Ecological Synthesis of Precious Metal Nanoparticles: Harnessing the Potential of Marine Algae Biomass
Abstract
1. Introduction
2. Precious Metal Nanoparticles: Characteristics and Applications
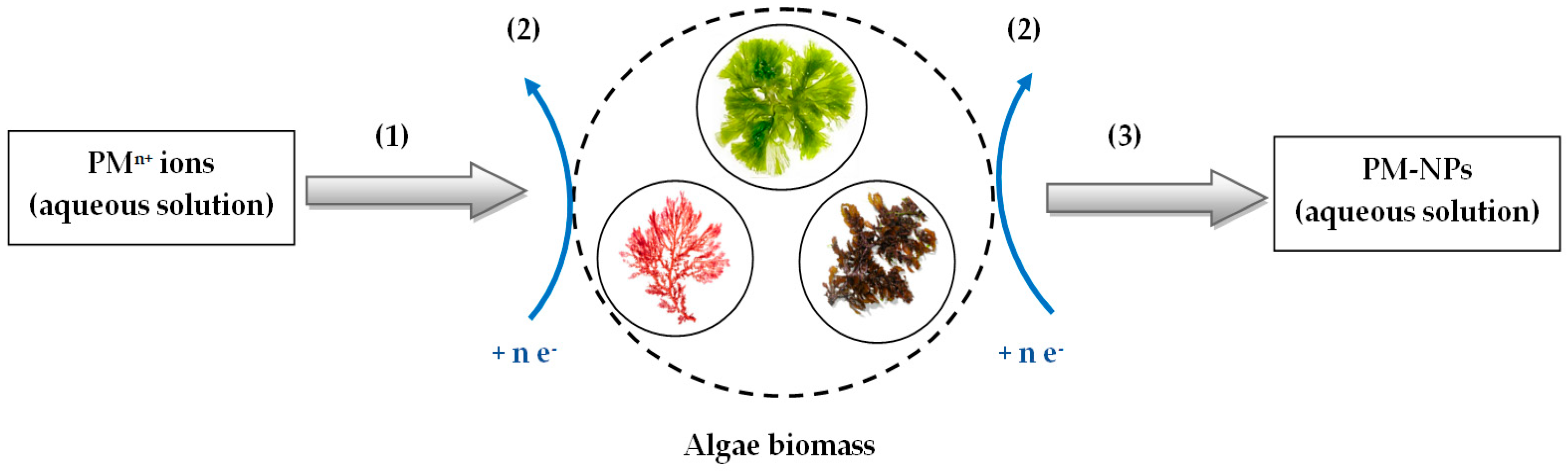
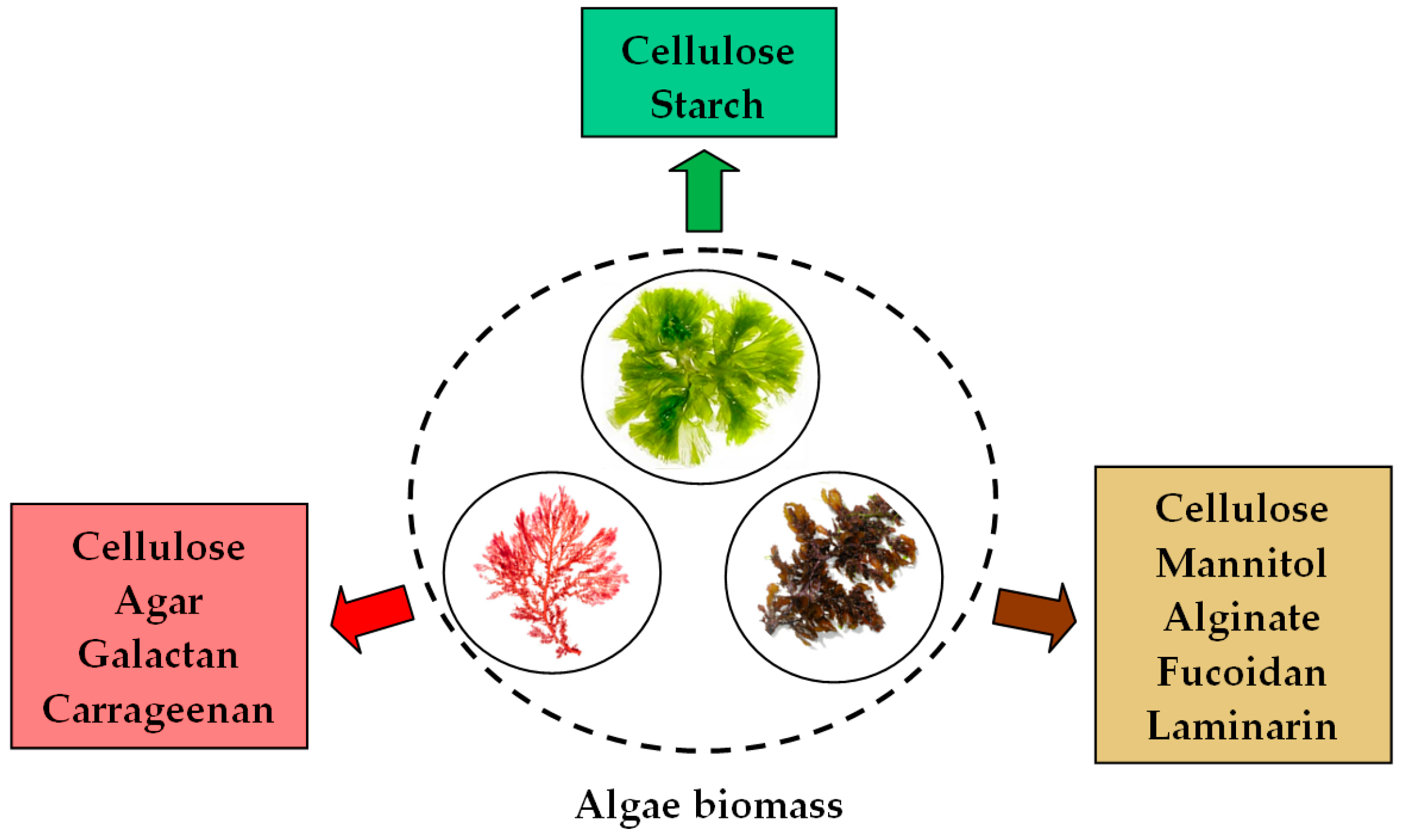
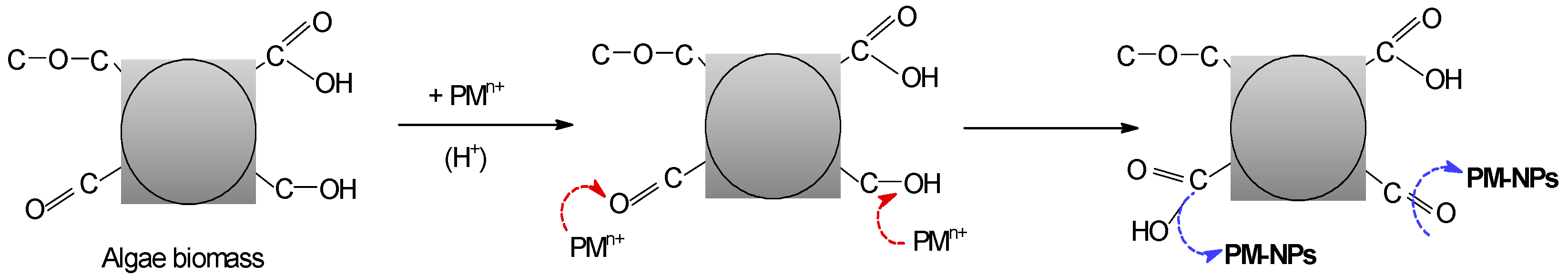
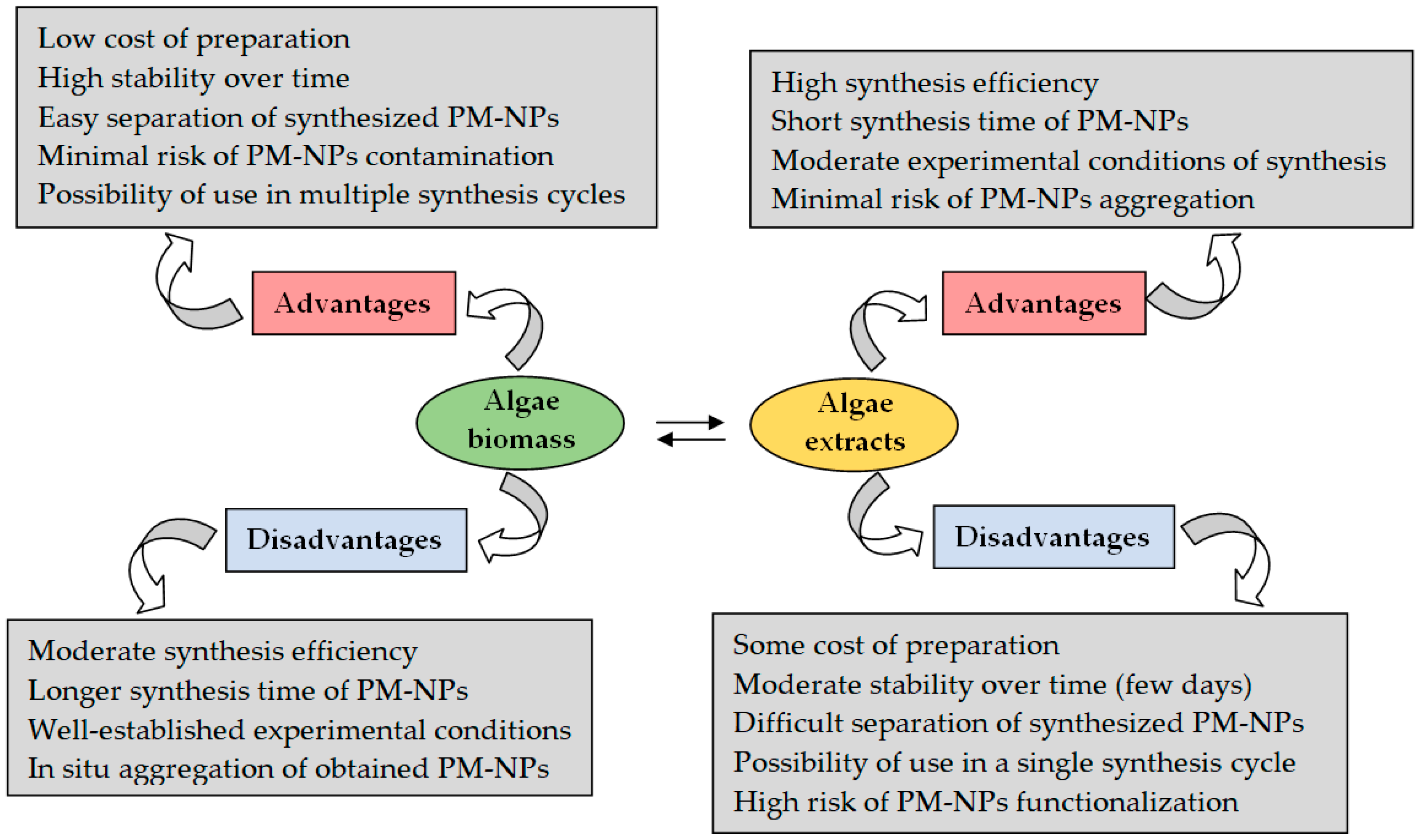
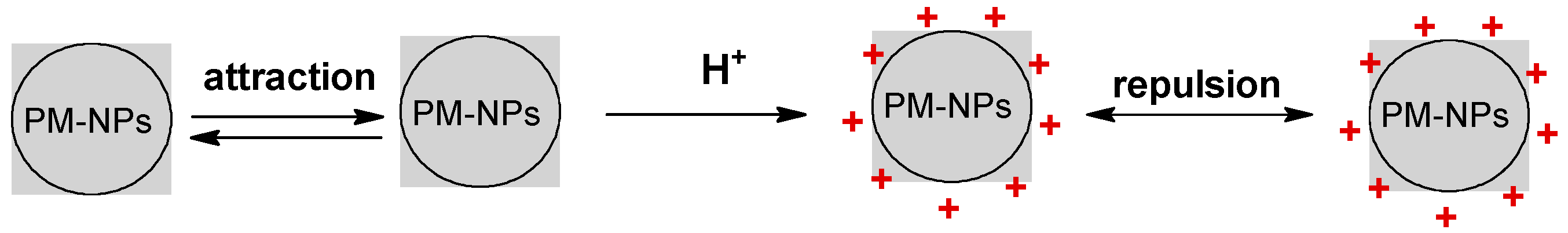
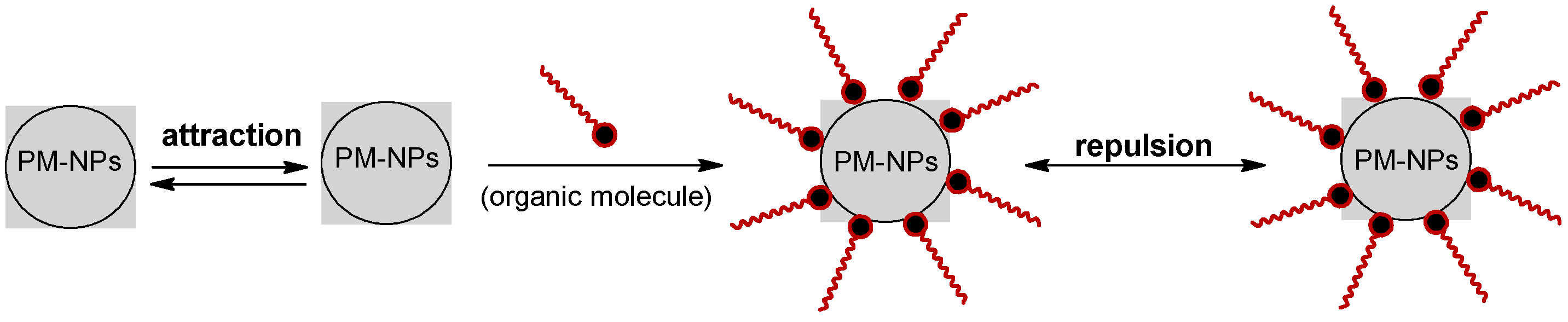
3. Synthesis Mechanism of PM-NPs Using Algae Biomass
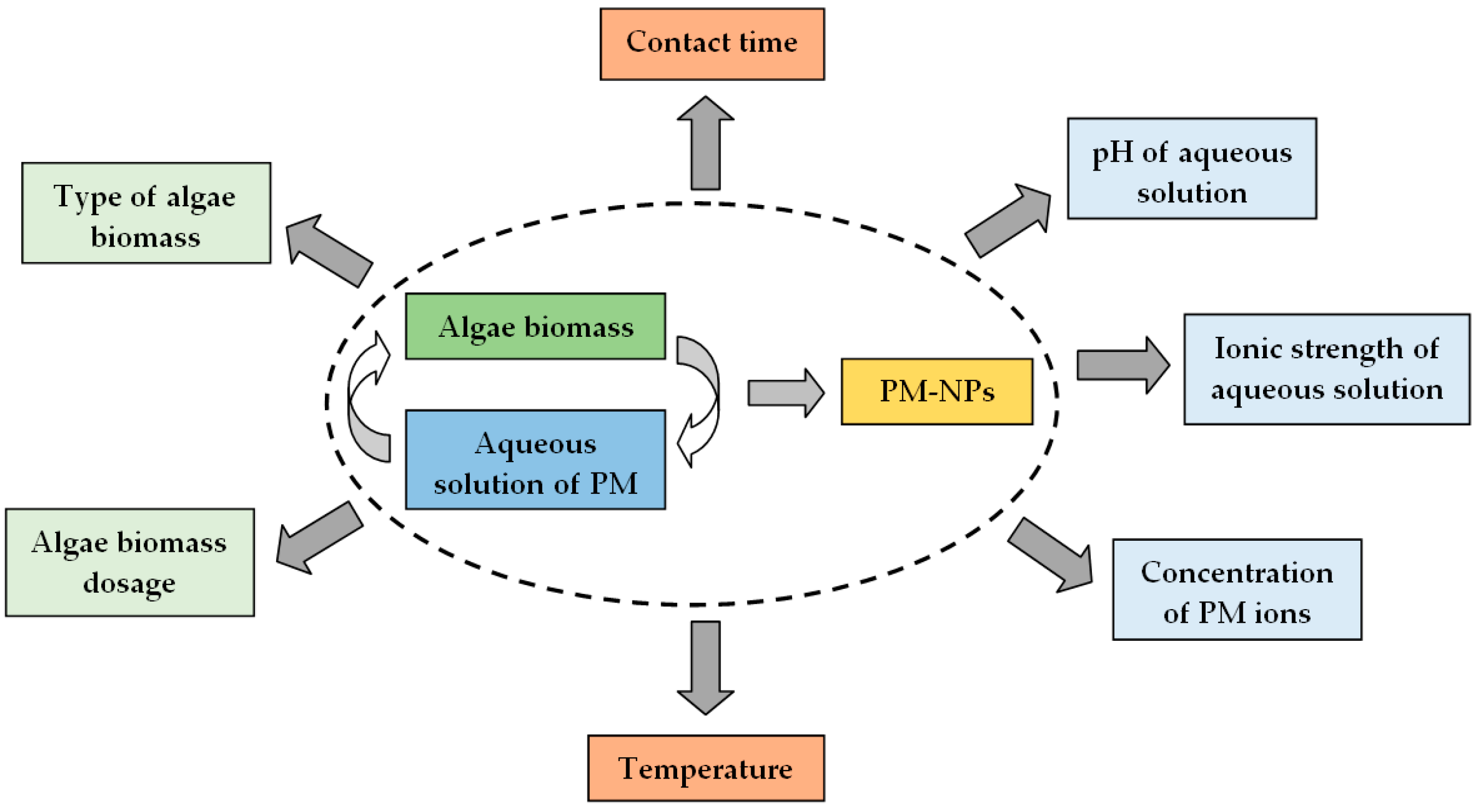
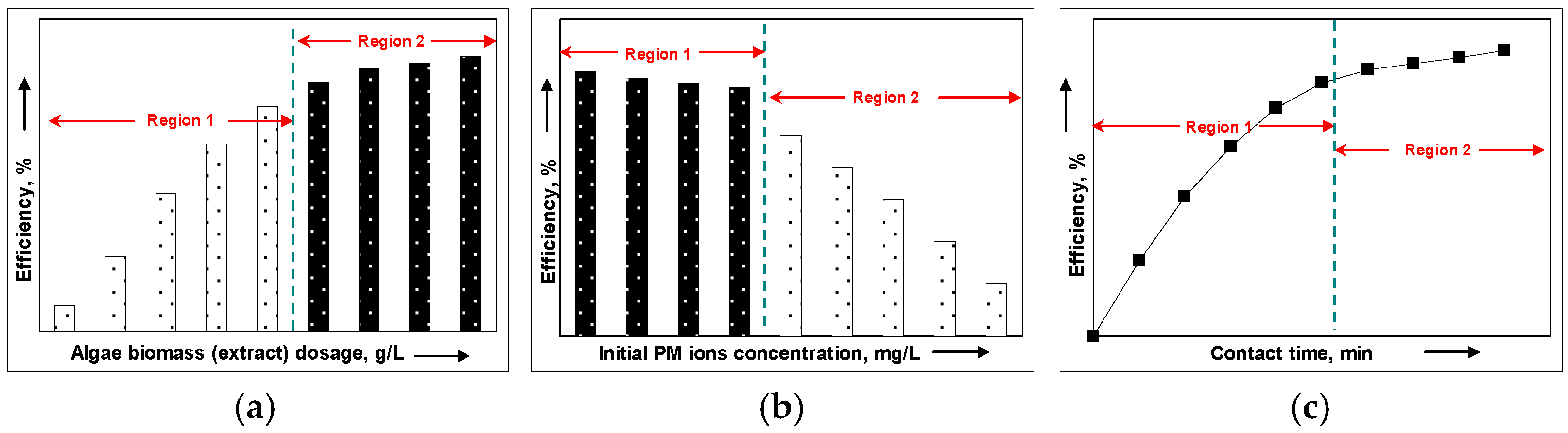
4. Experimental Factors Influencing the Synthesis of PM-NPs
4.1. pH of Aqueous Solution
4.2. Algae Biomass (Extract) Dosage
4.3. Concentration of PM Ions in Aqueous Solution
4.4. Contact Time
4.5. Temperature
5. Characterization Methods of PM-NPs
5.1. Identification Methods
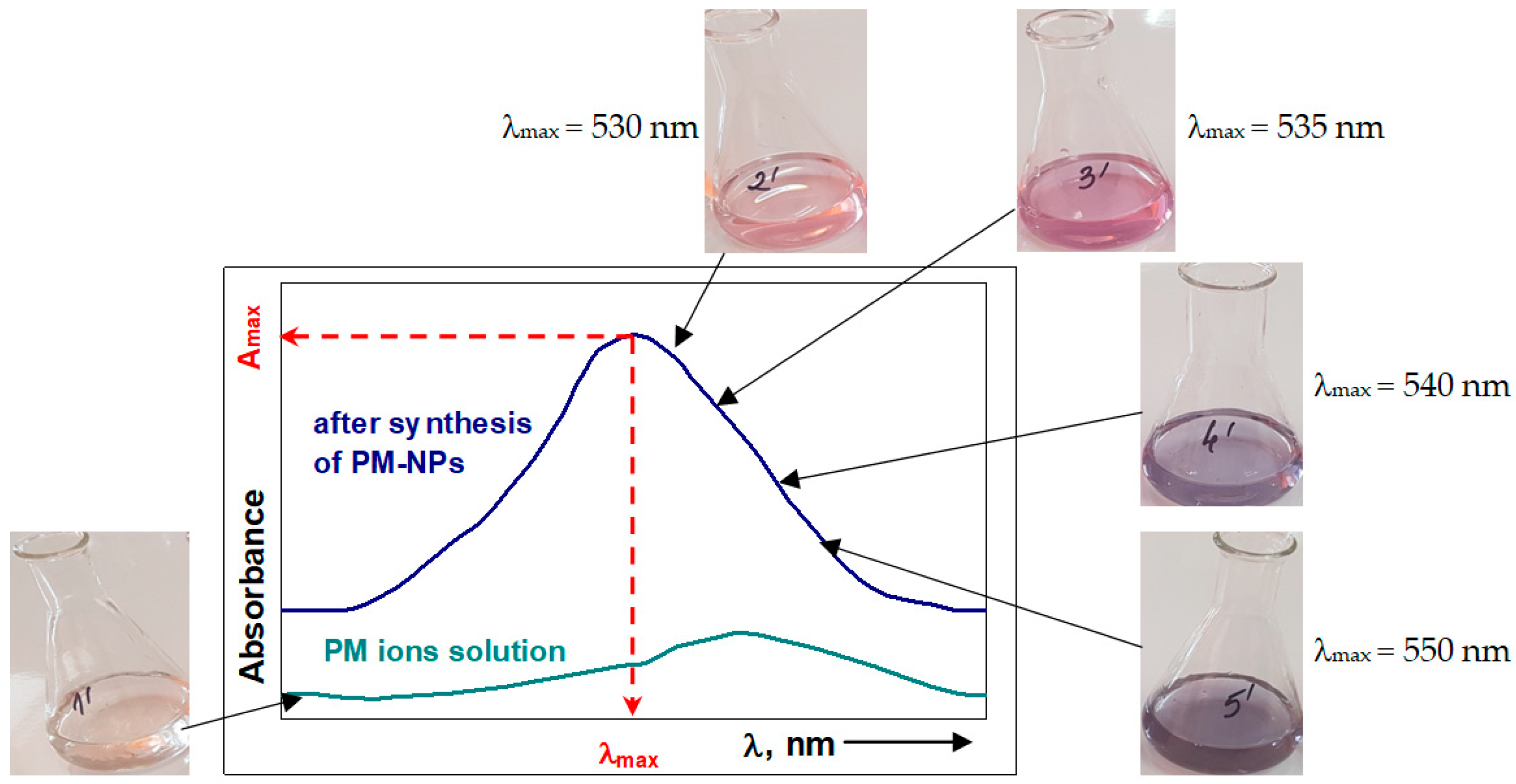
5.1.1. Visual—Color Change
5.1.2. UV–Vis Spectrophotometry
5.1.3. Zeta Potential Measurements
5.2. Structural Characterization Methods
5.2.1. Scanning Electron Microscopy (SEM)
5.2.2. Transmission Electron Microscopy (TEM)
5.2.3. Scanning Force Microscopy (SFM)
5.2.4. X-Ray Diffraction (XRD)
5.2.5. Dynamic Light Scattering (DLS)
5.3. Composition Characterization Methods
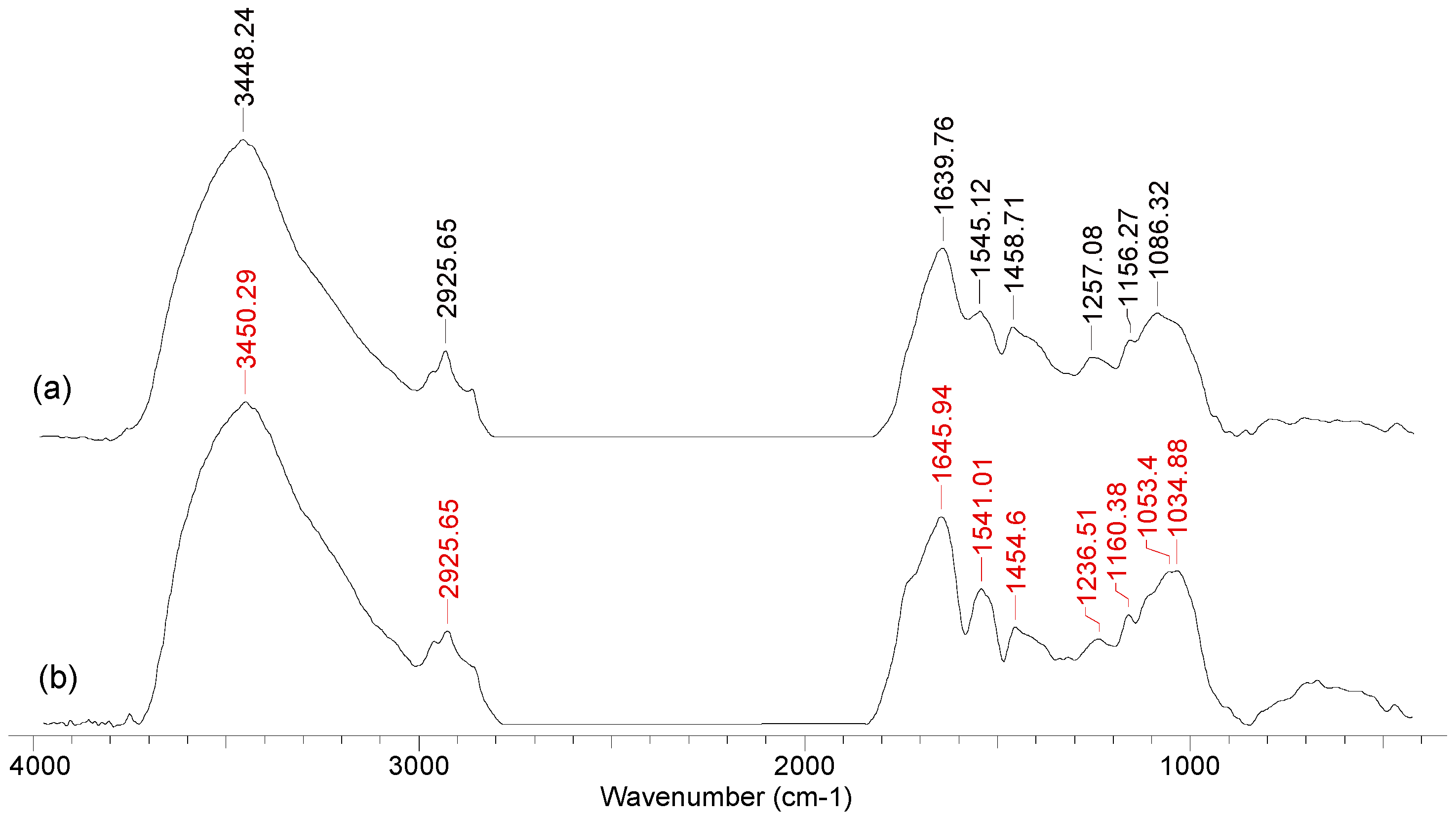
5.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
5.3.2. Raman Spectroscopy and Surface-Enhanced Raman Spectroscopy (SERS)
5.3.3. Energy Dispersive X-Ray Spectroscopy (EDX)
6. Challenges and Future Research
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramaraj, N.; Thiripuranathar, G.; Ekanayake, S.; Attanayake, K.; Marapana, U. Phyco-synthesized inorganic nanoparticles and their biomedical applications. RSC Sustain. 2025, 3, 2567–2581. [Google Scholar] [CrossRef]
- Mehta, J.; Gomaa, A.M.A.; Singh, G.P.; Assiri, M.A.; Bauddh, K. Phytoremediation of metal nanoparticles from the contaminated water bodies: A review. Egypt. J. Chem. 2024, 67, 31–48. [Google Scholar] [CrossRef]
- Li, Y.K.; Yang, T.; Chen, M.L.; Wang, J.H. Recent Advances in Nanomaterials for Analysis of Trace Heavy Metals. Crit. Rev. Anal. Chem. 2021, 51, 353–372. [Google Scholar] [CrossRef]
- Srivastava, N.; Choudhary, M.; Singhal, G.; Bhagyawant, S.S. SEM studies of saponin silver nanoparticles isolated from leaves of Chenopodium album L. for in vitro anti-acne activity. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 333–341. [Google Scholar] [CrossRef]
- Terra, A.L.M.; Kosinski, R.D.C.; Moreira, J.B.; Costa, J.A.V.; Morais, M.G.D. Microalgae biosynthesis of silver nanoparticles for application in the control of agricultural pathogens. J. Environ. Sci. Health Part B 2019, 54, 709–716. [Google Scholar] [CrossRef]
- Jacob, J.M.; Ravindran, R.; Narayanan, M.; Samuel, S.M.; Pugazhendhi, A.; Kumar, G. Microalgae: A prospective low cost green alternative for nanoparticle synthesis. Curr. Opin. Environ. Sci. Health 2021, 20, 100–163. [Google Scholar] [CrossRef]
- Borah, D.; Das, N.; Das, N.; Bhattacharjee, A.; Sarmah, P.; Ghosh, K.; Chandel, M.; Rout, J.; Pandey, P.; Ghosh, N.N.; et al. Alga-mediated facile green synthesis of silver nanoparticles: Photophysical, catalytic and antibacterial activity. Appl. Organomet. Chem. 2020, 34, e5597. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green”synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Narasimhalu, P.; Joseph, A.I.J.; Pugazhendhi, A. Synthesis of silver nanoparticle from X-ray film and its application in production of biofuel from jatropha oil. Int. J. Energy Res. 2021, 45, 17378–17388. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Sarkar, D.; Sasmal, S. A Review of Green Synthesis of Metal Nanoparticles Using Algae. Front. Microbiol. 2021, 12, 693899. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Lespes, G.; Faucher, S.; Slaveykova, V.I. Natural nanoparticles, anthropogenic nanoparticles, where is the frontier? Front. Environ. Sci. 2020, 8, 71. [Google Scholar] [CrossRef]
- Ponnuchamy, K.; Jacob, J.A. Metal nanoparticles from marine seaweeds—A review. Nanotechnol. Rev. 2016, 5, 589–600. [Google Scholar] [CrossRef]
- Roy, A.; Pandit, C.; Gacem, A.; Alqahtani, M.S.; Bilal, M.; Islam, S.; Hossain, J.; Jameel, M. Biologically Derived Gold Nanoparticles and Their Applications. Bioinorg. Chem. Appl. 2022, 8184217. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Bhargava, A.; Gupta, R.; Jain, N.; Panwar, J. Synthesis and Applications of Noble Metal Nanoparticles: A Review. Adv. Sci. Eng. Med. 2017, 9, 527–544. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Hachem, K.; Ansari, M.J.; Saleh, R.O.; Kzar, H.H.; Al-Gazally, M.E.; Altimari, U.S.; Hussein, S.A.; Mohammed, H.T.; Hammid, A.T.; Kianfar, E. Methods of Chemical Synthesis in the Synthesis of Nanomaterial and Nanoparticles by the Chemical Deposition Method: A Review. BioNanoScience 2022, 12, 1032–1057. [Google Scholar] [CrossRef]
- Gautam, A.; Komal, P.; Gautam, P.; Sharma, A.; Kumar, N.; Jung, J.P. Recent Trends in Noble Metal Nanoparticles for Colorimetric Chemical Sensing and Micro-Electronic Packaging Applications. Metals 2021, 11, 329. [Google Scholar] [CrossRef]
- Li, H.; Hua, Y.; Zhang, X.; Di, L. Rapid Synthesis of Noble Metal Colloids by Plasma–Liquid Interactions. Materials 2024, 17, 987. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoo, T.Y.; Bootharaju, M.S.; Kim, J.H.; Chung, D.Y.; Hyeon, T. Noble Metal-Based Multimetallic Nanoparticles for Electrocatalytic Applications. Adv. Sci. 2021, 9, 2104054. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Bjelajac, A.; Phillipe, A.-M.; Guillot, J.; Fleming, Y.; Chemin, J.-B.; Choquet, P.; Bulou, S. Gold nanoparticles synthesis and immobilization by atmospheric pressure DBD plasma torch method. Nanoscale Adv. 2023, 5, 2573–2582. [Google Scholar] [CrossRef]
