Humics-Functionalized Iron(III) Oxyhydroxides as Promising Nanoferrotherapeutics: Synthesis, Characterization, and Efficacy in Iron Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Iron(III) Oxyhydroxide Nanoparticles Stabilized with Humic Substances
2.2.1. Preparation of Freshly Precipitated Iron(III) Hydroxide
2.2.2. Production of Iron(III) Oxyhydroxide Nanoparticles Stabilized with Humic Substances
2.2.3. Isolation of Fe(III)-HS Samples by Alcohol Precipitation
2.3. Characterization of Iron(III) Oxyhydroxide Nanoparticles Stabilized with Humic Substances
2.4. Cytotoxicity Assessment
2.5. Cellular Iron Uptake
2.6. Statistical Analysis
3. Results
3.1. Synthesis of Nanostructured Iron(III) Oxyhydroxides and Their Elemental Composition
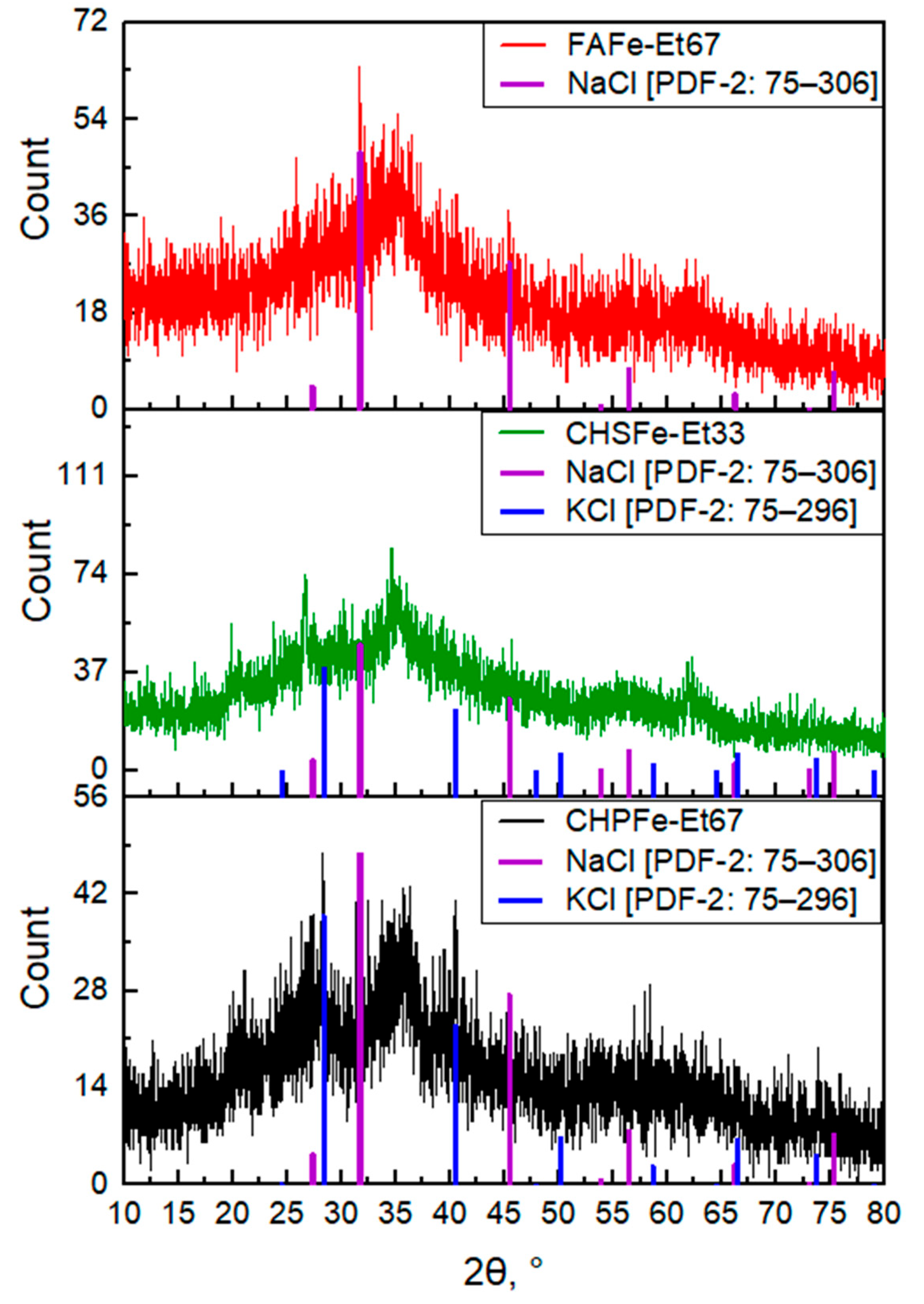
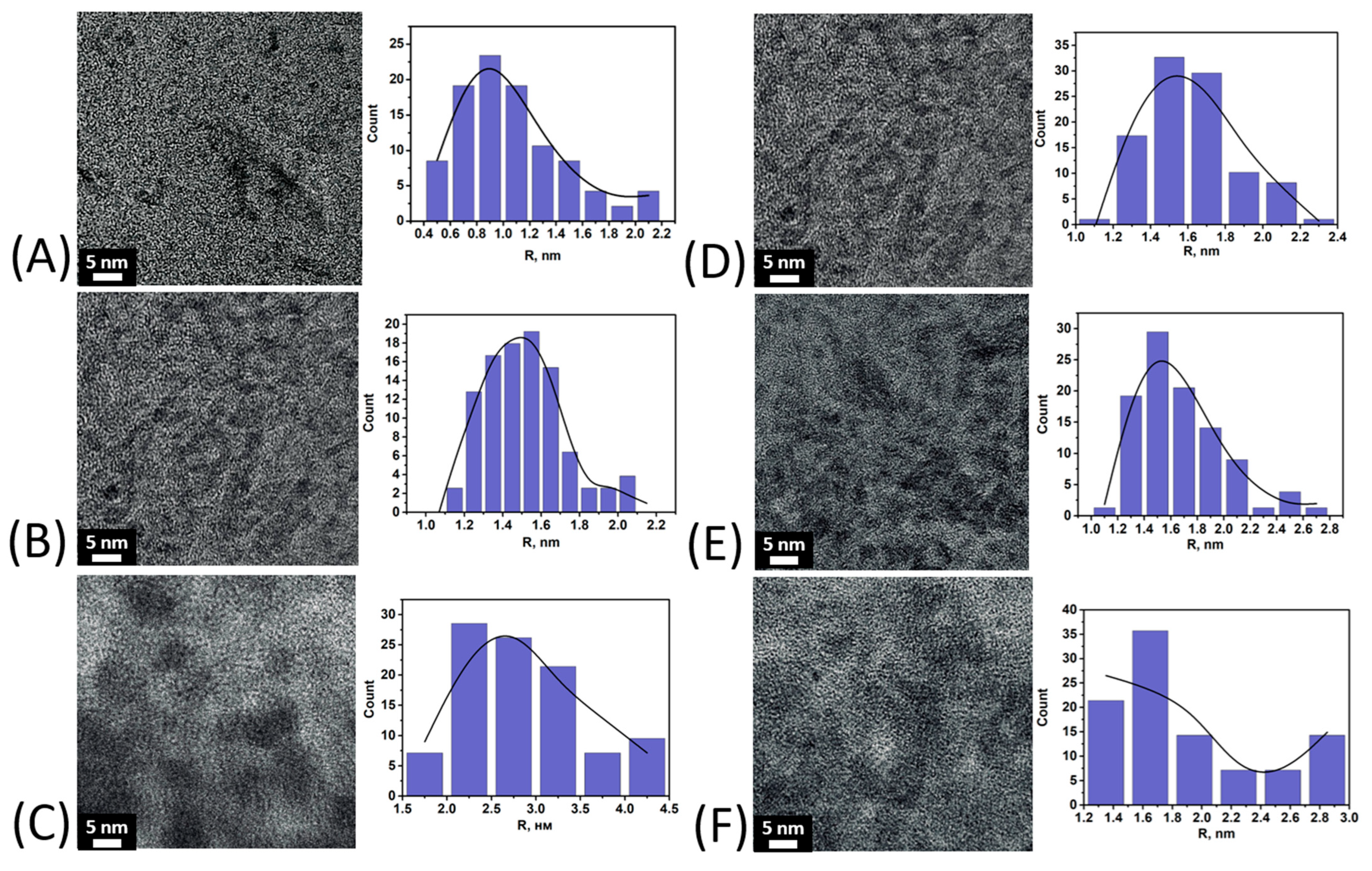
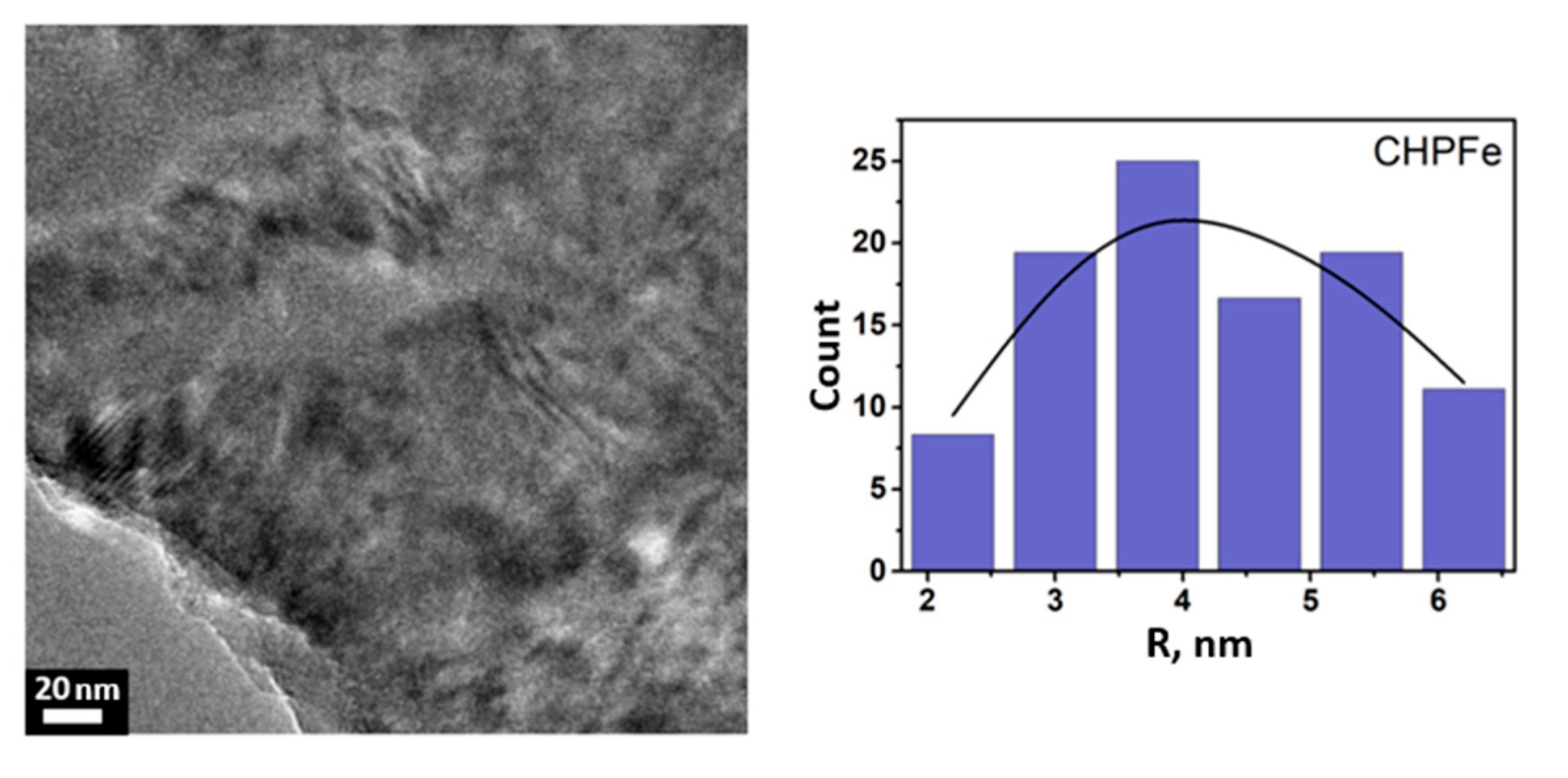
3.2. Characterization of Fe(III) Oxyhydroxide Nanoparticles Stabilized with Humic Substances
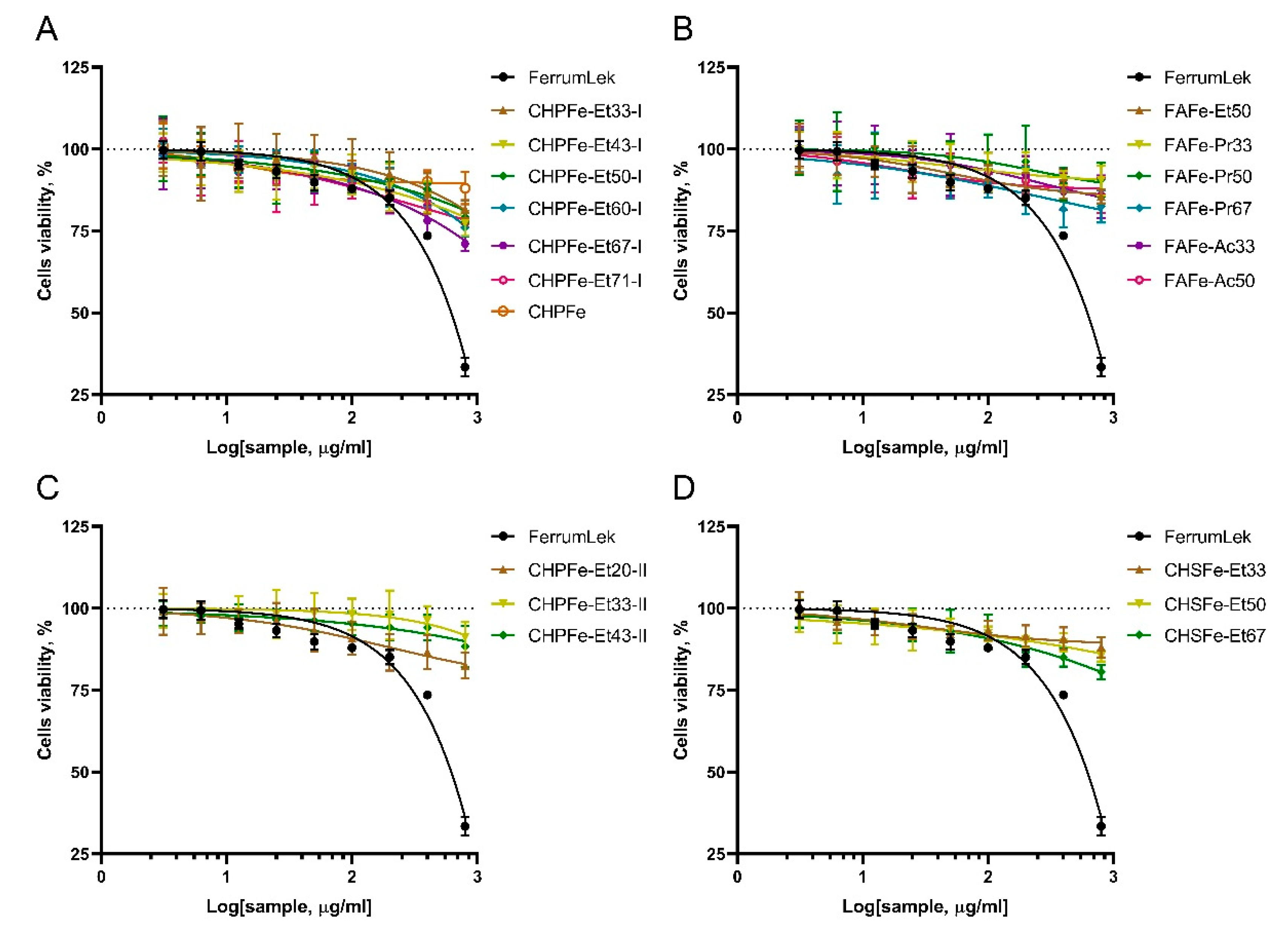
3.3. Cytotoxicity Assessment of Fe(III) Oxyhydroxide Nanoparticles Stabilized with Humic Substances
3.4. Fe(III) Oxyhydroxide Nanoparticles Stabilized with Humic Substances Iron Bioavailability Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Sharma, E.; Marley, A.; Samaan, M.A.; Brookes, M.J. Iron deficiency anaemia: Pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022, 9, e000759. [Google Scholar] [CrossRef]
- Wang, L.; Liang, D.; Huangfu, H.; Shi, X.; Liu, S.; Zhong, P.; Luo, Z.; Ke, C.; Lai, Y. Iron deficiency: Global trends and projections from 1990 to 2050. Nutrients 2024, 16, 3434. [Google Scholar] [CrossRef]
- Al-Bayyari, N.; Al Sabbah, H.; Hailat, M.; AlDahoun, H.; Abu-Samra, H. Dietary diversity and iron deficiency anemia among a cohort of singleton pregnancies: A cross-sectional study. BMC Public Health 2024, 24, 1840. [Google Scholar] [CrossRef]
