Effect of Metal Modification of Activated Carbon on the Hydrogen Adsorption Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Activated Carbon
2.3. Modification of AC
2.4. Structural Characterisation
2.5. Hydrogen Adsorption Experiments
3. Results and Discussion
3.1. Characterization
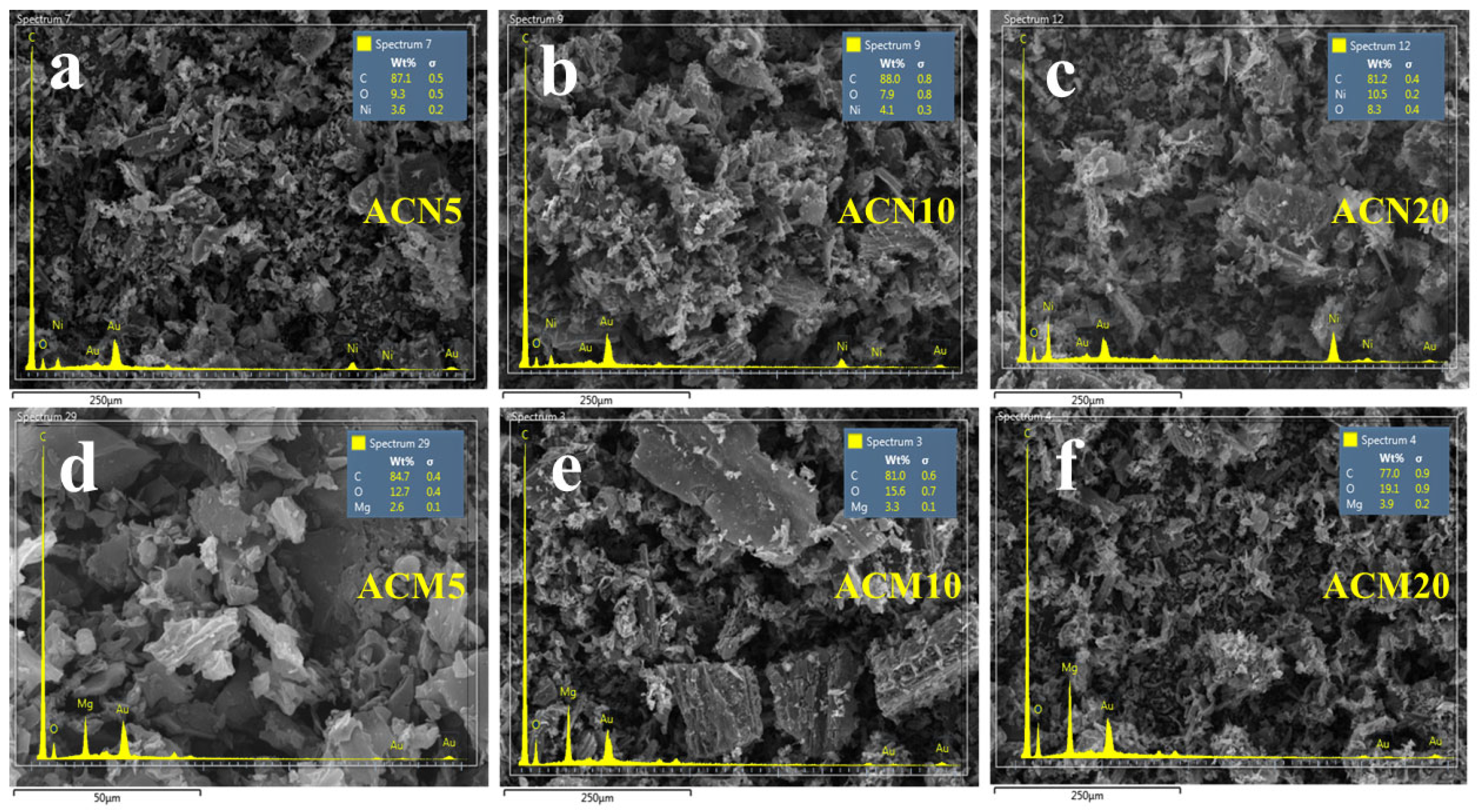
3.1.1. SEM Images and Elemental Analysis of Modified Activated Carbon
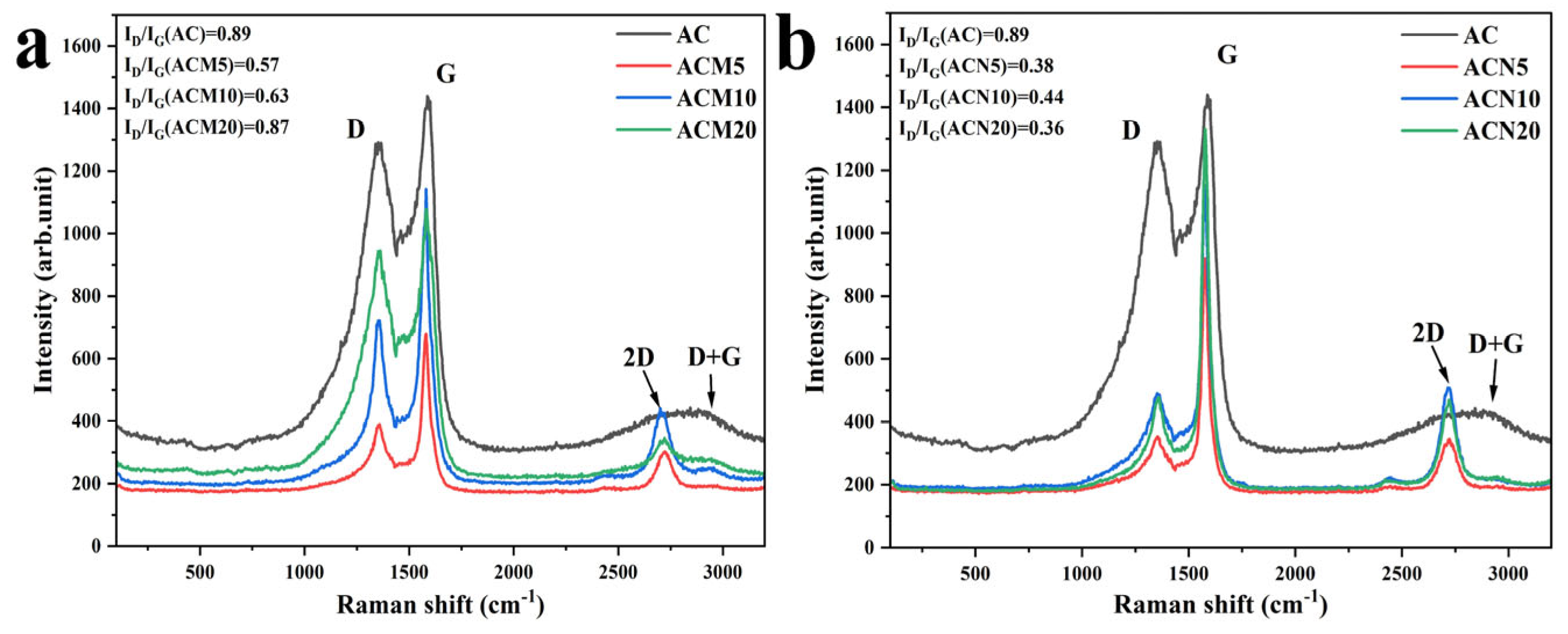
3.1.2. Raman Spectroscopy Analysis
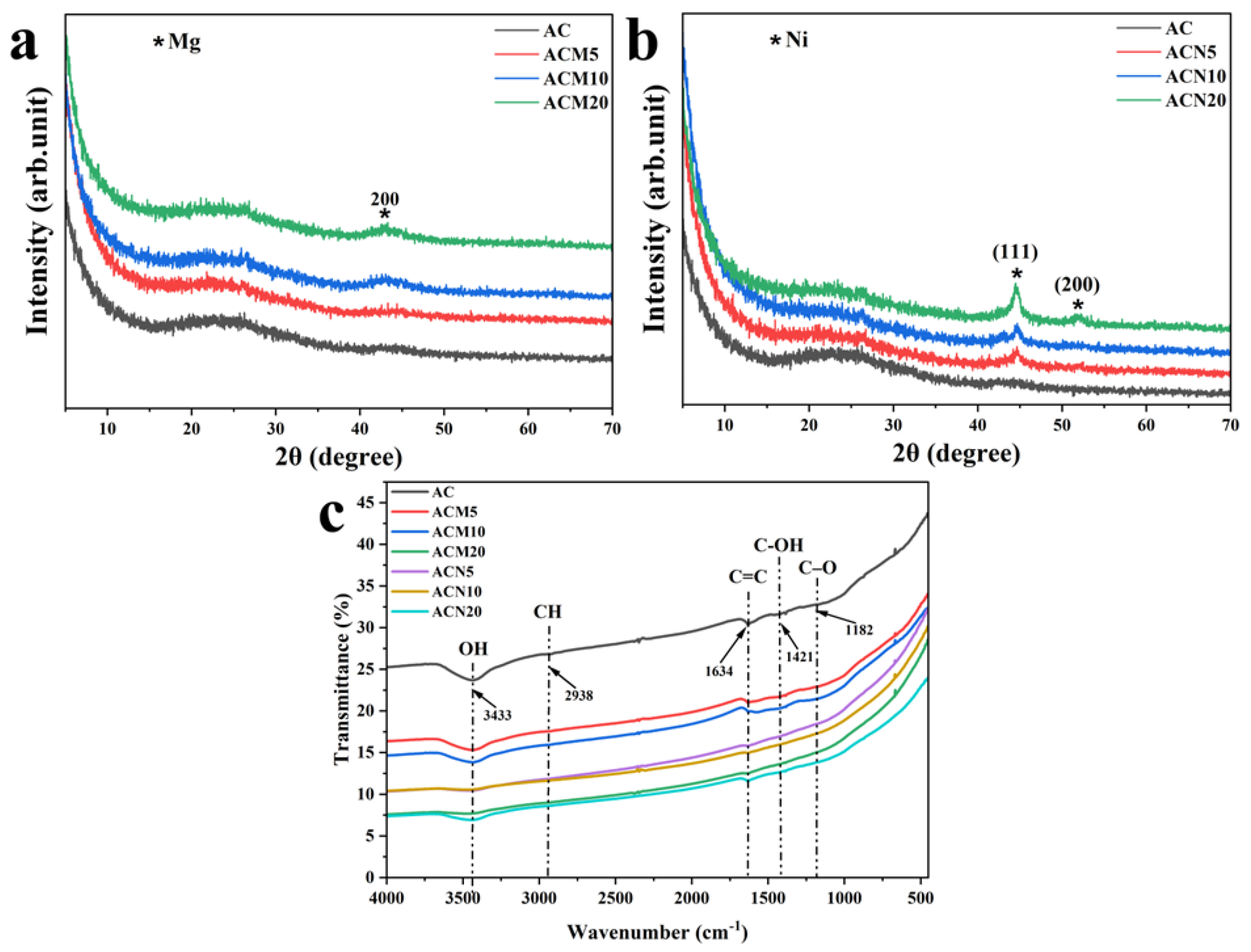
3.1.3. X–Ray Diffraction Analysis
3.1.4. FTIR and Textural Characterization
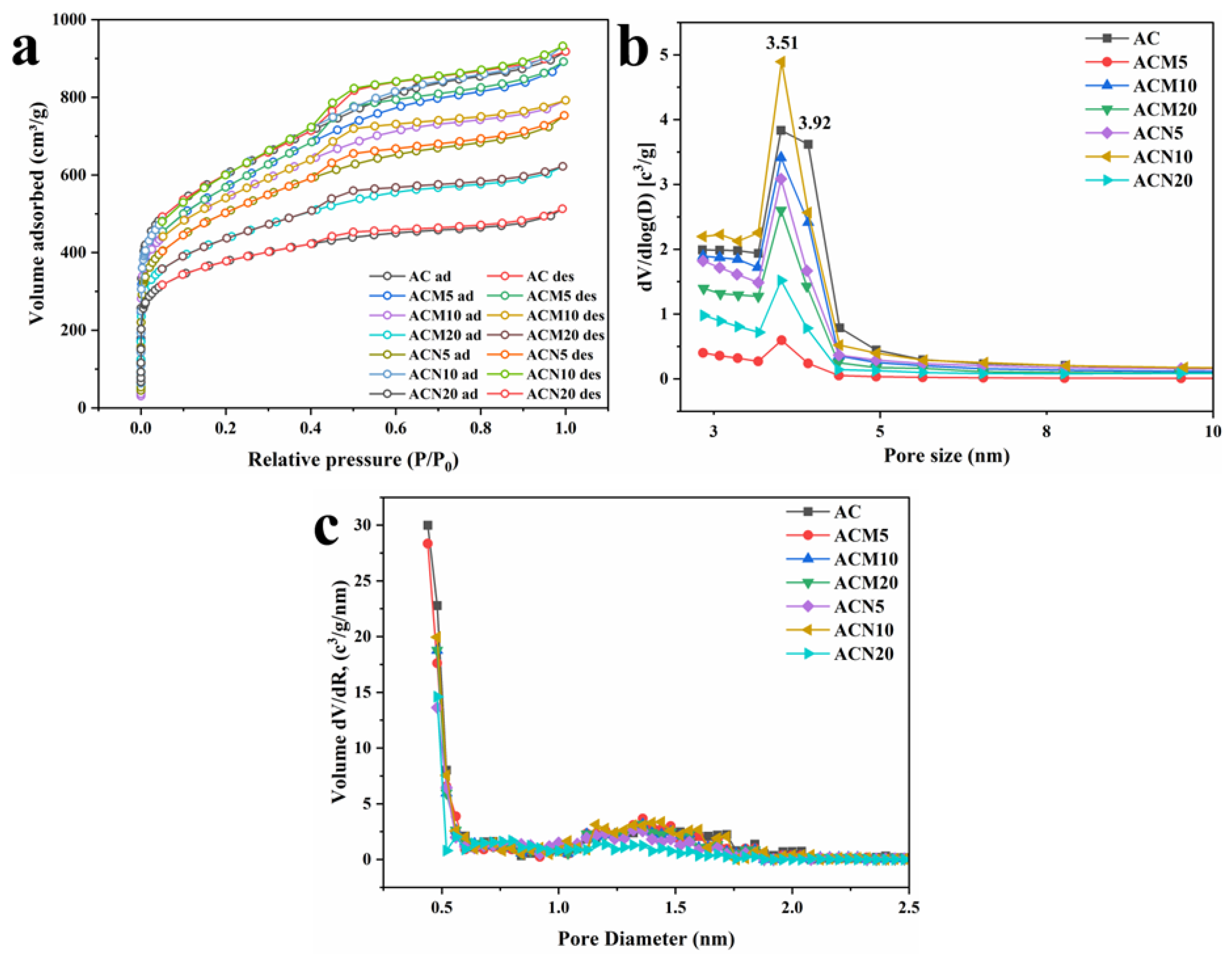
3.1.5. Nitrogen Adsorption–Desorption Isotherms and Pore Structure Analysis
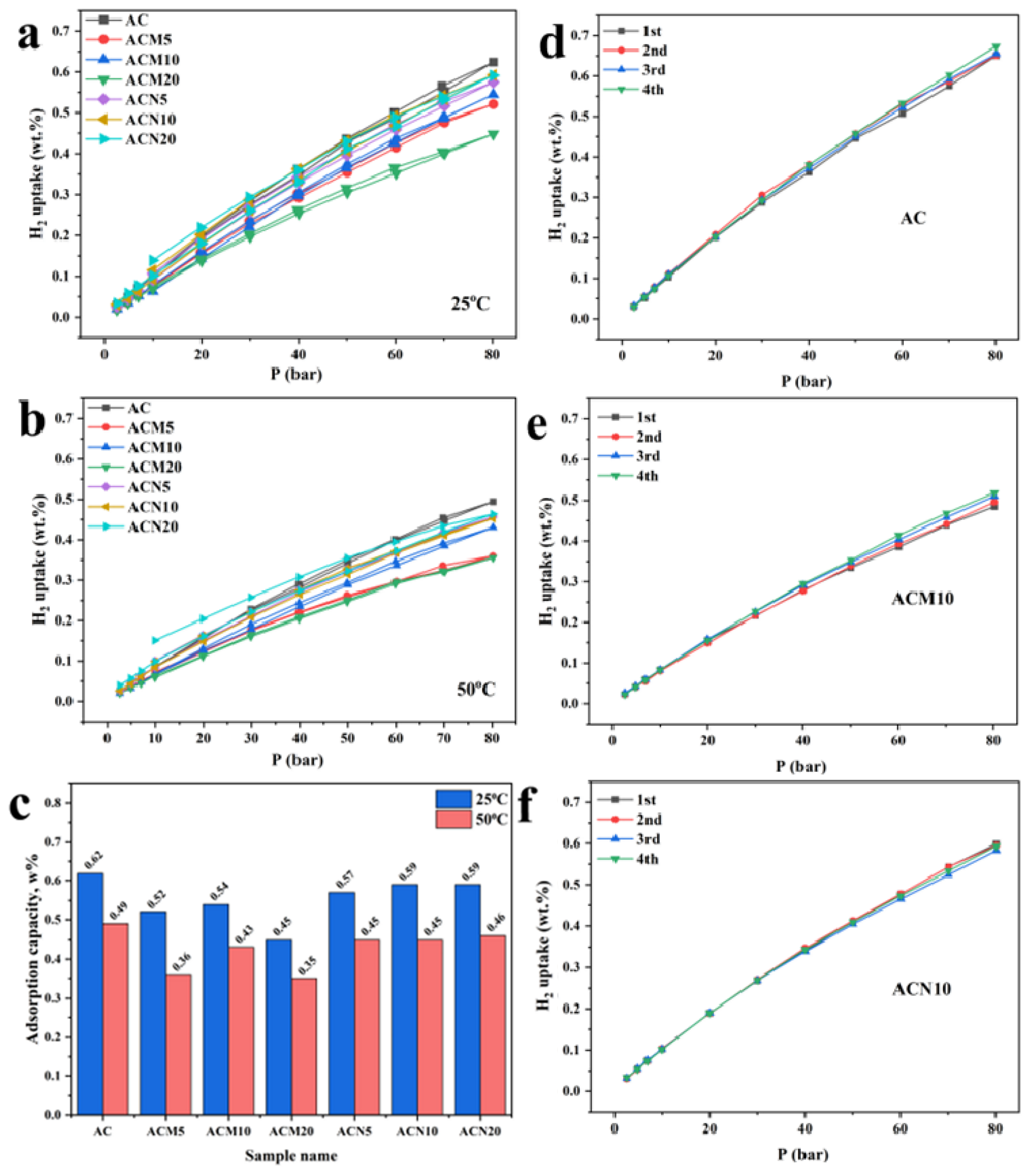
3.2. H2 Adsorption Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| AC | activated carbon; |
| ACM5/ACM10/ACM20 | activated carbon modified with Mg at loads of 5/10/20 wt%; |
| ACN5/ACN10/ACN20 | activated carbon modified with Ni at loads of 5/10/20 wt%; |
| SBET | BET specific surface area (m2/g); |
| Vμ | micropore volume (cm3/g); |
| Vm | mesopore volume (cm3/g); |
| q | adsorption capacity (wt%); |
| HPVA | High Pressure Volumetric Analyzer; |
| ΔHads | adsorption enthalpy (kJ/mol); |
| ΔGads | Gibbs free energy of adsorption (kJ/mol); |
| ΔSads | entropy of adsorption (kJ/mol); |
| ID/IG | ratio of intensities of D- and G-peaks (Raman). |
References
- Kamran, M.; Turzyński, M. Exploring Hydrogen Energy Systems: A Comprehensive Review of Technologies, Applications, Prevailing Trends, and Associated Challenges. J. Energy Storage 2024, 96, 112601. [Google Scholar] [CrossRef]
