Carbyne-Enriched Carbon Coatings on Silicon Chips as Biosensing Surfaces with Stable-over-Time Biomolecule Binding Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
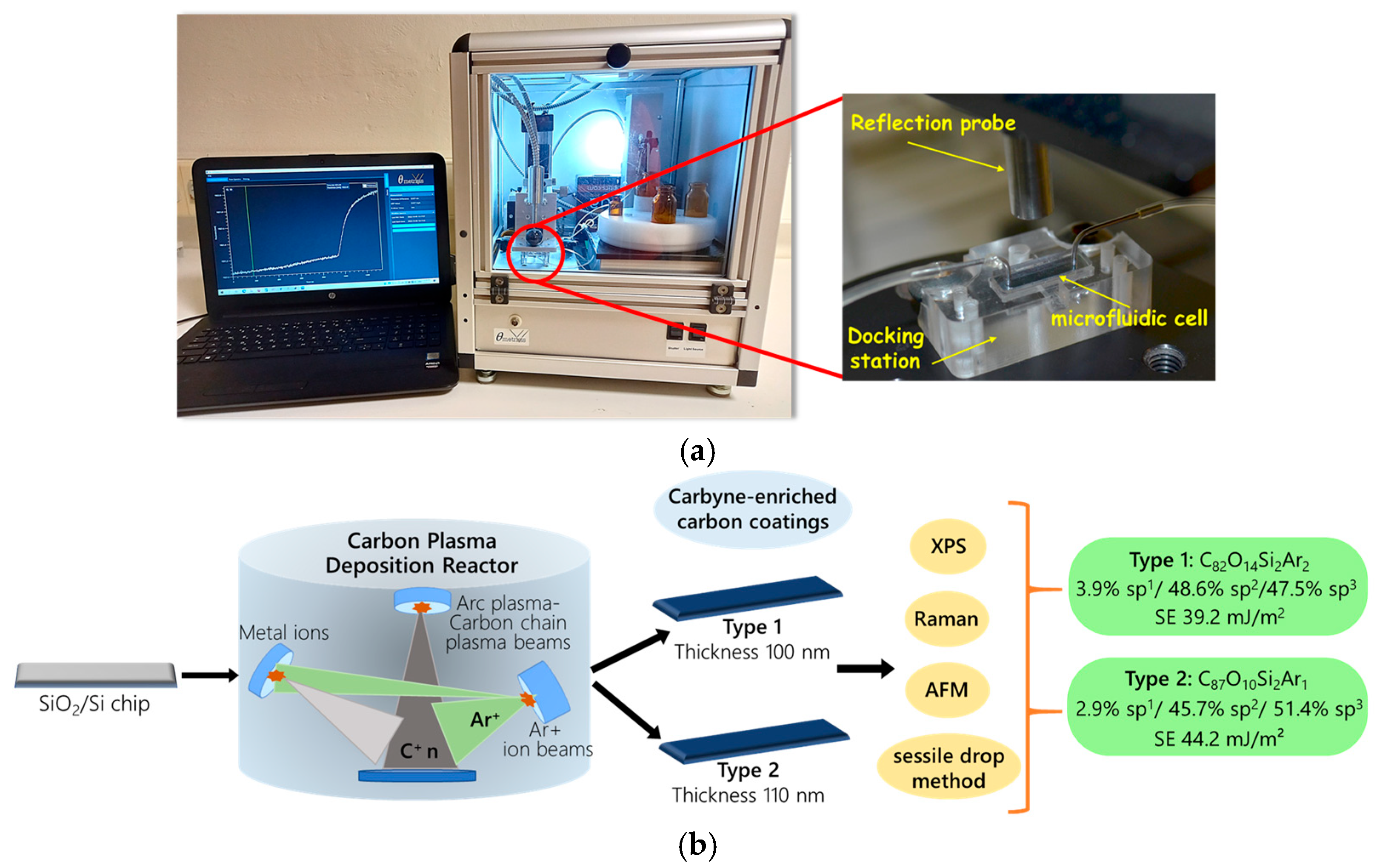
2.2. WLRS Instrumentation
2.3. Deposition and Characterization of Carbyne-Enriched Carbon Layer on SiO2/Si Chips
2.4. Modification of SiO2/Si Chips with APTES