- Wu, N.; Ye, E.; Ba, Y.; Caikai, S.; Ba, B.; Li, L.; Zhu, Q. The global burden of maternal disorders attributable to iron deficiency related sub-disorders in 204 countries and territories: An analysis for the Global Burden of Disease study. Front. Public Health 2024, 12, 1406549. [Google Scholar] [CrossRef]
- Dhurde, V.S.; Patel, A.B.; Locks, L.M.; Hibberd, P.L. Anaemia prevalence, its determinants and profile of micronutrient status among rural school adolescent girls aged 14–19 years: A cross-sectional study in Nagpur district, Maharashtra, India. Public Health Nutr. 2024, 27, e248. [Google Scholar] [CrossRef]
- Pastore, P.; Roverso, M.; Tedesco, E.; Micheletto, M.; Mantovan, E.; Zanella, M.; Benetti, F. Comparative evaluation of intestinal absorption and functional value of iron dietary supplements and drug with different delivery systems. Molecules 2020, 25, 5989. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of lactoferrin oral administration in the treatment of anemia and anemia of inflammation in pregnant and non-pregnant women: An interventional study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron deficiency anaemia revisited. J. Intern. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef]
- Perminova, I.V. From green chemistry and nature-like technologies towards ecoadaptive chemistry and technology. Pure Appl. Chem. 2019, 91, 851–864. [Google Scholar] [CrossRef]
- Trofimova, E.S.; Zykova, M.V.; Danilets, M.G.; Ligacheva, A.A.; Sherstoboev, E.Y.; Tsupko, A.V.; Mikhalyov, D.A.; Belousov, M.V. Immunomodulating properties of humic acids extracted from oligotrophic Sphagnum magellanicum peat. Bull. Exp. Biol. Med. 2021, 170, 461–465. [Google Scholar] [CrossRef]
- Zykova, M.V.; Schepetkin, I.A.; Belousov, M.V.; Krivoshchekov, S.V.; Logvinova, L.A.; Bratishko, K.A.; Yusubov, M.S.; Romanenko, S.V.; Quinn, M.T. Physicochemical characterization and antioxidant activity of humic acids isolated from peat of various origins. Molecules 2018, 23, 753. [Google Scholar] [CrossRef]
