Single- vs. Multi-Walled Carbon Nanotubes: Differential Cellular Stress and Lipid Metabolism Effects in Macrophage Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbon Nanotubes
2.2. Characterization of CNT
2.3. Cell Culture
2.4. Cytotoxicity and Mitochondrial Activity Assays
Dose Selection and Experimental Replicates
2.5. RNA Analysis and Microarrays
2.5.1. RNA Isolation and Quantification
2.5.2. Microarray Expression Profiling
2.5.3. Transcriptomic Data Analysis
2.6. Protein Analysis
2.6.1. Sample Preparation for Proteomics
2.6.2. Mass Spectrometry
2.6.3. Data Analysis
2.7. May-Grünwald Giemsa (MGG) Staining
3. Results
3.1. Characterization of CNT
3.2. Cytotoxic Effects of Carbon Nanotubes on NR8383 and dTHP-1 Cells
3.2.1. Cell Viability of Alveolar Macrophages (NR8383)
3.2.2. Cell Viability of dTHP-1 Cells
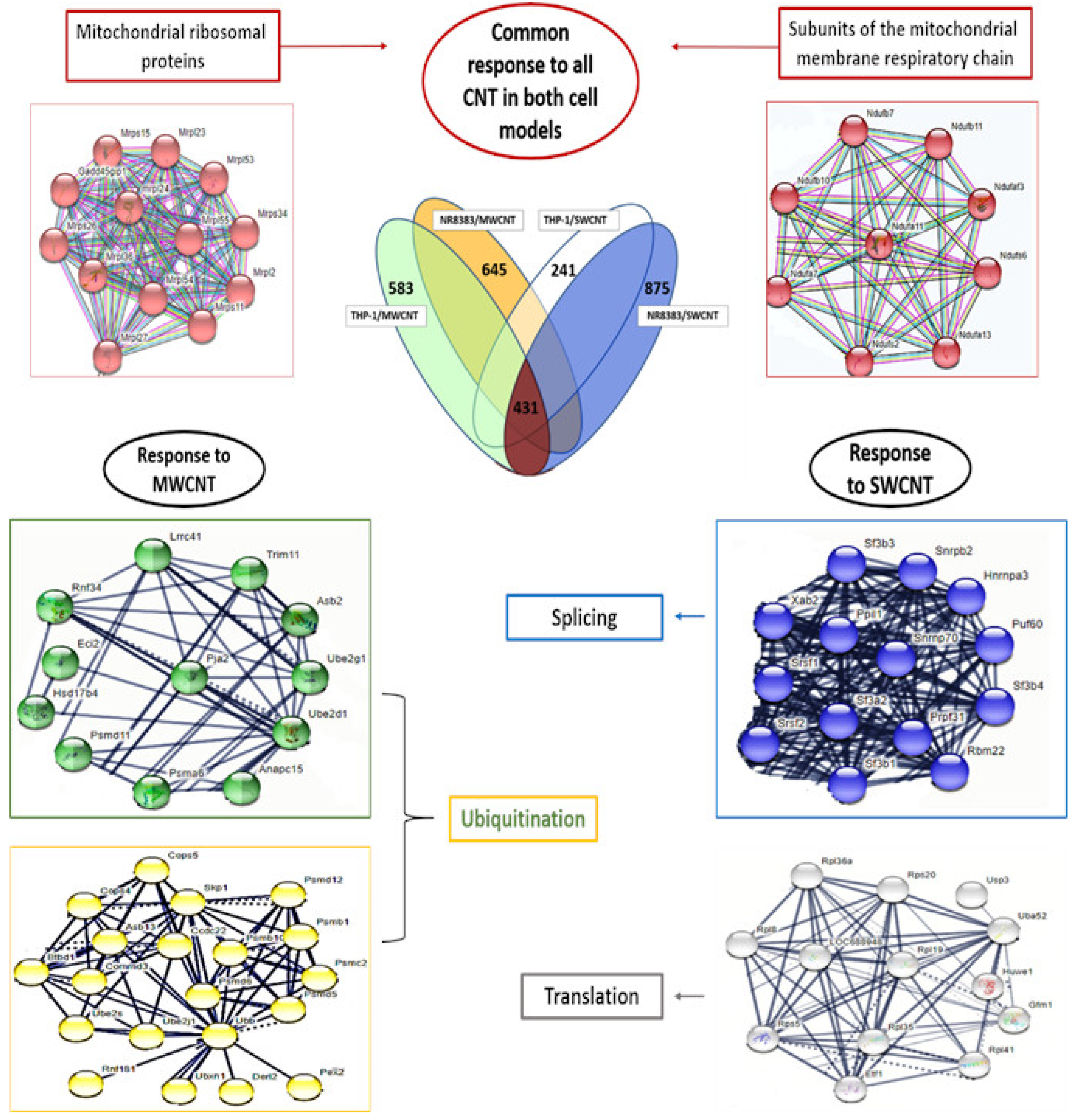
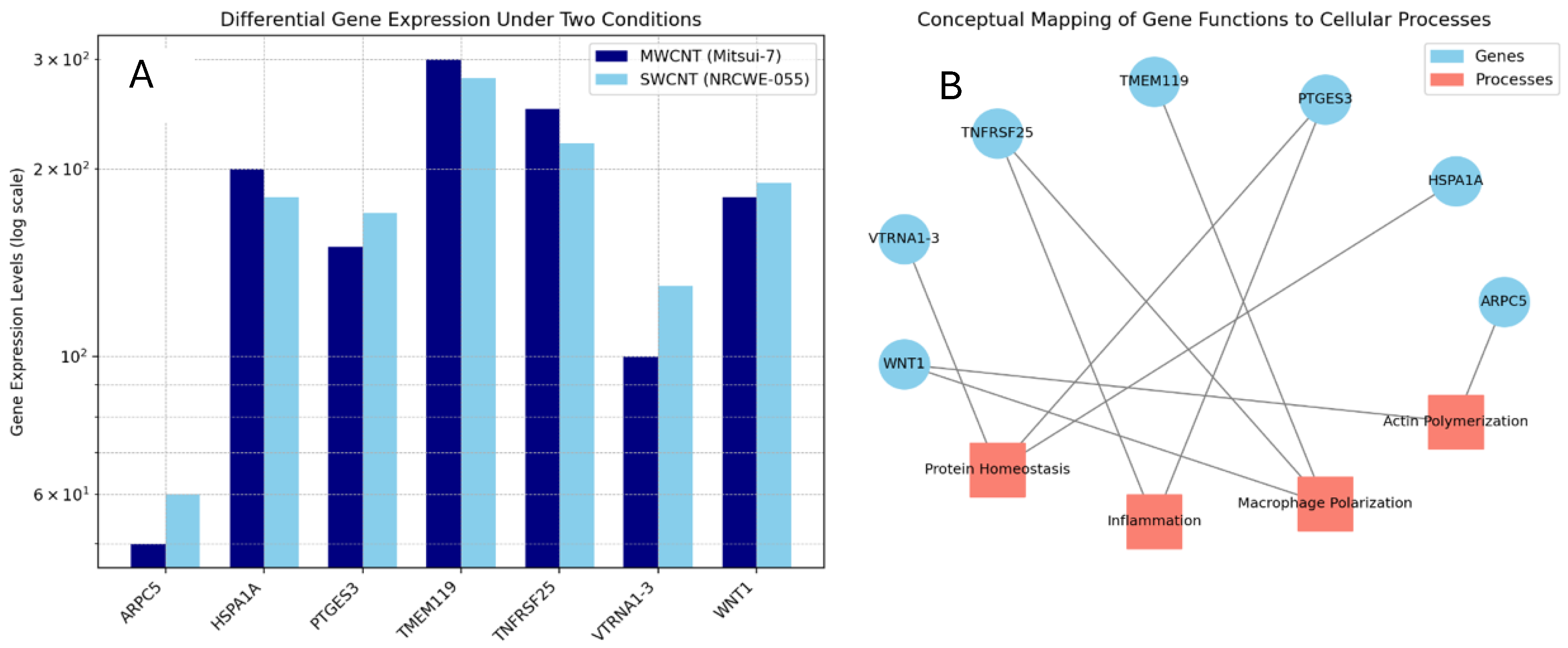
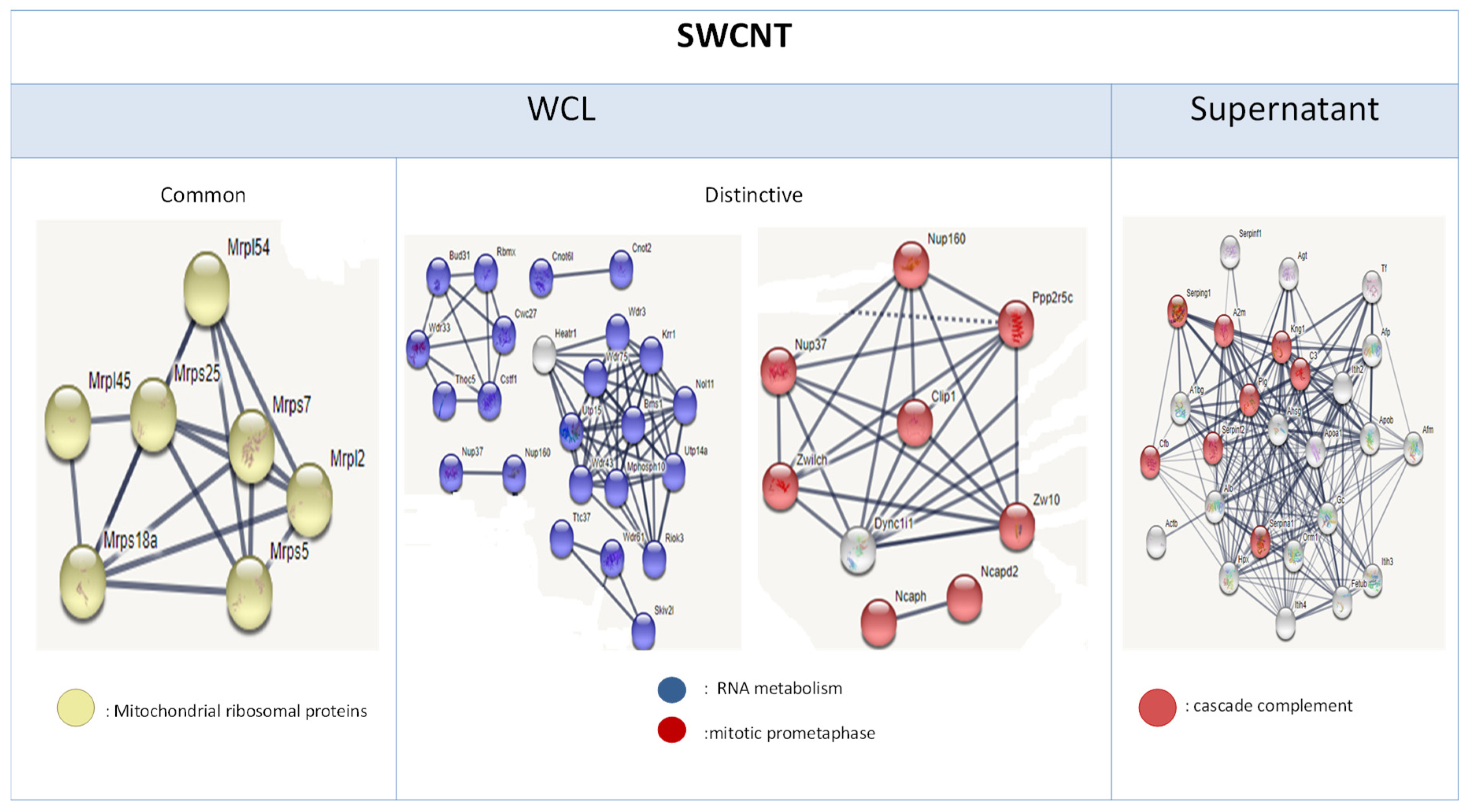
3.3. Genome Modulation
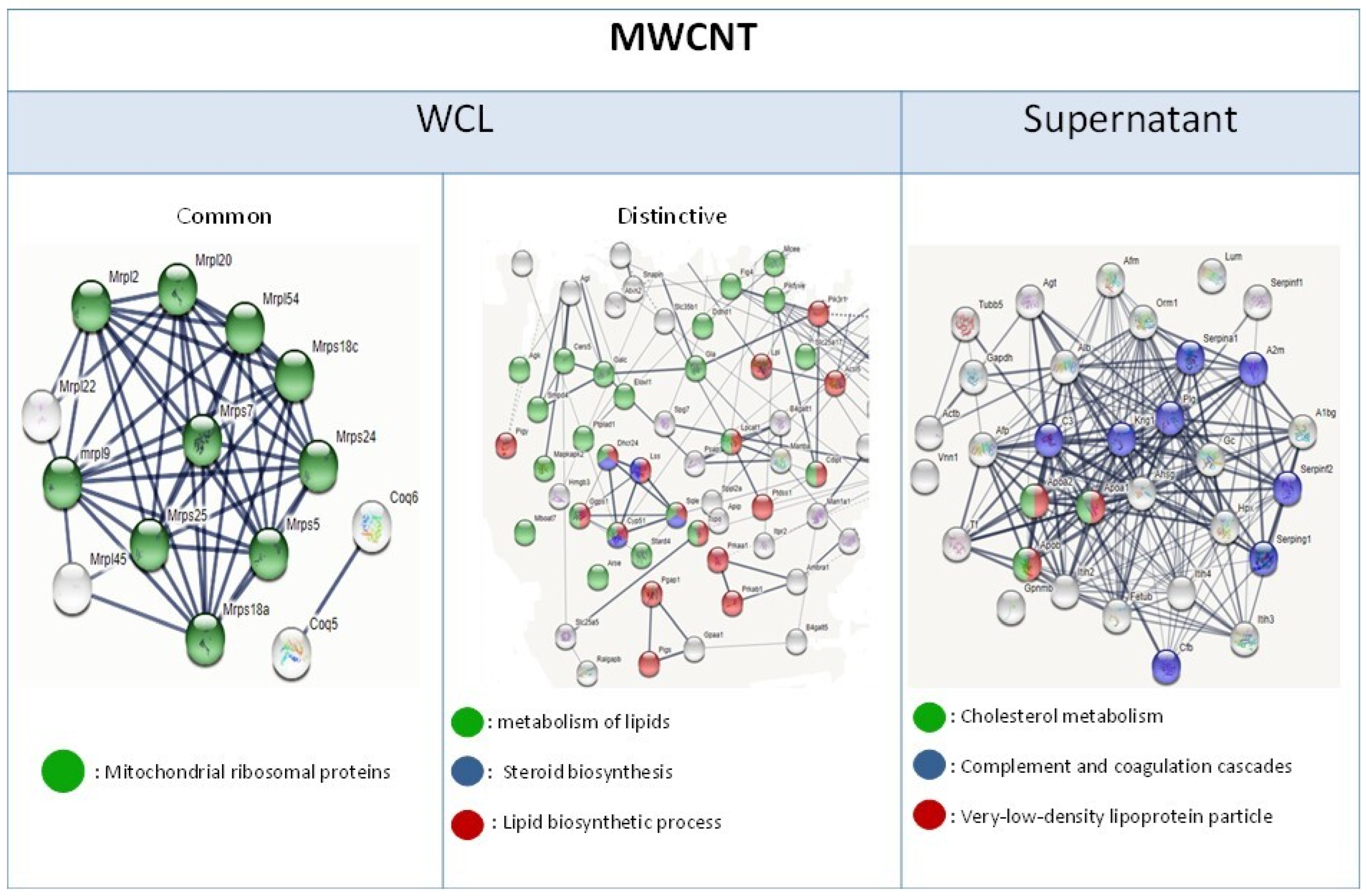
3.4. Proteome Modulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNT | carbon nanotubes |
| DEGs | differentially expressed genes |
| DEPs | differentially expressed proteins |
| FBS | fetal bovine serum |
| HA | human albumin |
| HS | human surfactant |
| MWCNT | multi-walled carbon nanotubes |
| PMA | phorbol 12-myristate 13-acetate |
| SWCNT | single-walled carbon nanotubes |
References
- Saliev, T. The Advances in Biomedical Applications of Carbon Nanotubes. C J. Carbon Res. 2019, 5, 29. [Google Scholar] [CrossRef]
- Gao, S.; Xu, B.; Sun, J.; Zhang, Z. Nanotechnological advances in cancer: Therapy a comprehensive review of carbon nanotube applications. Front. Bioeng. Biotechnol. 2024, 12, 1351787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef]
- Zeng, Z.; Fang, C.; Zhang, Y.; Chen, C.X.; Zhang, Y.F.; Zhang, K. Mitochondria-Targeted Nanocarriers Promote Highly Efficient Cancer Therapy: A Review. Front. Bioeng. Biotechnol. 2021, 9, 784602. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.K.; Priya, V.; Setia, A.; Mehata, A.K.; Mohan, S.; Albratty, M.; Najmi, A.; Meraya, A.M.; Makeen, H.A.; Tambuwala, M.M.; et al. Mitochondrial targeting theranostic nanomedicine and molecular biomarkers for efficient cancer diagnosis and therapy. Biomed. Pharmacother. 2022, 153, 113451. