Study on the Structure-Luminescence Relationship and Anti-Counterfeiting Application of (Ca,Sr)-Al-O Composite Fluorescent Materials
Abstract
1. Introduction
- (i)
- the difficulty in precisely controlling trap density and energy distribution, which limits the persistence of afterglow;
- (ii)
- the insufficient tunability of emission properties under multi-wavelength excitation; and
- (iii)
- the poor lattice stability under high-temperature conditions, which restricts their application in high-performance functional inks
- (i)
- Interface defect engineering: By regulating the lattice mismatch between (Ca,Sr)3Al2O6 and SrAl2O4, additional electron traps are formed, thereby improving energy storage capacity;
- (ii)
- Enhanced structural stability: The cubic (Ca,Sr)3Al2O6 matrix suppresses grain boundary collapse and maintains trap integrity during high-temperature synthesis.
2. Experimental Process
2.1. Experimental Reparation
2.2. Material Preparation Process
2.3. Characterization of Materials
3. Test Result Analysis
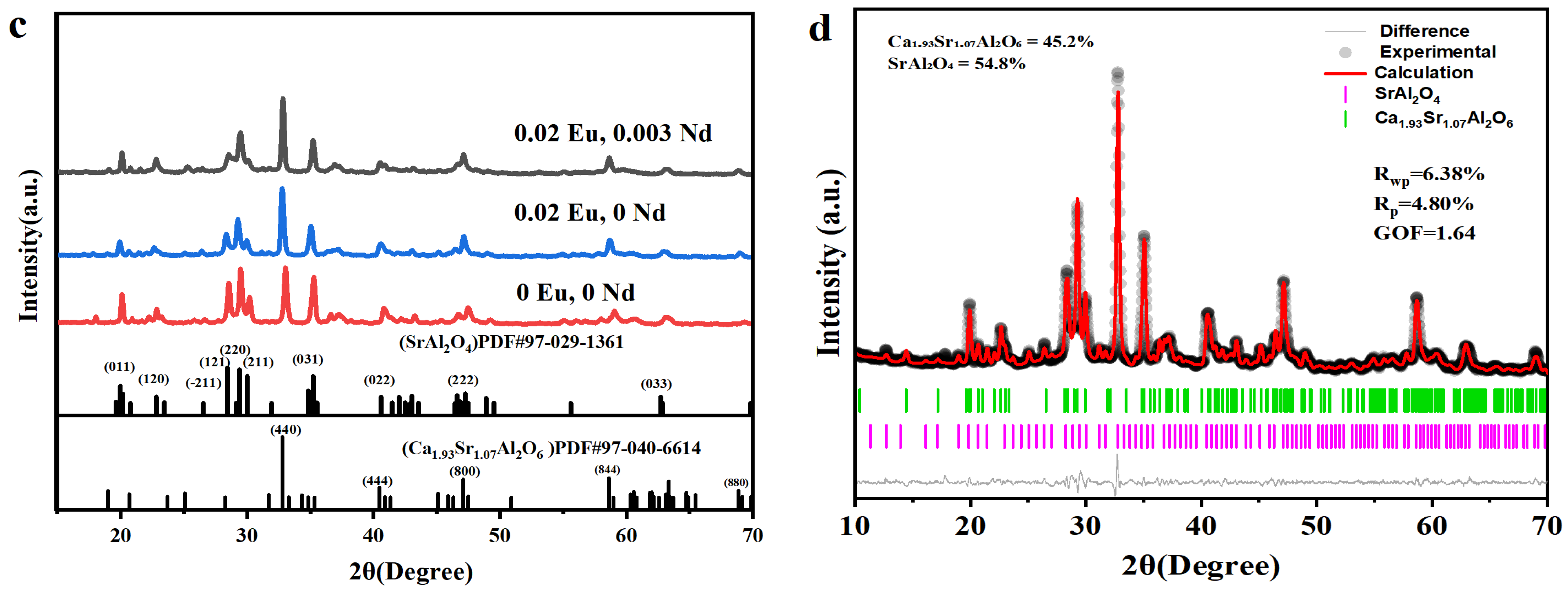
3.1. Phase Composition and Structural Evolution
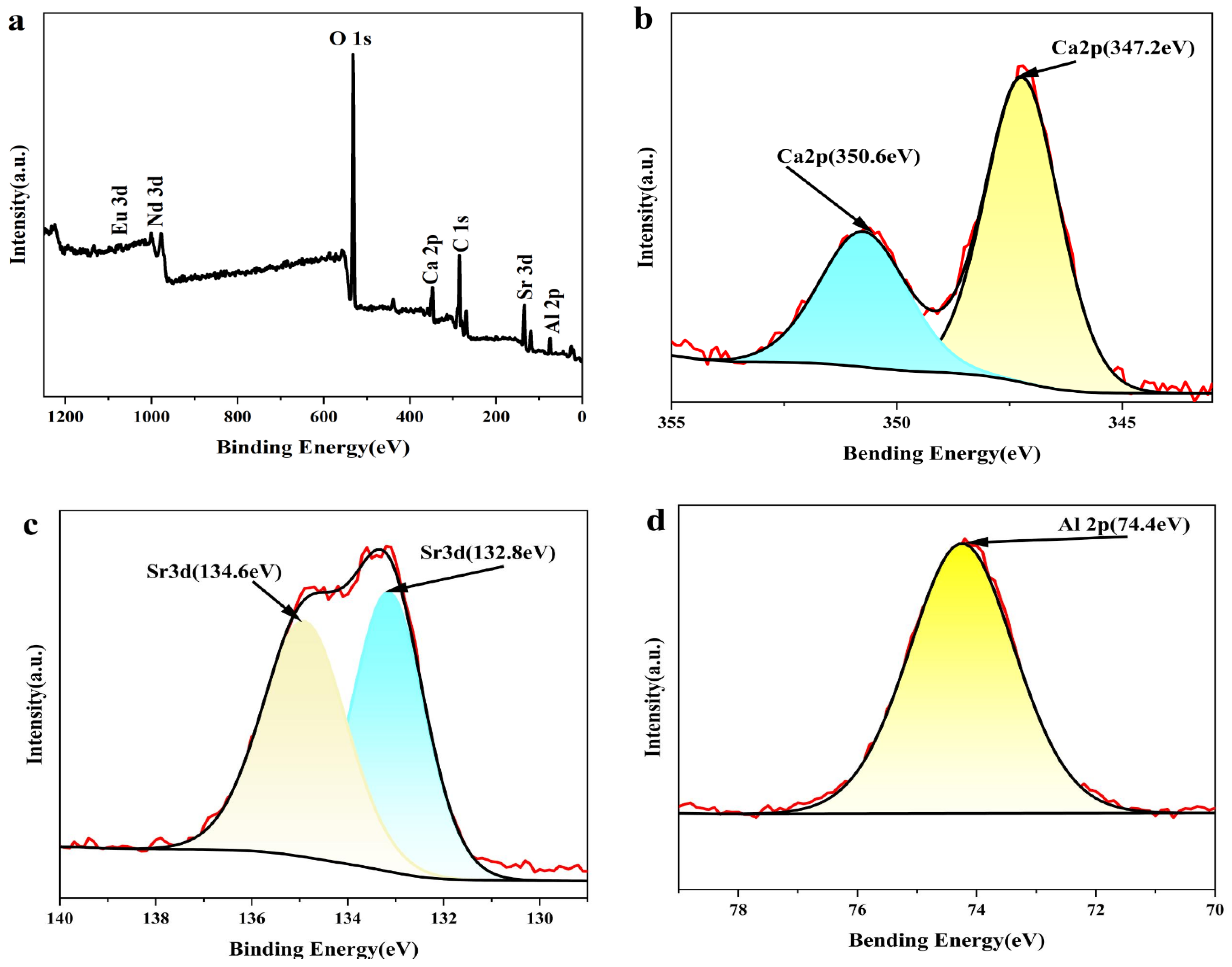
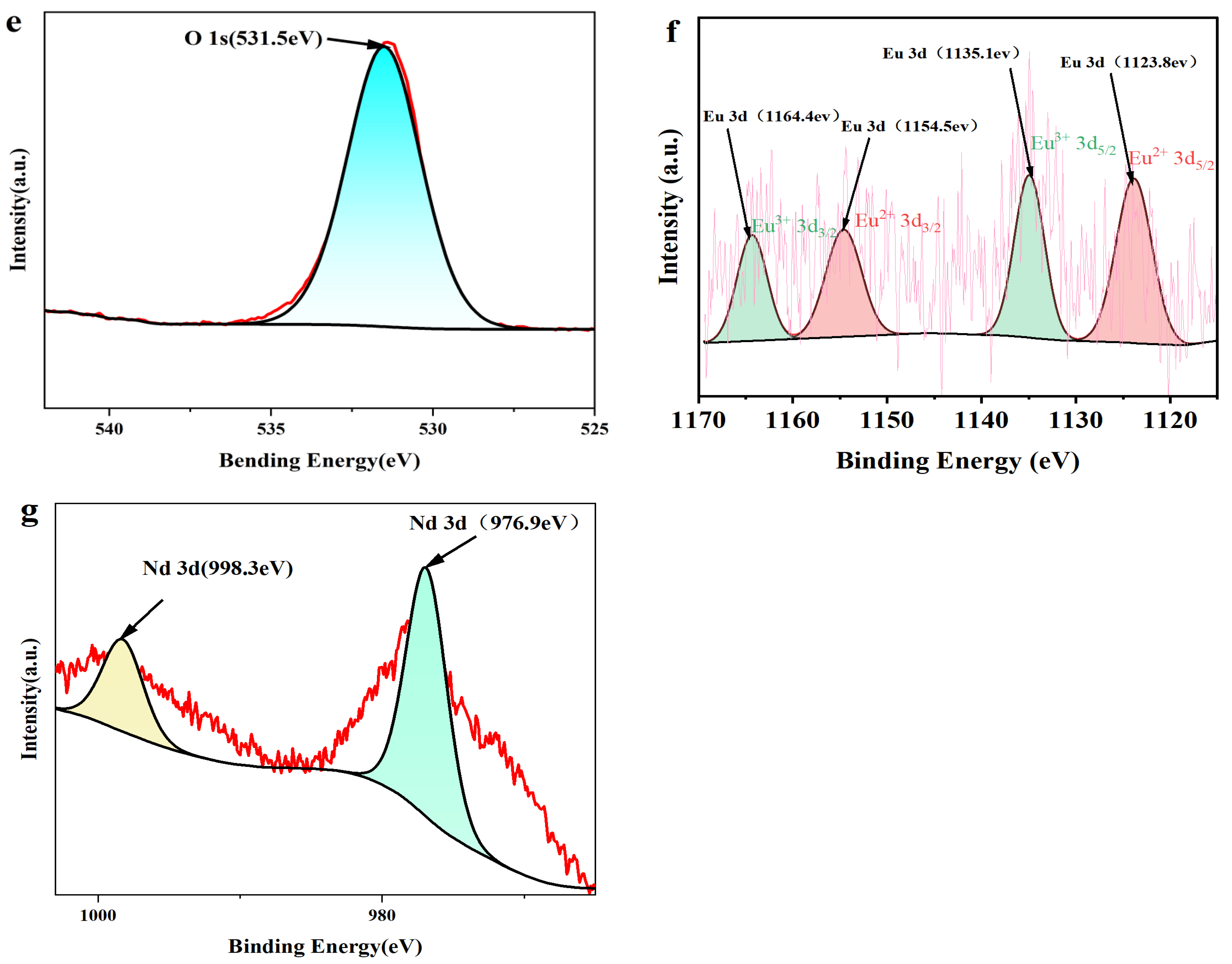
3.2. Elemental Composition Analysis
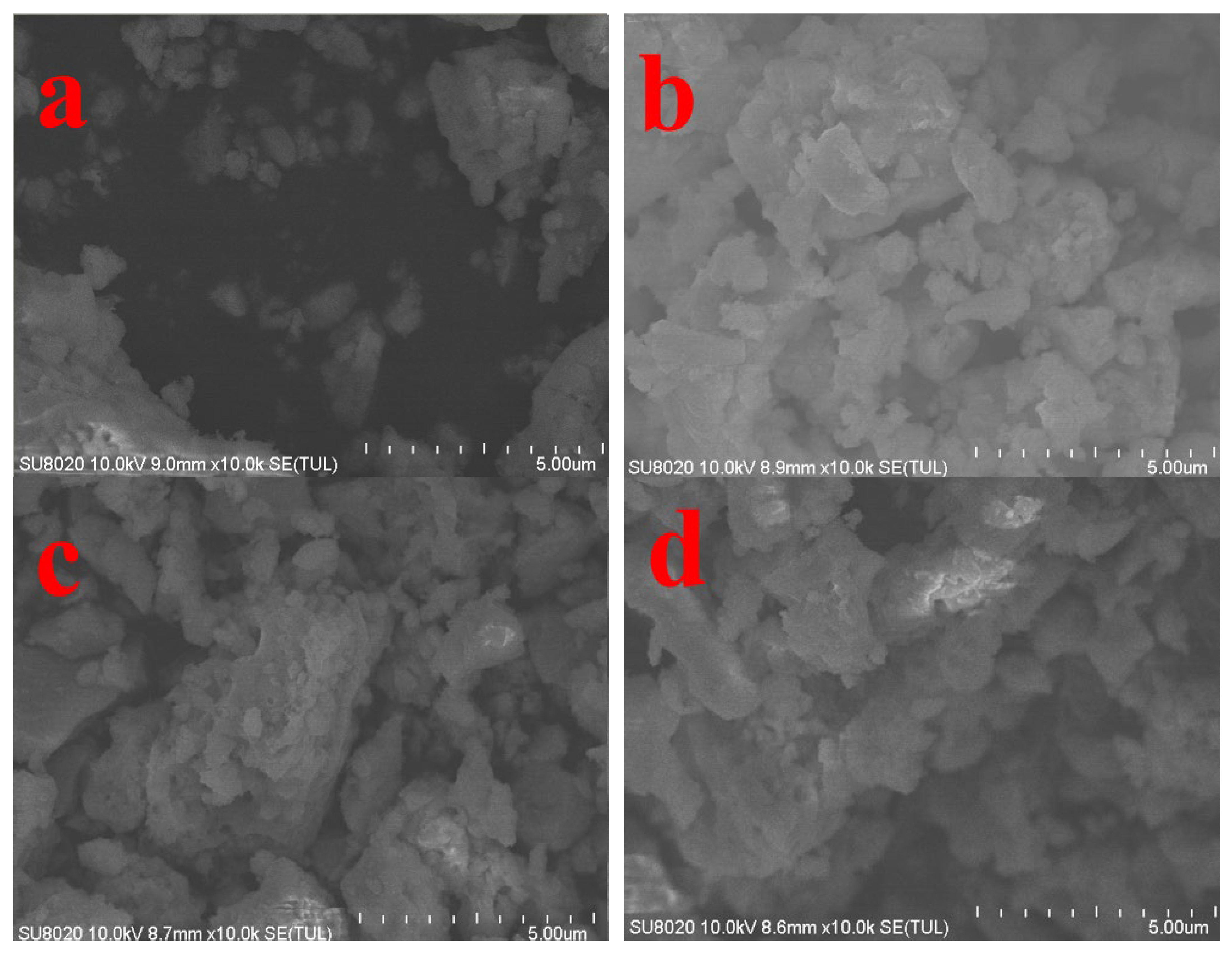
3.3. SEM Sintering Condition Analysis
3.4. Luminescence Performance Analysis
3.5. UV-Vis Diffuse Reflectance and Apparent Optical Onset
3.6. Fluorescence Lifetime Test Analysis
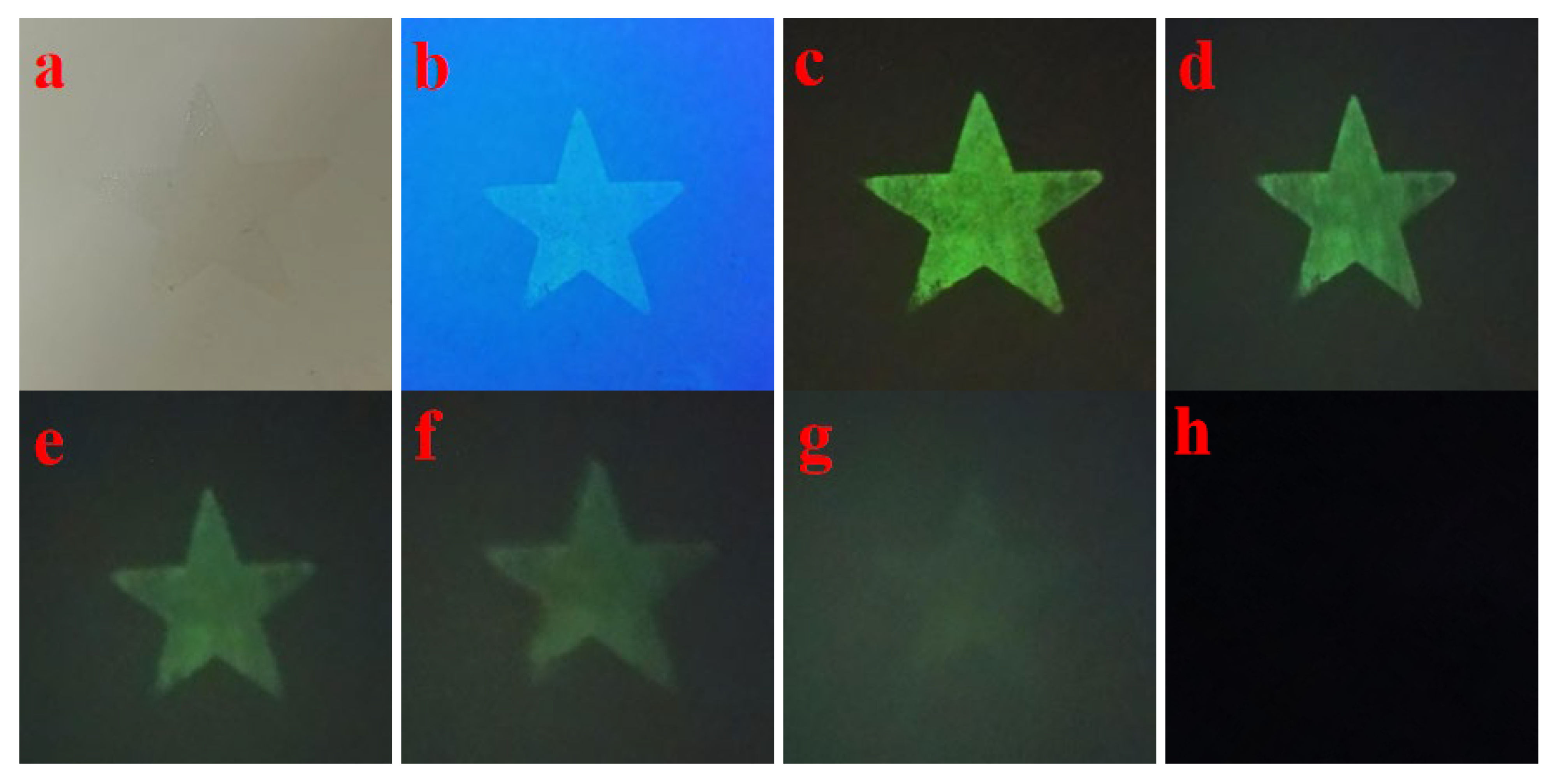
3.7. Afterglow Life Time Test
4. Application and Effect Testing
4.1. Ink Preparation and Screen Printing
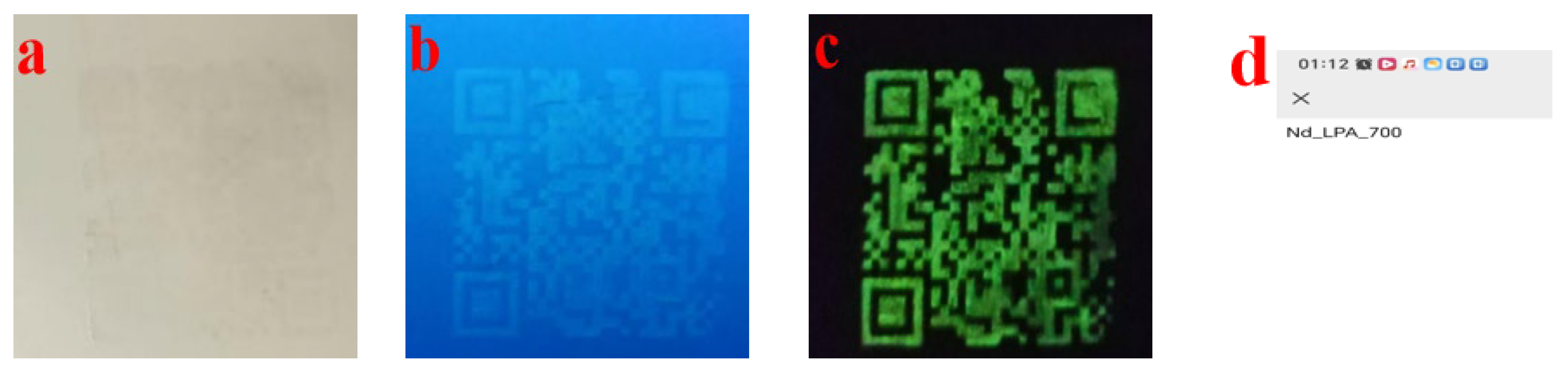
4.2. Anti-Counterfeiting Application Performance
