Preparation and Enhanced Catalytic Performance of a Polyhedral BiVO4-Nanoparticle-Modified ZnO Flower-like Nanorod Structure Composite Material
Abstract
1. Introduction
2. Experiment
2.1. Preparation of ZnO Flower-like Nanorods
2.2. Preparation of BiVO4@ZnO Heterojunction Composites
2.3. Characterization
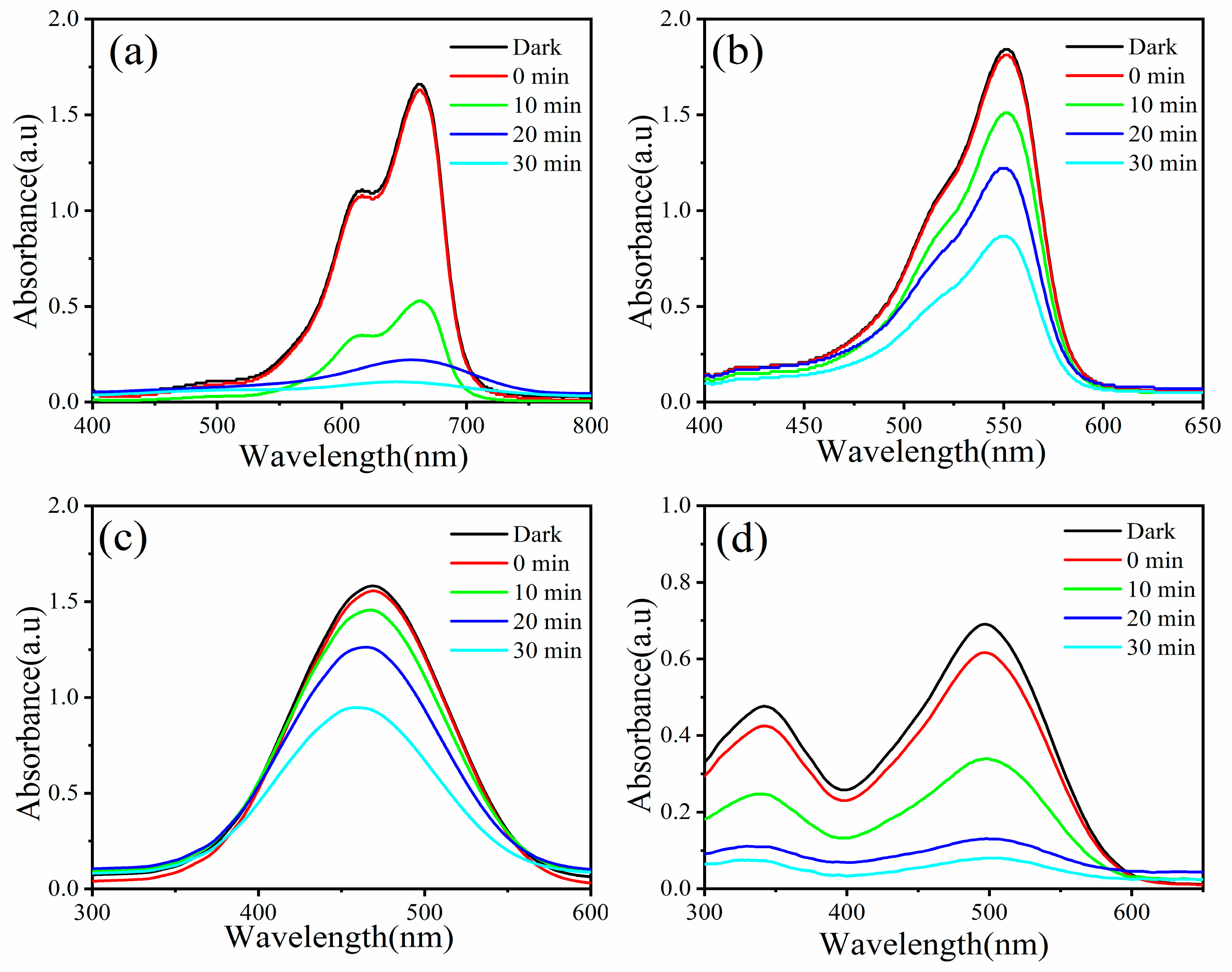
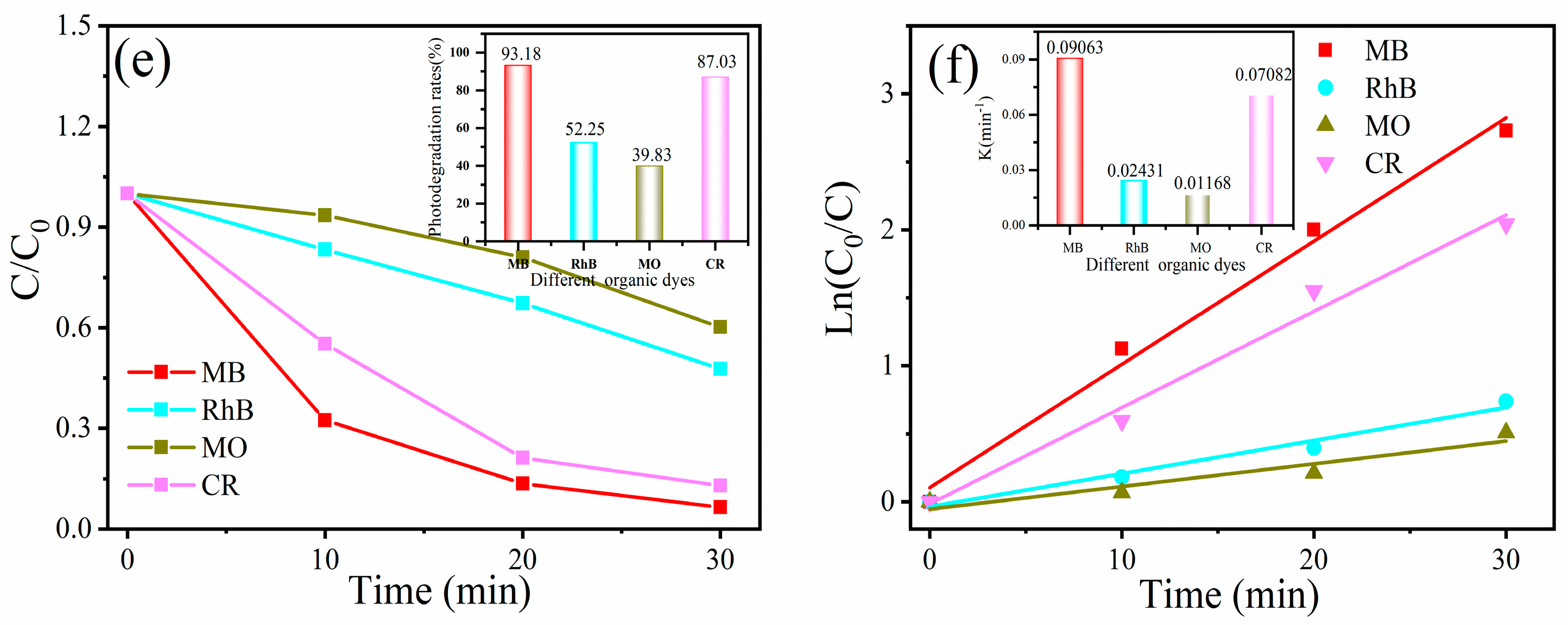
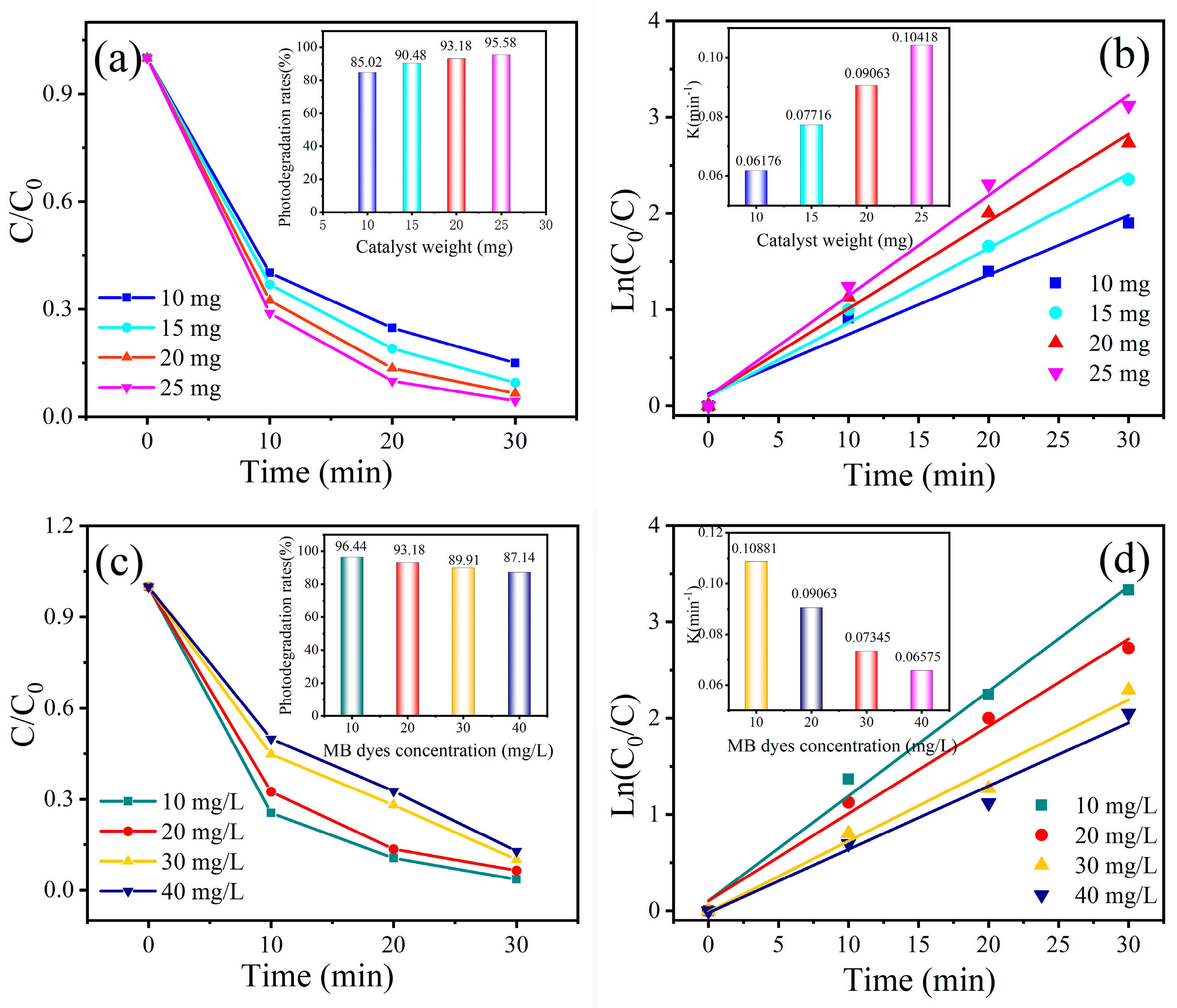
2.4. Evaluation of Photocatalytic Performance of MB, RhB, MO, and CR Dyes
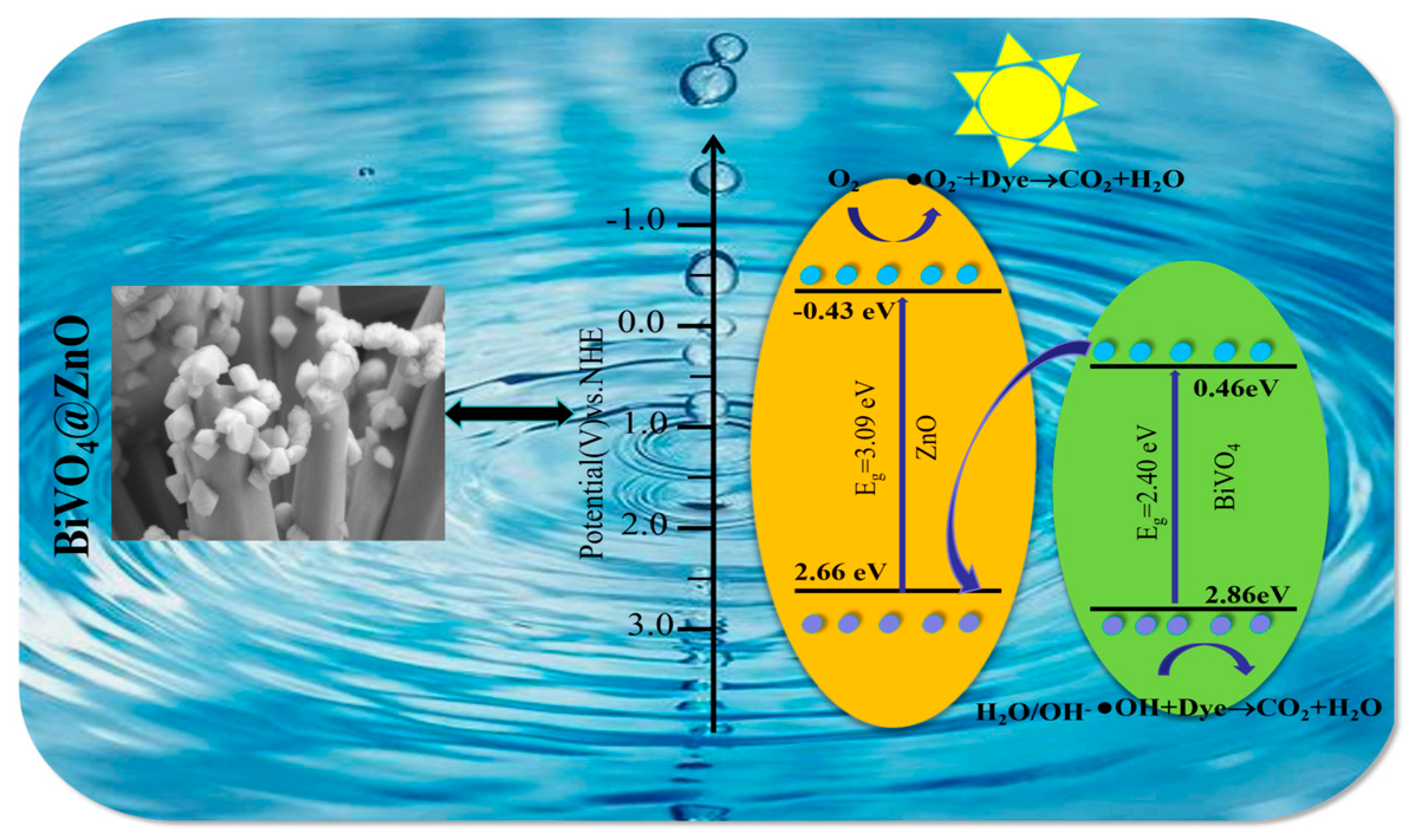
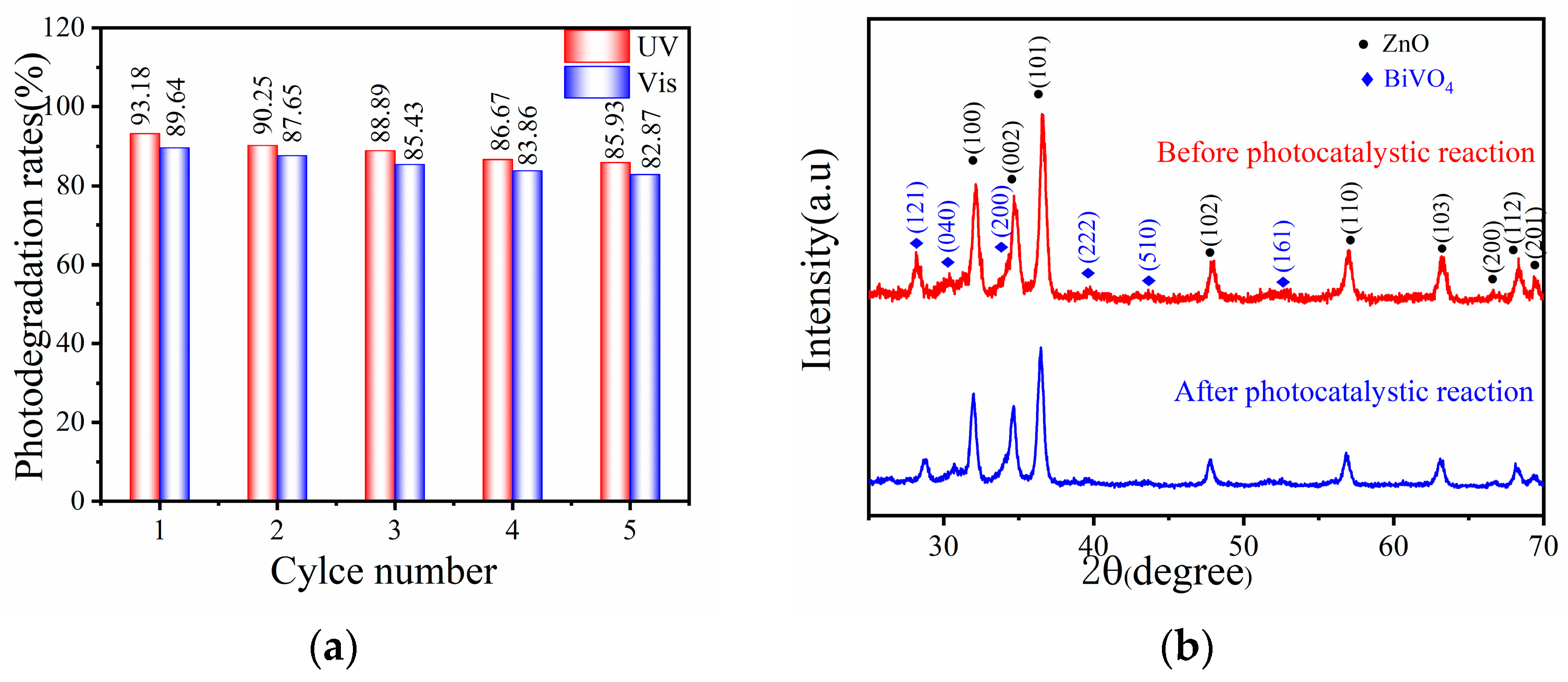
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Li, J.; Zhang, Q.; Guo, D. Constructing a novel CuS/Cu2S Z-scheme heterojunction for highly-efficiency NIR light-driven antibacterial activity. Appl. Surf. Sci. 2023, 624, 156848. [Google Scholar] [CrossRef]
- Xu, M.Z.; Ji, H.J.; Zheng, L.; Li, W.W.; Wang, J.; Wang, H.X.; Luo, L.; Lu, Q.B.; Gan, X.T.; Liu, Z.; et al. Reconfiguring nucleation for CVD growth of twisted bilayer MoS2 with a wide range of twist angles. Nat. Commun. 2024, 15, 562. [Google Scholar] [CrossRef]
- Tang, R.; Zeng, H.; Deng, Y.; Xiong, S.; Li, L.; Zhou, Z.; Wang, J.; Tang, L. Dual modulation on peroxymonosulfate activation site and photocarrier separation in carbon nitride for efficient photocatalytic organics degradation: Efficacy and mechanism evaluation. Appl. Catal. B Environ. 2023, 336, 122918. [Google Scholar] [CrossRef]
- Xu, M.Z.; Ji, H.J.; Zhang, W.W.; Luo, L.; Chen, M.D.; Liu, Z.; Gan, X.; Wang, X.; Huang, W. CVD Synthesis of Twisted Bilayer WS2 with Tunable Second Harmonic Generation. Adv. Mater. 2024, 36, 2313638. [Google Scholar] [PubMed]
- Liaqat, M.; Khalid, N.R.; Iqbal, T.; Maryam, I.; Tanveer, M.; Hussain, M.K.; Pham, P.V.; Ali, A.M.; Sayed, M.A. Synthesis of heterojunction BiVO4/MnO2 graphene ternary nanocomposites with enhanced photocatalytic activities through degradation of rhodamine B and tetracycline hydrochlorid. Chin. J. Phys. 2024, 91, 406–420. [Google Scholar] [CrossRef]