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, S.; Zhao, G.; Fu, X.; Xie, X.; Huang, Y.; Cheng, X.; Wei, J.; Liu, H.; Lai, Z. Long-term intravenous administration of carboxylated single-walled carbon nanotubes induces persistent accumulation in the lungs and pulmonary fibrosis via the nuclear factor-kappa B pathway. Int. J. Nanomed. 2016, 12, 263–277, Erratum in Int. J. Nanomed. 2017, 12, 1515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shvedova, A.A.; Kisin, E.; Murray, A.R.; Johnson, V.J.; Gorelik, O.; Arepalli, S.; Hubbs, A.F.; Mercer, R.R.; Keohavong, P.; Sussman, N.; et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: Inflammation, fibrosis, oxidative stress, and mutagenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L552–L565. [Google Scholar] [CrossRef]
- Vietti, G.; Lison, D.; van den Brule, S. Mechanisms of lung fibrosis induced by carbon nanotubes: Towards an Adverse Outcome Pathway (AOP). Part. Fibre Toxicol. 2016, 13, 11. [Google Scholar] [CrossRef]
- Barna, B.P.; Malur, A.; Thomassen, M.J. Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis. Int. J. Mol. Sci. 2021, 22, 3705. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Naghinejad, M.; Kasaeian, A.; Lohrasbi, A.; Shahraeini, S.S.; Zomorodbakhsh, S. The Applications of Carbon Nanotubes in the Diagnosis and Treatment of Lung Cancer: A Critical Review. Int. J. Nanomed. 2020, 15, 7063–7078. [Google Scholar] [CrossRef]
- Lucas, J.H.; Wang, Q.; Muthumalage, T.; Rahman, I. Multi-Walled Carbon Nanotubes (MWCNTs) Cause Cellular Senescence in TGF-β Stimulated Lung Epithelial Cells. Toxics 2021, 9, 144. [Google Scholar] [CrossRef]
- Chortarea, S.; Zerimariam, F.; Barosova, H.; Septiadi, D.; Clift, M.; Petri-Fink, A.; Rothen-Rutishauser, B. Profibrotic Activity of Multiwalled Carbon Nanotubes Upon Prolonged Exposures in Different Human Lung Cell Types. Appl. Vitr. Toxicol. 2019, 5, 47–61. [Google Scholar] [CrossRef]
- Svadlakova, T.; Hubatka, F.; Turanek Knotigova, P.; Kulich, P.; Masek, J.; Kotoucek, J.; Macak, J.; Motola, M.; Kalbac, M.; Kolackova, M.; et al. Proinflammatory Effect of Carbon-Based Nanomaterials: In Vitro Study on Stimulation of Inflammasome NLRP3 via Destabilisation of Lysosomes. Nanomaterials 2020, 10, 418. [Google Scholar] [CrossRef]