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuang, Q.; Hou, X.; Du, C.; Wang, X.; Gao, D. Recent Advances in the Anti-Counterfeiting Applications of Long Persistent Phosphors. Phys. Chem. Chem. Phys. 2023, 25, 17759–17768. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, Y.; Zhao, X.; He, Y.; Pei, J.; Xing, Y.; Wang, S.; Zhang, J. Aluminate Long Afterglow Luminescent Materials in Road Marking Field Research Progress and Development: A Review. Buildings 2024, 14, 2152. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Huo, X.; Meng, X.; Wang, Y.; Suo, H.; Li, P. Anti-Counterfeiting Application of Persistent Luminescence Materials and Its Research Progress. Laser Photon. Rev. 2024, 18, 2300751. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, H.; Qian, J.; Li, Z.; Hu, X.; Guo, Y.; Fu, Y.; Zhu, H.; Nai, W.; Yang, Z.; et al. Rare Earth Ion Doped Luminescent Materials: A Review of Up/Down Conversion Luminescent Mechanism, Synthesis, and Anti-Counterfeiting Application. Photonics 2023, 10, 1014. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B.; Salami-Kalajahi, M. Photoluminescent and Chromic Nanomaterials for Anticounterfeiting Technologies: Recent Advances and Future Challenges. ACS Nano 2020, 14, 14417–14492. [Google Scholar] [CrossRef]
- Dorenbos, P. Energy of the First 4f7 → 4f65d Transition of Eu2+ in Inorganic Compounds. J. Lumin. 2003, 104, 239–260. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4:Eu2+,Dy3+. J. Electrochem. Soc. 1996, 143, 2670–2673. [Google Scholar] [CrossRef]
- Lee, W.; Kim, S.; Lee, S.; Kim, S. Technical note: Usability evaluation of the modified CIE1976 Uniform-Chromaticity scale for assessing image quality of visual display monitors. Hum. Factors Ergon. Manuf. Serv. Ind. 2003, 13, 85–95. [Google Scholar] [CrossRef]
