Nano-Heterojunction NO2 Gas Sensor Based on n-ZnO Nanorods/p-NiO Nanoparticles Under UV Illumination at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Sensing Materials
2.1.1. Hydrothermal Synthesis of ZnO Nanorods
2.1.2. Formation of p-NiO NPs/n-ZnO NRs Heterojunctions
2.1.3. Electrode Formation
2.2. Characterizations
2.3. Gas Sensing Measurements
3. Results and Discussion
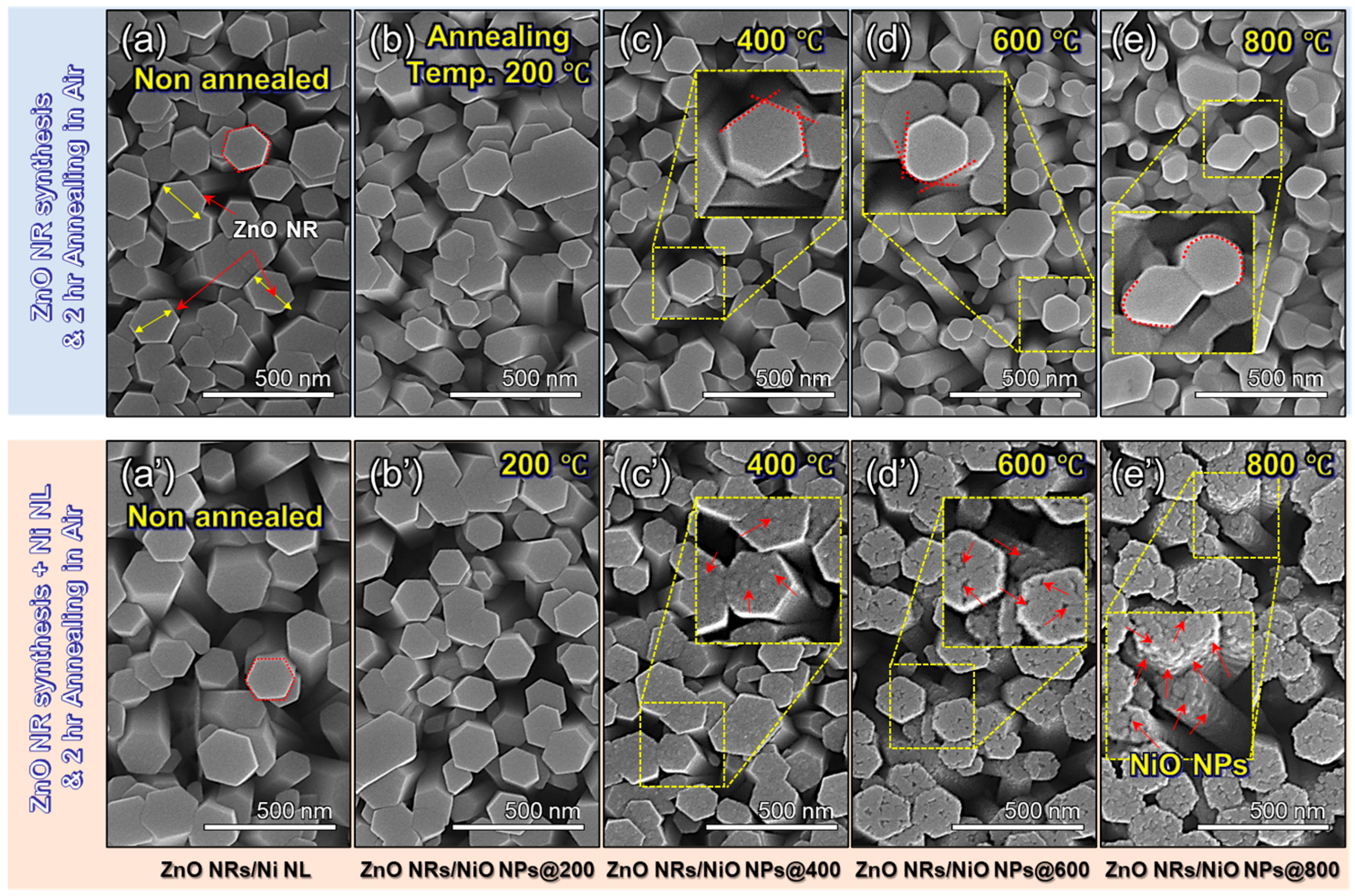
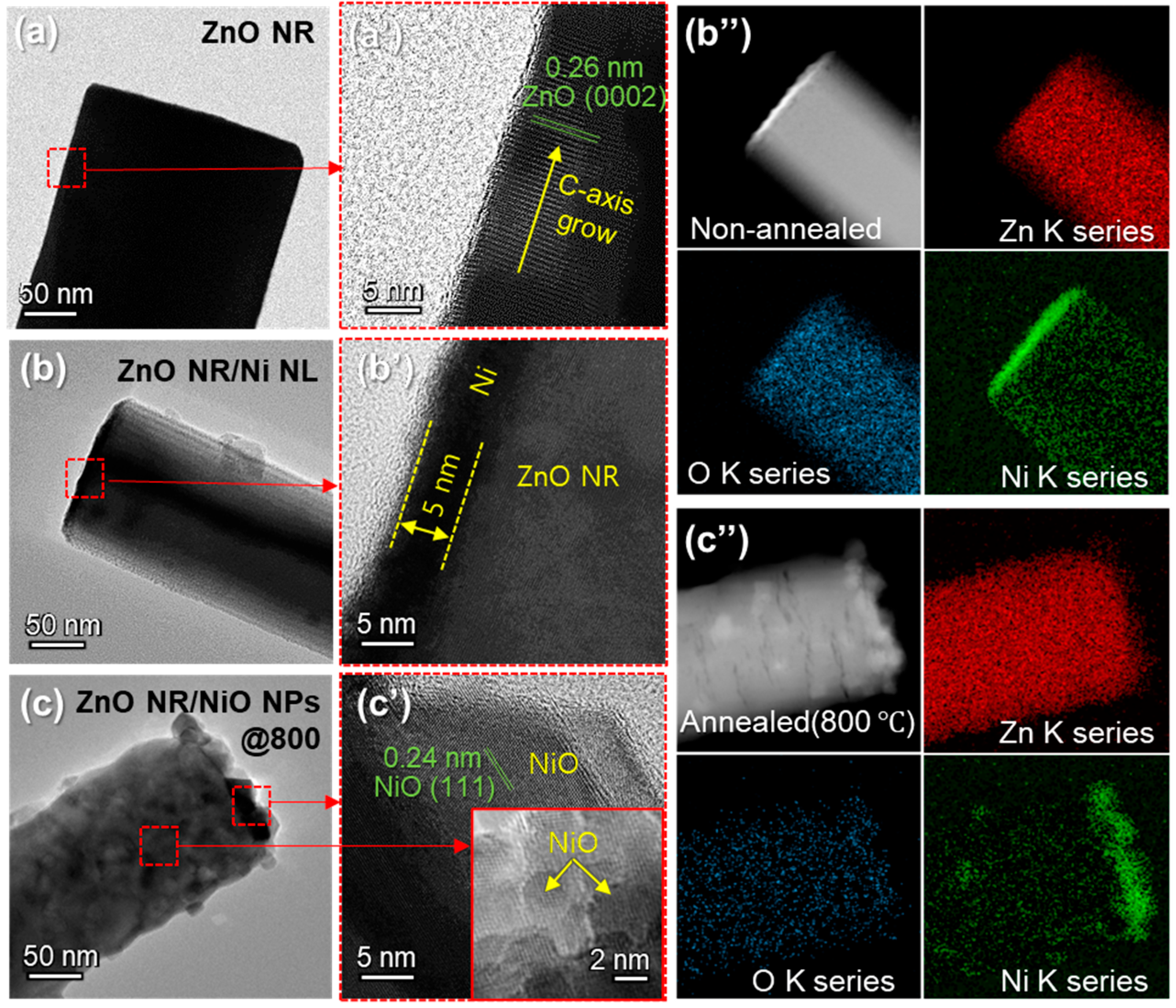
3.1. Material Characterizations
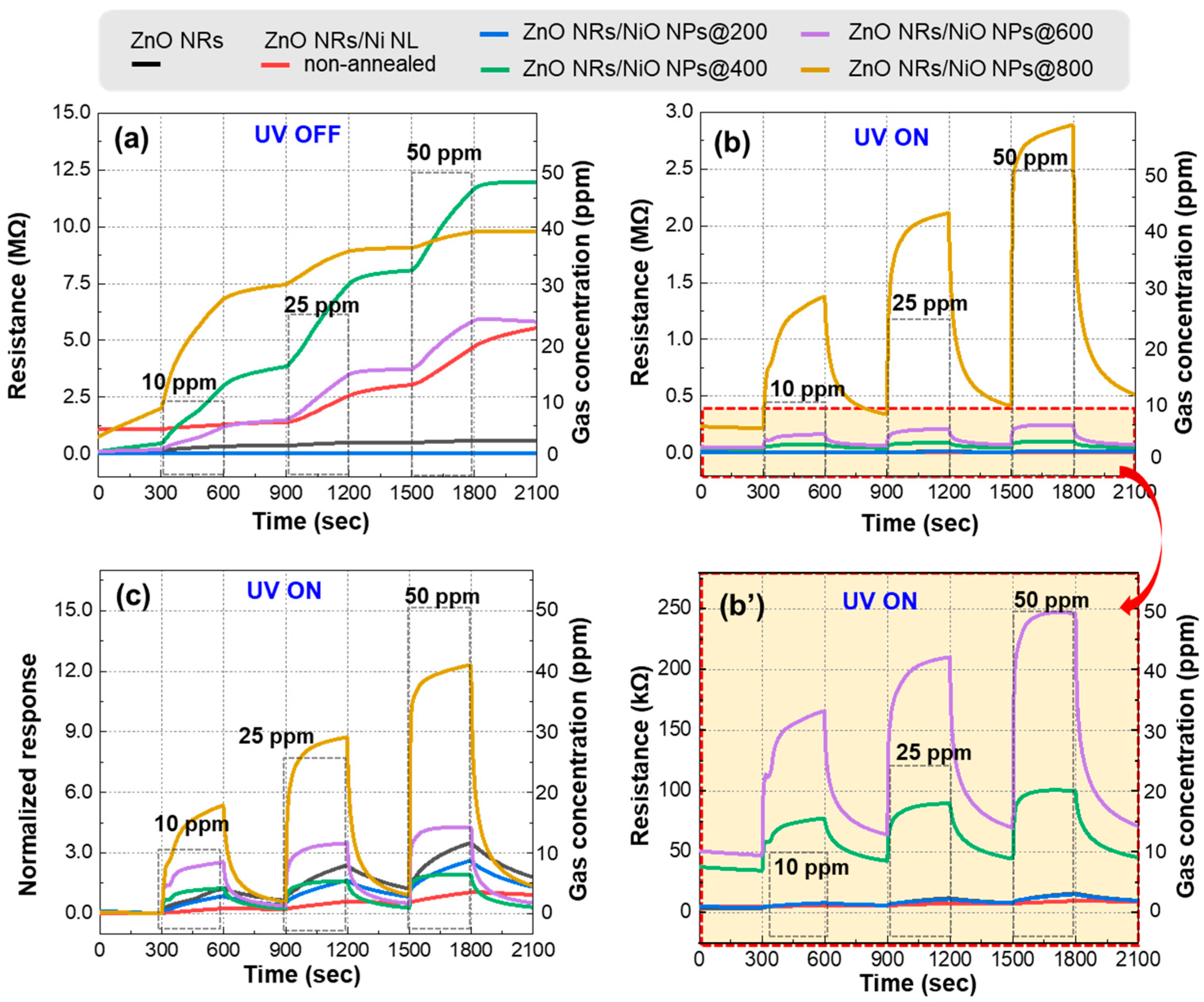
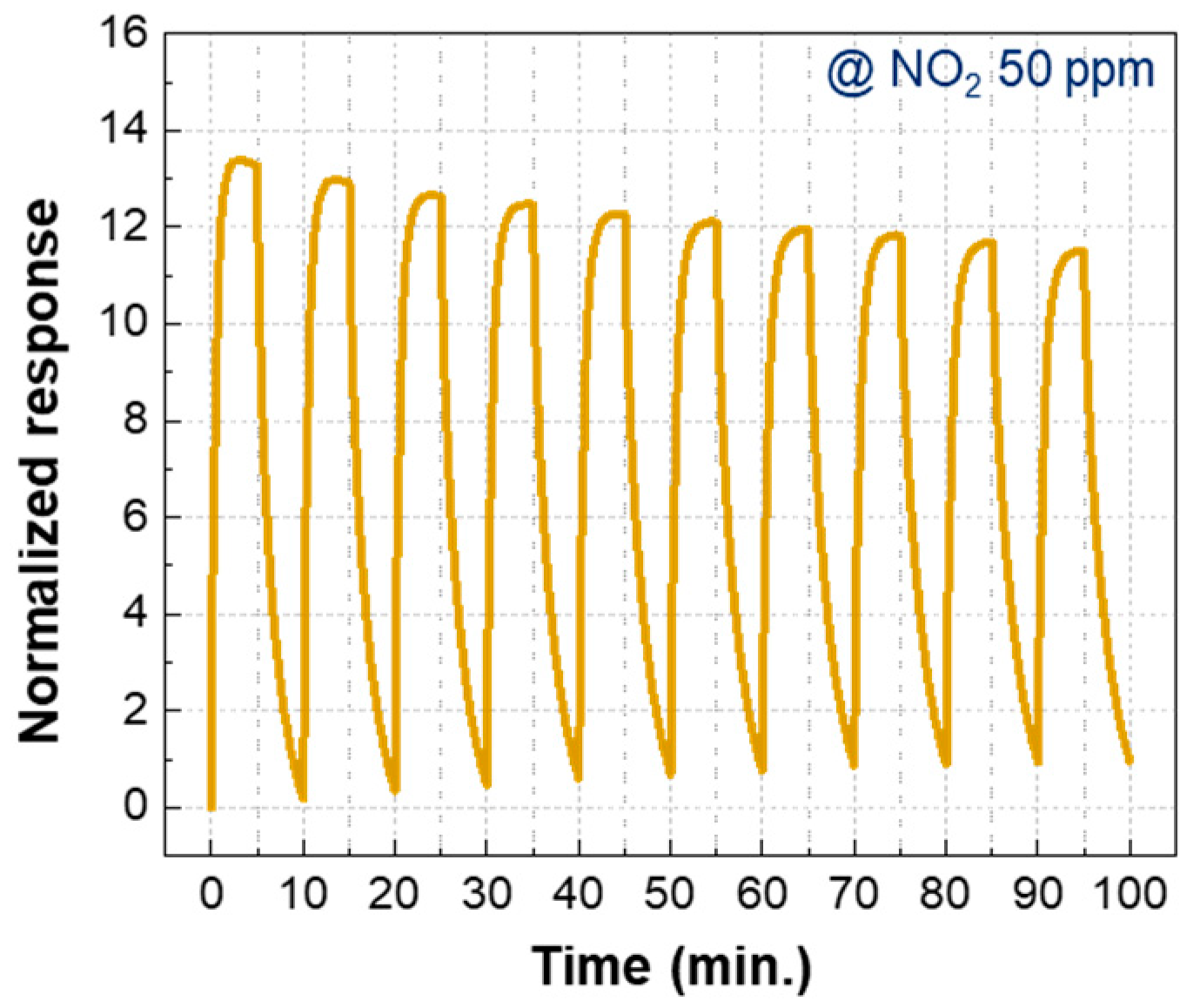
3.2. Gas Sensing Properties
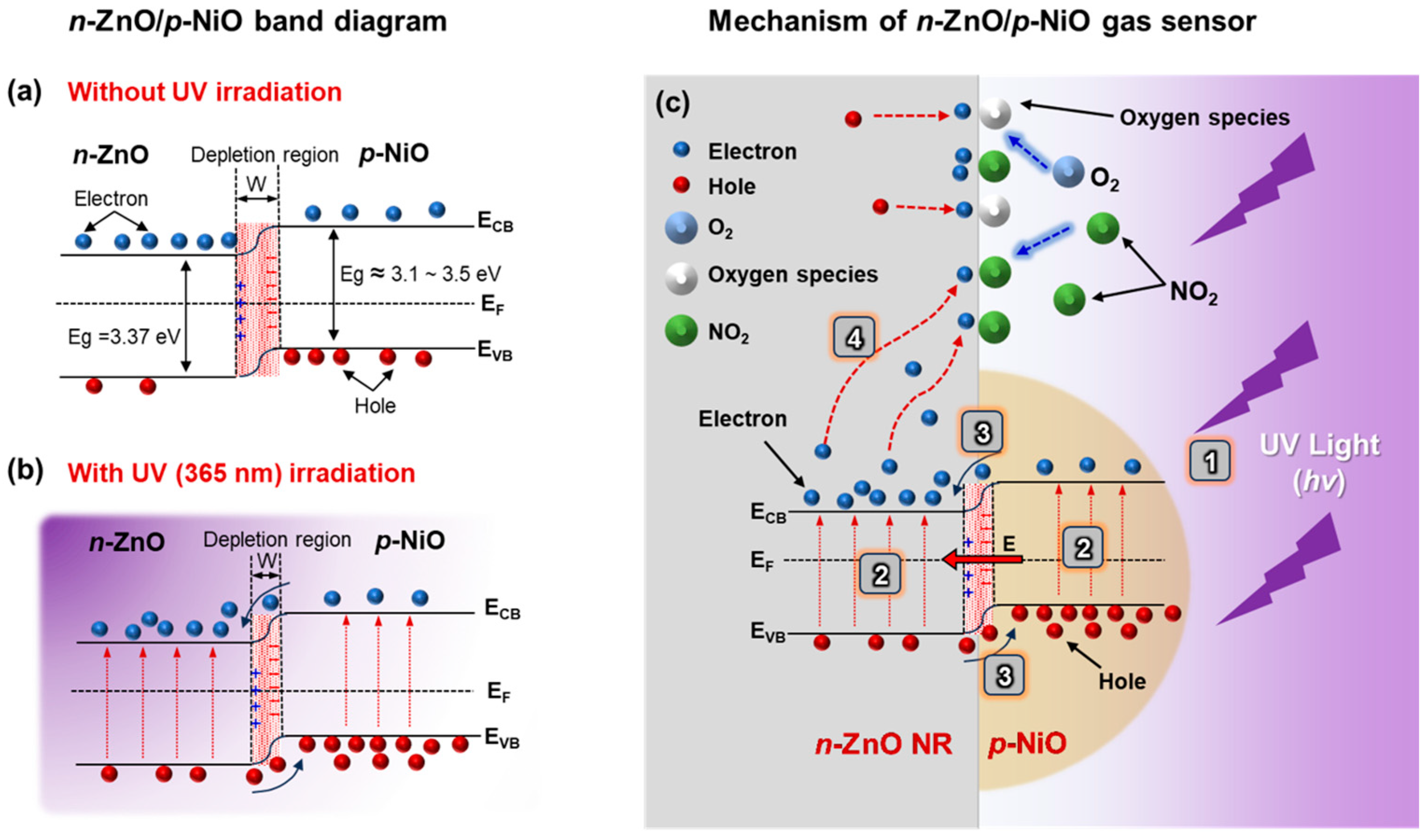
3.3. Gas Sensing Mechanisms
3.3.1. Gas Sensing Mechanism of ZnO with UV Activation
3.3.2. UV Photoactivation in n-ZnO/p-NiO NHJs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latza, U.; Gerdes, S.; Baur, X. Effects of nitrogen dioxide on human health: Systematic review of experimental and epidemiological studies conducted between 2002 and 2006. Int. J. Hyg. Environ. Health 2009, 212, 271–287. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Chougule, M.A.; Dalavi, D.S.; Mali, S.; Patil, P.S.; Moholkar, A.V.; Agawane, G.L.; Kim, J.H.; Sen, S.; Patil, V.B. Novel method for fabrication of room temperature polypyrrole–ZnO nanocomposite NO2 sensor. Measurement 2012, 45, 1989–1996. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Umar, A.; Nadargi, J.D.; Lokare, S.A.; Akbar, S.; Mulla, I.S.; Suryavanshi, S.S.; Bhandari, N.L.; Chaskar, M.G. Gas sensors and factors influencing sensing mechanism with a special focus on MOS sensors. J. Mater. Sci. 2023, 58, 559–582. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, X.; Zhou, Y.; He, Y.; Zang, Z. Copper-based metal oxides for chemiresistive gas sensors. J. Mater. Chem. C 2022, 10, 16218–16246. [Google Scholar] [CrossRef]
- Chai, H.; Zheng, Z.; Liu, K.; Xu, J.; Wu, K.; Luo, Y.; Liao, H.; Debliquy, M.; Zhang, C. Stability of metal oxide semiconductor gas sensors: A review. IEEE Sens. J. 2022, 22, 5470–5481. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Oh, E.; Choi, H.-Y.; Jung, S.-H.; Cho, S.; Kim, J.C.; Lee, K.-H.; Kang, S.-W.; Kim, J.; Yun, J.-Y.; Jeong, S.-H. High-performance NO2 gas sensor based on ZnO nanorod grown by ultrasonic irradiation. Sens. Actuators B 2009, 141, 239–243. [Google Scholar] [CrossRef]
- Wang, X.; Sun, F.; Duan, Y.; Yin, Z.; Luo, W.; Huang, Y.; Chen, J. Highly sensitive, temperature-dependent gas sensor based on hierarchical ZnO nanorod arrays. J. Mater. Chem. C 2015, 3, 11397–11405. [Google Scholar] [CrossRef]
- Kim, J.-W.; Porte, Y.; Ko, K.Y.; Kim, H.; Myoung, J.-M. Micropatternable double-faced ZnO nanoflowers for flexible gas sensor. ACS Appl. Mater. Interfaces 2017, 9, 32876–32886. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, H.; Fei, T.; Zhang, T. Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B 2014, 202, 272–278. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Xie, C.; Wu, J.; Zeng, D.; Liao, Y. A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceram. Int. 2012, 38, 503–509. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, K.-K.; Park, S. Room temperature detection of NO2 gas under UV irradiation based on Au nanoparticle-decorated porous ZnO nanowires. J. Mater. Res. Technol. 2020, 9, 16289–16302. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuators B 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kwon, S.-H.; Lee, W.-S.; Im, K.-G.; Kim, T.-H.; Noh, B.-R.; Park, S.; Oh, S.; Kim, K.-K. Ultraviolet photoactivated room temperature NO2 gas sensor of ZnO hemitubes and nanotubes covered with TiO2 nanoparticles. Nanomaterials 2020, 10, 462. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Kim, T.-H.; Kim, S.-M.; Oh, S.; Kim, K.-K. Ultraviolet light-emitting diode-assisted highly sensitive room temperature NO2 gas sensors based on low-temperature solution-processed ZnO/TiO2 nanorods decorated with plasmonic Au nanoparticles. Nanoscale 2021, 13, 12177–12184. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yang, J.-E.; Park, Y.-S.; Park, M.; Kim, S.-J.; Kim, K.-K. Convergence gas sensors with one-dimensional nanotubes and Pt nanoparticles based on ultraviolet photonic energy for room-temperature NO2 gas sensing. Nanomaterials 2023, 13, 2780. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, Y.; Tang, Y.; Yu, F. UV-activated ZnO–NiO heterojunction sensor for ethanol gas detection at low working temperature. Mater. Sci. Semicond. Process. 2024, 169, 107925. [Google Scholar] [CrossRef]