- Fujita, K.; Obara, S.; Maru, J.; Endoh, S. Cytotoxicity profiles of multi-walled carbon nanotubes with different physico-chemical properties. Toxicol. Mech. Methods 2020, 30, 477–489. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, Y.; Luo, Y.; Guo, H.; Huang, C.; Peng, J.; Cao, Y. Multi-walled carbon nanotubes (MWCNTs) transformed dTHP-1 macrophages into foam cells: Impact of pulmonary surfactant component dipalmitoylphosphatidylcholine. J. Hazard. Mater. 2020, 392, 122286. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, D.; Xu, Y.; Zhao, J.; Hu, G.; Yue, T. Dual role of pulmonary surfactant corona in modulating carbon nanotube toxicity and benzo[a]pyrene bioaccessibility. J. Hazard. Mater. 2023, 457, 131753. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Swerhone, G.D.W.; Dynes, J.J.; Hitchcock, A.P.; Korber, D.R. Complex organic corona formation on carbon nanotubes reduces microbial toxicity by suppressing reactive oxygen species production. Environ. Sci. Nano 2015, 3, 181–189. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Inman, A.O.; Wang, Y.Y.; Nemanich, R.J. Surfactant effects on carbon nanotube interactions with human keratinocytes. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 293–299. [Google Scholar] [CrossRef]
- Sweeney, S.; Leo, B.F.; Chen, S.; Abraham-Thomas, N.; Thorley, A.J.; Gow, A.; Schwander, S.; Zhang, J.J.; Shaffer, M.S.P.; Chung, K.F.; et al. Pulmonary surfactant mitigates silver nanoparticle toxicity in human alveolar type-I-like epithelial cells. Colloids Surf. B Biointerfaces 2016, 145, 167–175. [Google Scholar] [CrossRef]
- Du, Z.; Chen, S.; Cui, G.; Yang, Y.; Zhang, E.; Wang, Q.; Lavin, M.F.; Yeo, A.J.; Bo, C.; Zhang, Y.; et al. Silica nanoparticles induce cardiomyocyte apoptosis via the mitochondrial pathway in rats following intratracheal instillation. Int. J. Mol. Med. 2019, 43, 1229–1240. [Google Scholar] [CrossRef]
- Safar, R.; Doumandji, Z.; Saidou, T.; Ferrari, L.; Nahle, S.; Rihn, B.H.; Joubert, O. Cytotoxicity and global transcriptional responses induced by zinc oxide nanoparticles NM 110 in PMA-differentiated THP-1 cells. Toxicol. Lett. 2019, 308, 65–73. [Google Scholar] [CrossRef]
- Reed, L.R.; Muench, A. A simple method for estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Schmid, O.; Cassee, F.R. On the pivotal role of dose for particle toxicology and risk assessment: Exposure is a poor surrogate for delivered dose. Part. Fibre Toxicol. 2017, 14, 52. [Google Scholar] [CrossRef]
