Internalization of Lipid-Coated Gold Nanocomposites and Gold Nanoparticles by Mouse SC-1 Fibroblasts in Monolayer and Spheroids
Abstract
1. Introduction
2. Materials and Methods
2.1. Physicochemical Characterization of Nanoparticles
2.2. Cell Lines and Spheroid Preparation
2.3. The Preparation of Cell and Spheroid Samples in the Dynamics of Incubation with Nanoparticles
2.3.1. Monolayer
2.3.2. Spheroids
2.4. Processing of the Samples for TEM Studies
2.5. Scanning Electron Microscopy
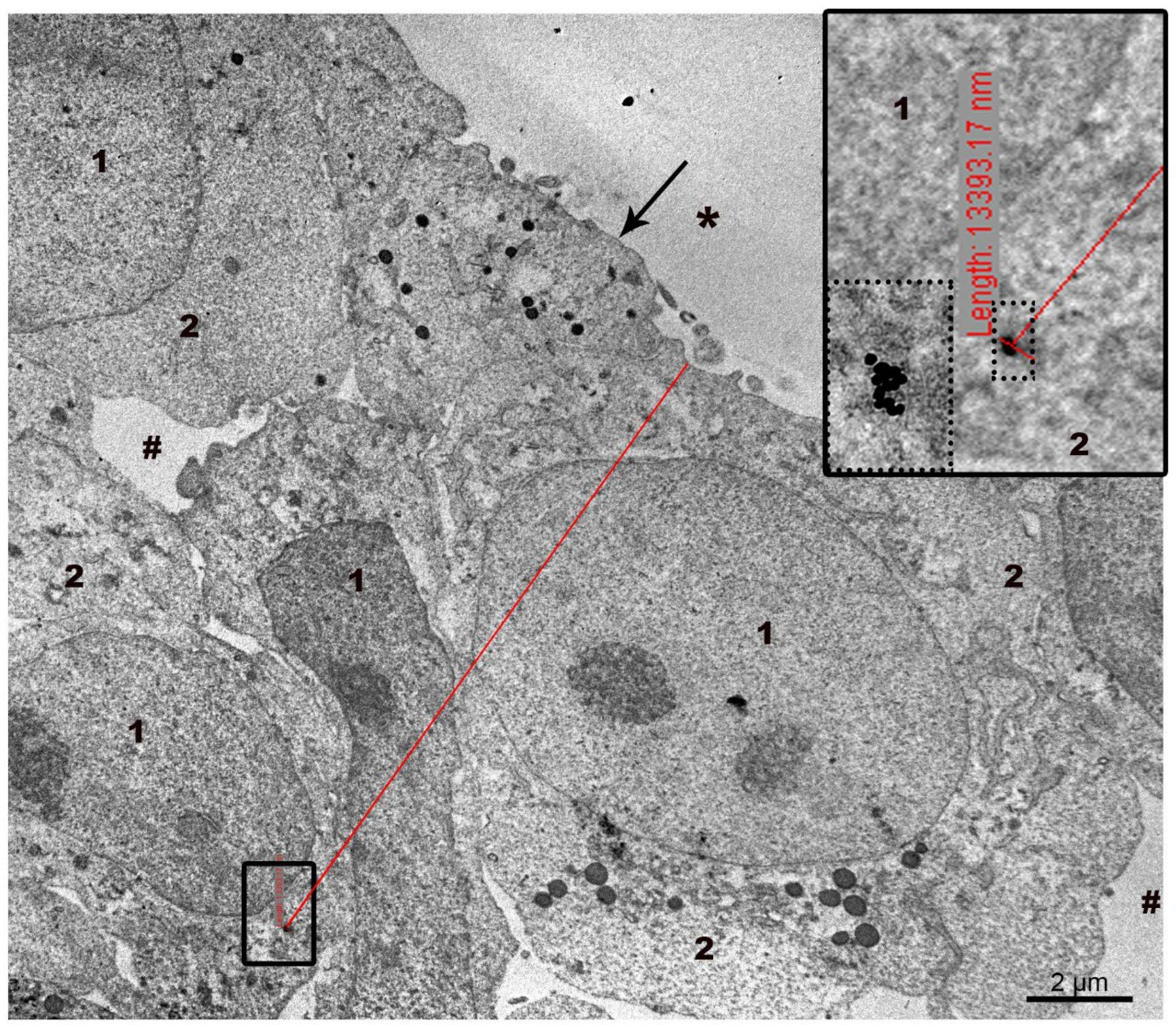
2.6. Measurements
3. Results and Discussions
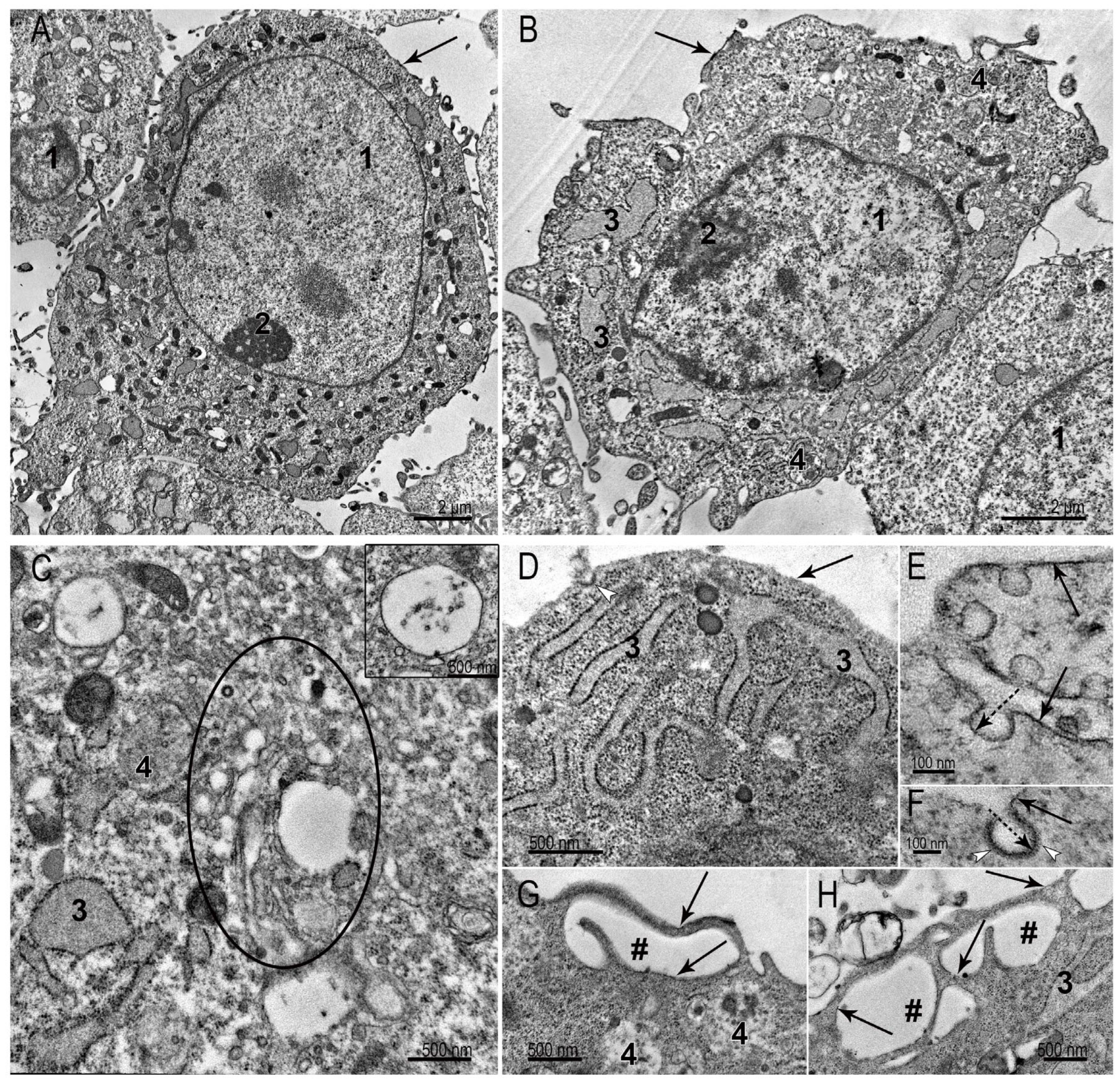
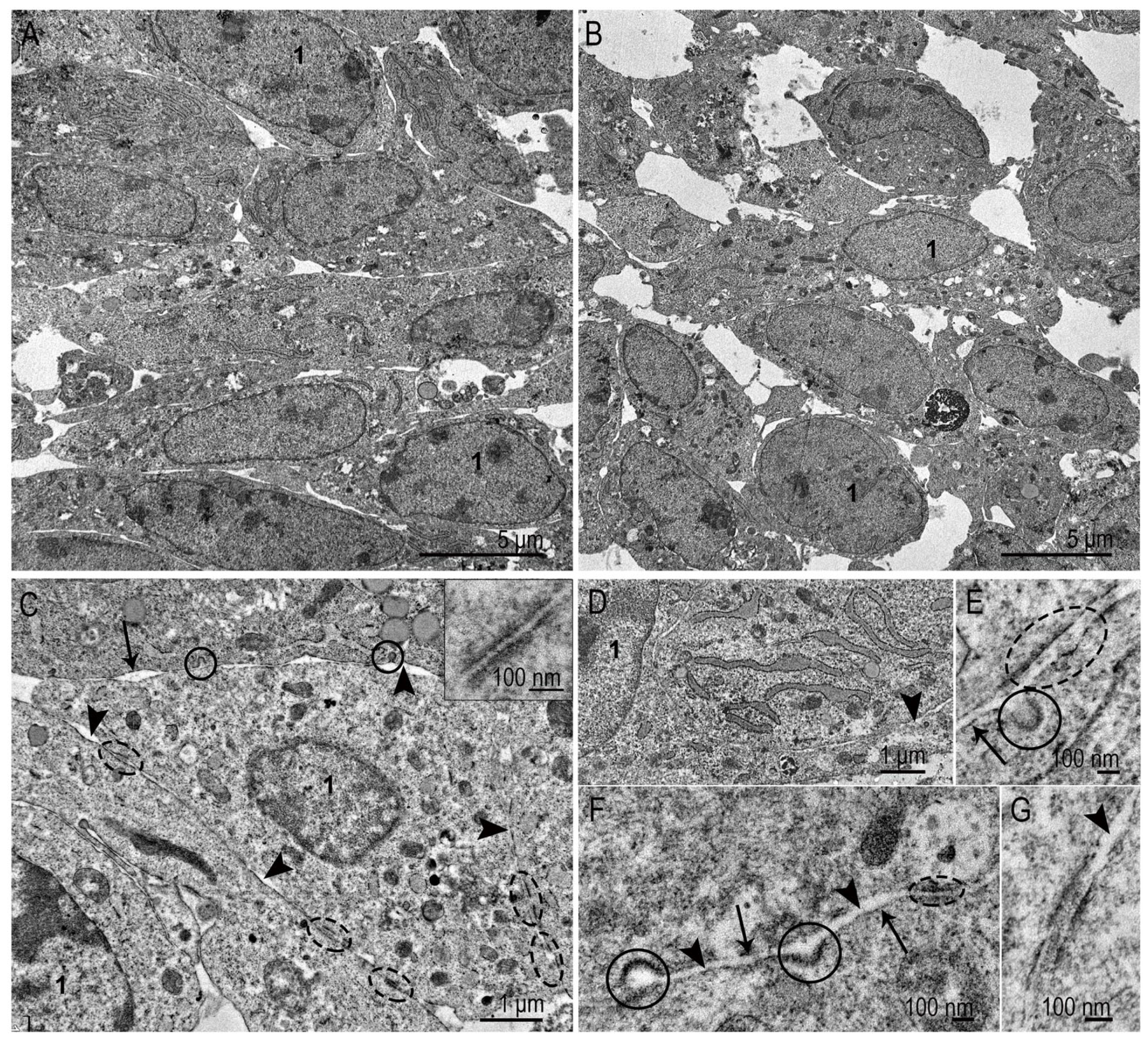
3.1. Ultrastructure of SC-1 Fibroblasts in Monolayer
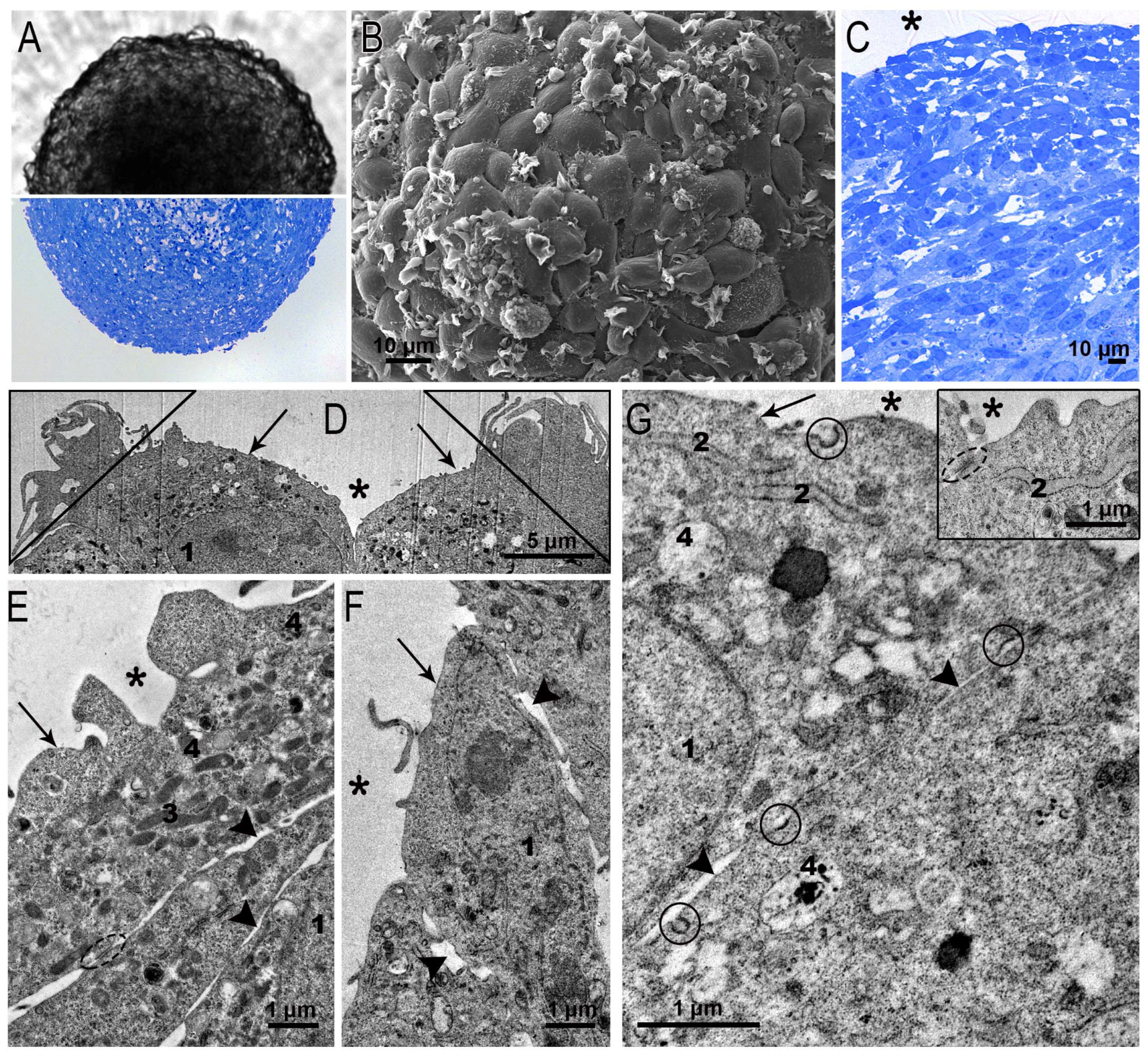
3.2. Ultrastructure of SC-1 Fibroblasts in Spheroids
3.2.1. Spheroid’s Superficial Layer
3.2.2. Ultrastructure of SC-1 Cells in Spheroid’s “Body”
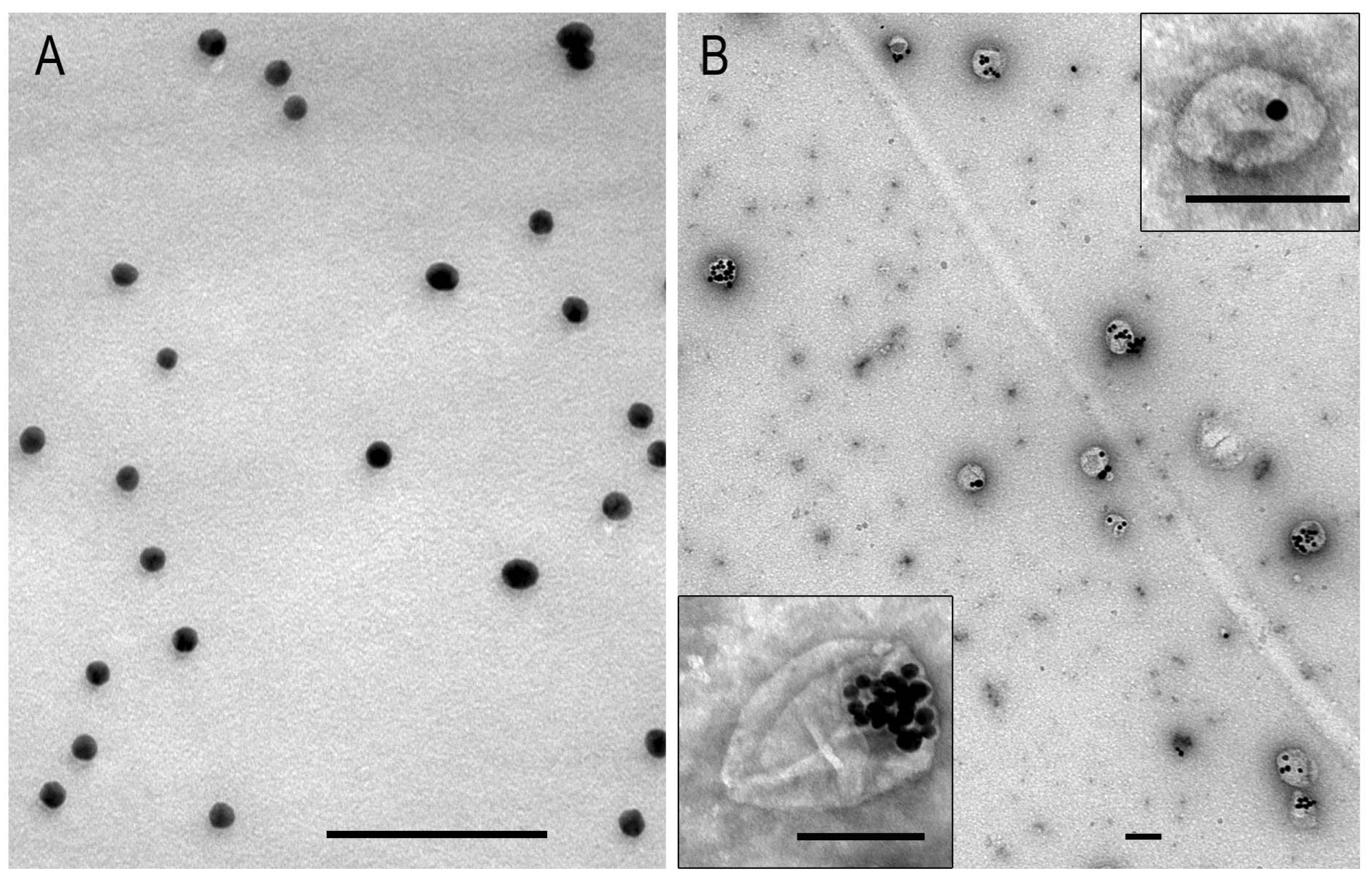
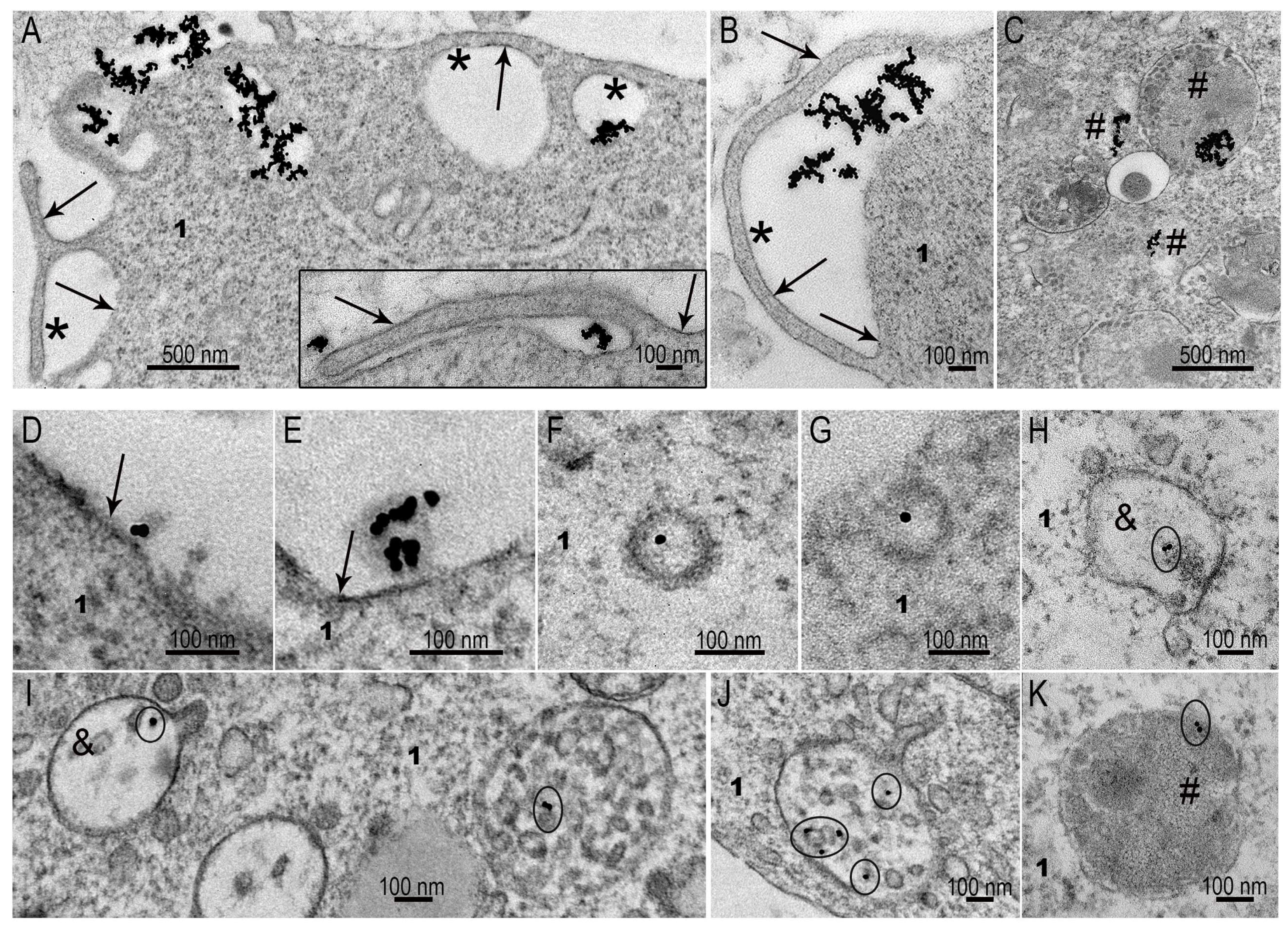
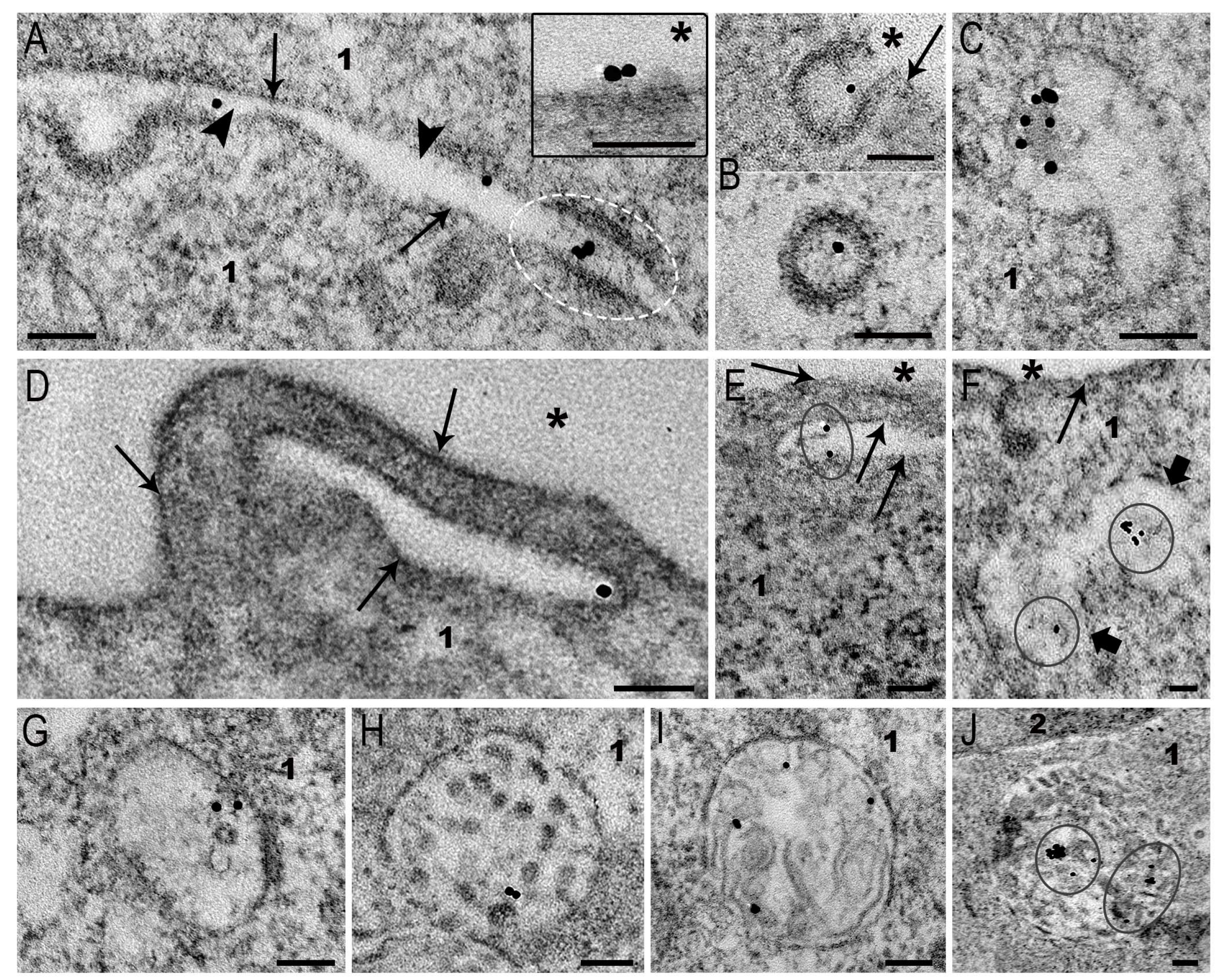
3.3. Interaction of AuNPs and MLNCs with SC-1 Cells in a Monolayer
3.4. Interaction of AuNPs with Spheroid SC-1 Cells