- Badoni, A.; Prakash, J. Noble metal nanoparticles and graphene oxide based hybrid nanostructures for antibacterial applications: Recent advances, synergistic antibacterial activities, and mechanistic approaches. Micro Nano Eng. 2024, 22, 100239. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Jadoun, S.; Chauhan, N.P.S.; Zarrintaj, P.; Barani, M.; Varma, R.S.; Chinnam, S.; Rahdar, A. Synthesis of nanoparticles using microorganisms and their applications: A review. Environ. Chem. Lett. 2022, 20, 3153–3197. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Alkhateeb, M.A.; Alfassam, H.E.; Momenah, M.A.; Bin-Meferij, M.M. Algal-Derived Synthesis of Silver Nanoparticles Using the Unicellular ulvophyte sp. MBIC10591: Optimisation, Characterisation, and Biological Activities. Molecules 2023, 28, 279. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. AnOverview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahlany, S.T.G.; Niamah, A.K.; Verma, D.K.; Prabhakar, P.; Patel, A.R.; Thakur, M.; Singh, S. Applications of Green Synthesis of Nanoparticles Using Microorganisms in Food and Dairy Products: Review. Processes 2025, 13, 1560. [Google Scholar] [CrossRef]
- Chowdhury, N.K.; Choudhury, R.; Gogoi, B.; Chang, C.M.; Pandey, R.P. Microbial Synthesis of Gold Nanoparticles and their Application. Curr. Drug Targets 2022, 23, 752–760. [Google Scholar] [CrossRef]
- Hu, Z.T.; Chen, Y.; Fei, Y.F.; Loo, S.L.; Chen, G.; Hu, M.; Song, Y.; Zhao, J.; Zhang, Y.; Wang, J. An overview of nanomaterial-based novel disinfection technologies for harmful microorganisms: Mechanism, synthesis, devices and application. Sci. Total Environ. 2022, 837, 155720. [Google Scholar] [CrossRef]
- Yamal, G.; Singh, M.; Pardha-Saradhi, P.; Rao, K.S. Roots of Pennisetum sp.possess the competence to generate nanoparticles of noble metals. Indian J. Biochem. Biophys. 2022, 59, 461–467. [Google Scholar]
- Mickymaray, S. One-step Synthesis of Silver Nanoparticles Using Saudi Arabian Desert Seasonal Plant Sisymbrium irio and Antibacterial Activity Against Multidrug-Resistant Bacterial Strains. Biomolecules 2019, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Jha, A. Plant Extract Mediated Synthesis of Metal Nanoparticles, their Characterization and Applications: A Green Approach. Curr. Green Chem. 2021, 8, 185–202. [Google Scholar] [CrossRef]
- Nhani, G.B.B.; Di Filippo, L.D.; de Paula, G.A.; Mantovanelli, V.R.; da Fonseca, P.P.; Tashiro, F.M.; Monteiro, D.C.; Fonseca-Santos, B.; Duarte, J.L.; Chorilli, M. High-Tech Sustainable Beauty: Exploring Nanotechnology for the Development of Cosmetics Using Plant and Animal By-Products. Cosmetics 2024, 11, 112. [Google Scholar] [CrossRef]
- Almeida, M.; Santos, D.; Soares, B.B.; Farias De Sousa, L.; Alves, E.C. Cleaner Production Alternatives for a Cosmetics Industry in Southern Bahia. Indep. J. Manag. Prod. 2021, 12, 1068–1086. [Google Scholar] [CrossRef]
- Al-Ealayawi, Z.A.; Al-Dulaimy, A.F.Z. Marine Algae and Applications to Plant Nutrition: A review. IOP Conf. Series Earth Environ. Sci. 2023, 1158, 042004. [Google Scholar] [CrossRef]
- Arora, N.; Lo, E.; Legall, N.; Philippidis, G.P. A Critical Review of Growth Media Recycling to Enhance the Economics and Sustainability of Algae Cultivation. Energies 2023, 16, 5378. [Google Scholar] [CrossRef]
- Loke Show, P. Global market and economic analysis of microalgae technology: Status and perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar] [CrossRef]
- Li, S.N.; Wang, R.; Ho, S.H. Algae-mediated biosystems for metallic nanoparticle production: From synthetic mechanisms to aquatic environmental applications. J. Hazard. Mater. 2021, 420, 126625. [Google Scholar] [CrossRef]
- Sampath, S.; Madhavan, Y.; Muralidharan, M.; Sunderam, V.; Lawrance, A.V.; Muthupandian, S. A review on algal mediated synthesis of metal and metal oxide nanoparticles and their emerging biomedical potential. J. Biotechnol. 2022, 360, 92–109. [Google Scholar] [CrossRef]
- Ojha, S.; Khan, A.; Sahoo, C.R.; Mohapatra, R.K.; Tripathi, D.K.; Mukherjee, M.; Guldhe, A.; Nayak, M. A Review on Biosynthesis of Nanoparticles via Microalgal Technology and Their Biomedical Applications. BioNanoScience 2025, 15, 225. [Google Scholar] [CrossRef]
- Moraes, L.C.; Figueiredo, R.C.; Ribeiro-Andrade, R.; Pontes-Silva, A.V.; Arantes, M.L.; Giani, A.; Figueredo, C.C. High diversity of microalgae as a tool for the synthesis of different silver nanoparticles: A species-specific green synthesis. Colloid Interface Sci. Commun. 2021, 42, 100420. [Google Scholar] [CrossRef]
- Ruzik, L. Microalgae with active biological metal-nanoparticles as a novel food. Biosynthesis, characterization and bioavailability investigation—Review. Trend Food Sci. Technol. 2023, 139, 104127. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Sharma, K.; Chetri, S.P.; Vashishtha, A.; Singh, P.; Kumar, R.; Rathi, B.; Agrawal, V. Algae as crucial organisms in advancing nanotechnology: A systematic review. J. Appl. Phycol. 2016, 28, 1759–1774. [Google Scholar] [CrossRef]
- Vincy, W.; Mahathalana, T.J.; Sukumaran, S.; Jeeva, S. Algae as a source for synthesis of nanoparticles-a review. Int. J. Latest Trends Eng. Technol. 2017, 5–9. [Google Scholar]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Rana, N.F.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef]
- Slepicka, P.; Slepicková Kasálková, N.; Siegel, J.; Kolská, Z.; Švorcík, V. Methods of Gold and Silver Nanoparticles Preparation. Materials 2020, 13, 1. [Google Scholar] [CrossRef]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef]
- Dean, J.A. Handbook of Analytical Chemistry; Mc-Grow Hill Inc.: New York, NY, USA, 1995. [Google Scholar]
- Jin, R. The impacts of nanotechnology on catalysis by precious metal nanoparticles. Nanotechnol. Rev. 2012, 1, 31–56. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Wang, Z.; Ming, N. Shape-Selective Synthesis of Gold Nanoparticles with Controlled Sizes, Shapes, and Plasmon Resonances. Adv. Funct. Mater. 2007, 17, 3295–3303. [Google Scholar] [CrossRef]