- Doumandji, Z.; Safar, R.; Lovera-Leroux, M.; Nahle, S.; Cassidy, H.; Matallanas, D.; Rihn, B.; Ferrari, L.; Joubert, O. Protein and lipid homeostasis altered in rat macrophages after exposure to metallic oxide nanoparticles. Cell Biol. Toxicol. 2020, 36, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Kumarathasan, P.; Breznan, D.; Das, D.; Salam, M.A.; Siddiqui, Y.; MacKinnon-Roy, C.; Guan, J.; de Silva, N.; Simard, B.; Vincent, R. Cytotoxicity of carbon nanotube variants: A comparative in vitro exposure study with A549 epithelial and J774 macrophage cells. Nanotoxicology 2015, 9, 148–161. [Google Scholar] [CrossRef]
- Wörle-Knirsch, J.L.; Pulskamp, K.; Krug, H.F. Oops They Did It Again! Carbon Nanotubes Hoax Scientists in Viability Assays. Nano Lett. 2006, 6, 1261–1268. [Google Scholar] [CrossRef]
- Kim, J.S.; Yu, I.J. Single-wall carbon nanotubes (SWCNT) induce cytotoxicity and genotoxicity produced by reactive oxygen species (ROS) generation in phytohemagglutinin (PHA)-stimulated male human peripheral blood lymphocytes. J. Toxicol. Environ. Health A 2014, 77, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Castranova, V.; Mishra, A.; Chen, B.; Mercer, R.R.; Schwegler-Berry, D.; Rojanasakul, Y. Dispersion of single-walled carbon nanotubes by a natural lung surfactant for pulmonary in vitro and in vivo toxicity studies. Part. Fibre Toxicol. 2010, 7, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenblatt, M.N.; Burns, J.R.; Duncan, V.E.; Hughes, J.A. Infection of the macrophage cell line NR8383 with Mycobacterium tuberculosis (H37Ra) leads to an increase in oligodeoxynucleotide accumulation. Antisense Nucleic Acid Drug Dev. 2000, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Cheng, H.C.; Bogdanova, A.; Bogdanov, A., Jr. Magnetically labeled cells can be detected by MR imaging. J. Magn. Reson. Imaging 1997, 7, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, J.E.; Fischel-Ghodsian, N.; Mougey, E.B.; O’Brien, T.W. Mitochondrial ribosomal proteins: Candidate genes for mitochondrial disease. Genet. Med. 2004, 6, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, J.; Gao, L.; Huang, Y.; Liao, G.; Cao, Y. Induction of lipid droplets in THP-1 macrophages by multi-walled carbon nanotubes in a diameter-dependent manner: A transcriptomic study. Toxicol. Lett. 2020, 332, 65–73. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Weissman, A.M. Ubiquitylation in ERAD: Reversing to go forward? PLoS Biol. 2011, 9, e1001038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DuRose, J.B.; Scheuner, D.; Kaufman, R.J.; Rothblum, L.I.; Niwa, M. Phosphorylation of eukaryotic translation initiation factor 2alpha coordinates rRNA transcription and translation inhibition during endoplasmic reticulum stress. Mol. Cell. Biol. 2009, 29, 4295–4307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.; Chang, S.; Long, J.; Li, J.; Li, X.; Cao, Y. The toxicity of multi-walled carbon nanotubes (MWCNTs) to human endothelial cells: The influence of diameters of MWCNTs. Food Chem. Toxicol. 2019, 126, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Ma, W.; Yu, Z.; Liu, H.; Cao, Y. Multi-walled carbon nanotubes (MWCNTs) promoted lipid accumulation in dTHP-1 macrophages through modulation of endoplasmic reticulum (ER) stress. Nanotoxicology 2019, 13, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Ranalletta, M.; Wang, N.; Han, S.; Terasaka, N.; Li, R.; Welch, C.; Tall, A.R. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Investig. 2007, 117, 3900–3908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen, A.M.; Graham, A. Mitochondrial function is involved in regulation of cholesterol efflux to apolipoprotein (apo)A-I from murine RAW 264.7 macrophages. Lipids Health Dis. 2012, 11, 169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.G.; Yang, Y. Sirtuins in glucose and lipid metabolism. Oncotarget 2017, 8, 1845–1859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheng, S.; Kang, Y.; Guo, Y.; Pu, Q.; Cai, M.; Tu, Z. Overexpression of Sirt3 inhibits lipid accumulation in macrophages through mitochondrial IDH2 deacetylation. Int. J. Clin. Exp. Pathol. 2015, 8, 9196–9201. [Google Scholar] [PubMed] [PubMed Central]
- Möst, J.; Spötl, L.; Mayr, G.; Gasser, A.; Sarti, A.; Dierich, M.P. Formation of multinucleated giant cells in vitro is dependent on the stage of monocyte to macrophage maturation. Blood 1997, 89, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Tomita, T.; Toyosaki-Maeda, T.; Maeda-Tanimura, M.; Tsuboi, H.; Takeuchi, E.; Kaneko, M.; Shi, K.; Takahi, K.; Myoui, A.; et al. Comparison of the activities of multinucleated bone-resorbing giant cells derived from CD14-positive cells in the synovial fluids of rheumatoid arthritis and osteoarthritis patients. Rheumatology 2004, 43, 435–441. [Google Scholar] [CrossRef] [PubMed]