- Abdullah, F.H.; Bakar, N.; Bakar, M.A. Current advancements on the fabrication, modification, and industrial application of zinc oxide as photocatalyst in the removal of organic and inorganic contaminants in aquatic systems. J. Hazard. Mater. 2022, 424, 127416. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Wen, M.; Pan, H.; Shen, S. Solvothermal preparation of spindle hierarchical ZnO and its photocatalytic and gas sensing properties. Physica B 2021, 602, 412545. [Google Scholar] [CrossRef]
- Jayamadhava, P.; Sudhakara, A.; Ramesha, S.; Nataraja, G. Synthesise of ZnO nano particle as an alternative catalyst for Photocatalytic degradation of brilliant red azo dye. Am. J. Environ. Prot. 2014, 3, 318–322. [Google Scholar] [CrossRef]
- Jaramillo-Paez, C.; Sanchez-Cid, P.; Navío, J.A.; Hidalgo, M.C. A comparative assessment of the UV-photocatalytic activities of ZnO synthesized by different routes. J. Environ. Chem. Eng. 2018, 6, 7161–7171. [Google Scholar]
- Shi, C.; Zhang, L.; Shi, Z.; Ma, J.; Wang, Z. Cellulose template designed porous ZnO based catalysts with different valence copper for solar photocatalytic CO2 conversion. Ind. Crop. Prod. 2022, 186, 115223. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Liu, J.; Zhang, Z.Y.; Zhang, W.H.; Wang, A.Y.; Tian, F.; Zhao, W.; Yan, J. Hydrothermal synthesis of brush-like ZnO NWAs@CC composites with enhanced photocatalytic and field emission performance. Mater. Sci. Semicond. Process. 2021, 135, 106043. [Google Scholar] [CrossRef]
- Preda, N.; Costas, A.; Enculescu, M.; Enculescu, I. Biomorphic 3D fibrous networks based on ZnO, CuO and ZnO–CuO composite nanostructures prepared from eggshell membranes. Mater. Chem. Phys. 2020, 240, 122205. [Google Scholar] [CrossRef]
- Yadav, R.; Chundawat, T.S.; Rawat, P.; Rao, G.K.; Vaya, D. Photocatalytic degradation of malachite green dye by ZnO and ZnO–β-cyclodextrin nanocomposite. Bull. Mater. Sci. 2021, 44, 250. [Google Scholar]