- Li, J.; Lou, Z.; Li, B. Nanostructured materials with localized surface plasmon resonance for photocatalysis. Chin. Chem. Lett. 2022, 33, 1154–1168. [Google Scholar] [CrossRef]
- Wang, L.; Kafshgari, M.H.; Meunier, M. Optical Properties and Applications of Plasmonic-Metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, G.; Zhang, Z.; Han, Y.; Guan, G.; Yang, W.; Han, M.Y. Intrinsic Optical Properties and Emerging Applications of Gold Nanostructures. Adv. Mater. 2023, 35, 2206700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Zhu, Y.K.; Feng, J.; Ge, Z.H. Precious metal nanoparticles dispersing toward highly enhanced mechanical and thermoelectric properties of copper sulfides. J. Alloys Compd. 2022, 892, 162035. [Google Scholar] [CrossRef]
- Kus-Liskiewicz, M.; Fickers, P.; Tahar, I.B. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Vikulova, E.S.; Sergeevichev, D.S.; Guselnikova, T.Y.; Korolkov, I.V.; Fedorenko, A.D.; Nasimov, D.A.; Vasilieva, M.B.; Chepeleva, E.V.; Zherikova, K.V.; et al. Heterostructures Based on Noble Metal Films with Ag and Au Nanoparticles: Fabrication, Study of In Vivo Biocompatibility and Antibacterial Activity. Coatings 2023, 13, 1269. [Google Scholar] [CrossRef]
- Pechyen, C.; Ponsanti, K.; Tangnorawich, B.; Ngernyuang, N. Waste fruit peel e Mediated green synthesis of biocompatible gold nanoparticles. J. Mater. Res. Technol. 2021, 14, 2982–2991. [Google Scholar] [CrossRef]
- Talarska, P.; Boruczkowski, M.; Zurawski, J. Current Knowledge of Silver and Gold Nanoparticles in Laboratory Research—Application, Toxicity, Cellular Uptake. Nanomaterials 2021, 11, 2454. [Google Scholar] [CrossRef]
- Behzad, F.; Naghib, S.M.; Kouhbanani, M.A.J.; Tabatabaei, S.N.; Zare, Y.; Rhee, K.Y. An overview of the plant-mediated green synthesis of noble metal nanoparticles for antibacterial applications. J. Ind. Eng. Chem. 2021, 94, 92–104. [Google Scholar] [CrossRef]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, L.; Li, J.; Doyle-Davis, K.; Li, R.; Wang, Z.; Sun, X. Advanced Support Materials and Interactions for Atomically Dispersed Noble-Metal Catalysts: From Support Effects to Design Strategies. Adv. Energy Mater. 2022, 12, 2102556. [Google Scholar] [CrossRef]
- McDarby, S.P.; Wang, C.J.; King, M.E.; Personick, M.L. An Integrated Electrochemistry Approach to the Design and Synthesis of Polyhedral Noble Metal Nanoparticles. J. Am. Chem. Soc. 2020, 142, 21322–21335. [Google Scholar] [CrossRef] [PubMed]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, F.M.M. Characterization of Labeled Gold Nanoparticles for Surface-Enhanced Raman Scattering. Molecules 2022, 27, 892. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Q.; Cao, J.; Li, H.; Bian, Z. Precious metal recovery. Joule 2021, 5, 3097–3115. [Google Scholar] [CrossRef]
- Nadaf, S.J.; Jadhav, N.R.; Naikwadi, H.S.; Savekar, P.L.; Sapkal, I.D.; Kambli, M.M.; Desai, I.A. Green synthesis of gold and silver nanoparticles: Updates on research, patents, and future prospects. OpenNano 2022, 8, 100076. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.E.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Premarathna, K.S.D.; Lau, S.Y.; Chiong, T.; Show, P.L.; Vithanage, M.; Lam, M.K. Greening up the fight against emerging contaminants: Algae-based nanoparticles for water remediation. Clean Technol. Environ. Policy 2024. [Google Scholar] [CrossRef]
- Carreira, A.R.F.; Veloso, T.; Macario, I.P.E.; Pereira, J.L.; Ventura, S.P.M.; Passos, H.; Coutinho, J.A.P. The role of biomass elemental composition and ion-exchange in metal sorption by algae. Chemosphere 2023, 314, 137675. [Google Scholar] [CrossRef] [PubMed]
- Znad, H.; Awual, R.; Martini, S. The Utilization of Algae and Seaweed Biomass for Bioremediation of Heavy Metal-Contaminated Wastewater. Molecules 2022, 27, 1275. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.M.P.; Silva, C.S.; Delgado, V.M.S.; Tonelli, F.C.P. Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators. Green Proc. Synth. 2023, 12, 20230008. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, P.B.; Genova, J.; Chamati, H. Green Synthesis of Gold Nanoparticles: An Eco-Friendly Approach. Chemistry 2022, 4, 345–369. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.B.; Lastra, M.; Rodríguez-Argüelles, M.C. Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: Its activity in colon cancer cells. Colloids Surf. B. Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef]
- Mata, Y.; Torres, E.; Blazquez, M.; Ballester, A.; González, F.; Munoz, J. Gold (III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J. Hazard. Mater. 2009, 166, 612–618. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Kannan, C.; Annadurai, G. Green synthesis of silver nanoparticles using marine brown algae Turbinaria conoides and its antibacterial activity. Int. J. Pharma Bio Sci. 2012, 3, 502–510. [Google Scholar]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef]
- Naveena, B.E.; Prakash, S. Biological synthesis of gold nanoparticles using marine algae Gracilaria corticata and its application as a potent antimicrobial and antioxidant agent. Asian J. Pharm. Clin. Res. 2013, 6, 179–182. [Google Scholar]
- Bhimba, B.; Kumari, P. Phytosynthesis of silver nanoparticles from the extracts of seaweed Ulva lactuca and its antimicrobial activity. Int. J. Pharma Bio Sci. 2014, 5, 666–677. [Google Scholar]
- Manikandakrishnan, M.; Palanisamy, S.; Vinosha, M.; Kalanjiaraja, B.; Mohandoss, S.; Manikandan, R.; Tabarsa, M.; You, S.G.; Prabhu, N.M. Facile green route synthesis of gold nanoparticles using Caulerpa racemosa for biomedical applications. J. Drug Deliv. Sci. Technol. 2019, 54, 101345. [Google Scholar] [CrossRef]
- Apostică, A.G.; Bulgariu, L. Utilization of Marine Algae Biomass for Eco friendly Obtaining of Gold Nanoparticles. In Proceedings of the 11th IEEE International Conference on E-Health and Bioengineering—EHB 2023, Bucharest, Romania, 9–10 November 2023. IEEE-979-8-3503-2887-5/23. [Google Scholar]