- Askaruly, K.; Idrissov, N.; Abdisattar, A.; Azat, S.; Kuli, Z.; Yeleuov, M.; Malchik, F.; Daulbayev, C.; Yszhan, Y.; Sarsembayeva, B.; et al. Utilizing Rice Husk-Derived Si/C Composites to Enhance Energy Capacity and Cycle Sustainability of Lithium-Ion Batteries. Diam. Relat. Mater. 2024, 149, 111631. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Serik, A.; Baratov, A.; Abdikarimova, U.; Idrissov, N.; Bissenova, M.; Daulbayev, C. Efficient Photocatalytic Hydrogen Evolution via Cocatalyst Loaded Al-Doped SrTiO3. Eurasian Chem.-Technol. J. 2024, 26, 133–140. [Google Scholar] [CrossRef]
- Serik, A.; Idrissov, N.; Baratov, A.; Dikov, A.; Kislitsin, S.; Daulbayev, C.; Kuspanov, Z. Recent Progress in Photocatalytic Applications of Electrospun Nanofibers: A Review. Molecules 2024, 29, 4824. [Google Scholar] [CrossRef] [PubMed]
- Bosu, S.; Rajamohan, N. Recent Advancements in Hydrogen Storage—Comparative Review on Methods, Operating Conditions and Challenges. Int. J. Hydrogen Energy 2024, 52, 352–370. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Serik, A.; Matsko, N.; Bissenova, M.; Issadykov, A.; Yeleuov, M.; Daulbayev, C. Efficient Photocatalytic Degradation of Methylene Blue via Synergistic Dual Co-Catalyst on SrTiO3@Al under Visible Light: Experimental and DFT Study. J. Taiwan Inst. Chem. Eng. 2024, 165, 105806. [Google Scholar] [CrossRef]
- Yelishenkov, A.; Kulsartov, T.; Chikhray, Y.; Kenzhina, I.; Zaurbekova, Z.; Askerbekov, S.; Kenzhin, Y.; Udartsev, S.; Podoinikov, M.; Shaimerdenov, A. Experiments on Investigation of Hydrogen Interaction with Titanium Beryllide Be2ti. 2025. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5153235 (accessed on 22 September 2025).