- Kadi, M.W.; McKinney, D.; Mohamed, R.M.; Mkhalid, I.A.; Sigmund, W. Fluorine doped zinc oxide nanowires: Enhanced photocatalysts degrade malachite green dye under visible light conditions. Ceram. Int. 2016, 42, 4672–4678. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Shawky, A. CNT supported Mn-doped ZnO nanoparticles: Simple synthesis and improved photocatalytic activity for degradation of malachite green dye under visible light. Appl. Nanosci. 2018, 8, 1179–1188. [Google Scholar] [CrossRef]
- Li, H.; Ding, J.; Cai, S.; Zhang, W.; Zhang, X.; Wu, T.; Wang, C.; Foss, M.; Yang, R. Plasmon-enhanced photocatalytic properties of Au/ZnO nanowires. Appl. Surf. Sci. 2022, 583, 152539. [Google Scholar] [CrossRef]
- He, K.; Zeng, Z.T.; Chen, A.W.; Zeng, G.M.; Xiao, R.; Xu, P.; Huang, Z.Z.; Shi, J.B.; Hu, L.; Chen, G.Q. Advancement of Ag-graphene based nanocomposites: An overview of synthesis and its applications. Small 2018, 15, 1800871–1800884. [Google Scholar] [CrossRef]
- Wang, W.W.; Zhu, Y.J.; Yang, L.X. ZnO–SnO2 hollow spheres and hierarchical nanosheets: Hydrothermal preparation, formation mechanism, and photocatalytic properties. Adv. Funct. Mater. 2007, 17, 59–64. [Google Scholar]
- Liang, X.; Wang, P.; Gao, Y.; Huang, H.; Tong, F.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Dai, Y.; et al. Design and synthesis of porous M-ZnO/CeO2 microspheres as efficient plasmonic photocatalysts for nonpolar gaseous molecules oxidation: Insight into the role of oxygen vacancy defects and M = Ag, Au nanoparticles. Appl. Catal. B Environ. 2020, 260, 118151. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Xie, L. Synthesis and sonophotocatalytic activities of ZnO\BiVO4\Co3O4 composites. Chem. Phys. Lett. 2021, 775, 138660. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Liu, J.; Zhang, Z.Y.; Zhang, W.H.; Wang, A.Y.; Tian, F.; Zhao, W.; Yan, J.F. Green synthesis of CuO nanoparticles-loaded ZnO nanowires arrays with enhanced photocatalytic activity. Mater. Chem. Phys. 2021, 267, 124703. [Google Scholar]
- Jiang, J.; Wang, G.; Shao, Y.; Wang, J.; Zhou, S.; Su, Y. Step-scheme ZnO@ZnS hollow microspheres for improved photocatalytic H2 production performance. Chin. J. Catal. 2022, 43, 329–338. [Google Scholar] [CrossRef]