- McInnes, A.; Rennick, D.M. Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J. Exp. Med. 1988, 167, 598–611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Enelow, R.I.; Sullivan, G.W.; Carper, H.T.; Mandell, G.L. Induction of multinucleated giant cell formation from in vitro culture of human monocytes with interleukin-3 and interferon-gamma: Comparison with other stimulating factors. Am. J. Respir. Cell Mol. Biol. 1992, 6, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.T.; Schepetkin, I.A. Role of NADPH oxidase in formation and function of multinucleated giant cells. J. Innate Immun. 2009, 1, 509–526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McClean, C.M.; Tobin, D.M. Macrophage form, function, and phenotype in mycobacterial infection: Lessons from tuberculosis and other diseases. Pathog. Dis. 2016, 74, ftw068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russell, D.G.; Cardona, P.J.; Kim, M.J.; Allain, S.; Altare, F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009, 10, 943–948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puissegur, M.P.; Lay, G.; Gilleron, M.; Botella, L.; Nigou, J.; Marrakchi, H.; Mari, B.; Duteyrat, J.L.; Guerardel, Y.; Kremer, L.; et al. Mycobacterial lipomannan induces granuloma macrophage fusion via a TLR2-dependent, ADAM9- and beta1 integrin-mediated pathway. J. Immunol. 2007, 178, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.J.; Glogauer, M.; McCulloch, C.A. An Overview of the Derivation and Function of Multinucleated Giant Cells and Their Role in Pathologic Processes. Am. J. Pathol. 2019, 189, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Calabrese, E.J.; Nascarella, M.A. Exposure to nanoparticles and hormesis. Dose Response 2010, 8, 501–517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huaux, F.; Lo Re, S.; Giordano, G.; Uwambayinema, F.; Devosse, R.; Yakoub, Y.; Panin, N.; Palmai-Pallag, M.; Rabolli, V.; Delos, M.; et al. IL-1α induces CD11b(low) alveolar macrophage proliferation and maturation during granuloma formation. J. Pathol. 2015, 235, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.J.; Dobbs, L.G. Alveolar Epithelium and Pulmonary Surfactant. Murray Nadel’s Textb. Respir. Med. 2016, 1, 134–149.e5. [Google Scholar] [CrossRef] [PubMed Central]