- Navaid, H.B.; Emadi, H.; Watson, M. A Comprehensive Literature Review on the Challenges Associated with Underground Hydrogen Storage. Int. J. Hydrogen Energy 2023, 48, 10603–10635. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen Storage in Carbon Materials—A Review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- De Rose, E.; Bartucci, S.; Poselle Bonaventura, C.; Conte, G.; Agostino, R.G.; Policicchio, A. Effects of Activation Temperature and Time on Porosity Features of Activated Carbons Derived from Lemon Peel and Preliminary Hydrogen Adsorption Tests. Colloids Surf. A Physicochem. Eng. Asp. 2023, 672, 131727. [Google Scholar] [CrossRef]
- Serik, A.; Kuspanov, Z.; Daulbayev, C. Cost-Effective Strategies and Technologies for Green Hydrogen Production. Renew. Sustain. Energy Rev. 2026, 226, 116242. [Google Scholar] [CrossRef]
- Sadeq, A.M.; Homod, R.Z.; Hussein, A.K.; Togun, H.; Mahmoodi, A.; Isleem, H.F.; Patil, A.R.; Moghaddam, A.H. Hydrogen Energy Systems: Technologies, Trends, and Future Prospects. Sci. Total Environ. 2024, 939, 173622. [Google Scholar] [CrossRef]
- Mohana, P.; Isacfranklin, M.; Yuvakkumar, R.; Ravi, G.; Kungumadevi, L.; Arunmetha, S.; Han, J.H.; Hong, S.I. Facile Synthesis of Ni-MgO/CNT Nanocomposite for Hydrogen Evolution Reaction. Nanomaterials 2024, 14, 280. Available online: https://www.preprints.org/manuscript/202312.1992/v1 (accessed on 30 June 2025). [CrossRef] [PubMed]