- Rajasulochana, P.; Dhamotharan, R.; Murugakoothan, P.; Murugesan, S.; Krishnamoorthy, P. Biosynthesis and characterization of gold nanoparticles using the alga Kappaphycus alvarezii. Int. J. Nanosci. 2010, 9, 511–516. [Google Scholar] [CrossRef]
- Ganesan, V.; Aruna Devi, J.; Astalakshmi, A.; Nima, P.; Thangaraja, A. Eco-friendly synthesis of silver nanoparticles using a seaweed, Kappaphycus alvarezii (Doty) Doty ex PC Silva. Int. J. Eng. Adv. Technol. 2013, 2, 559–563. [Google Scholar]
- Chellapandian, C.; Ramkumar, B.; Puja, P.; Shanmuganathan, R.; Pugazhendhi, A.; Kumar, P. Gold nanoparticles using red seaweed Gracilaria verrucosa: Green synthesis, characterization and biocompatibility studies. Proc. Biochem. 2019, 80, 58–63. [Google Scholar] [CrossRef]
- Fatima, R.; Priya, M.; Indurthi, L.; Radhakrishnan, V.; Sudhakaran, R. Biosynthesis of silver nanoparticles using red algae Portieria hornemannii and its antibacterial activity against fish pathogens. Microb. Pathog. 2020, 138, 103780. [Google Scholar] [CrossRef]
- Sathiyaraj, G.; Manikandakrishnan, M.V.M.; Sangeetha, D.; Sonaimuthu, S.P.M.; Manikandan, R.; You, S.G.; Prabhu, N.M. Bio-directed synthesis of Pt-nanoparticles from aqueous extract of red algae Halymenia dilatata and their biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126434. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Sherif, M.H.; Malarkodi, C.; Ponnanikajamideen, M.; Arasu, M.V.; Al-Dhabi, N.A.; Roopan, S.M. Cytotoxicity behaviour of response surface model optimized gold nanoparticles by utilizing fucoidan extracted from Padina tetrastromatica. J. Mol. Struct. 2021, 1228, 129440. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Prakash, S.; Ahila, N.K.; Vinoj, G.; Selvam, S.; Kumar, G.; Kannapiran, E.; Rajendran, R.B. Synthesis of platinum nanoparticles using seaweed Padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications. Biomed. Pharmacother. 2017, 92, 479–490. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Diego-González, L.; Lastra-Valdor, M.; Cavazza, A.; Bigi, F.; Rodríguez-Argüelles, M.C.; Grimaldi, M.; Simón-Vázquez, R. Immunomodulatory and Antitumoral Activity of Gold Nanoparticles Synthesized by Red Algae Aqueous Extracts. Mar. Drugs 2022, 20, 182. [Google Scholar] [CrossRef]
- Madani, M.; Hosny, S.; Alshangiti, D.M.; Nady, N.; Alkhursani, S.A.; Alkhaldi, H.; Al-Gahtany, S.A.; Ghobashy, M.M.; Gaber, G.A. Green Synthesis of Nanoparticles for Varied Applications: Green Renewable Resources and Energy-Efficient Synthetic Routes. Nanotechnol. Rev. 2022, 11, 731–759. [Google Scholar] [CrossRef]
- Montasser, M.S.; Younes, A.M.; Hegazi, M.M.; Dashti, N.H.; El-Sharkawey, A.E.; Beall, G.W. A Novel Eco-friendly Method of Using Red Algae (Laurencia papillosa) to Synthesize Gold Nanoprisms. J. Nanomed. Nanotechnol. 2016, 7, 3. [Google Scholar]
- Koçer, A.T.; Özçimen, D. Eco-friendly synthesis of silver nanoparticles from macroalgae: Optimization, characterization and antimicrobial activity. Biomass Convers. Biorefin. 2025, 15, 1995–2006. [Google Scholar] [CrossRef]
- Algotiml, R.; Gab-Alla, A.; Seoudi, R.; Abulreesh, H.H.; El-Readi, M.Z.; Elbanna, K. Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci. Rep. 2022, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.H.; Yuan, Z.Q.; Li, G.K. Preparation of phytosterols and phytol from edible marine algae by microwave-assisted extraction and high-speed counter-current chromatography. Sep. Purif. Technol. 2013, 104, 284–289. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Fernandes, M.; Machado, R.; Gomes, A.C.; Cavazza, A.; Sampaio, P.; Bigi, F.; Rodríguez-Argüelles, M.C. Valorisation of the Invasive Macroalgae Undaria pinnatifida (Harvey) Suringar for the Green Synthesis of Gold and Silver Nanoparticles with Antimicrobial and Antioxidant Potential. Mar. Drugs 2023, 21, 397. [Google Scholar] [CrossRef]
- Aboeita, N.M.; Fahmy, S.A.; El-Sayed, M.M.H.; El-Said Azzazy, H.M.; Shoeib, T. Enhanced Anticancer Activity of Nedaplatin Loaded onto Copper Nanoparticles Synthesized Using Red Algae. Pharmaceutics 2022, 14, 418. [Google Scholar] [CrossRef]
- Viswanathan, S.; Palaniyandi, T.; Shanmugam, R.; Karunakaran, S.; Pandi, M.; Wahab, M.R.A.; Baskar, G.; Rajendran, B.K.; Sivaji, A.; Moovendhan, M. Synthesis, characterization, cytotoxicity, and antimicrobial studies of green synthesized silver nanoparticles using red seaweed Champia parvula. Biomass Convers. Biorefin. 2024, 14, 7387–7400. [Google Scholar] [CrossRef]
- Alarif, W.M.; Shaban, Y.A.; Orif, M.I.; Ghandourah, M.A.; Alorfi, H.S.; Tadros, H.R.Z. Green Synthesis of TiO2 Nanoparticles Using Natural Marine Extracts for Antifouling Activity. Mar. Drugs 2023, 21, 62. [Google Scholar] [CrossRef]
- Abbasi Tarighat, M.; Ghorghosheh, F.H.; Abdi, G. Fe3O4@SiO2-Ag nanocomposite colorimetric sensor for determination of arginine and ascorbic acid based on synthesized small size AgNPs by Cystoseria algae extract. Mater. Sci. Eng. 2022, B283, 115855. [Google Scholar] [CrossRef]
- Patel, D.; Patel, B.; Yadav, V.K.; Sudhakar, M.P.; Alharbi, S.A.; Salmen, S.H.; Patel, I.; Choudhary, N.; Patel, A. Silver nanoparticles synthesized from marine algae Spatoglossum asperum: Antioxidant properties and seed germination enhancement. J. Hazard. Mater. Adv. 2024, 16, 100478. [Google Scholar] [CrossRef]
- Anwar, S.J.; Ul Haq Bhat, I.; Yusoff, H.M.; Razali, M.H.; Kadir, M.A.; Ern, L.K. Brown algae-based preparation, characterization and application of Pd nanocatalyst for enhanced reductive azo dye degradation. Clean. Eng. Technol. 2021, 4, 100172. [Google Scholar] [CrossRef]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.J.M.; Biswal, S.L.; Bharti, B. Active Colloids as Models, Materials, and Machines. Annu. Rev. Chem. Biomol. Eng. 2023, 14, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Mulder, D.W.; Phiri, M.M.; Jordaan, A.; Vorster, B.C. Modified HEPES one-pot synthetic strategy for gold nanostars. R. Soc. Open Sci. 2019, 6, 190160. [Google Scholar] [CrossRef]