3.5. Interaction of MLNCs with Spheroid SC-1 Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hafeez, M.N.; Celia, C.; Petrikaite, V. Challenges towards Targeted Drug Delivery in Cancer Nanomedicines. Processes 2021, 9, 1527. [Google Scholar] [CrossRef]
- Bromma, K.; Beckham, W.; Chithrani, D.B. Utilizing two-dimensional monolayer and three-dimensional spheroids to enhance radiotherapeutic potential by combining gold nanoparticles and docetaxel. Cancer Nano 2023, 14, 80. [Google Scholar] [CrossRef]
- Ferreira, D.; Fernandes, A.R.; Baptista, P.V. Mild hyperthermia via gold nanoparticles and visible light irradiation for enhanced siRNA and ASO delivery in 2D and 3D tumour spheroids. Cancer Nanotechnol. 2024, 15, 19. [Google Scholar] [CrossRef]
- Bano, L.; Rihan; Siddiqui, Y.; Alam, M.; Abbas, K. Therapeutic potential of gold nanoparticles in cancer therapy: A comparative insight into synthesis overview and cellular mechanisms. Med. Oncol. 2025, 42, 320. [Google Scholar] [CrossRef]
- Epanchintseva, A.V.; Poletaeva, J.E.; Dovydenko, I.S.; Chelobanov, B.P.; Pyshnyi, D.V.; Ryabchikova, E.I.; Pyshnaya, I.A. A Lipid-Coated Nanoconstruct Composed of Gold Nanoparticles Noncovalently Coated with Small Interfering RNA: Preparation, Purification and Characterization. Nanomaterials 2021, 11, 2775. [Google Scholar] [CrossRef] [PubMed]
- Epanchintseva, A.V.; Poletaeva, J.E.; Dome, A.S.; Dovydenko, I.S.; Pyshnaya, I.A.; Ryabchikova, E.I. Chemical Modifications Influence the Number of siRNA Molecules Adsorbed on Gold Nanoparticles and the Efficiency of Downregulation of a Target Protein. Nanomaterials 2022, 12, 4450. [Google Scholar] [CrossRef] [PubMed]
- Poletaeva, J.; Dovydenko, I.; Epanchintseva, A.; Korchagina, K.; Pyshnyi, D.; Apartsin, E.; Ryabchikova, E.; Pyshnaya, I. Non-Covalent Associates of siRNAs and AuNPs Enveloped with Lipid Layer and Doped with Amphiphilic Peptide for Efficient siRNA Delivery. Int. J. Mol. Sci. 2018, 19, 2096. [Google Scholar] [CrossRef]
- Epanchintseva, A.V.; Poletaeva, J.E.; Bakhno, I.A.; Belov, V.V.; Grigor’eva, A.E.; Baranova, S.V.; Ryabchikova, E.I.; Dovydenko, I.S. Fixation and Visualization of Full Protein Corona on Lipid Surface of Composite Nanoconstruction. Nanomaterials 2023, 13, 3094. [Google Scholar] [CrossRef]