- Czakkel, O.; Nagy, B.; Dobos, G.; Fouquet, P.; Bahn, E.; László, K. Static and Dynamic Studies of Hydrogen Adsorption on Nanoporous Carbon Gels. Int. J. Hydrogen Energy 2019, 44, 18169–18178. [Google Scholar] [CrossRef]
- Yu, Y.; Ji, Y.; Zhang, S.; Wang, S.; Chen, Y.; Yong, H.; Liu, B.; Zhang, Y. Microstructure Characteristics, Hydrogen Storage Thermodynamic and Kinetic Properties of Mg–Ni–Y Based Hydrogen Storage Alloys. Int. J. Hydrogen Energy 2022, 47, 27059–27070. [Google Scholar] [CrossRef]
- Ren, W.; Zhuang, X.; Liu, Z.; Li, S. Hydrogen Adsorption Performance of Cu-BTC/Graphene Aerogel Composite: A Combined Experimental and Computational Study. Int. J. Hydrogen Energy 2021, 46, 13097–13105. [Google Scholar] [CrossRef]
- Department of Energy. Hydrogen Storage. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed on 30 June 2025).
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A Review on Metal Hydride Materials for Hydrogen Storage. J. Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. A Review on Current Trends in Potential Use of Metal-Organic Framework for Hydrogen Storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Cruz Junior, O.F.; Sreńscek-Nazzal, J. Design of Highly Microporous Activated Carbons Based on Walnut Shell Biomass for H2 and CO2 Storage. Carbon 2023, 201, 633–647. [Google Scholar] [CrossRef]
- Yu, Z.; Deschamps, J.; Hamon, L.; Karikkethu Prabhakaran, P.; Pré, P. Hydrogen Adsorption and Kinetics in MIL-101(Cr) and Hybrid Activated Carbon-MIL-101(Cr) Materials. Int. J. Hydrogen Energy 2017, 42, 8021–8031. [Google Scholar] [CrossRef]
- Yahia, M.B.; Wjihi, S. Study of the Hydrogen Physisorption on Adsorbents Based on Activated Carbon by Means of Statistical Physics Formalism: Modeling Analysis and Thermodynamics Investigation. Sci. Rep. 2020, 10, 16118. [Google Scholar] [CrossRef]