- Gao, P.; Wang, J.; Wu, J.; Xu, Q.; Yang, L.; Liu, Q.; Qi, Y.; Li, Z. Preparation of SrAl2O4:Eu2+,Dy3+ Powder by Combustion Method and Application in Anticounterfeiting. Coatings 2023, 13, 808. [Google Scholar] [CrossRef]
- Peng, T.; Yang, H.; Pu, X.; Hu, B.; Jiang, Z.; Yan, C. Combustion Synthesis and Photoluminescence of SrAl2O4:Eu,Dy Phosphor Nanoparticles. Mater. Lett. 2023, 331, 133510. [Google Scholar] [CrossRef]
- Chang, C.K.; Xu, J.; Jiang, L.; Mao, D.L.; Ying, W.J. Luminescence of Long-Lasting CaAl2O4:Eu2+,Nd3+ Phosphor by Co-Precipitation Method. Mater. Chem. Phys. 2006, 98, 509–513. [Google Scholar]
- Synthesis of Sr4Al14O25:Eu2+,Dy3+ Phosphor Nanometer Powders by Combustion Processes and Its Optical Properties. Mater. Sci. Eng. B 2006, 133, 200–204. [CrossRef]
- Song, H.; Chen, D.; Tang, W.; Peng, Y. Synthesis of SrAl2O4:Eu2+,Dy3+,Gd3+ Phosphor by Combustion Method and Its Phosphorescence Properties. Displays 2008, 29, 41–44. [Google Scholar] [CrossRef]
- Gültekin, S.; Yıldırım, S.; Yılmaz, O.; Keskin, İ.Ç.; Katı, M.İ.; Çelik, E. Structural and Optical Properties of SrAl2O4:Eu2+/Dy3+ Phosphors Synthesized by Flame Spray Pyrolysis Technique. J. Lumin. 2019, 206, 59–69. [Google Scholar] [CrossRef]
- Xie, W.; Quan, J.; Wu, H.; Shao, L.; Zou, C.; Zhang, J.; Shi, X.; Wang, Y. Structure and luminescence properties of SrAl2O4:Eu2+, Dy3+ by Ba2+ and Ca2+ co-doping. J. Alloys Compd. 2012, 514, 97–102. [Google Scholar] [CrossRef]
- Guo, H.; Yang, H.; Dang, S.; Zhang, S.; Hou, H. Structural, Elastic, Electronic, Dynamic, and Thermal Properties of SrAl2O4 with an Orthorhombic Structure under Pressure. Molecules 2024, 29, 5192. [Google Scholar] [CrossRef]