- Gorohovs, M.; Dekhtyar, Y. Surface Functionalization of Nanoparticles for Enhanced Electrostatic Adsorption of Biomolecules. Molecules 2025, 30, 3206. [Google Scholar] [CrossRef]
- Gerasimovich, E.; Karaulov, A.; Nabiev, I.; Sukhanova, A. Protein Adsorption on Nano- and Microparticles: Dependence on Morphological and Physicochemical Properties of Particles and Effect on Particle-Cell Interactions. Nanomaterials 2025, 15, 1013. [Google Scholar] [CrossRef]
- Gatti, L.; Chirizzi, C.; Rotta, G.; Milesi, P.; Sancho-Albero, M.; Sebastian, V.; Mondino, A.; Santamaria, J.; Metrangolo, P.; Chaabane, L.; et al. Pivotal role of the protein corona in the cell uptake of fluorinated nanoparticles with increased sensitivity for (19)F-MR imaging. Nanoscale Adv. 2023, 5, 3749–3760. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Baimanov, D.; Guo, Z.; Gao, Q.; Chen, C.; Cardoso, M.B.; Wang, L. In Situ Analysis for Protein Corona: From Morphology, Composition, Structure to Dynamic Process. Small Methods 2025, 9, e2500348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ren, M.; Shi, J.; Wang, H.; Bai, J.; Du, W.; Xiang, B. Engineering the protein corona: Strategies, effects, and future directions in nanoparticle therapeutics. Biomed. Pharmacother. 2024, 175, 116627. [Google Scholar] [CrossRef]