- Hsu, M.H.; Chang, C.J.; Weng, H.T. Efficient H2 production using Ag2S-coupled ZnO@ZnS core–shell nanorods decorated metal wire mesh as an immobilized hierarchical photocatalyst. ACS Sustain. Chem. Eng. 2016, 4, 1381–1391. [Google Scholar] [CrossRef]
- Wejdan, T.A.; Shawky, A.; Mahmoud, M.H.H. S-scheme CuO/ZnO p-n heterojunctions for endorsed photocatalytic reduction of mercuric ions under visible light. Inorg. Chem. Commun. 2022, 143, 109778. [Google Scholar]
- Yu, F.; Ren, J.; Zhang, J.; Chen, H.; Tian, X.; Feng, C.; Li, C.; Zhang, J.; Tang, X.; Hou, X. Structural and optical properties of polyhedral N-doped ZnO@BiVO4 nanocomposite photocatalyst derived from ZIF-8. Vacuum 2024, 220, 112814. [Google Scholar]
- Zhu, Z.; Hsieh, C.; Chiang, Z.; Lin, Y.; Wu, R. Nanoarchitectonics of three-dimensional ZnO–BiVO4 for trace nitrogen dioxide gas detection. Ceram. Int. 2022, 48, 7706–7714. [Google Scholar]
- Ghazal, N.; Mohamed, S.A.; Hameed, M.F.O.; Obayya, S.S.A.; Nazer, H.A.E.; Madkour, M. Surface and optoelectronic impacts of ZnO/BiVO4/MWCNT nanoheterostructure toward photodegradation of water contaminants. Surf. Interfaces 2022, 33, 102278. [Google Scholar] [CrossRef]
- Liaqat, M.; Younas, A.; Iqbal, T.; Afsheen, S.; Zubair, M.; Kamran, S.K.S.; Syed, A.; Bahkali, A.H.; Wong, L.S. Synthesis and characterization of ZnO/BiVO4 nanocomposites as heterogeneous photocatalysts for antimicrobial activities and waste water treatment. Mater. Chem. Phys. 2024, 315, 128923. [Google Scholar]
- Bai, S.L.; Jia, S.Y.; Zhao, Y.Y.; Feng, Y.J.; Luo, R.X.; Li, D.Q.; Chen, A.F. NiFePB-modified ZnO/BiVO4 photoanode for PEC water oxidation. Dalton. T. 2023, 52, 5760–5770. [Google Scholar]
- Jia, X.; Liu, T.; Feng, S.; Yu, S.; Zhang, J.; Yang, J.; Wang, S.; Li, Y.; Song, H. Construction of ZnO@ZIF-CoZn bimetallic core-shell nanospheres for enhanced ethanol sensing performance. Vacuum 2023, 215, 112271. [Google Scholar]