- Brik, M.G.; Ma, C.G.; Yamamoto, T.; Piasecki, M.; Popov, A.I. First-Principles Methods as a Powerful Tool for Fundamental and Applied Research in the Field of Optical Materials. In Phosphor Handbook: Experimental Methods for Phosphor Evaluation and Characterization; Shionoya, S., Yen, W.M., Yamamoto, H., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–26. [Google Scholar]
- Wang, Z.; Liu, Q.; Wang, J.; Qi, Y.; Li, Z.; Li, J.; Zhang, Z.; Wang, X.; Li, C.; Wang, R. Study on the Luminescence Performance and Anti-Counterfeiting Application of Eu2+,Nd3+ Co-Doped SrAl2O4 Phosphor. Nanomaterials 2024, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Kshatri, D.S.; Khare, A. Optical properties of rare earth doped strontium aluminate (SAO) phosphors: A review. Opt. Spectrosc. 2014, 117, 769–783. [Google Scholar] [CrossRef]
- Clabau, F.; Rocquefelte, X.; Jobic, S.; Deniard, P.; Whangbo, M.-H.; Garcia, A.; Le Mercier, T. Mechanism of Phosphorescence Appropriate for the Long-Lasting Phosphors Eu2+-Doped SrAl2O4 with Codopants Dy3+ and B3+. Chem. Mater. 2005, 17, 3904–3912. [Google Scholar] [CrossRef]
- Lin, L.; Shi, R. The Effect of Sr2+ on Luminescence of Ce3+-Doped (Ca,Sr)2Al2SiO7. Inorg. Chem. 2017, 56, 12476–12484. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, M.; Tang, Y.; Hunter, E.C.; Battle, P.D.; Hadermann, J. Structural and Magnetic Properties of A2LaFe2SbO9 (A = Ca,Sr,Ba). J. Solid State Chem. 2021, 295, 121914. [Google Scholar] [CrossRef]
- Hölsä, J.; Jungner, H.; Lastusaari, M.; Niittykoski, J. Persistent Luminescence of Eu2+-Doped Alkaline Earth Aluminates, MAl2O4:Eu2+. J. Alloys Compd. 2001, 323–324, 326–330. [Google Scholar] [CrossRef]
- Komai, S.; Hirano, M.; Ohtsu, N. Spectral Analysis of Sr 3d XPS Spectrum in Sr-Containing Hydroxyapatite. Surf. Interface Anal. 2020, 52, 823–828. [Google Scholar] [CrossRef]