- Boselli, L.; Castagnola, V.; Armirotti, A.; Benfenati, F.; Pompa, P.P. Biomolecular Corona of Gold Nanoparticles: The Urgent Need for Strong Roots to Grow Strong Branches. Small 2024, 20, e2306474. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Beigi, H.; Hayashi, Y.; Zeuthen, C.M.; Eskandari, H.; Scavenius, C.; Juul-Madsen, K.; Vorup-Jensen, T.; Enghild, J.J.; Sutherland, D.S. Mapping and identification of soft corona proteins at nanoparticles and their impact on cellular association. Nat. Commun. 2020, 11, 4535. [Google Scholar] [CrossRef] [PubMed]
- Sharapova, T.N.; Ivanova, O.K.; Prasolov, V.S.; Romanova, E.A.; Sashchenko, L.P.; Yashin, D.V. Innate immunity protein Tag7 (PGRP-S) activates lymphocytes capable of Fasl-Fas-dependent contact killing of virus-infected cells. IUBMB Life 2017, 69, 971–977. [Google Scholar] [CrossRef]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef]
- Tagliatti, E.; Cortese, K. Imaging Endocytosis Dynamics in Health and Disease. Membranes 2022, 12, 393. [Google Scholar] [CrossRef]
- Griffiths, G.; Gruenberg, J.; Marsh, M.; Wohlmann, J.; Jones, A.T.; Parton, R.G. Nanoparticle entry into cells; the cell biology weak link. Adv. Drug Deliv. Rev. 2022, 188, 114403. [Google Scholar] [CrossRef]
- Fobian, S.-F.; Petzer, M.; Vetten, M.; Steenkamp, V.; Gulumian, M.; Cordier, W. Mechanisms facilitating the uptake of carboxyl–polythene glycol-functionalized gold nanoparticles into multicellular spheroids. J. Pharm. Pharmacol. 2022, 74, 1282–1295. [Google Scholar] [CrossRef]
- Akhtar, S.; Zuhair, F. Advancing Nanomedicine Through Electron Microscopy: Insights Into Nanoparticle Cellular Interactions and Biomedical Applications. Int. J. Nanomed. 2025, 20, 2847–2878. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Chelobanov, B.; Poletaeva, J.; Epanchintseva, A.; Tupitsyna, A.; Pyshnaya, I.; Ryabchikova, E. Ultrastructural Features of Gold Nanoparticles Interaction with HepG2 and HEK293 Cells in Monolayer and Spheroids. Nanomaterials 2020, 10, 2040. [Google Scholar] [CrossRef]