- Trofimova, E.S.; Zykova, M.V.; Ligacheva, A.A.; Danilets, M.G.; Sherstoboev, E.Y.; Logvinova, L.A.; Belousov, M.V. Anti-allergic properties of humic acids isolated from Pine-Sphagnum-Cotton sedge peat. Bull. Exp. Biol. Med. 2022, 172, 324–327. [Google Scholar] [CrossRef]
- Buyko, E.E.; Zykova, M.V.; Ivanov, V.V.; Bratishko, K.A.; Ufandeev, A.A.; Grigorieva, I.O.; Tsupko, A.V.; Mikhalyov, D.A.; Perminova, I.V.; Belousov, M.V. Antioxidant activity of silver-containing bionanocompositions based on humic substances in cell culture. Drug Dev. Regist. 2021, 10, 46–53. [Google Scholar] [CrossRef]
- Cieschi, M.T.; Caballero-Molada, M.; Menéndez, N.; Naranjo, M.A.; Lucena, J.J. Long-term effect of a leonardite iron humate improving Fe nutrition as revealed in silico, in vivo, and in field experiments. J. Agric. Food Chem. 2017, 65, 6554–6563. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; Sellitto, V.M.; Rizzardo, C.; Tomasi, N.; Pinton, R.; Cesco, S. Characteristics of insoluble, high molecular weight iron-humic substances used as plant iron sources. Soil Sci. Soc. Am. J. 2012, 76, 1246–1256. [Google Scholar] [CrossRef]
- Kovács, K.; Czech, V.; Fodor, F.; Solti, A.; Lucena, J.J.; Santos-Rosell, S.; Hernández-Apaolaza, L. Characterization of Fe–leonardite complexes as novel natural iron fertilizers. J. Agric. Food Chem. 2013, 61, 12200–12210. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Goldt, A.E.; Sorkina, T.A.; Perminova, I.V.; Pankratov, D.A.; Goodilin, E.A.; Tretyakov, Y.D. Constrained growth of anisotropic magnetic δ-FeOOH nanoparticles in the presence of humic substances. CrystEngComm 2012, 14, 8097–8100. [Google Scholar] [CrossRef]