- Abdulkadir, B.A.; Ismail, M.; Setiabudi, H.D. Enhancing Hydrogen Adsorption through Optimized Magnesium Dispersion on Fibrous Nano-Silica Scaffold: Kinetic and Thermodynamic Studies. Microporous Mesoporous Mater. 2024, 378, 113232. [Google Scholar] [CrossRef]
- Thaweelap, N.; Plerdsranoy, P.; Poo-arporn, Y.; Khajondetchairit, P.; Suthirakun, S.; Fongkaew, I.; Hirunsit, P.; Chanlek, N.; Utke, O.; Pangon, A.; et al. Ni-Doped Activated Carbon Nanofibers for Storing Hydrogen at Ambient Temperature: Experiments and Computations. Fuel 2021, 288, 119608. [Google Scholar] [CrossRef]
- Khalit, W.N.A.W.; Marliza, T.S.; Asikin-Mijan, N.; Gamal, M.S.; Saiman, M.I.; Ibrahimef, M.L.; Taufiq-Yap, Y.H. Development of Bimetallic Nickel-Based Catalysts Supported on Activated Carbon for Green Fuel Production. RSC Adv. 2020, 10, 37218–37232. Available online: https://pubs.rsc.org/en/content/articlelanding/2020/ra/d0ra06302a (accessed on 30 June 2025). [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Wu, H.; Cheng, C.; Guo, Z.; Zhang, C. Carbohydrate-Based Activated Carbon with High Surface Acidity and Basicity for Nickel Removal from Synthetic Wastewater. RSC Adv. 2015, 5, 52048–52056. [Google Scholar] [CrossRef]
- Khalil, A.K.A.; Dweiri, F.; Almanassra, I.W.; Chatla, A.; Atieh, M.A. Mg-Al Layered Double Hydroxide Doped Activated Carbon Composites for Phosphate Removal from Synthetic Water: Adsorption and Thermodynamics Studies. Sustainability 2022, 14, 6991. Available online: https://www.mdpi.com/2071-1050/14/12/6991 (accessed on 30 June 2025). [CrossRef]