- Del Pozo, M.A.; Lolo, F.N.; Echarri, A. Caveolae: Mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Curr. Opin. Cell Biol. 2021, 68, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Arora, P.; Lee, W.; McCulloch, C. Biochemical and functional characterization of intercellular adhesion and gap junctions in fibroblasts. Am. J. Physiol. Cell Physiol. 2000, 279, C147–C157. [Google Scholar] [CrossRef] [PubMed]
- Kosheleva, N.V.; Efremov, Y.M.; Shavkuta, B.S.; Zurina, I.M.; Zhang, D.; Zhang, Y.; Minaev, N.V.; Gorkun, A.A.; Wei, S.; Shpichka, A.I.; et al. Cell spheroid fusion: Beyond liquid drops model. Sci. Rep. 2020, 10, 12614. [Google Scholar] [CrossRef]
- Salmenperä, P.; Karhemo, P.R.; Räsänen, K.; Laakkonen, P.; Vaheri, A. Fibroblast spheroids as a model to study sustained fibroblast quiescence and their crosstalk with tumor cells. Exp. Cell Res. 2016, 345, 17–24. [Google Scholar] [CrossRef]
- Dirand, Z.; Tissot, M.; Chatelain, B.; Viennet, C.; Rolin, G. Is Spheroid a Relevant Model to Address Fibrogenesis in Keloid Research? Biomedicines 2023, 11, 2350. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Cell Junctions. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26857/ (accessed on 5 July 2025).