- Khaokhajorn, C.; Amornpitoksuk, P.; Randorn, C.; Rattana, T.; Suwanboon, S. One-pot synthesis of In2O3/ZnO nanocomposite photocatalysts and their enhanced photocatalytic activity against cationic and anionic dye pollutants. Inorg. Chem. Commun. 2023, 157, 111392. [Google Scholar] [CrossRef]
- Meena, P.L.; Surela, A.K.; Chhachhia, L.K. Investigating photocatalytic activity of biosynthesized ZnO nanoparticles against organic dye pollutants under visible light. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 338, 126214. [Google Scholar]
- Lv, Y.Y.; Liu, J.; Zhang, Z.Y.; Zhang, W.H.; Wang, A.Y.; Tian, F. Two-step liquid phase synthesis of ZnO@CuO core–shell heterojunction nanorods arrays composites photodetectors with the enhanced UV photoelectric performances. Opt. Laser Technol. 2024, 168, 109958. [Google Scholar]
- Shen, Z.Y.; Wang, J.T.; Chiang, W.H.; Hsiao, C.N.; Wuu, D.S.; Chen, G.Y.; Wang, T.W.; Liu, P.L.; Chu, J.P.; Horng, R.H. Study on performance improvement of zinc oxide gas sensor with silver nanoparticles surface modification. Sens. Actuators B Chem. 2025, 438, 137803. [Google Scholar]
- Nguyen, V.H.; Bui, Q.T.P.; Vo, D.V.N.; Lim, K.T.; Bach, L.G.; Do, S.T.; Nguyen, T.V.; Doan, V.D.; Nguyen, T.D. Effective Photocatalytic Activity of Sulfate-Modified BiVO4 for the Decomposition of Methylene Blue Under LED Visible Light. Materials 2019, 12, 2681. [Google Scholar] [CrossRef] [PubMed]
- Kumar, E.T.D.; Easwaramoorthi, S.; Rao, J.R. Gold-reduced graphene oxide intimated BiVO4-ZnO mixed oxide composite with leveraged charge carrier transport under solar radiation. Opt. Mater. 2023, 142, 114054. [Google Scholar] [CrossRef]
- Ye, Z.; Xiao, X.; Chen, J.; Wang, Y. Fabrication of BiVO4/BiOBr composite with enhanced photocatalytic activity by a CTAB-assisted polyol method. J. Photochem. Photobiol. A Chem. 2019, 368, 153–161. [Google Scholar] [CrossRef]
- Han, C.; Yang, M.Q.; Weng, B.; Xu, Y.J. Improving the photocatalytic activity and anti-photocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys. Chem. Chem. Phys. 2014, 16, 16891–16903. [Google Scholar] [CrossRef]