- Cortés-Adasme, E.; Castillo, R.; Conejeros, S.; Fuentes, M.; Valenzuela, M.A. Behavior of Eu Ions in SrSnO3: Optical Properties, XPS Experiments and DFT Calculations. J. Alloys Compd. 2019, 771, 162–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Xu, C.; Li, Y.; Seo, H.J. Photoluminescence and Abnormal Reduction of Eu3+ Ions in CaAl2O4:Eu Nanophosphors. Phys. B Condens. Matter 2015, 472, 6–10. [Google Scholar] [CrossRef]
- Chernov, V.; Salas-Castillo, P.; Díaz-Torres, L.A.; López-Morales, M.A.; Cruz-Zaragoza, E. Thermoluminescence and Infrared Stimulated Luminescence in Long Persistent Monoclinic SrAl2O4:Eu2+,Dy3+ and SrAl2O4:Eu2+,Nd3+ Phosphors. Opt. Mater. 2019, 92, 46–52. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Ianculescu, A.; Preda, S.; Negrila, C.; Secu, M. Photoluminescence and Thermoluminescence Properties of the Sr3Al2O6:Eu3+/Eu2+,Tb3+ Persistent Phosphor. J. Lumin. 2019, 214, 116540. [Google Scholar] [CrossRef]
- Kim, D. Recent Developments in Lanthanide-Doped Alkaline Earth Aluminate Phosphors. Nanomaterials 2021, 11, 723. [Google Scholar] [CrossRef]
- Gedekar, K.A.; Wankhede, S.P.; Moharil, S.V.; Belekar, R.M. d–f Luminescence of Ce3+ and Eu2+ Ions in BaAl2O4, SrAl2O4 and CaAl2O4 Phosphors. J. Adv. Ceram. 2017, 6, 341–350. [Google Scholar] [CrossRef]
- Dorenbos, P. f→d Transition Energies of Divalent Lanthanides in Inorganic Compounds. J. Phys. Condens. Matter 2003, 15, 575–594. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Qu, B.; Zhang, B.; Wang, L.; Zhou, R.; Zeng, X.C. Mechanistic Study of the Persistent Luminescence of CaAl2O4:Eu2+,Nd3+. Chem. Mater. 2015, 27, 2195–2202. [Google Scholar] [CrossRef]
- Yamamoto, H.; Matsuzawa, T. Mechanism of Long Phosphorescence of SrAl2O4:Eu2+,Dy3+ and CaAl2O4:Eu2+,Nd3+. J. Lumin. 1997, 72, 287–289. [Google Scholar] [CrossRef]