- Kay, J.G.; Murray, R.Z.; Pagan, J.K.; Stow, J.L. Cytokine secretion via cholesterol-rich lipid raft-associated SNAREs at the phagocytic cup. J. Biol. Chem. 2006, 281, 11949–11954. [Google Scholar] [CrossRef] [PubMed]
- Desai, B.N.; Leitinger, N. Purinergic and calcium signaling in macrophage function and plasticity. Front. Immunol. 2014, 5, 580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohradanova-Repic, A.; Machacek, C.; Charvet, C.; Lager, F.; Le Roux, D.; Platzer, R.; Leksa, V.; Mitulovic, G.; Burkard, T.R.; Zlabinger, G.J.; et al. Extracellular Purine Metabolism Is the Switchboard of Immunosuppressive Macrophages and a Novel Target to Treat Diseases with Macrophage Imbalances. Front. Immunol. 2018, 9, 852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samie, M.A.; Xu, H. Lysosomal exocytosis and lipid storage disorders. J. Lipid Res. 2014, 55, 995–1009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Meng, J.; Yang, M.; Jia, F.; Xu, Z.; Kong, H.; Xu, H. Immune responses of BALB/c mice to subcutaneously injected multi-walled carbon nanotubes. Nanotoxicology 2011, 5, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Ndika, J.D.T.; Sund, J.; Alenius, H.; Puustinen, A. Elucidating differential nano-bio interactions of multi-walled andsingle-walled carbon nanotubes using subcellular proteomics. Nanotoxicology 2018, 12, 554–570. [Google Scholar] [CrossRef] [PubMed]
- Butcher, S.E. The spliceosome and its metal ions. Met. Ions Life Sci. 2011, 9, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Mouton, A.J.; DeLeon-Pennell, K.Y.; Rivera Gonzalez, O.J.; Flynn, E.R.; Freeman, T.C.; Saucerman, J.J.; Garrett, M.R.; Ma, Y.; Harmancey, R.; Lindsey, M.L. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res. Cardiol. 2018, 113, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berger, A.; Tran, A.H.; Paige, C.J. Co-regulated decrease of Neurokinin-1 receptor and Hemokinin-1 gene expression in monocytes and macrophages after activation with pro-inflammatory cytokines. J. Neuroimmunol. 2007, 187, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084, Erratum in Front. Immunol. 2020, 11, 234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fontana, M.F.; Baccarella, A.; Pancholi, N.; Pufall, M.A.; Herbert, D.R.; Kim, C.C. JUNB is a key transcriptional modulator of macrophage activation. J. Immunol. 