- Yneni, V.; Punugoti, T.; Kala, N.; Anidarapu, N.; Vangalapati, M. Modelling and Optimization of Methylene Blue Adsorption onto Magnesium Oxide Nanoparticles Loaded onto Activated Carbon (MgONP-AC): Response Surface Methodology and Artificial Neural Networks. Mater. Today Proc. 2019, 18, 4932–4941. [Google Scholar] [CrossRef]
- Khalil, A.K.A.; Almanassra, I.W.; Chatla, A.; Ihsanullah, I.; Laoui, T.; Ali Atieh, M. Insights into the Adsorption of Lead Ions by Mg-Al LDH Doped Activated Carbon Composites: Implications for Fixed Bed Column and Batch Applications. Chem. Eng. Sci. 2023, 281, 119192. [Google Scholar] [CrossRef]
- How, G.T.S.; Pandikumar, A.; Ming, H.N.; Ngee, L.H. Highly Exposed {001} Facets of Titanium Dioxide Modified with Reduced Graphene Oxide for Dopamine Sensing. Sci. Rep. 2014, 4, 5044. [Google Scholar] [CrossRef]
- Galaburda, M.; Kovalska, E.; Hogan, B.T.; Baldycheva, A.; Nikolenko, A.; Dovbeshko, G.I.; Oranska, O.I.; Bogatyrov, V.M. Mechanochemical Synthesis of Carbon-Stabilized Cu/C, Co/C and Ni/C Nanocomposites with Prolonged Resistance to Oxidation. Sci. Rep. 2019, 9, 17435. [Google Scholar] [CrossRef]
- Koo-amornpattana, W.; Phadungbut, P.; Kunthakudee, N.; Jonglertjunya, W.; Ratchahat, S. Innovative Metal Oxides (CaO, SrO, MgO) Impregnated Waste-Derived Activated Carbon for Biohydrogen Purification. Sci. Rep. 2023, 13, 4705. Available online: https://www.nature.com/articles/s41598-023-31723-4 (accessed on 30 June 2025). [CrossRef]
