogen Evolution. ACS Catal. 2013, 3, 166–169. [Google Scholar] [CrossRef]


| Photoanode | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Modal Pore Diamater (nm) |
|---|---|---|---|
| NiOx | 56 | 0.12 | 7.8 |
| NiFeOx | 38 | 0.09 | 8.5 |
| NiWO4 | 22 | 0.06 | 10.2 |
| Photocathode | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|
| Ni | 0.6 | <0.002 |
| NiCu | 0.8 | <0.003 |
| NiMo | 0.5 | <0.002 |
| Photoanode | Cdl (mF cm−2) | ECSA (cm2) |
|---|---|---|
| bare | 0.6 | 15 |
| NiOx | 2.4 | 60 |
| NiFeOx | 1.6 | 40 |
| NiWO4 | 1 | 25 |
| Photocathode | Cdl (mF cm−2) | ECSA (cm2) |
|---|---|---|
| bare | 0.8 | 20 |
| Ni | 1.2 | 30 |
| NiCu | 1.8 | 45 |
| NiMo | 3 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, M.A.; Giaquinta, R.; Arnese, C.; Frontera, P.; Macario, A.; Malara, A.; Trocino, S. Experimental Investigation of Nickel-Based Co-Catalysts for Photoelectrochemical Water Splitting Using Hematite and Cupric Oxide Nanostructured Electrodes. Nanomaterials 2025, 15, 1551. https://doi.org/10.3390/nano15201551
Mancuso MA, Giaquinta R, Arnese C, Frontera P, Macario A, Malara A, Trocino S. Experimental Investigation of Nickel-Based Co-Catalysts for Photoelectrochemical Water Splitting Using Hematite and Cupric Oxide Nanostructured Electrodes. Nanomaterials. 2025; 15(20):1551. https://doi.org/10.3390/nano15201551
Chicago/Turabian StyleMancuso, Maria Aurora, Rossana Giaquinta, Carmine Arnese, Patrizia Frontera, Anastasia Macario, Angela Malara, and Stefano Trocino. 2025. "Experimental Investigation of Nickel-Based Co-Catalysts for Photoelectrochemical Water Splitting Using Hematite and Cupric Oxide Nanostructured Electrodes" Nanomaterials 15, no. 20: 1551. https://doi.org/10.3390/nano15201551
APA StyleMancuso, M. A., Giaquinta, R., Arnese, C., Frontera, P., Macario, A., Malara, A., & Trocino, S. (2025). Experimental Investigation of Nickel-Based Co-Catalysts for Photoelectrochemical Water Splitting Using Hematite and Cupric Oxide Nanostructured Electrodes. Nanomaterials, 15(20), 1551. https://doi.org/10.3390/nano15201551