- Ning, L.; Huang, X.; Huang, Y.; Tanner, P.A. Origin of the Green Persistent Luminescence of Eu-Doped SrAl2O4 from a Multiconfigurational: Ab Initio Study of 4f7→4f65d1 Transitions. J. Mater. Chem. C 2018, 6, 6637–6640. [Google Scholar] [CrossRef]
- Chithambo, M.L. Thermoluminescence of the Main Peak in SrAl2O4:Eu2+,Dy3+: Spectral and Kinetics Features of Secondary Emission Detected in the Ultra-Violet Region. Radiat. Meas. 2017, 96, 29–41. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q. Decay Model of Eu2+ and Eu2+,Dy3+ Substituted SrAl2O4. J. Phys. Chem. Solids 2019, 124, 151–156. [Google Scholar] [CrossRef]
- Rezende, M.V.S.; Andrade, A.B. The Effect of the Host Composition on the Lifetime Decay Properties of Barium/Strontium Aluminates Compounds. J. Appl. Phys. 2014, 115, 103510. [Google Scholar] [CrossRef]
- Jiang, L.; Chang, C. Luminescent Properties of Ca2MgSi2O7 Phosphor Activated by Eu2+,Dy3+ and Nd3+. Opt. Mater. 2004, 27, 51–55. [Google Scholar] [CrossRef]
- Aitasalo, T.; Durygin, A.; Hölsä, J.; Lastusaari, M.; Niittykoski, J.; Suchocki, A. Low Temperature Thermoluminescence Properties of Eu2+ and R3+ Doped CaAl2O4. J. Alloys Compd. 2004, 380, 4–8. [Google Scholar] [CrossRef]
- Ruiz-Torres, R.; Chernov, V.; Salas-Castillo, P.; Zúñiga-Rivera, N.J.; Diaz-Torres, L.A.; Meléndrez, R.; Barboza-Flores, M. Effect of thermal treatment on luminescence properties of long persistent CaAl2O4:Eu2+,Dy3+ synthesized by combustion method. Opt. Mater. 2020, 101, 109763. [Google Scholar] [CrossRef]
- Chen, X.Y.; Bao, S.P.; Wu, Y.C. Controlled Synthesis and Luminescent Properties of Eu2+ (Eu3+), Dy3+-Doped Sr3Al2O6 Phosphors by Hydrothermal Treatment and Postannealing Approach. J. Solid State Chem. 2010, 183, 2004–2011. [Google Scholar] [CrossRef]