- Millstone, J.E.; Hurst, S.J.; Métraux, G.S.; Cutler, J.I.; Mirkin, C.A. Colloidal Gold and Silver Triangular Nanoprisms. Small 2009, 5, 646–664. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Garcia-Leis, A.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Silver Nanostars with High SERS Performance. J. Phys. Chem. C 2013, 117, 7791–7795. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Huang, C.Z.; Xia, Y. Synthesis of Ag Nanocubes 18–32 nm in Edge Length: The Effects of Polyol on Reduction Kinetics, Size Control, and Reproducibility. J. Am. Chem. Soc. 2013, 135, 1941–1951. [Google Scholar] [CrossRef]
- Nagao, H.; Ichiji, M.; Hirasawa, I. Synthesis of Platinum Nanoparticles by Reductive Crystallization Using Polyethyleneimine. Chem. Eng. Technol. 2017, 40, 1242–1246. [Google Scholar] [CrossRef]
- Herricks, T.; Chen, J.; Xia, Y. Polyol Synthesis of Platinum Nanoparticles: Control of Morphology with Sodium Nitrate. Nano Lett. 2004, 4, 2367–2371. [Google Scholar] [CrossRef]
- Walbrück, K.; Kuellmer, F.; Witzleben, S.; Guenther, K. Synthesis and Characterization of PVP-Stabilized Palladium Nanoparticles by XRD, SAXS, SP-ICP-MS, and SEM. J. Nanomater. 2019, 2019, 4758108. [Google Scholar] [CrossRef]
- Iben Ayad, A.; Belda Marín, C.; Colaco, E.; Lefevre, C.; Méthivier, C.; Ould Driss, A.; Landoulsi, J.; Guénin, E. “Water soluble” palladium nanoparticle engineering for C–C coupling, reduction and cyclization catalysis. Green Chem. 2019, 21, 6646–6657. [Google Scholar] [CrossRef]
- Miranda, E.G.A.; Tofanello, A.; Brito, A.M.M.; Lopes, D.M.; Albuquerque, L.J.C.; de Castro, C.E.; Costa, F.N.; Giacomelli, F.C.; Ferreira, F.F.; Araújo-Chaves, J.C.; et al. Effects of Gold Salt Speciation and Structure of Human and Bovine Serum Albumins on the Synthesis and Stability of Gold Nanostructures. Frontiers Chem. 2016, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Pargar, F.; Koleva, D. Polarization Behaviour of Silver in Model Solutions. Int. J. Struct. Civ. Eng. Res. 2017, 6, 172–176. [Google Scholar] [CrossRef]
- Murakami, Y.; Hiraiwa, K.; Sasaki, Y.; Fujiwara, I.; Tagashira, S. Surfactant Gel Adsorption of Platinum(II), (IV) and Palladium(II) as Chloro complexes and Kinetic Separation of Palladium from Platinum Using EDTA. Anal. Sci. 2007, 23, 1147–1149. [Google Scholar] [CrossRef]
- Lucaci, A.R.; Bulgariu, D.; Bulgariu, L. Green Synthesis of Gold Nanoparticles Using Marine Red Algae Biomass. In Proceedings of the 9th IEEE International Conference on E-Health and Bioengineering—EHB 2021, Iasi, Romania, 18–19 November 2021. IEEE 978-1-6654-4000-4/21. [Google Scholar]
- Palaniyandi, T.; Viswanathan, S.; Prabhakaran, P.; Baskar, G.; Wahab, M.R.A.; Sivaji, A.; Ravi, M.; Rajendran, B.K.; Moovendhan, M.; Surendran, H.; et al. Green synthesis of gold nanoparticles using Halymenia pseudofloresii extracts and their antioxidant, antimicrobial, and anti-cancer activities. Biomass Convers. Biorefin. 2023, 15, 21733–21744. [Google Scholar] [CrossRef]
- Venkatesan, J.; Manivasagan, P.; Kim, S.K.; Kirthi, A.V.; Marimuthu, S.; Rahuman, A.A. Marine algae-mediated synthesis of gold nanoparticles using a novel Ecklonia cava. Bioprocess Biosyst. Eng. 2014, 37, 1591–1597. [Google Scholar] [CrossRef]
- Singaravelu, G.; Arockiamary, J.S.; Kumar, V.G.; Govindaraju, K. Anovel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef]
- Dhas, T.S.; Kumar, V.G.; Abraham, L.S.; Karthick, V.; Govindaraju, K. Sargassum myriocystum mediated biosynthesis of gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 97–101. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Mahadevan, A.; Sathishkumar, M.; Pavagadhi, S.; Balasubramanian, R. Biosynthesis of Au(0) from Au(III) via biosorption and bioreduction using brown marine alga Turbinaria conoides. Chem. Eng. J. 2011, 167, 223–227. [Google Scholar] [CrossRef]
- Lodeiro, P.; Sillanpää, M. Gold recovery from artificial seawater using synthetic materials and seaweed biomass to induce gold nanoparticles formation in batch and column experiments. Mar. Chem. 2013, 152, 11–19. [Google Scholar] [CrossRef]
- Dogmaz, S.; Cavas, L. Biohydrogen production via green silver nanoparticles synthesized through biomass of Ulva lactuca bloom. Bioresour. Technol. 2023, 379, 129028. [Google Scholar] [CrossRef] [PubMed]
- Aboelfetoh, E.F.; El-Shenody, R.A.; Ghobara, M.M. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): Reaction optimization, catalytic and antibacterial activities. Environ. Monit. Assess. 2017, 189, 349. [Google Scholar] [CrossRef] [PubMed]
- Massironi, A.; Morelli, A.; Grassi, L.; Puppi, D.; Braccini, S.; Maisetta, G.; Esin, S.; Batoni, G.; Pina, C.D.; Chiellini, F. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: Application to green synthesis of silver nanoparticles. Carbohydr. Polym. 2019, 203, 310–321. [Google Scholar] [CrossRef]
- Azizi, S.; Namvar, F.; Mahdavi, M.; Ahmad, M.B.; Mohamad, R. Biosynthesis of Silver Nanoparticles Using Brown Marine Macroalga, Sargassum muticum Aqueous Extract. Materials 2013, 6, 5942–5950. [Google Scholar] [CrossRef] [PubMed]
- Sivagnanam, S.P.; Getachew, A.T.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Green synthesis of silver nanoparticles from deoiled brown algal extract via Box-Behnken based design and their antimicrobial and sensing properties. Green Process. Synth. 2017, 6, 147–160. [Google Scholar] [CrossRef]
- Vinayagam, R.; Nagendran, V.; Goveas, L.C.; Narasimhan, M.K.; Varadavenkatesan, T.; Chandrasekar, N.; Selvaraj, R. Structural characterization of marine macroalgae derived silver nanoparticles and their colorimetric sensing of hydrogen peroxide. Mater. Chem. Phys. 2024, 313, 128787. [Google Scholar] [CrossRef]