- Takezawa, T.; Mori, Y.; Yonaha, T.; Yoshizato, K. Characterization of morphology and cellular metabolism during the spheroid formation by fibroblasts. Exp. Cell Res. 1993, 208, 430–441. [Google Scholar] [CrossRef]
- Tan, Y.; Suarez, A.; Garza, M.; Khan, A.A.; Elisseeff, J.; Coon, D. Human fibroblast-macrophage tissue spheroids demonstrate ratio-dependent fibrotic activity for in vitro fibrogenesis model development. Biomater. Sci. 2020, 8, 1951–1960. [Google Scholar] [CrossRef]
- Douglas, O.; Halverson, J.R.; Faletto, D.; Fisher, R.; Anver, M.; Rong, S.; May, J.A.; Tsarfaty, I.; Blair, D.G. A Mouse fibroblast line cycles between monolayer and spheroid forms, regulates met and hgf expression, and releases an attachment and growth-promoting substance. Cell Biol. Int. 1999, 23, 257–274. [Google Scholar] [CrossRef]
- Shearer, L.J.; Petersen, N.O. Internalization of Phospholipid-Coated Gold Nanoparticles. Crystals 2019, 9, 544. [Google Scholar] [CrossRef]
- Pratiwi, F.W.; Peng, C.C.; Wu, S.H.; Kuo, C.W.; Mou, C.-Y.; Tung, Y.-C.; Chen, P. Evaluation of Nanoparticle Penetration in the Tumor Spheroid Using Two-Photon Microscopy. Biomedicines 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Cesar-Silva, D.; Pereira-Dutra, F.S.; Giannini, A.L.M.; de Almeida, C.J.G. The Endolysosomal System: The Acid Test for SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 4576. [Google Scholar] [CrossRef]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug delivery to solid tumors: The predictive value of the multicellular tumor spheroid model for nanomedicine screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef]
- Sobańska, Z.; Domeradzka-Gajda, K.; Szparaga, M.; Grobelny, J.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Celichowski, G.; Zapór, L.; Kowalczyk, K.; Stępnik, M. Comparative analysis of biological effects of molybdenum(IV) sulfide in the form of nano- and microparticles on human hepatoma HepG2 cells grown in 2D and 3D models. Toxicol. In Vitro 2020, 68, 104931. [Google Scholar] [CrossRef]
- Huang, K.; Ma, H.; Liu, L.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-Dependent Localization and Penetration of Ultrasmall Gold Nanoparticles in Cancer Cells, Multicellular Spheroids, and Tumors in Vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Xie, Z.; Centurion, F.; Sun, B.; Paterson, D.J.; Chang-Hao Tsao, S.; Chu, D.; Shen, Y.; Mao, G.; et al. Size-Dependent Penetration of Nanoparticles in Tumor Spheroids: A Multidimensional and Quantitative Study of Transcellular and Paracellular Pathways. Small 2024, 20, 2304693. [Google Scholar] [CrossRef]
- Sujai, P.T.; Joseph, M.M.; Saranya, G.; Nair, J.B.; Murali, V.P.; Maiti, K.K. Surface charge modulates the internalization vs. penetration of gold nanoparticles: Comprehensive scrutiny on monolayer cancer cells, multicellular spheroids and solid tumors by SERS modality. Nanoscale 2020, 12, 6971–6975. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poletaeva, J.E.; Chelobanov, B.P.; Epanchintseva, A.V.; Tupitsyna, A.V.; Dovydenko, I.S.; Ryabchikova, E.I. Internalization of Lipid-Coated Gold Nanocomposites and Gold Nanoparticles by Mouse SC-1 Fibroblasts in Monolayer and Spheroids. Nanomaterials 2025, 15, 1419. https://doi.org/10.3390/nano15181419
Poletaeva JE, Chelobanov BP, Epanchintseva AV, Tupitsyna AV, Dovydenko IS, Ryabchikova EI. Internalization of Lipid-Coated Gold Nanocomposites and Gold Nanoparticles by Mouse SC-1 Fibroblasts in Monolayer and Spheroids. Nanomaterials. 2025; 15(18):1419. https://doi.org/10.3390/nano15181419
Chicago/Turabian StylePoletaeva, Julia E., Boris P. Chelobanov, Anna V. Epanchintseva, Anastasiya V. Tupitsyna, Ilya S. Dovydenko, and Elena I. Ryabchikova. 2025. "Internalization of Lipid-Coated Gold Nanocomposites and Gold Nanoparticles by Mouse SC-1 Fibroblasts in Monolayer and Spheroids" Nanomaterials 15, no. 18: 1419. https://doi.org/10.3390/nano15181419
APA StylePoletaeva, J. E., Chelobanov, B. P., Epanchintseva, A. V., Tupitsyna, A. V., Dovydenko, I. S., & Ryabchikova, E. I. (2025). Internalization of Lipid-Coated Gold Nanocomposites and Gold Nanoparticles by Mouse SC-1 Fibroblasts in Monolayer and Spheroids. Nanomaterials, 15(18), 1419. https://doi.org/10.3390/nano15181419