- Sorkina, T.A.; Polyakov, A.Y.; Kulikova, N.A.; Goldt, A.E.; Philippova, O.I.; Aseeva, A.A.; Perminova, I.V. Nature-inspired soluble iron-rich humic compounds: New look at the structure and properties. J. Soils Sediments 2014, 14, 261–268. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Polyakov, A.Y.; Lebedev, V.A.; Abroskin, D.P.; Volkov, D.S.; Pankratov, D.A.; Klein, O.I.; Senik, S.V.; Sorkina, T.A.; Garshev, A.V.; et al. Key roles of size and crystallinity of nanosized iron hydr(oxides) stabilized by humic substances in iron bioavailability to plants. J. Agric. Food Chem. 2017, 65, 11157–11169. [Google Scholar] [CrossRef]
- Cieschi, M.T.; Polyakov, A.Y.; Lebedev, V.A.; Volkov, D.S.; Pankratov, D.A.; Veligzhanin, A.A.; Lucena, J.J. Eco-friendly iron-humic nanofertilizers synthesis for the prevention of iron chlorosis in soybean (Glycine max) grown in calcareous soil. Front. Plant Sci. 2019, 10, 413. [Google Scholar] [CrossRef]
- Turan, M.; Ekinci, M.; Kul, R.; Kocaman, A.; Argin, S.; Zhirkova, A.M.; Perminova, I.V.; Yildirim, E. Foliar applications of humic substances together with Fe/Nano Fe to increase the iron content and growth parameters of Spinach (Spinacia oleracea L.). Agronomy 2022, 12, 2044. [Google Scholar] [CrossRef]
- Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.D.; Brandão, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-based nanoformulations for drug delivery: An ongoing perspective. Pharmaceutics 2024, 16, 1376. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wang, S.; Li, Y.; Qu, H. Separation characteristics of ethanol precipitation for the purification of the water extract of medicinal plants. Sep. Purif. Technol. 2013, 107, 273–280. [Google Scholar] [CrossRef]
- Grover, P.K.; Ryall, R.L. Critical appraisal of salting-out and its implications for chemical and biological sciences. Chem. Rev. 2005, 105, 1–10. [Google Scholar] [CrossRef]