- Govindaraju, K.; Kiruthiga, V.; Kumar, V.G.; Singaravelu, G. Extracellular Synthesis of Silver Nanoparticles by a Marine Alga, Sargassum wightii grevilli and Their Antibacterial Effects. J. Nanosci. Nanotechnol. 2009, 9, 5497–5501. [Google Scholar] [CrossRef]
- Şahin, M.; Arslan, Y.; Tomul, F.; Akgül, F.; Akgül, R. Green synthesis of metal nanoparticles from Codium macroalgae for wastewater pollutants removal by adsorption. CLEAN-Soil Air Water 2024, 52, 2300187. [Google Scholar]
- Viana, T.; Henriques, B.; Ferreira, N.; Pinto, R.J.B.; Monteiro, F.L.S.; Pereira, E. Insight into the mechanisms involved in the removal of toxic, rare earth, and platinum elements from complex mixtures by Ulva sp. Chem. Eng. J. 2023, 453, 139630. [Google Scholar] [CrossRef]
- Shargh, A.Y.; Sayadi, M.H.; Heidari, A. Green Biosynthesis of Palladium Oxide Nanoparticles Using Dictyota indica Seaweed and its application for adsorption. J. Water Environ. Nanotechnol. 2018, 3, 337–347. [Google Scholar]
- Sonbol, H.; Ameen, F.; Al Yahya, S.; Almansob, A.; Alwakee, S. Padina boryana mediated green synthesis of crystalline palladium nanoparticles as potential nanodrug against multidrug resistant bacteria and cancer cells. Sci. Rep. 2021, 11, 5444. [Google Scholar] [CrossRef] [PubMed]
- Edo, G.I.; Mafe, A.N.; Ali, A.B.M.; Akpoghelie, P.O.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Ismael, S.A.; Essaghah, A.E.A.; Ahmed, D.S.; et al. Green Biosynthesis of Nanoparticles Using Plant Extracts: Mechanisms, Advances, Challenges, and Applications. BioNanoScience 2025, 15, 267. [Google Scholar] [CrossRef]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Alderhami, S.A. Revisiting the green syn thesis of nanoparticles: Uncovering influences of plant extracts as reducing agents for enhanced synthesis efficiency and its bio medical applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar]
- Jacob, R.H.; Shanab, S.M.; Shalaby, E.A. Algal biomass nanoparticles: Chemical characteristics, biological actions, and applications. Biomass Convers. Biorefin. 2023, 13, 11441–11455. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.H.; Kim, J.H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Doak, J.; Gupta, R.K.; Manivannan, K.; Ghosh, K.; Kahol, P.K. Effect of particle size distributions on absorbance spectra of gold nanoparticles. Physica E 2010, 42, 1605–1609. [Google Scholar] [CrossRef]
- Haider, M.J.; Mehdi, M.S. Study of morphology zeta potential analyzer for the silver nanoparticles. Int. J. Sci. Eng. Res. 2014, 5, 381–385. [Google Scholar]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Aguirre, F.M.A.; Bazán-Díaz, L.; Mendoza-Cruz, R.; Gómez-Rodríguez, A.; Zorrilla-Cangas, C.; Herrera-Becerra, R. Nano Phase Characterization by Transmission Electron Microscopy: Experimental and Simulation. Mater. Sci. Appl. 2015, 6, 935–942. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties applications toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Santosh Kumar, S.R.; Bongale, M.M.; Sachidanandam, M.; Maurya, G.; Yuvraj, M.; Sarwade, P.P. A Review on Green Synthesized Metal Nanoparticles Applications. J. Res. Appl. Sci. Biotechol. 2024, 3, 80–100. [Google Scholar] [CrossRef]
- Ingham, B. X-ray scattering characterisation of nanoparticles. Crystallogr. Rev. 2015, 21, 229–303. [Google Scholar] [CrossRef]
- Mori, T.; Hegmann, T. Determining the composition of gold nanoparticles: A compilation of shapes, sizes, and calculations using geometric considerations. J. Nanoparticle Res. 2016, 18, 295. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M. Measurement of nanoparticle by light scattering techniques. TrAC Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Charles, B.; Cher, M.T.; Jeng, C.K. FTIR spectroscopy as a tool for nano-material characterization. Inf. Biophys. Technol. 2010, 53, 434–438. [Google Scholar]
- Menon, S.; Rajeshkumar, S.; Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour.-Effic. Technol. 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Vijayan, S.R.; Santhiyagu, P.; Singamuthu, M.; Kumari Ahila, N.; Jayaraman, R.; Ethiraj, K. Synthesis and characterization of silver and gold nanoparticles using aqu eous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014, 2014, 938272. [Google Scholar] [CrossRef]
- Ghodake, G.; Lee, D.S. Biological synthesis of gold nanoparticles using the aqueous extract of the brown algae Laminaria japonica. J. Nanoelectron. Optoelectron. 2011, 6, 268–271. [Google Scholar] [CrossRef]
- Singh, M.; Kalaivani, R.; Manikandan, S.; Sangeetha, N.; Kumaraguru, A.K. Facile green synthesis of variable metallic gold nanoparticle using Padina gymnospora, a brown marine macroalga. Appl. Nanosci. 2013, 3, 145–151. [Google Scholar] [CrossRef]
- Varun, S.; Sudha, S.; Kumar, P.S. Biosynthesis of gold nanoparticles from aqueous extract of Dictyota Bartayresiana and their antifungal activity. Indian J. Adv. Chem. Sci. 2014, 2, 190–193. [Google Scholar]
- Ramakrishna, M.; Babu, D.R.; Gengan, R.M.; Chandra, S.; Rao, G.N. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostruct. Chem. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; Muñoz, J.A.; González, F.; Ballester, A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 2013, 7, 109–116. [Google Scholar] [CrossRef]
- Dhanalakshmi, P.K.; Azeez, R.; Rekha, R.; Poonkodi, S.; Nallamuthu, T. Synthesis of silver nanoparticles using green and brown seaweeds. Phykos 2012, 42, 39–45. [Google Scholar]
- Rahimi, Z.; Yousefzadi, M.; Noori, A. Green synthesis of silver nanoparticles using Ulva flexousa from the Persian Gulf, Iran. J. Persian Gulf 2014, 5, 9–16. [Google Scholar]
- Sinha, S.N.; Paul, D.; Halder, N.; Sengupta, D.; Patra, S.K. Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl. Nanosci. 2015, 5, 703–709. [Google Scholar] [CrossRef]