- Tan, W.; Hu, G.J.; Tuo, L.M.; Zuo, W.Y.; Liu, Q.; Tong, H.J.; Zhu, Z.J.; Shi, B.F. Construction of metal–organic framework ZIF-8-derived heterostructured ZnO/BiVO4 for enhanced photocatalytic degradation of carbamazepine. New J. Chem. 2025, 49, 2803. [Google Scholar]
- Yan, L.; Zhao, W.; Liu, Z. 1D ZnO/BiVO4 heterojunction photoanodes for efficient photoelectrochemical water splitting. Dalton Trans. 2016, 45, 11346–11352. [Google Scholar] [CrossRef]
- Gindose, T.G.; Gebreslassie, G.; Godeto, Y.G.; Hailegebreal, T.D.; Atisme, T.B.; Zereffa, E.A. Synthesis of PVA-assisted MnO2-CuO-ZnO-g-C3N4 quaternary nanocomposite for the degradation of methylene blue from industrial wastewater. Heliyon 2024, 10, 40983. [Google Scholar] [CrossRef]
- Boutaleb, N.; Al-Senani, G.M.; Al-Qahtani, S.D.; Benyoucef, A.; Alkoudsi, B.D. Investigation and properties of PAni-coated CuO–ZnO–MnO quaternary hybrid as electrode material for supercapacitor application. Colloids Surf. A 2025, 718, 136867. [Google Scholar] [CrossRef]
- Ahmad, M.; Yousaf, M.; Cai, W.; Zhao, Z.P. Formulation of heterometallic ZIF-8@Cu/Ni/ZnO@CNTs heterostructure photocatalyst for Ultra-Deep desulphurization of coal and fuels. Chem. Eng. J. 2023, 453, 139846. [Google Scholar] [CrossRef]
- Liaqat, M.; Riaz, K.N.; Iqbal, T.; Nabi, G.; Rizwan, M.; Shakil, M. Fabrication of novel BiVO4/Bi2O3 heterostructure with superior visible light induced photocatalytic properties. Nanotechnology 2022, 34, 015711. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.Y.; Yang, H.; Zhang, J.; Lin, B.; Xu, J. Adjustable double cation vacancies MnSex/FeSex p-n heterojunction catalysts on improving multifunctional photocatalytic and photoelectrochemical performance. Sep. Purif. Technol. 2025, 355, 129642. [Google Scholar] [CrossRef]
- Islam, M.T.; Roni, M.N.P.; Ali, M.Y.; Islam, M.R.; Hossan, M.S.; Rahman, M.H.; Zahid, A.A.S.M.; Alam, M.N.E.; Hanif, M.A.; Akhtar, M.S. Selectivity of Sol-Gel and Hydrothermal TiO2 Nanoparticles towards Photocatalytic Degradation of Cationic and Anionic Dyes. Molecules 2023, 28, 6834. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, T.S.; Bajaj, H.C.; Tayade, R.J. Preferential adsorption behavior of methylene blue dye onto surface hydroxyl group enriched TiO2 nanotube and its photocatalytic regeneration. J. Colloid Interface Sci. 2014, 433, 104–114. [Google Scholar] [CrossRef]