- Chang, R.; Xiong, L.; Li, M.; Liu, J.; Wang, Y.; Chen, H.-H.; Sun, Q. Fractionation of debranched starch with sifferent molecular weights via edible alcohol precipitation. Food Hydrocoll. 2018, 83, 430–437. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Fanzaga, M.; Bollati, C.; Ranaldi, G.; Sucato, S.; Fustinoni, S.; Roda, G.; Lammi, C. Bioavailability assessment of an iron formulation using differentiated human intestinal Caco-2 Cells. Foods 2023, 12, 3016. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Kelimu, A.; Korma, S.A.; Cacciotti, I.; Xiang, H.; Cui, C. Novel iron-chelating peptide from egg yolk: Preparation, characterization, and iron transportation. Food Chem. X 2023, 18, 100692. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, H.; Yu, X.; Wang, Q.; Li, X.; Zhang, R.; Feng, J. Plasma non-transferrin-bound iron uptake by the small intestine leads to intestinal injury and intestinal flora dysbiosis in an iron overload mouse model and Caco-2 cells. Sci. China Life Sci. 2023, 66, 2041–2055. [Google Scholar] [CrossRef] [PubMed]
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef]
- Ostertag, F.; Grimm, V.J.; Hinrichs, J. Iron saturation and binding capacity of lactoferrin—Development and validation of a colorimetric protocol for quality control. Food Chem. 2025, 463, 141365. [Google Scholar] [CrossRef]
- Olson, B.J.; Markwell, J. Assays for determination of protein concentration. Curr. Protoc. Protein Sci. 2007, 38, A.3A.1–A.3A.29. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Zhu, G.; Eminov, A.; Okada, K. Template-free hydrothermal synthesis of hollow α-FeOOH urchin-like spheres and their conversion to α-Fe2O3 under low-temperature thermal treatment in air. J. Clust. Sci. 2013, 24, 97–106. [Google Scholar] [CrossRef]
- Bowles, J. The iron oxides: Structure, properties, reactions, occurrence and uses (Review). Miner. Mag. 1997, 61, 740–741. [Google Scholar] [CrossRef]