- Pinjarkar, H.; Gaikwad, S.; Ingle, A.P.; Gade, A.; Rai, M. Phycofabrication of silver nanoparticles and their antibacterial activity against human pathogens. Adv. Mater. Lett. 2016, 7, 1010–1014. [Google Scholar] [CrossRef]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef]
- de Aragao, A.P.; de Oliveira, T.M.; Quelemes, P.V.; Perfeito, M.L.G.; Araujo, M.C.; Santiago, J.D.A.S.; Cardoso, V.S.; Quaresma, P.; de Almeida, J.R.D.S.; da Silva, D.A. Green synthesis of silver nanoparticles using the seaweed Gracilaria birdiae and their antibacterial activity. Arab. J. Chem. 2019, 12, 4182–4188. [Google Scholar] [CrossRef]
- Govindaraju, K.; Krishnamoorthy, K.; Alsagaby, S.A.; Singaravelu, G.; Premanathan, M. Green synthesis of silver nanoparticles for selective toxicity towards cancer cells. IET Nanobiotechnol. 2015, 9, 325–330. [Google Scholar] [CrossRef]
- Palanisamy, S.; Anjali, R.; Jeneeta, S.; Mohandoss, S.; Keerthana, D.; Shin, I.S.; You, S.G.; Prabhu, N.M. An effective bio-inspired synthesis of platinum nanoparticles using Caulerpa sertularioides and investigating their antibacterial and antioxidant activities. Bioprocess Biosyst. Eng. 2023, 46, 105–118. [Google Scholar] [CrossRef]
- Prasad, B.; Padmesh, T. Seaweed (Sargassum ilicifolium) assisted green synthesis of palladium nanoparticles. Int. J. Sci. Eng. Res. 2014, 5, 229–231. [Google Scholar]
- Momeni, S.; Nabipour, I. A simple green synthesis of palladium nanoparticles with Sargassum alga and their electrocatalytic activities towards hydrogen peroxide. App. Biochem. Biotechnol. 2015, 176, 1937–1949. [Google Scholar] [CrossRef] [PubMed]


| Precious Metal | Au | Ag | Pt | Pd |
|---|---|---|---|---|
| Atomic number (Z) | 79 | 47 | 78 | 46 |
| Atomic weight (A) | 196.97 | 107.87 | 195.08 | 106.42 |
| Oxidation state | +3, +1 | +1 | +2, +4 | +2, +4 |
| Electronegativity (Pauling scale) | 2.54 | 1.93 | 2.28 | 2.20 |
| Standard redox potential, V | +1.5000 | +0.7994 | +1.2000 | +0.9200 |
| Density (at 20 °C), g/cm3 | 19.283 | 10.503 | 21.452 | 12.007 |
| Meting point, °C | 1064.18 | 961.78 | 1768.3 | 1554.9 |
| Atomic radius, pm | 144.0 | 144.0 | 139.0 | 137.0 |
| Ionic radius, pm | 85.0 | 126.0 | 62.5 | 64.0 |
| Precious Metal | Chemical Reaction | log β |
|---|---|---|
| Au | Au3+ (H+) + 4 Cl− → AuCl4− | 24.49 |
| Ag | Ag+ (H+) + Cl− → AgCl ↓ | 1.1 × 10−10 * |
| Pt | Pt2+ (H+) + 4 Cl− → PtCl42− | 13.99 |
| Pd | Pd2+ (H+) + 4 Cl− → PdCl42− | 27.20 |
| Marine Algae | Type of Algae | PM-NPs | Notable Features | Reference |
|---|---|---|---|---|
| Ulva lactuca | Green algae | Au, Ag | Successfully used in eco-friendly synthesis | [84] |
| Caulerpa racemose | Green algae | Ag | Spherical and triangle stable nanoparticles | [85] |
| Cladophora vagabunda | Green Algae | Au | High efficiency, long stable nanoparticles | [86] |
| Kappaphycus alvarezii | Red algae | Au, Ag | Stable and monodisperse nanoparticles | [87,88] |
| Gracilaria edulis | Red algae | Au | Produces uniform and stable nanoparticles | [89] |
| Galaxaura elongata | Red algae | Au | High efficiency, stable and spherical nanoparticles | [12] |
| Portieria hornemannii | Red algae | Ag | Stable and monodisperse nanoparticles | [90] |
| Halymenia dilatata | Red algae | Pt | Stable and monodisperse nanoparticles | [91] |
| Fucus vesiculosus | Brown algae | Au | Active functional groups (carboxyl, phenol); increased efficiency | [80] |
| Padina pavonica | Brown algae | Au | Efficient and rapid synthesis | [92] |
| Padina gymnospora | Brown algae | Pt | Efficient and rapid synthesis | [93] |
| Algae | g, Biomass | Solvent | T, °C | t, min | Reference |
|---|---|---|---|---|---|
| Laurencia papillosa | 5.0 | distilled water | 70–80 | 5 | [96] |
| Ulva lactuca | 1.0 | distilled water | 70–80 | 45 | [97] |
| Halopteris scoparia | |||||
| Ulva rigida | 10 | distilled water | 70 | 15 | [98] |
| Gracilaria foliifera | |||||
| Cystoseira myrica | |||||
| Undaria pinnatifida | 15.0 | 1.5 mol/L ethanol | microwave | 200 | [99] |
| Sargassum fusiform | |||||
| Undaria pinnatifida | distilled water | 100 | 15 | [100] | |
| Pterocladia capillacea | 5.0 | distilled water | ultrasonication | 240 | [101] |
| Champia parvula | 1.0 | distilled water | 60 | 20 | [102] |
| Bostrychia tenella | 100 | methanol | Room temperature | - | [103] |
| Laurencia obtusa | |||||
| Cystoseria sp. | 2.0 | distilled water | 60 | 20 | [104] |
| Spatoglossum asperum | 5.0 | distilled water | 60 | 20 | [105] |
| Saragassum cervicorne | 5.0 | distilled water | 85–90 | 60 | [106] |
| PM-NPs | Stabilizing Agent | Size, nm | Shape | Reference |
|---|---|---|---|---|
| Au | Sodium citrate | 3.5–4.0 | Spherical | [107] |
| Poly-vinyl-pyrrolidone | 37.0 ± 2.0 | Nano-stars | [109] | |
| Cetyltrimethylammonium bromide | 144.0 ± 25.0 | Nano-prisms | [110] | |
| Ag | Sodium citrate | 10.0–200.0 | Spherical | [111] |
| Citric acid | cca. 300 | Nano-stars | [112] | |
| Poly-vinyl-pyrrolidone | 15.0–35.0 | Cubic | [113] | |
| Pt | Poly-ethylen-imine | 4.9 | Spherical | [114] |
| 5.2 | Cubic | |||
| Poly-vinyl-pyrrolidone | 3.0–30.0 | Octahedral | [115] | |
| Pd | Poly-vinyl-pyrrolidone | 5.0–15.0 | Spherical | [116] |
| Phosphonic acids | 50.0 | Nanodendrites | [117] |
| PM-NPs | Algae Biomass | pH | Biomass Dose | PM Ions Concentration, mg/L | Contact Time, min | Temperature, °C | Reference |
|---|---|---|---|---|---|---|---|
| Au | Ulva lactuca | 2.0 | 4.0 g/L | 40.0 | 1440 | 22 | [86] |
| Cladophora vagabunda | 2.0 | 4.0 g/L | 40.0 | 1440 | 22 | ||
| Callithamnion corymbosum | 4.0 | 4.0 g/L | 240.0 | 60 | 21 | [121] | |
| Halymenia pseudoforesii | - | 1.0 g/L | 150.0 | 20 | 60 | [122] | |
| Fucus vesiculosus | 7.0 | 1.0 g/L | 100.0 | 480 | 23 | [80] | |
| Ecklonia cava (extract) | - | 1.0 g/mL | 50.0 | 10 | 80 | [123] | |
| Sargassum wightii | - | 1.0 g/L | 200.0 | 720 | 25 | [124] | |
| Sargassum muticum | - | 1.0 g/L | 200.0 | 15 | 76 | [125] | |
| Undaria pinnatifida (extract) | - | 1.0 g/mL | 50.0 | 1440 | 100 | [94] | |