- Chokradjaroen, C.; Watanabe, H.; Ishii, T.; Ishizaki, T. Simultaneous Synthesis of Graphite-like and Amorphous Carbon Materials via Solution Plasma and Their Evaluation as Additive Materials for Cathode in Li–O2 Battery. Sci. Rep. 2021, 11, 6261. [Google Scholar] [CrossRef]
- Demiral, İ.; Samdan, C.; Demiral, H. Enrichment of the Surface Functional Groups of Activated Carbon by Modification Method. Surf. Interfaces 2021, 22, 100873. [Google Scholar] [CrossRef]
- Mopoung, S.; Dejang, N. Activated Carbon Preparation from Eucalyptus Wood Chips Using Continuous Carbonization–Steam Activation Process in a Batch Intermittent Rotary Kiln. Sci. Rep. 2021, 11, 13948. [Google Scholar] [CrossRef] [PubMed]
- Tipo, R.; Chaichana, C.; Noda, R.; Chaiklangmuang, S. Influence of Coal Treatments on the Ni Loading Mechanism of Ni-Loaded Lignite Char Catalysts. RSC Adv. 2021, 11, 35624–35643. [Google Scholar] [CrossRef] [PubMed]
- Adio, S.O.; Ganiyu, S.A.; Usman, M.; Abdulazeez, I.; Alhooshani, K. Facile and Efficient Nitrogen Modified Porous Carbon Derived from Sugarcane Bagasse for CO2 Capture: Experimental and DFT Investigation of Nitrogen Atoms on Carbon Frameworks. Chem. Eng. J. 2020, 382, 122964. [Google Scholar] [CrossRef]
- Ramirez-Vidal, P.; Sdanghi, G.; Celzard, A.; Fierro, V. High Hydrogen Release by Cryo-Adsorption and Compression on Porous Materials. Int. J. Hydrogen Energy 2022, 47, 8892–8915. [Google Scholar] [CrossRef]
- Worth, J.D.; Seddon, A.M.; Ting, V.P.; Faul, C.F.J. Polytriphenylamine Conjugated Microporous Polymers as Versatile Platforms for Tunable Hydrogen Storage. Small 2025, 21, e2407292. Available online: https://onlinelibrary.wiley.com/doi/10.1002/smll.202407292 (accessed on 30 June 2025).