- Suguna, A.; Prabhu, S.; Selvaraj, M.; Geerthana, M.; Silambarasan, A.; Navaneethan, M.; Ramesh, R.; Sridevi, C. Annealing effect on photocatalytic activity of ZnO nanostructures for organic dye degradation. J. Mater. Sci. Mater. Electron. 2022, 33, 8868–8879. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Mi, R.; Deng, C.; Gao, P. An environment-benign method for the synthesis of p-NiO/n-ZnO heterostructure with excellent performance for gas sensing and photocatalysis. Sens. Actuators B 2014, 191, 537–544. [Google Scholar] [CrossRef]
- Che, L.; Pan, J.; Cai, K.; Cong, Y.; Lv, S.-W. The construction of p–n heterojunction for enhancing photocatalytic performance in environmental application: A review. Sep. Purif. Technol. 2023, 315, 123708. [Google Scholar] [CrossRef]
- Moumen, A.; Kumarage, G.C.W.; Comin, E. P-type metal oxide semiconductor thin films: Synthesis and chemical sensor applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Dong, G.; Ma, L.; Ma, J. NiO/ZnO p–n heterostructures and their gas sensing properties for reduced operating temperature. RSC Adv. 2016, 6, 109091–109098. [Google Scholar] [CrossRef]