| Turbinaria conoides | 2.0 | 2.0 g/L | 100.0 | 60 | - | [126] | |
| Sargassum muticum | 2.6–3.2 | 4.0 g/L | 50.0 | 75 | - | [127] | |
| Ag | Ulva lactuca (extract) | 11.0 | 10 mg/mL | 150.0 | 60 | 25 | [128] |
| Ulva lactuca | 3.0 | - | 50–100 | 60 | 25 | [97] | |
| Caulerpa serrulata | 4.1 | 1.0 g/L | 150.0 | 1440 | 27 | [129] | |
| Ulva armoricana | 3.0 | 0.5 g/L | 100.0 | 360 | 20 | [130] | |
| Portieria hornemannii (extract) | - | 5.0 mL | 150.0 | 1440 | 25 | [90] | |
| Sargassum muticum (extract) | 5.6 | 1.0 g/mL | 55.0 | 30 | 35 | [131] | |
| Undaria pinnatifida (extract) | - | 0.5 g/mL | 25.0 | 1440 | 100 | [94] | |
| Saccharina japonica (extract) | - | 50.0 mL | 150–170 | 45 | 40 | [132] | |
| Sargassum spp. (extract) | - | 25.0 mL | 25.0 | 60 | 80 | [133] | |
| Sargassum wightti | - | 1.0 g/L | 150.0 | 1440 | 25 | [134] | |
| Pt | Padina gymnospora (extract) | - | 10.0 mL | 100.0 | 1440 | 100 | [93] |
| Codium sp. (extract) | - | 10.0 mL | 200.0 | 120 | 45 | [135] | |
| Ulva sp. | 7.8–8.0 | 3.0 g/L | 0.1 | 720 | 20 | [136] | |
| Pd | Codium sp. (extract) | - | 10.0 mL | 100.0 | 120 | 45 | [135] |
| Dictyota indica (extract) | 8.0 | 20.0 mL | 120.0 | 120 | 60 | [137] | |
| Padina boryana (extract) | - | 5.0 mL | 120.0 | 120 | 60 | [138] |
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| SEM | - evaluation of morphology, surface distribution - fast analysis - 3D images of the surface | - moderate resolution - required conductive coating | [146] |
| TEM | - evaluation of size, shape, and internal structure - very high resolution - detailed images | - high cost - complex sample preparation | [147] |
| SFM | - nanoscale topography - does not require vacuum - can analyze sample in liquid media | - longer analysis time - small scan area | [142] |
| XRD | - identification of structure and degree of crystallinity | - does not detect amorphous PM-NPs - requires sufficient amount of sample | [148] |
| DLS | - evaluation of the average size and the size distribution - short working time, simplicity - non-invasive method -allows direct analysis of suspensions | - high sensitivity to impurities - difficulties in analyzing polydisperse samples - does not provide morphological details | [149] |
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| FTIR | - rapid and easy to use method - identification of functional groups - identifies surface modifications | - limited sensitivity for small PM-NPs | [153] |
| Raman | - reduced cost and complexity in sample preparation - non-destructive method, complementary to FTIR | - moderate sensitivity - possible interferences | [155] |
| SERS | - very high sensitivity - reduced interferences - destructive method | - high cost and complexity in sample preparation | [154] |
| EDX | - allows the determination of elemental composition - short working time - non-destructive method -integrable with SEM/TEM | - high sensitivity to impurities - low sensitivity to light elements | [156] |
| PM | Algae Biomass | Characterization Method | Size, nm | Morphology | Reference |
|---|---|---|---|---|---|
| Au | Sargassum sp. | UV–Vis, AFM, TEM, XRD, FTIR | 300–400 | hexagonal, truncated triangular | [125] |
| Laminaria japonica | UV–Vis, TEM, XRD, FTIR | 15–20 | spherical | [157] | |
| Fucus vesiculosus | XRD, SEM, EDS, TEM, FTIR | 20–50 | spherical | [80] | |
| Padina gymnospora | UV–Vis, XRD, AFM, TEM, FTIR | 8–21 | spherical | [158] | |
| Dictyota bartayresiana | UV–Vis, FTIR, SEM | poly-size | spherical | [159] | |
| Sargassum tenerrimum | UV–Vis, Zeta potential, TEM, FTIR, DLS | 5–45 | polymorphic | [160] | |
| Chondrus crispus | UV–Vis, TEM, SEM, EDX, FTIR | 30–50 | spherical, polyhedral | [161] | |
| Galaxaura elongata | Zeta potential, TEM, FTIR | 3.85–77.13 | triangular, hexagonal | [12] | |
| Ecklonia cava | UV–Vis, XRD, SEM, TEM, FTIR, EDX | 20–50 | spherical, triangular | [123] | |
| Ag | Ulva reticulata | UV–Vis, FTIR, SEM, XRD | 40–50 | spherical | [162] |
| Ulva lactuca | UV–Vis, Zeta potential, FTIR, SEM, XRD | 48.9 | spherical | [84] | |
| Ulva flexousa | UV–Vis, XRD, FTIR, TEM | 2–32 | spherical | [163] | |
| Pithophora oedogonia | UV–Vis, EDX, SEM, DLS, FTIR | 25–44 | cubical, hexagonal | [164] | |
| Spirogyra sp. | UV–Vis, FTIR, TEM | 40–80 | spherical | [165] | |
| Caulerpa serrulata | UV–Vis, FTIR, XRD, TEM | 10 ± 2 | spherical | [129] | |
| Caulerpa racemosa | UV–Vis, XRD, TEM, FTIR | 5–25 | face-centered cubic | [166] | |
| Gracilaria birdiae | UV–Vis, Zeta potential, TEM, FTIR, DLS | 20.30 | spherical | [167] | |
| Sargassum vulgare | TEM, XRD, TEM, FTIR, EDX | 10.00 | spherical | [168] | |
| Pt | Padina gymnospora | UV–Vis, XRD, SEM, TEM, EDX | 5–50 | octahedral | [93] |
| Caulerpa sertularioide | UV–Vis, XRD, SEM, TEM, DLS, FTIR, EDX | 6–22 | spherical | [169] | |
| Codium sp. | UV–Vis, SEM, TEM, FTIR, EDX | 15.97 | cubic | [135] | |
| Pd | Sargassum ilicifolium | UV–Vis, SEM | 60–80 | spherical | [170] |
| Sargassum bovinum | UV–Vis, TEM, XRD, EDX, FTIR | 5–10 | octahedral | [171] | |
| Codium sp. | UV–Vis, SEM, TEM, FTIR, EDX | 11.38 | hexagonal | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulgariu, L. Ecological Synthesis of Precious Metal Nanoparticles: Harnessing the Potential of Marine Algae Biomass. Nanomaterials 2025, 15, 1492. https://doi.org/10.3390/nano15191492
Bulgariu L. Ecological Synthesis of Precious Metal Nanoparticles: Harnessing the Potential of Marine Algae Biomass. Nanomaterials. 2025; 15(19):1492. https://doi.org/10.3390/nano15191492
Chicago/Turabian StyleBulgariu, Laura. 2025. "Ecological Synthesis of Precious Metal Nanoparticles: Harnessing the Potential of Marine Algae Biomass" Nanomaterials 15, no. 19: 1492. https://doi.org/10.3390/nano15191492
APA StyleBulgariu, L. (2025). Ecological Synthesis of Precious Metal Nanoparticles: Harnessing the Potential of Marine Algae Biomass. Nanomaterials, 15(19), 1492. https://doi.org/10.3390/nano15191492