- Michel, F.; Ehm, L.; Antao, S.; Lee, P.; Chupas, P.; Liu, G.; Strongin, D.; Schoonen, M.; Phillips, B.; Parise, J. The structure of ferrihydrite, a nanocrystalline material. Science 2007, 316, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Dilantika, C. Iron absorption and its influencing factors to prevent iron deficiency. J. Indones. Spec. Nutr. 2023, 1, 10–21. [Google Scholar] [CrossRef]
- Piskin, E.; Cianciosi, D.; Gules, S.; Tomas, M.; Capanoglu, E. Iron absorption: Factors, limitations, and improvement methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, M.; Salvat, V.; Pizarro, F.; Olivares, M. Smaller iron particle size improves bioavailability of hydrogen-reduced iron-fortified bread. Nutr. Res. 2006, 26, 235–239. [Google Scholar] [CrossRef]
- Balsamo Crespo, E.; Reichelt-Brushett, A.; Smith, R.; Rose, A.; Batley, G. Improving the measurement of iron(III) bioavailability in freshwater samples: Methods and performance. Environ. Toxicol. Chem. 2023, 42, 303–316. [Google Scholar] [CrossRef]
- Čolić, M.; Kraljević Pavelić, S.; Peršurić, Ž.; Agaj, A.; Bulog, A.; Pavelić, K. Enhancing the bioavailability and activity of natural antioxidants with nanobubbles and nanoparticles. Redox Rep. 2024, 29, 2333619. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Raikwar, S. Enhancement of oral bioavailability of protein and peptide by polysaccharide-based nanoparticles. Protein Pept. Lett. 2024, 31, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Gangavarapu, A.; Tapia-Lopez, L.V.; Sarkar, B.; Pena-Zacarias, J.; Badruddoza, A.Z.M.; Nurunnabi, M. Lipid nanoparticles for enhancing oral bioavailability. Nanoscale 2024, 16, 18319–18338. [Google Scholar] [CrossRef] [PubMed]

| Solvent Content in the Mixture, % | Parallel Precipitation | Sequential Precipitation | ||
|---|---|---|---|---|
| Volume of Fe(III)-HS Mixture, mL | Solvent Volume, mL | Volume of Fe(III)-HS Mixture, mL | Solvent Volume, mL | |