- Uhlenbrock, S.; Scharfschwerdt, C.; Neumann, M.; Illing, G.; Freund, H.-J. The influence of defects on the Ni 2p and O 1s XPS of NiO. J. Phys. Condens. Matter 1992, 4, 7973–7978. [Google Scholar] [CrossRef]
- Chastain, J.; King Jr., R.C. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Connecticut, CT, USA, 1992; Volume 40, p. 25. [Google Scholar]
- Berlich, A.; Liu, Y.C.; Morgner, H. Growth of nickel nanoparticles on NiO/Ni (001): Evidence of adsorbed oxygen on metal particles by metastable induced electron spectroscopy (MIES). Surf. Sci. 2008, 602, 3737–3744. [Google Scholar] [CrossRef]
- Yang, Z.-G.; Zhu, L.-P.; Guo, Y.-M.; Tian, W.; Ye, Z.-Z.; Zhao, B.-H. Valence-band offset of p-NiO/n-ZnO heterojunction measured by X-ray photoelectron spectroscopy. Phys. Lett. A 2011, 375, 1760–1763. [Google Scholar] [CrossRef]
- Dubey, P.; Kaurav, N.; Devan, R.S.; Okram, G.S.; Kuo, Y.K. The effect of stoichiometry on the structural, thermal and electronic properties of thermally decomposed nickel oxide. RSC Adv. 2018, 8, 5882–5890. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Tomar, M.; Gupta, V. Postdeposition annealing of NiOx thin films: A transition from n-type to p-type conductivity for short wavelength optoelectronic devices. J. Mater. Res. 2013, 28, 723–732. [Google Scholar] [CrossRef]
- Chen, Z.; Dedova, T.; Spalatu, N.; Maticiuc, N.; Rusu, M.; Katerski, A.; Acik, I.O.; Unold, T.; Krunks, M. ZnO/NiO heterostructures with enhanced photocatalytic activity obtained by ultrasonic spraying of a NiO shell onto ZnO nanorods. Colloids Surf. A 2022, 648, 129366. [Google Scholar] [CrossRef]