- Dudrica, R.; Bortnic, R.; Souca, G.; Ciceo-Lucacel, R.; Stiufiuc, R.; Tetean, R. XPS on Nd0.6−xBixSr0.4MnO3 Nano Powders. Appl. Surf. Sci. 2019, 487, 17–21. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Ahmed, H.; Khattab, T.A. Recent Advances in Long-Persistent Luminescence in Rare-Earth-Doped Compounds. Prog. Opt. Sci. Photonics 2023, 25, 309–331. [Google Scholar]
- Armetta, F.; Lo Bianco, A.; Boiko, V.; Hreniak, D.; Saladino, M.L. Multimodal anti-counterfeiting inks: Modern use of an ancient pigment in synergy with a persistent phosphor. J. Mater. Chem. C 2024, 13, 1188–1197. [Google Scholar] [CrossRef]
- Neema, R.; Malla, M.S.; Sharma, P.K.; Mittal, M. Luminescence Studies of Rare-Earth Ce3+ and Dy3+ Doped SrAl2O4 Aluminate Phosphors. J. Mater. Sci. Mater. Electron. 2021, 32, 12318–12329. [Google Scholar] [CrossRef]
- Xie, Q.; Li, B.; He, X.; Zhang, M.; Chen, Y.; Zeng, Q. Correlation of Structure, Tunable Colors, and Lifetimes of (Sr, Ca, Ba)Al2O4:Eu2+, Dy3+ Phosphors. Materials 2017, 10, 1198. [Google Scholar] [CrossRef] [PubMed]


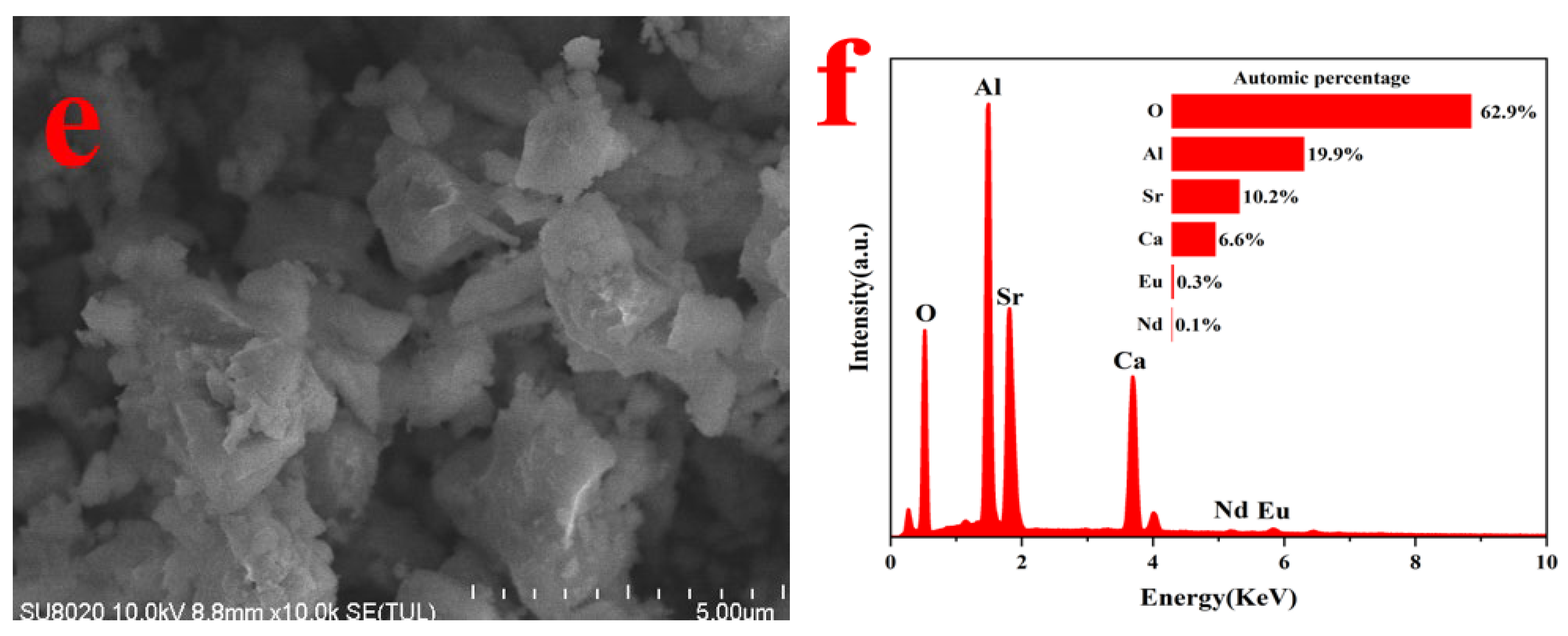
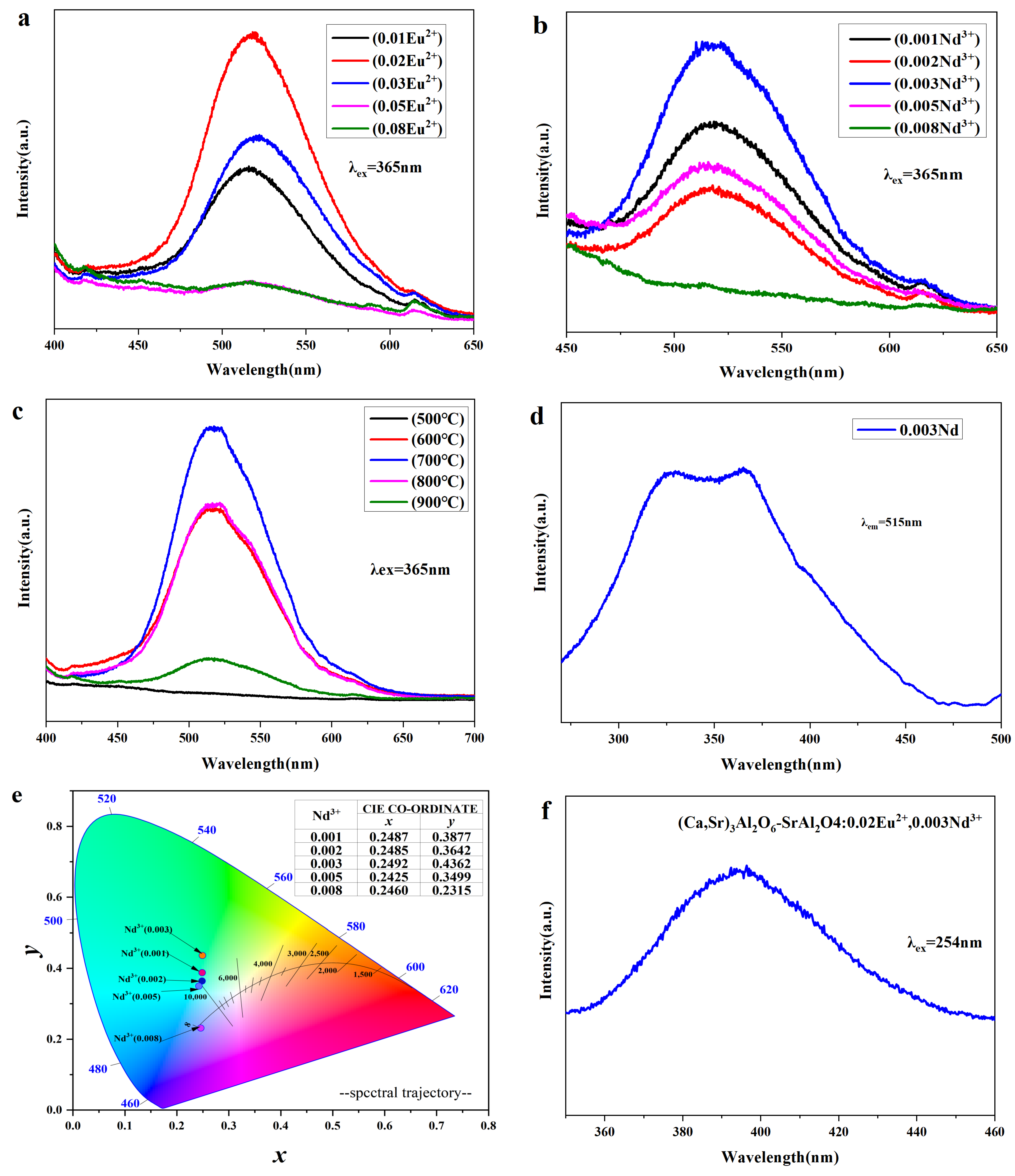

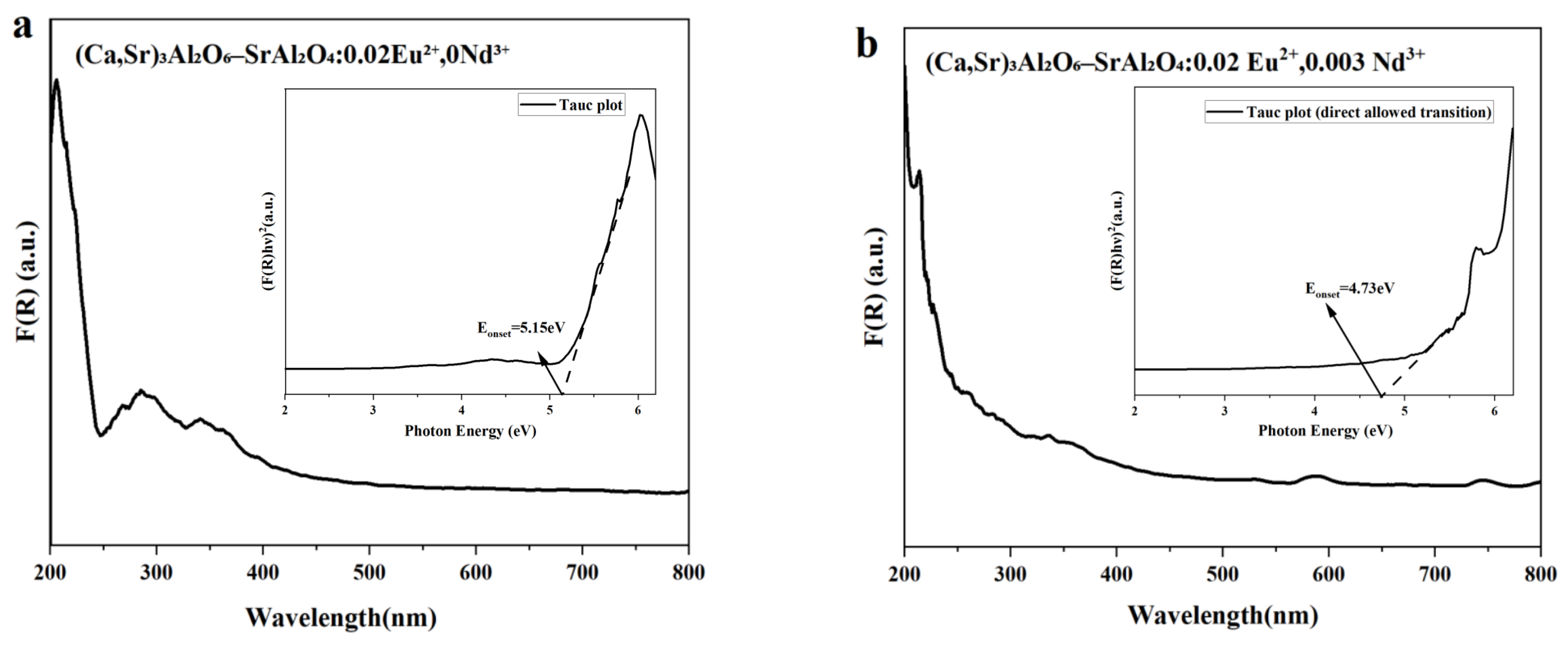
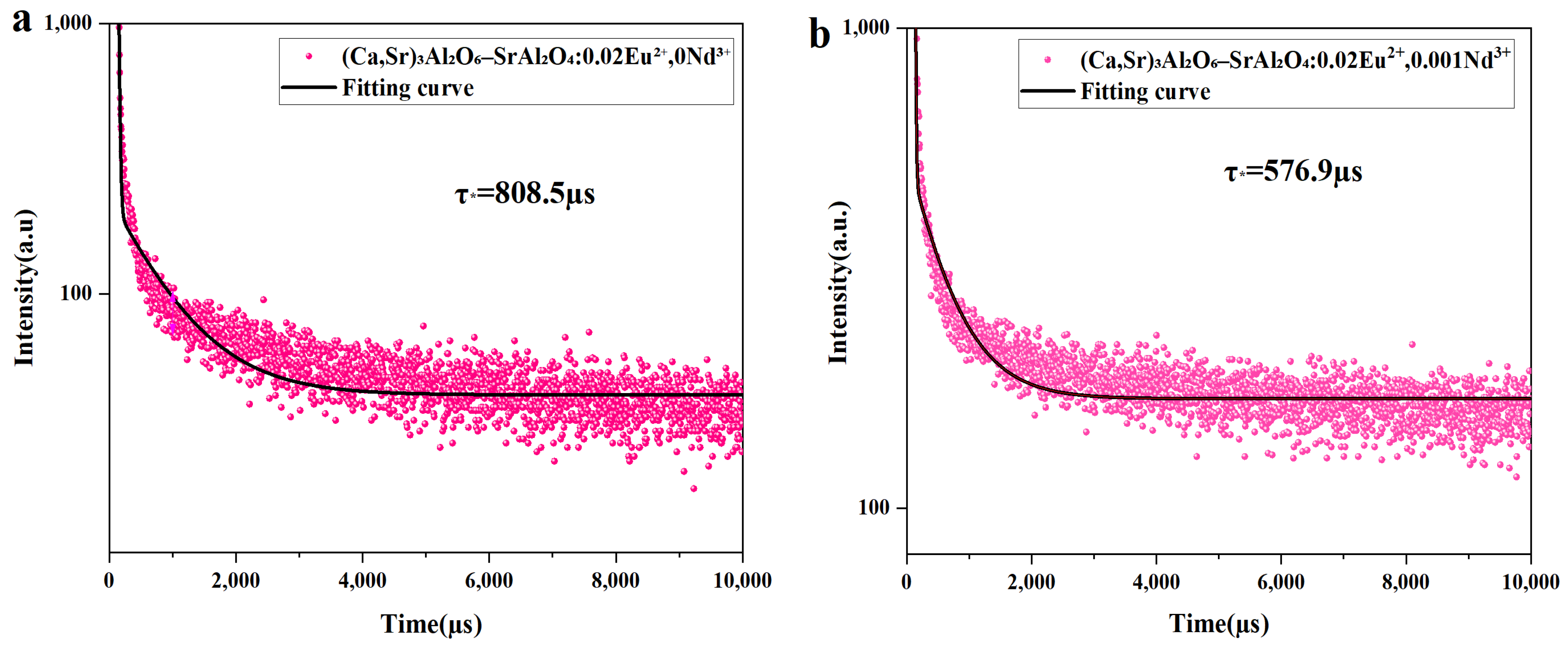






| Ca(NO3)2 | Sr(NO3)2 | Al(NO3)3 | Eu(NO3)3 | Nd(NO3)3 |
|---|---|---|---|---|
| 1 mmol/mL | 0.5 mmol/mL | 1 mmol/mL | 0.1 mmol/mL | 0.1 mmol/mL |
| Fluorescence Lifetime (µs) | ||||||
|---|---|---|---|---|---|---|
| Nd3+ | 0.000 | 0.001 | 0.002 | 0.003 | 0.005 | 0.008 |
| A1 | 1.43 | 8.38 | 4.69 | 5.57 | 3.35 | 1.79 |
| τ1 | 15.59 | 10.00 | 13.52 | 13.28 | 14.08 | 15.25 |
| A2 | 187.94 | 388.50 | 392.50 | 546.55 | 423.78 | 445.81 |