| 20 | 50 | 12.5 | 100 | 25 |
| 33 | 50 | 25 | 25 | |
| 43 | 50 | 37.5 | 25 | |
| 50 | 50 | 50 | 25 | |
| 60 | 50 | 75 | 50 | |
| 67 | 50 | 100 | 50 | |
| 71 | 50 | 125 | 50 | |
| Name * | Fe Content, % | Fe Yield, % | C Content, % | C Yield, % | S, g/L |
|---|---|---|---|---|---|
| CHPFe | 25.1 | 96 | 17.9 | 32 | 56.6 |
| CHPFe-Et20-I | 16.2 | 27 | 25.8 | 19 | 144.5 |
| CHPFe-Et33-I | 13.1 | 56 | 31.2 | 63 | 125.9 |
| CHPFe-Et43-I | 11.7 | 56 | 29.5 | 62 | 141.8 |
| CHPFe-Et50-I | 11.6 | 50 | 32.6 | 69 | 132.6 |
| CHPFe-Et60-I | 12.6 | 57 | 32.6 | 74 | 139.7 |
| CHPFe-Et67-I | 12.2 | 58 | 32.1 | 82 | 140.7 |
| CHPFe-Et71-I | 11.9 | 59 | 32.7 | 74 | 133.8 |
| CHPFe-Et20-II | 8.3 | 17 | 34.3 | 23 | 143.3 |
| CHPFe-Et33-II | 17.7 | 35 | 23.7 | 26 | 120.1 |
| CHPFe-Et43-II | 5.7 | 6 | 38.6 | 15 | 145.7 |
| FAFe-Et50 | 27.2 | 48 | 16.9 | 49 | - |
| FAFe-Et67 | 15.6 | 43 | 21.5 | 31 | - |
| FAFe-Pr33 | 33.5 | 72 | 15.9 | 22 | - |
| FAFe-Pr50 | 33.5 | 83 | 21.2 | 53 | - |
| FAFe-Pr67 | 12.7 | 77 | 28.0 | 57 | - |
| FAFe-Ac33 | 30.3 | 85 | 16.1 | 28 | - |
| FAFe-Ac50 | 23.4 | 85 | 20.6 | 47 | - |
| CHSFe-Et33 | 9.3 | 43 | - | - | - |
| CHSFe-Et50 | 9.6 | 54 | - | - | - |
| CHSFe-Et67 | 5.8 | 54 | - | - | - |
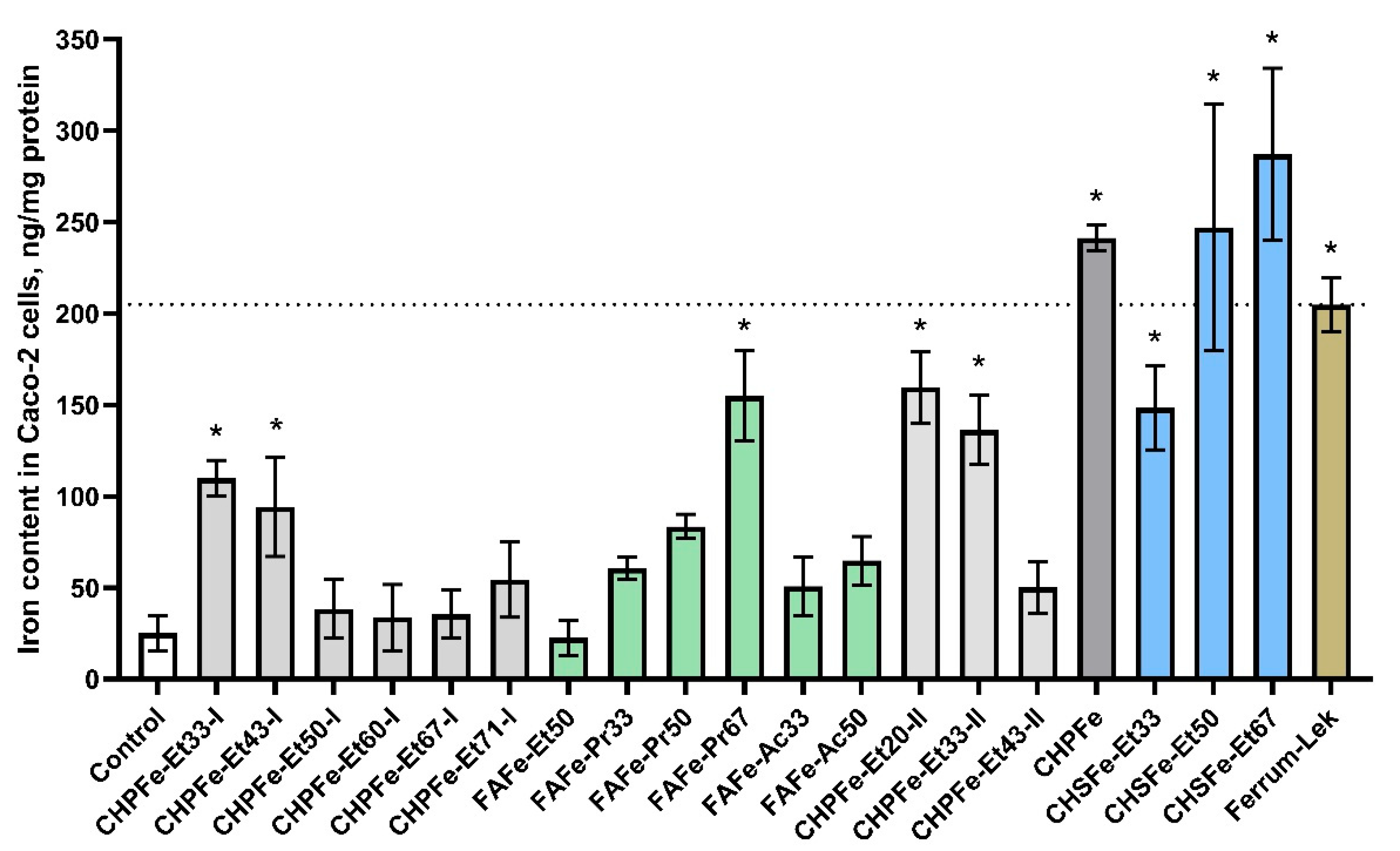
| Sample | Iron Content (ng/mg Protein) | Fold-Change vs. Control |
|---|---|---|
| Control | 25.1 ± 9.6 | 1.0 |
| CHPFe-Et33-I | 109.9 ± 9.8 | 5.6 |
| CHPFe-Et43-I | 94.3 ± 27.2 | 4.8 |
| CHPFe-Et50-I | 38.4 ± 16.0 | 2.0 |
| CHPFe-Et60-I | 33.7 ± 18.0 | 1.7 |
| CHPFe-Et67-I | 35.5 ± 13.1 | 1.8 |
| CHPFe-Et71-I | 54.4 ± 20.6 | 2.8 |
| FAFe-Et50 | 22.6 ± 9.6 | 1.1 |
| FAFe-Pr33 | 60.7 ± 6.1 | 3.1 |
| FAFe-Pr50 | 83.5 ± 6.7 | 4.2 |
| FAFe-Pr67 | 154.8 ± 24.7 | 7.9 |
| FAFe-Ac33 | 50.7 ± 16.0 | 2.6 |
| FAFe-Ac50 | 64.8 ± 13.2 | 4.8 |
| CHPFe-Et20-II | 159.7 ± 19.7 | 8.1 |