- Imran, M.; Coskun, H.; Khan, N.A.; Ouyang, J. Role of annealing temperature of nickel oxide (NiOx) as hole transport layer in work function alignment with perovskite. Appl. Phys. A 2021, 127, 117. [Google Scholar] [CrossRef]
- Navale, Y.H.; Navale, S.T.; Chougule, M.A.; Ramgir, N.S.; Patil, V.B. NO2 gas sensing properties of heterostructural CuO nanoparticles/ZnO nanorods. J. Mater. Sci. Mater. Electron. 2021, 32, 18178–18191. [Google Scholar] [CrossRef]
- Ganbavle, V.V.; Inamdar, S.I.; Agawane, G.L.; Kim, J.H.; Rajpure, K.Y. Synthesis of fast response, highly sensitive and selective Ni:ZnO based NO2 sensor. Chem. Eng. J. 2016, 286, 36–47. [Google Scholar] [CrossRef]
- Patil, V.L.; Vanalakar, S.A.; Tarwal, N.L.; Patil, A.P.; Dongale, T.D.; Kim, J.H.; Patil, P.S. Construction of Cu doped ZnO nanorods by chemical method for low temperature detection of NO2 gas. Sens. Actuators A 2019, 299, 111611. [Google Scholar] [CrossRef]
- Navale, Y.H.; Navale, S.T.; Stadler, F.J.; Ramgir, N.S.; Patil, V.B. Enhanced NO2 sensing aptness of ZnO nanowire/CuO nanoparticle heterostructure-based gas sensors. Ceram. Int. 2019, 45, 1513–1522. [Google Scholar] [CrossRef]