- Kou, T.; Chen, M.; Wu, F.; Smart, T.J.; Wang, S.; Wu, Y.; Zhang, Y.; Li, S.; Lall, S.; Zhang, Z.; et al. Carbon Doping Switching on the Hydrogen Adsorption Activity of NiO for Hydrogen Evolution Reaction. Nat. Commun. 2020, 11, 590. [Google Scholar] [CrossRef]
- Song, F.; Huang, L.; Ding, H.; Zhang, S.; Yu, J. In Situ Ni-Doped Hierarchically Porous Carbon Nanofibers Derived from Polyacrylonitrile/Pitch for Hydrogen Storage at Ambient Temperature. Sustainability 2023, 15, 8722. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Solis, C.; Ramírez de la Piscina, P.; Homs, N. Medium-Pressure Hydrogen Storage on Activated Carbon Derived from Biomass Conversion. Fuel 2024, 363, 130975. [Google Scholar] [CrossRef]
- Lee, J.H.; Suh, D.H. Entropy, Enthalpy, and Gibbs Free Energy Variations of 133Cs via CO2-Activated Carbon Filter and Ferric Ferrocyanide Hybrid Composites. Nucl. Eng. Technol. 2021, 53, 3711–3716. [Google Scholar] [CrossRef]
| Metal Loading | Mass of Mg (g) | Mass of Mg(NO3)2·6H2O (g) | Mass of Ni (g) | Mass of Ni(NO3)2·6H2O (g) |
|---|---|---|---|---|
| 5% | 0.1 | 1.05 | 0.1 | 0.5 |
| 10% | 0.2 | 2.1 | 0.2 | 0.99 |
| 20% | 0.4 | 4.2 | 0.4 | 1.98 |
| Sample | BET Surface Area (m2/g) | Langmuir Surface Area (m2/g) | VT (P/P0 = 0.99) (cm3/g) | Vμ (cm3/g) |
Vm (cm3/g) | V μ/ VT | Vm/VT |
Dp (nm) |
|---|---|---|---|---|---|---|---|---|
| AC | 2164 | 2245 | 1.43 | 0.22 | 0.93 | 0.16 | 0.65 | 3.46 |
| ACM5 | 2034 | 2077 | 1.38 | 0.15 | 0.94 | 0.11 | 0.68 | 3.44 |
| ACM10 | 1935 | 2005 | 1.23 | 0.18 | 0.81 | 0.15 | 0.66 | 3.28 |
| ACM20 | 1579 | 1635 | 0.97 | 0.16 | 0.57 | 0.16 | 0.59 | 3.52 |
| ACN5 | 1809 | 1859 | 1.17 | 0.13 | 0.72 | 0.11 | 0.61 | 3.36 |
| ACN10 | 2147 | 2406 | 1.44 | 0.16 | 1.03 | 0.11 | 0.71 | 3.52 |
| ACN20 | 1381 | 1450 | 0.79 | 0.16 | 1.86 | 0.2 | 0.5 | 3.72 |
| Sample | ΔHads (kJ/mol) | ΔGads (kJ/mol) | ΔSads (J/K·mol) |
|---|---|---|---|
| AC | −11.81 | −0.03 | 40.03 |
| ACM5 | −11.77 | −0.03 | 39.9 |
| ACM10 | −11.88 | −0.03 | 40.59 |
| ACM20 | −11.84 | −0.03 | 40.15 |
| ACN5 | −11.66 | −0.03 | 39.53 |
| ACN10 | −11.64 | −0.03 | 39.46 |
| ACN20 | −11.62 | −0.03 | 39.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrissov, N.; Aidarbekov, N.; Kuspanov, Z.; Askaruly, K.; Tsurtsumia, O.; Kuterbekov, K.; Zeinulla, Z.; Bekmyrza, K.; Kabyshev, A.; Kubenova, M.; et al. Effect of Metal Modification of Activated Carbon on the Hydrogen Adsorption Capacity. Nanomaterials 2025, 15, 1503. https://doi.org/10.3390/nano15191503
Idrissov N, Aidarbekov N, Kuspanov Z, Askaruly K, Tsurtsumia O, Kuterbekov K, Zeinulla Z, Bekmyrza K, Kabyshev A, Kubenova M, et al. Effect of Metal Modification of Activated Carbon on the Hydrogen Adsorption Capacity. Nanomaterials. 2025; 15(19):1503. https://doi.org/10.3390/nano15191503
Chicago/Turabian StyleIdrissov, Nurlan, Nursultan Aidarbekov, Zhengisbek Kuspanov, Kydyr Askaruly, Olga Tsurtsumia, Kairat Kuterbekov, Zhassulan Zeinulla, Kenzhebatyr Bekmyrza, Asset Kabyshev, Marzhan Kubenova, and et al. 2025. "Effect of Metal Modification of Activated Carbon on the Hydrogen Adsorption Capacity" Nanomaterials 15, no. 19: 1503. https://doi.org/10.3390/nano15191503
APA StyleIdrissov, N., Aidarbekov, N., Kuspanov, Z., Askaruly, K., Tsurtsumia, O., Kuterbekov, K., Zeinulla, Z., Bekmyrza, K., Kabyshev, A., Kubenova, M., & Serik, A. (2025). Effect of Metal Modification of Activated Carbon on the Hydrogen Adsorption Capacity. Nanomaterials, 15(19), 1503. https://doi.org/10.3390/nano15191503