- Guo, M.; Zhou, Z.; Yan, S.; Zhou, P.; Miao, F.; Liang, S.; Wang, J.; Cui, X. Bi2WO6-BiOCl heterostructure with enhanced photocatalytic activity for efficient degradation of oxytetracycline. Sci. Rep. 2020, 10, 18401. [Google Scholar] [CrossRef]
- Gao, Z.; Guan, X.J.; Zhang, T.; Dong, Y.C.; Zhu, B.; Zeng, W.G.; Ye, X.Y.; Guo, L.J. ZIF-8 Derived N-Doped Carbon for Selective Photocatalytic Degradation of Methylene Blue. Fuel 2024, 374, 132475. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Q.Q.; Li, K.K.; Zhang, Y.T.; Liu, E.Z.; Li, X. Recent advances on g-C3N4-based Z-scheme photocatalysts: Structural design and photocatalytic applications. Int. J. Hydrogen Energy 2022, 48, 196–231. [Google Scholar] [CrossRef]
- Zhou, G.H.; Meng, L.R.; Ning, X.; Yin, W.Q.; Hou, J.H.; Xu, Q.; Yi, J.J.; Wang, S.S.; Wang, X.Z. Switching charge transfer of g-C3N4/BiVO4 heterojunction from type II to Z-scheme via interfacial vacancy engineering for improved photocatalysis. Int. J. Hydrogen Energy 2022, 47, 8749–8760. [Google Scholar] [CrossRef]
- Kumar, A.; Khosla, A.; Sharma, S.K.; Dhiman, P.; Sharma, G.; Gnanasekaran, L.; Naushad, M.; Stadler, F.J. A review on S-scheme and dual S-scheme heterojunctions for photocatalytic hydrogen evolution, water detoxification and CO2 reduction. Fuel 2023, 333, 126267. [Google Scholar] [CrossRef]
- Wu, X.H.; Chen, C.Q.; Wang, J.; Li, J.M.; Wang, G.H. Review on S-Scheme Heterojunctions for Photocatalytic Hydrogen Evolution. Acta Phys. Chim. Sin. 2023, 39, 221201. [Google Scholar]
- Balachandran, S.; Prakash, N.; Thirumalai, K.; Muruganandham, M.; Sillanpää, M.; Swaminathan, M. Facile construction of heterostructured BiVO4–ZnO and its dual application of greater solar photocatalytic activity and self-cleaning property. Ind. Eng. Chem. Res. 2014, 53, 8346–8356. [Google Scholar]