| τ2 | 813.99 | 576.90 | 549.26 | 385.86 | 483.45 | 564.21 |
| τ* | 808.5 µs | 576.9 µs | 549.5 µs | 385.7 µs | 483.2 µs | 564.2 µs |
| Afterglow Lifetime (s) | ||
|---|---|---|
| Nd3+ | 0.003 | 0.008 |
| A1 | 7058.75 | 4472.70 |
| τ1 | 3.42 | 3.43 |
| A2 | 397.87 | 238.82 |
| τ2 | 60.05 | 85.61 |
| τave | 31.56 s | 50.37 s |
| Segment | 0–10 s | 10–30 s | 30–100 s | 100–120 s | Total |
|---|---|---|---|---|---|
| Integrated_Intensity | 28,626.48 | 9733.73 | 9305.71 | 1261.16 | 61,498.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Wang, J.; Qi, Y.; Hu, J.; Li, H.; Wang, C.; Cheng, X.; Pan, D.; Li, Z.; Li, J. Study on the Structure-Luminescence Relationship and Anti-Counterfeiting Application of (Ca,Sr)-Al-O Composite Fluorescent Materials. Nanomaterials 2025, 15, 1446. https://doi.org/10.3390/nano15181446
Lv J, Wang J, Qi Y, Hu J, Li H, Wang C, Cheng X, Pan D, Li Z, Li J. Study on the Structure-Luminescence Relationship and Anti-Counterfeiting Application of (Ca,Sr)-Al-O Composite Fluorescent Materials. Nanomaterials. 2025; 15(18):1446. https://doi.org/10.3390/nano15181446
Chicago/Turabian StyleLv, Jianhui, Jigang Wang, Yuansheng Qi, Jindi Hu, Haiming Li, Chuanming Wang, Xiaohan Cheng, Deyu Pan, Zhenjun Li, and Junming Li. 2025. "Study on the Structure-Luminescence Relationship and Anti-Counterfeiting Application of (Ca,Sr)-Al-O Composite Fluorescent Materials" Nanomaterials 15, no. 18: 1446. https://doi.org/10.3390/nano15181446
APA StyleLv, J., Wang, J., Qi, Y., Hu, J., Li, H., Wang, C., Cheng, X., Pan, D., Li, Z., & Li, J. (2025). Study on the Structure-Luminescence Relationship and Anti-Counterfeiting Application of (Ca,Sr)-Al-O Composite Fluorescent Materials. Nanomaterials, 15(18), 1446. https://doi.org/10.3390/nano15181446