- Liao, J.; Li, Z.; Wang, G.; Chen, C.; Lv, S.; Li, M. ZnO nanorod/porous silicon nanowire hybrid structures as highly-sensitive NO2 gas sensors at room temperature. Phys. Chem. Chem. Phys. 2016, 18, 4835–4841. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.; Li, X.; Xia, Y.; Yang, C. Synergistic effects of UV activation and surface oxygen vacancies on the room-temperature NO2 gas sensing performance of ZnO nanowires. Sens. Actuators B 2019, 298, 126858. [Google Scholar] [CrossRef]
- Karaduman, I.; Yıldız, D.E.; Sincar, M.M.; Acar, S. UV light activated gas sensor for NO2 detection. Mater. Sci. Semicond. Process. 2014, 28, 43–47. [Google Scholar] [CrossRef]
- Bai, S.; Han, J.; Meng, J.C.; Sun, L.; Sun, J.; Zhao, Y.; Tang, P.; Luo, R.; Li, D.; Chen, A. NiO/ZnO composite decorated on rGO for detection of NO2. Sens. Actuators B Chem. 2021, 339, 129720. [Google Scholar] [CrossRef]
- Tagliaferro, J.C.; Komorizono, A.A.; Pessoa, N.C.S.; Correia, R.S.; Bernardi, M.I.B.; Mastelaro, V.R. Influence of morphology and heterostructure formation on the NO2 gas sensing properties of the ZnO–NiO system. Talanta Open 2024, 10, 100388. [Google Scholar] [CrossRef]
- Ambi, R.R.; Mane, A.A.; Patil, V.B.; Mane, R.D. Highly porous hierarchical NiO coated ZnO p–n heterostructure for NO2 detection. Mater. Sci. Eng. B 2024, 300, 117066. [Google Scholar] [CrossRef]


| Material | Operating Temp. (°C) | NO2 Concentration (ppm) | Normalized Response | UV | Ref. |
|---|---|---|---|---|---|
| CuO/ZnO Heterostructure | 150 | 50 | 0.7 | X | [35] |
| 1.5 at % Ni-doped ZnO film | 200 | 100 | 4.82 | X | [36] |
| Cu-doped ZnO | 175 | 60 | 5.82 | X | [37] |