- Wannakan, K.; Khansamrit, K.; Senasu, T.; Nanan, S. Ultrasound-Assisted synthesis of a ZnO/BiVO4 S-scheme heterojunction photocatalyst for degradation of the reactive red 141 dye and oxytetracycline antibiotic. ACS Omega 2023, 8, 4835–4852. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Yu, X.Y.; Zhou, Y.J.; Lin, Y.; Wu, Y. Nanostructured ZnO/BiVO4 I-scheme heterojunctions for piezocatalytic degradation of organic dyes via harvesting ultrasonic vibration energy. Int. J. Min. Met. Mater. 2025, 32, 488–497. [Google Scholar]


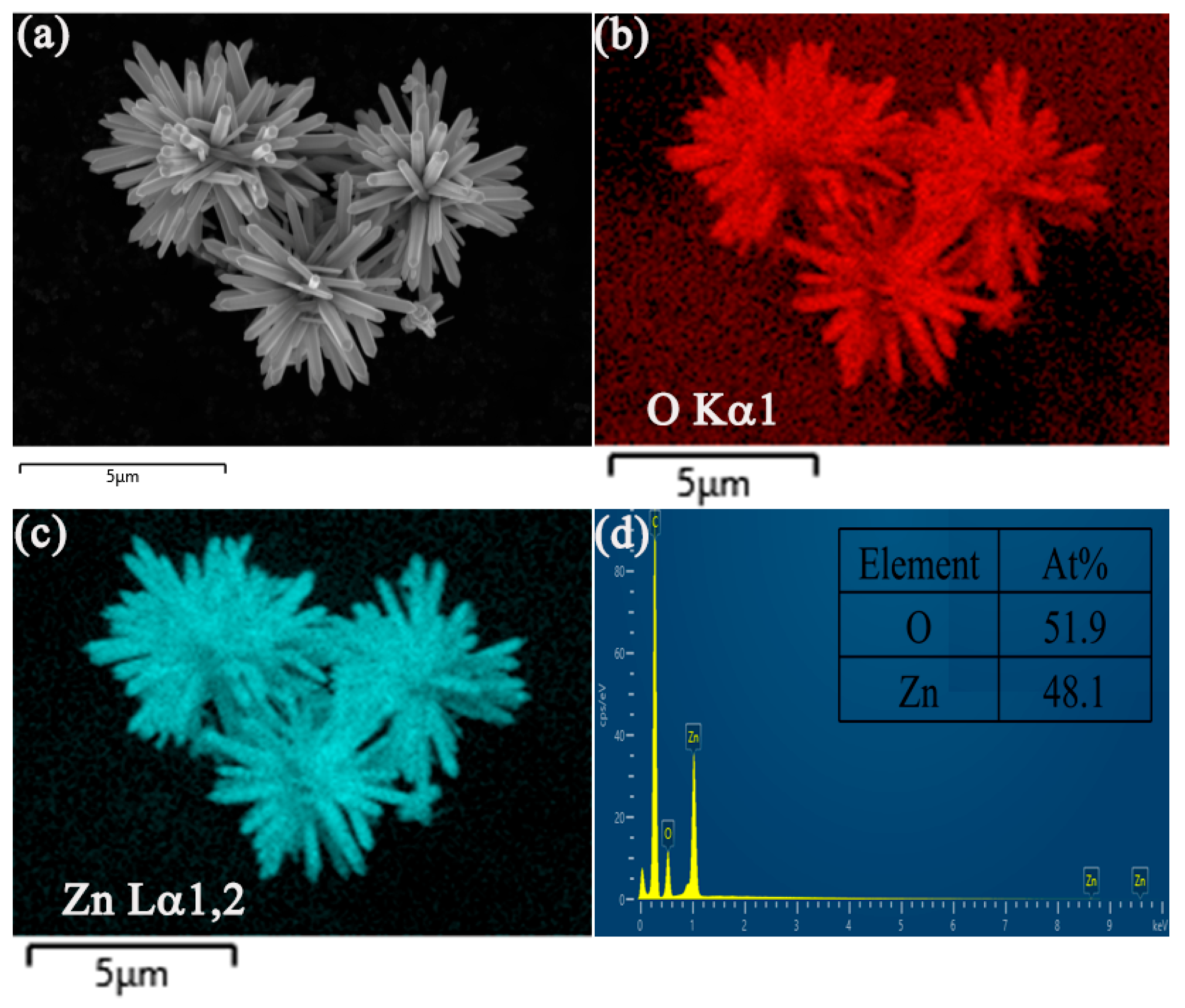
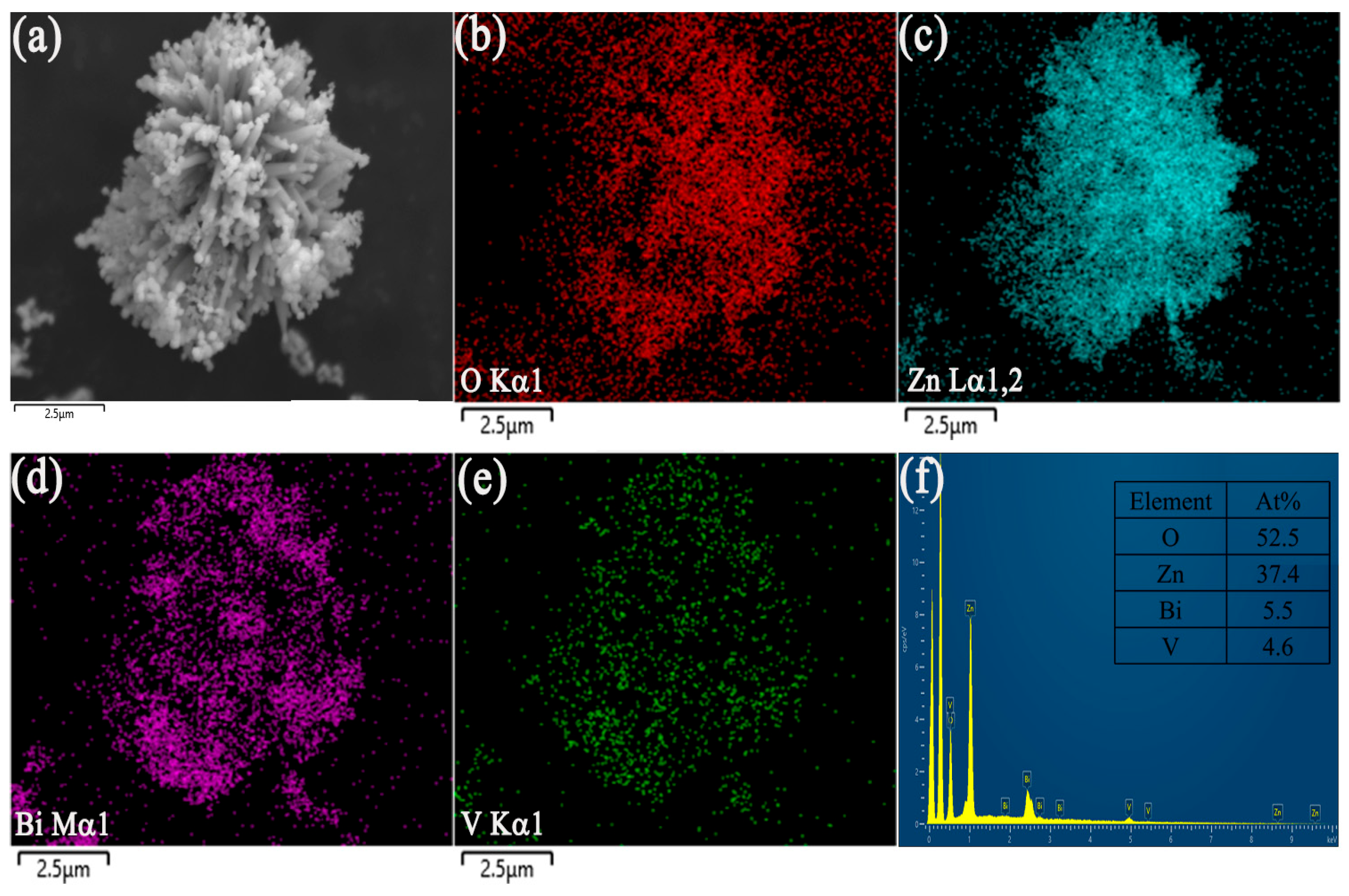
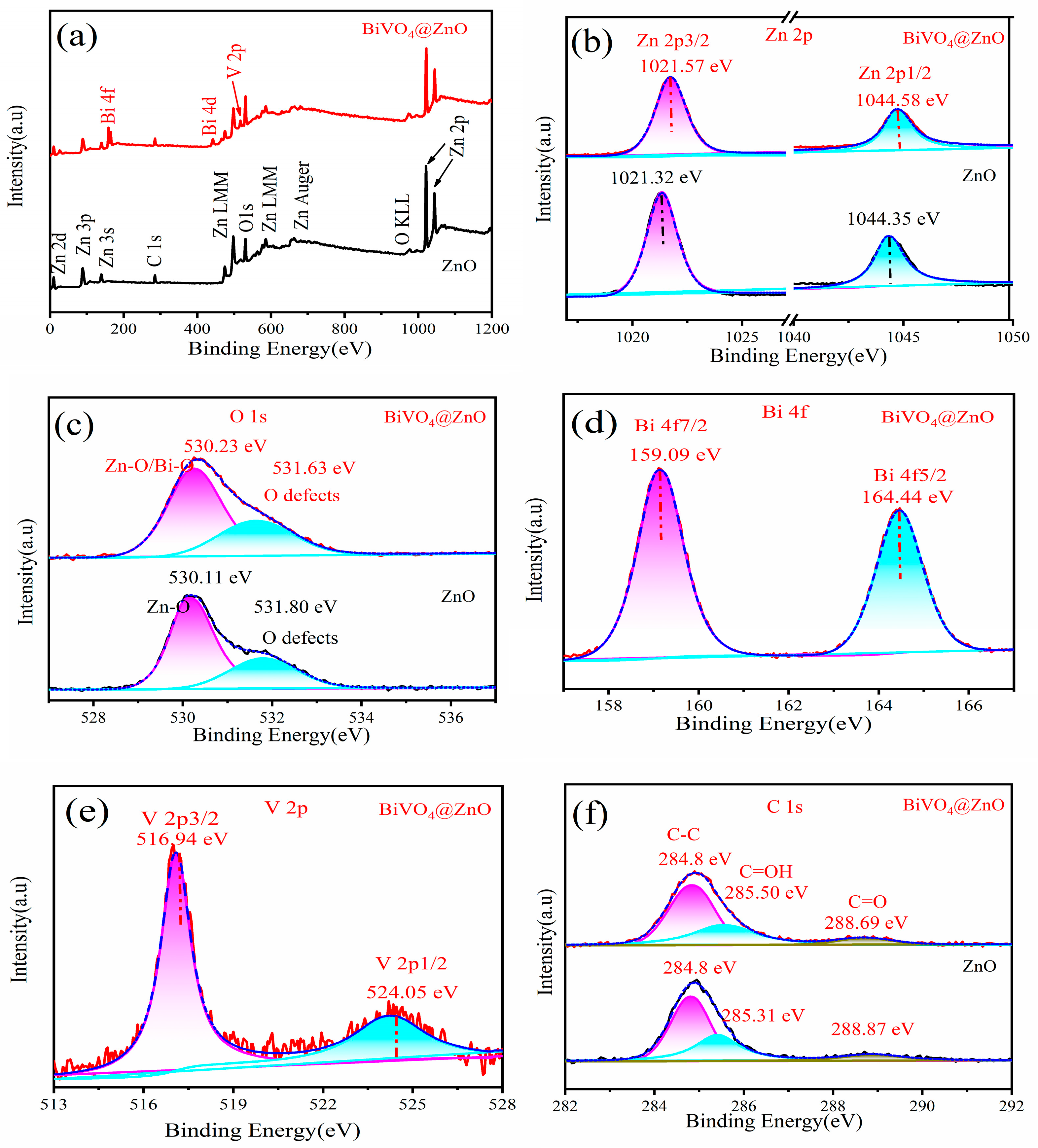
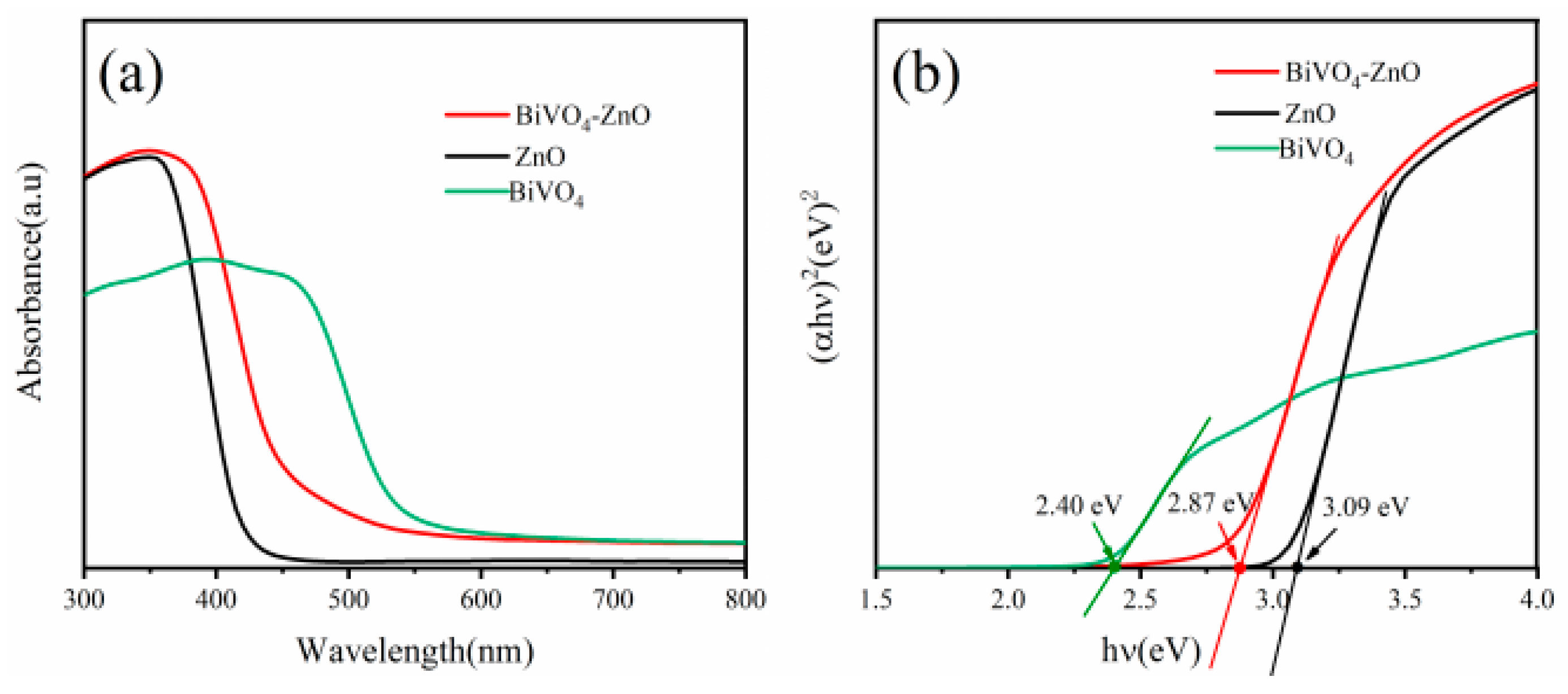
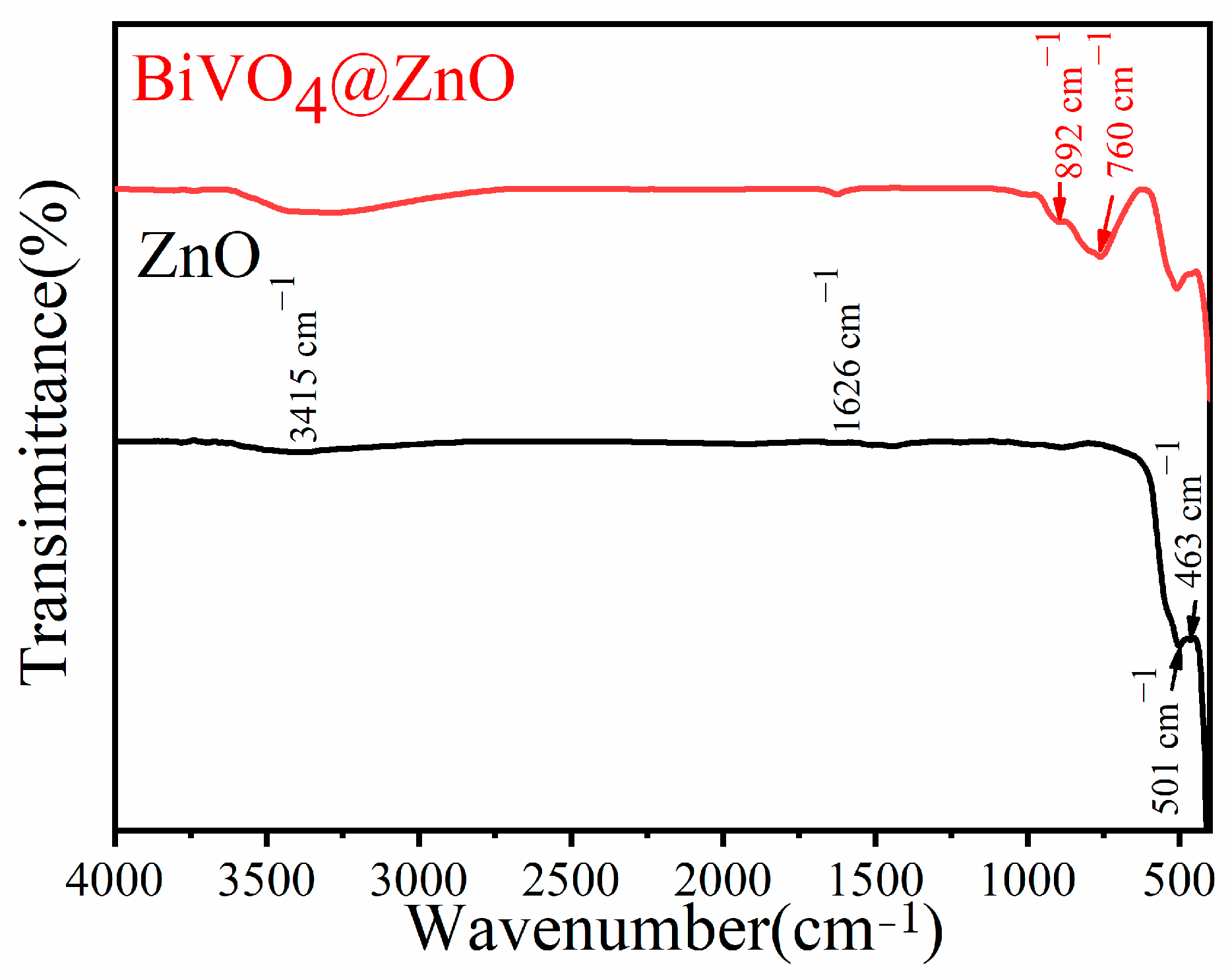
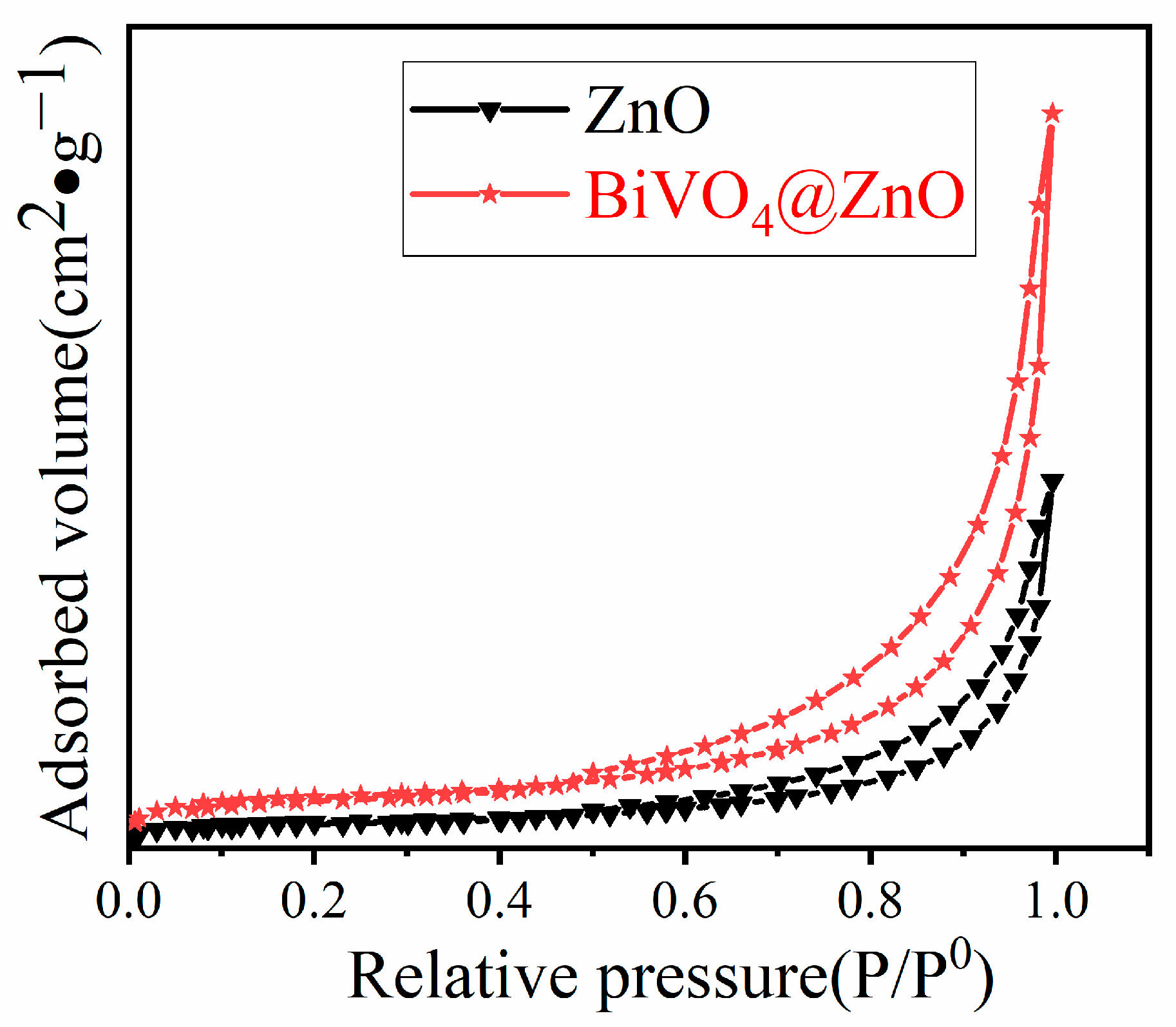
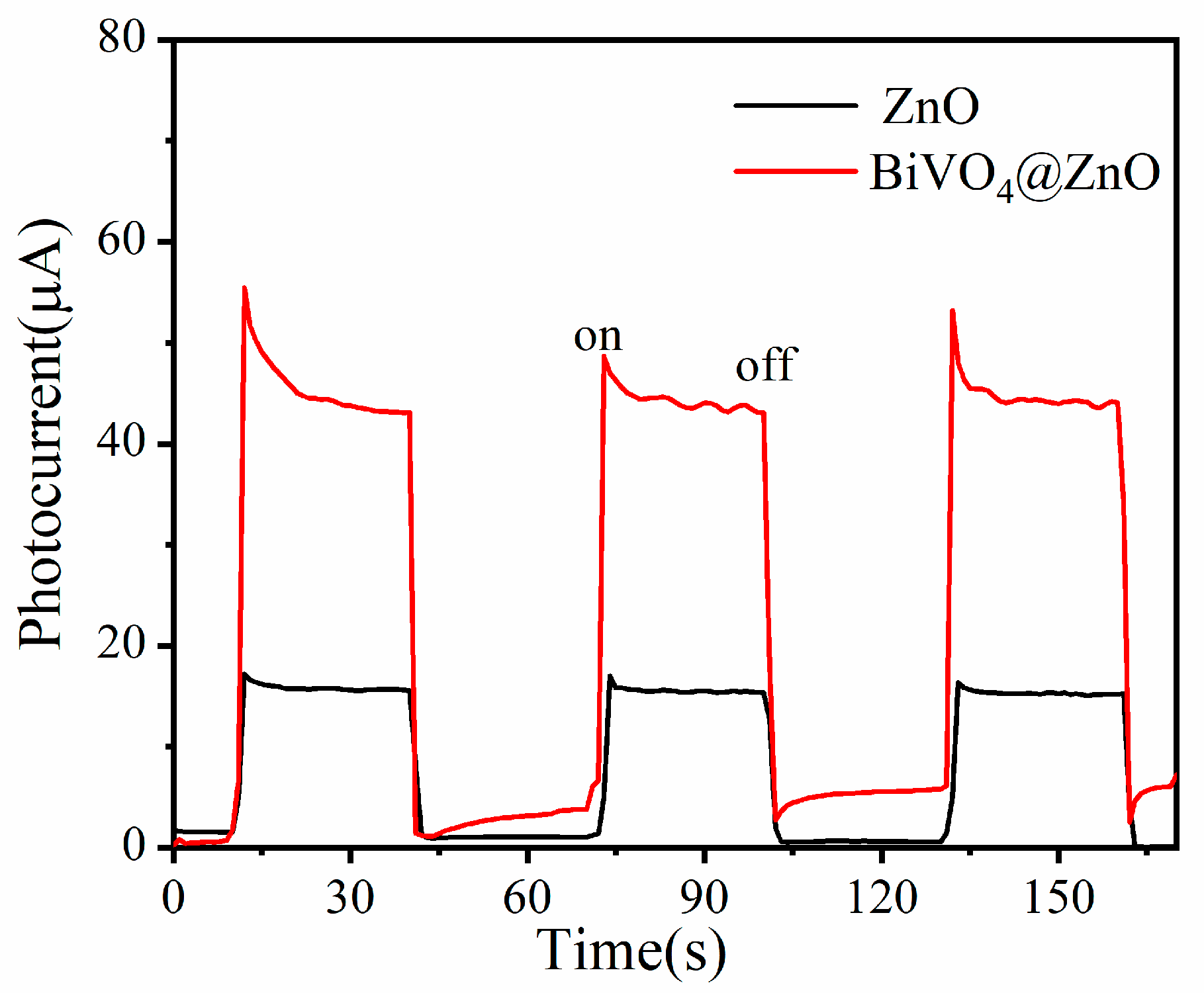
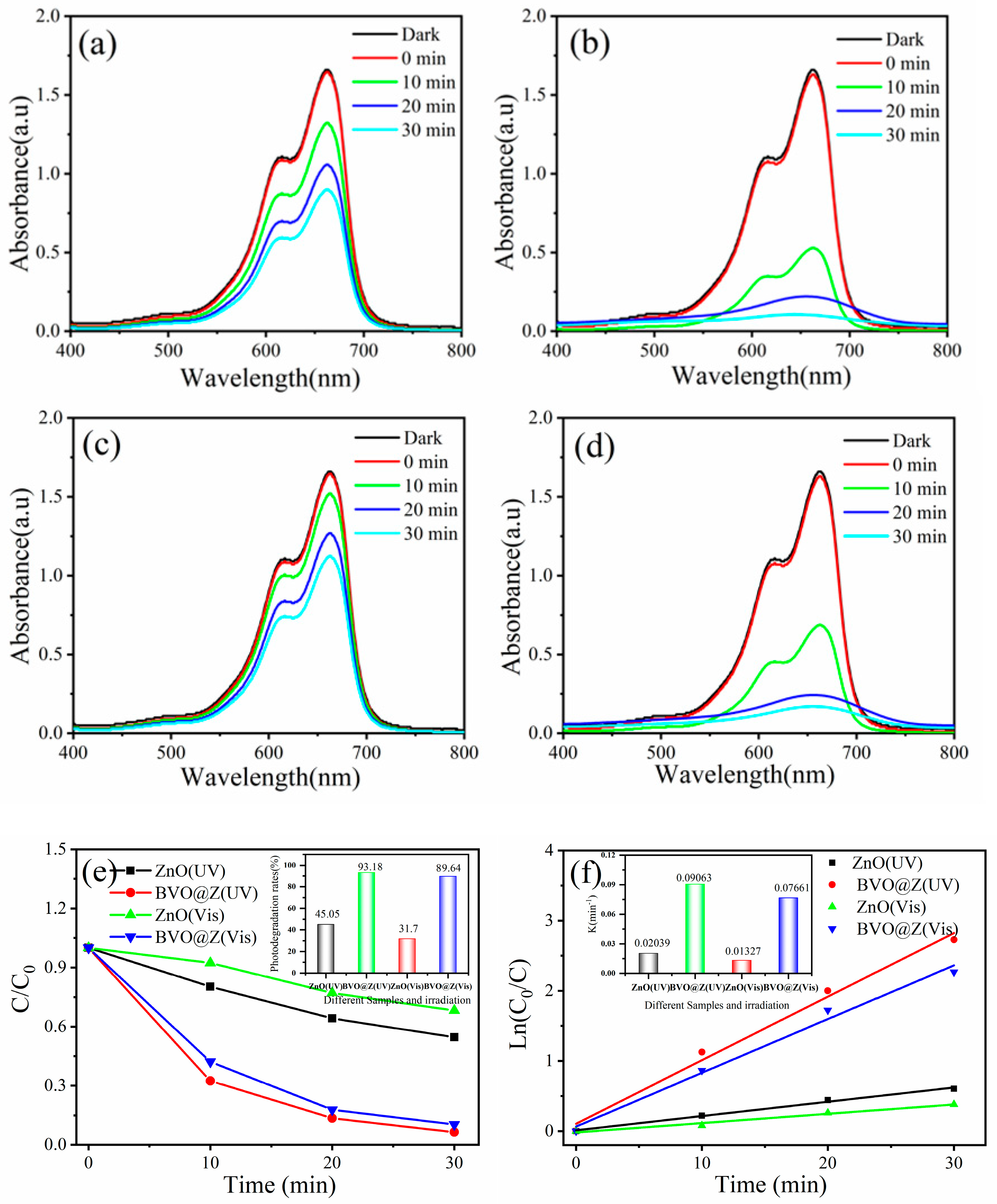
| Sample | BET Surface Area, (m2/g) |
| ZnO | 4.8432 |
| BiVO4@ZnO | 7.8308 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Li, N.; Liu, J.; Liu, Q.; Hui, X.; Li, Q. Preparation and Enhanced Catalytic Performance of a Polyhedral BiVO4-Nanoparticle-Modified ZnO Flower-like Nanorod Structure Composite Material. Nanomaterials 2025, 15, 1536. https://doi.org/10.3390/nano15191536
Lv Y, Li N, Liu J, Liu Q, Hui X, Li Q. Preparation and Enhanced Catalytic Performance of a Polyhedral BiVO4-Nanoparticle-Modified ZnO Flower-like Nanorod Structure Composite Material. Nanomaterials. 2025; 15(19):1536. https://doi.org/10.3390/nano15191536
Chicago/Turabian StyleLv, Yuanyuan, Neng Li, Jin Liu, Quanhui Liu, Xueqi Hui, and Qiang Li. 2025. "Preparation and Enhanced Catalytic Performance of a Polyhedral BiVO4-Nanoparticle-Modified ZnO Flower-like Nanorod Structure Composite Material" Nanomaterials 15, no. 19: 1536. https://doi.org/10.3390/nano15191536
APA StyleLv, Y., Li, N., Liu, J., Liu, Q., Hui, X., & Li, Q. (2025). Preparation and Enhanced Catalytic Performance of a Polyhedral BiVO4-Nanoparticle-Modified ZnO Flower-like Nanorod Structure Composite Material. Nanomaterials, 15(19), 1536. https://doi.org/10.3390/nano15191536