| CHPFe-Et33-II | 136.4 ± 19.0 | 6.9 |
| CHPFe-Et43-II | 50.2 ± 14.0 | 2.5 |
| CHPFe | 241.3 ± 6.9 | 12.2 |
| CHSFe-Et33 | 148.6 ± 23.1 | 7.5 |
| CHSFe-Et50 | 247.1 ± 67.6 | 12.5 |
| CHSFe-Et67 | 287.2 ± 47.0 | 14.6 |
| Ferrum-Lek | 204.9 ± 14.6 | 10.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhirkova, A.M.; Zykova, M.V.; Buyko, E.E.; Ushakova, K.A.; Ivanov, V.V.; Pankratov, D.A.; Udut, E.V.; Azarkina, L.A.; Bashirov, S.R.; Plotnikov, E.V.; et al. Humics-Functionalized Iron(III) Oxyhydroxides as Promising Nanoferrotherapeutics: Synthesis, Characterization, and Efficacy in Iron Delivery. Nanomaterials 2025, 15, 1400. https://doi.org/10.3390/nano15181400
Zhirkova AM, Zykova MV, Buyko EE, Ushakova KA, Ivanov VV, Pankratov DA, Udut EV, Azarkina LA, Bashirov SR, Plotnikov EV, et al. Humics-Functionalized Iron(III) Oxyhydroxides as Promising Nanoferrotherapeutics: Synthesis, Characterization, and Efficacy in Iron Delivery. Nanomaterials. 2025; 15(18):1400. https://doi.org/10.3390/nano15181400
Chicago/Turabian StyleZhirkova, Anastasiya M., Maria V. Zykova, Evgeny E. Buyko, Karina A. Ushakova, Vladimir V. Ivanov, Denis A. Pankratov, Elena V. Udut, Lyudmila A. Azarkina, Sergey R. Bashirov, Evgenii V. Plotnikov, and et al. 2025. "Humics-Functionalized Iron(III) Oxyhydroxides as Promising Nanoferrotherapeutics: Synthesis, Characterization, and Efficacy in Iron Delivery" Nanomaterials 15, no. 18: 1400. https://doi.org/10.3390/nano15181400
APA StyleZhirkova, A. M., Zykova, M. V., Buyko, E. E., Ushakova, K. A., Ivanov, V. V., Pankratov, D. A., Udut, E. V., Azarkina, L. A., Bashirov, S. R., Plotnikov, E. V., Pestryakov, A. N., Belousov, M. V., & Perminova, I. V. (2025). Humics-Functionalized Iron(III) Oxyhydroxides as Promising Nanoferrotherapeutics: Synthesis, Characterization, and Efficacy in Iron Delivery. Nanomaterials, 15(18), 1400. https://doi.org/10.3390/nano15181400