| ZnO nanowire /CuO nanoparticle | 150 | 50 | 0.89 | X | [38] |
| ZnO nanorod/Porous Silicon Nanowire | RT | 50 | 0.35 | X | [39] |
| ZnO nanorod /NiO nanoparticle | RT | 50 | 12.3 | O | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-S.; Kim, S.; Lee, J.; Jeong, J.-H.; Byun, S.-Y.; Shin, J.; Park, I.-K.; Kim, K.-K. Nano-Heterojunction NO2 Gas Sensor Based on n-ZnO Nanorods/p-NiO Nanoparticles Under UV Illumination at Room Temperature. Nanomaterials 2025, 15, 1426. https://doi.org/10.3390/nano15181426
Park Y-S, Kim S, Lee J, Jeong J-H, Byun S-Y, Shin J, Park I-K, Kim K-K. Nano-Heterojunction NO2 Gas Sensor Based on n-ZnO Nanorods/p-NiO Nanoparticles Under UV Illumination at Room Temperature. Nanomaterials. 2025; 15(18):1426. https://doi.org/10.3390/nano15181426
Chicago/Turabian StylePark, Yoon-Seo, Sohyeon Kim, Junyoung Lee, Jae-Hoon Jeong, Sung-Yun Byun, Jiyoon Shin, Il-Kyu Park, and Kyoung-Kook Kim. 2025. "Nano-Heterojunction NO2 Gas Sensor Based on n-ZnO Nanorods/p-NiO Nanoparticles Under UV Illumination at Room Temperature" Nanomaterials 15, no. 18: 1426. https://doi.org/10.3390/nano15181426
APA StylePark, Y.-S., Kim, S., Lee, J., Jeong, J.-H., Byun, S.-Y., Shin, J., Park, I.-K., & Kim, K.-K. (2025). Nano-Heterojunction NO2 Gas Sensor Based on n-ZnO Nanorods/p-NiO Nanoparticles Under UV Illumination at Room Temperature. Nanomaterials, 15(18), 1426. https://doi.org/10.3390/nano15181426