2015, 194, 177–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shrivastava, R.; Asif, M.; Singh, V.; Dubey, P.; Ahmad Malik, S.; Lone, M.U.; Tewari, B.N.; Baghel, K.S.; Pal, S.; Nagar, G.K.; et al. M2 polarization of macrophages by Oncostatin M in hypoxic tumor microenvironment is mediated by mTORC2 and promotes tumor growth and metastasis. Cytokine 2019, 118, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Hamerman, J.A.; Hayashi, F.; Schroeder, L.A.; Gygi, S.P.; Haas, A.L.; Hampson, L.; Coughlin, P.; Aebersold, R.; Aderem, A. Serpin 2a is induced in activated macrophages and conjugates to a ubiquitin homolog. J. Immunol. 2002, 168, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
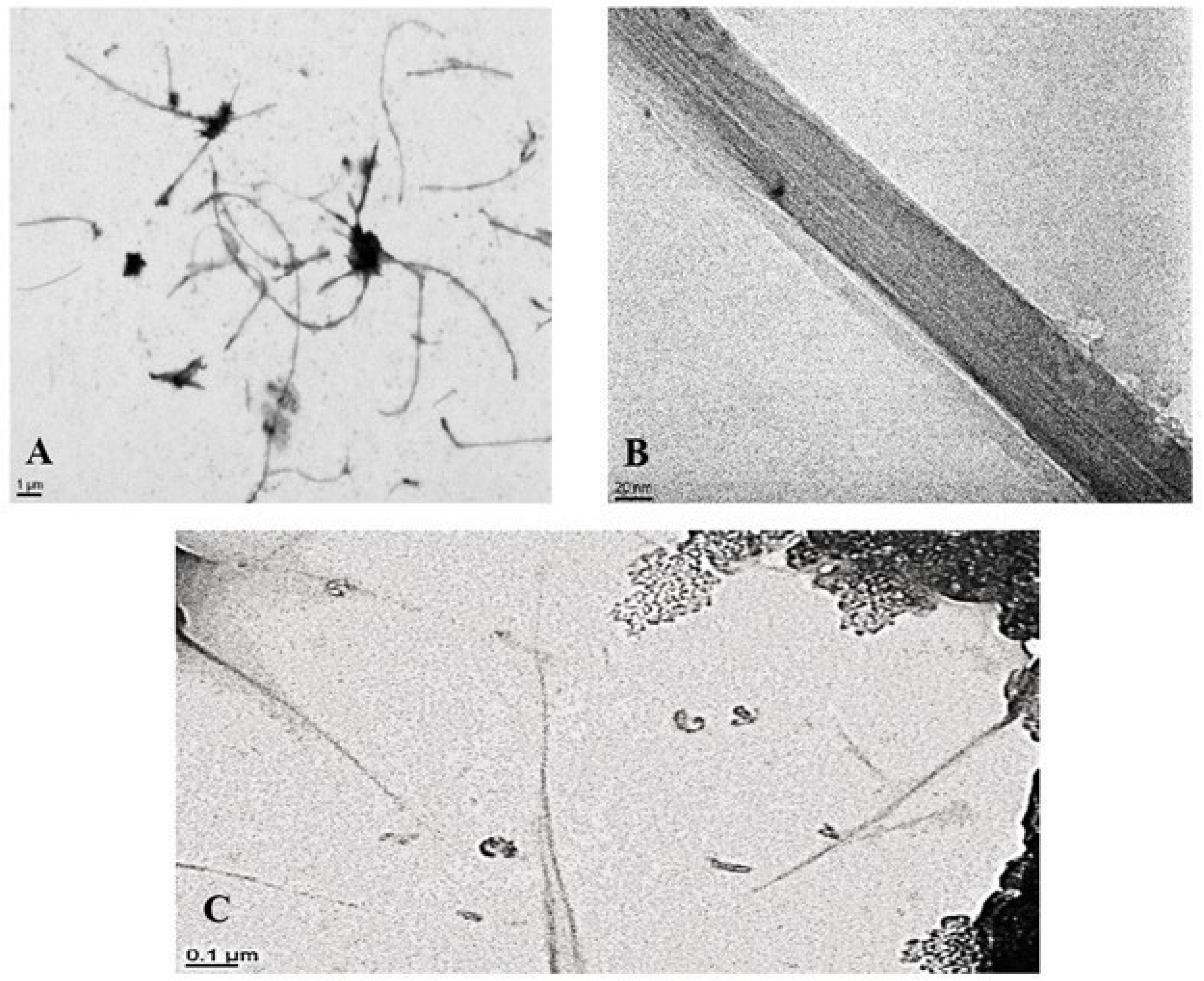



| Property | NRCWE-006 MWCNT | NRCWE-055 SWCNT | |
|---|---|---|---|
| Length (µm) | 3 ± 2 | 2 ± 1 | |
| Diameter (nm) | 65 ± 3 | 12 ± 2 | |
| Metal Impurities (%) | Fe2O3 | 1.11 | 60.74 |
| CoO | 0 | 19.27 | |
| Al2O3 | 0 | 0.14 | |
| MgO | 0.18 | 0.42 | |
| Other | 0.0068 | 19.43 | |
| Slope fluorescein/(cm2/mL) | 1302 | 5571 | |
| Surface Area/BET (m2/g) | 26 | 453 | |
| Hydrodynamic Diameter (nm) in FBS | 557 ± 63 | 601 ± 90 | |
| Hydrodynamic Diameter (nm) in HS + HA | 1023 ± 56 | 1129 ± 74 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahle, S.; Cassidy, H.; Matallanas, D.; Rihn, B.H.; Joubert, O.; Ferrari, L. Single- vs. Multi-Walled Carbon Nanotubes: Differential Cellular Stress and Lipid Metabolism Effects in Macrophage Models. Nanomaterials 2025, 15, 1401. https://doi.org/10.3390/nano15181401
Nahle S, Cassidy H, Matallanas D, Rihn BH, Joubert O, Ferrari L. Single- vs. Multi-Walled Carbon Nanotubes: Differential Cellular Stress and Lipid Metabolism Effects in Macrophage Models. Nanomaterials. 2025; 15(18):1401. https://doi.org/10.3390/nano15181401
Chicago/Turabian StyleNahle, Sara, Hilary Cassidy, David Matallanas, Bertrand H. Rihn, Olivier Joubert, and Luc Ferrari. 2025. "Single- vs. Multi-Walled Carbon Nanotubes: Differential Cellular Stress and Lipid Metabolism Effects in Macrophage Models" Nanomaterials 15, no. 18: 1401. https://doi.org/10.3390/nano15181401
APA StyleNahle, S., Cassidy, H., Matallanas, D., Rihn, B. H., Joubert, O., & Ferrari, L. (2025). Single- vs. Multi-Walled Carbon Nanotubes: Differential Cellular Stress and Lipid Metabolism Effects in Macrophage Models. Nanomaterials, 15(18), 1401. https://doi.org/10.3390/nano15181401