2.5. Immobilization of Anti-CRP Antibody and WLRS CRP Assay
2.6. Covalent Bonding of Amine-Terminated Biotin onto APTES-Modified Chips
2.7. Covalent Bonding of Amine-Terminated Biotin onto Chips with Carbyne-Enriched Carbon Coatings
3. Results and Discussion
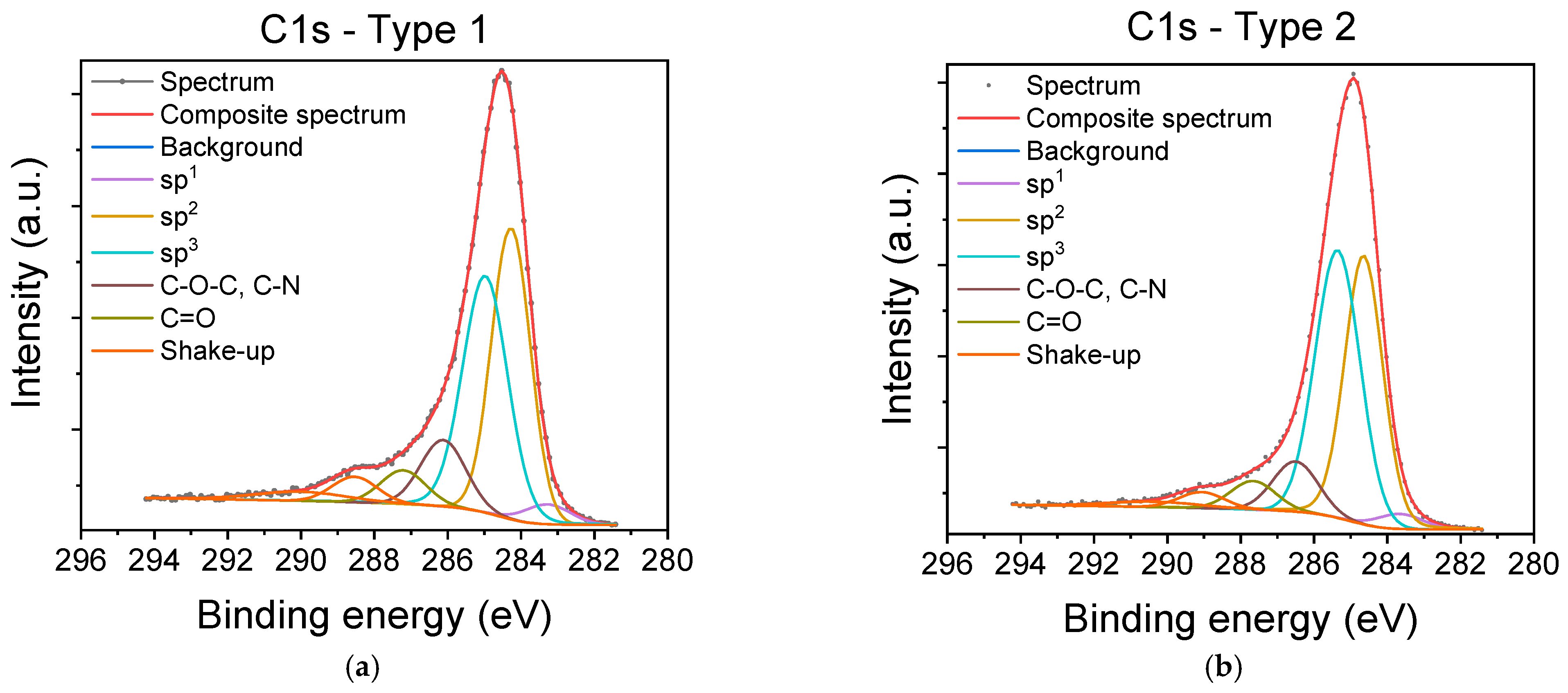
3.1. Characterization of Carbyne-Enriched Carbon Layers Deposited onto SiO2/Si Chips
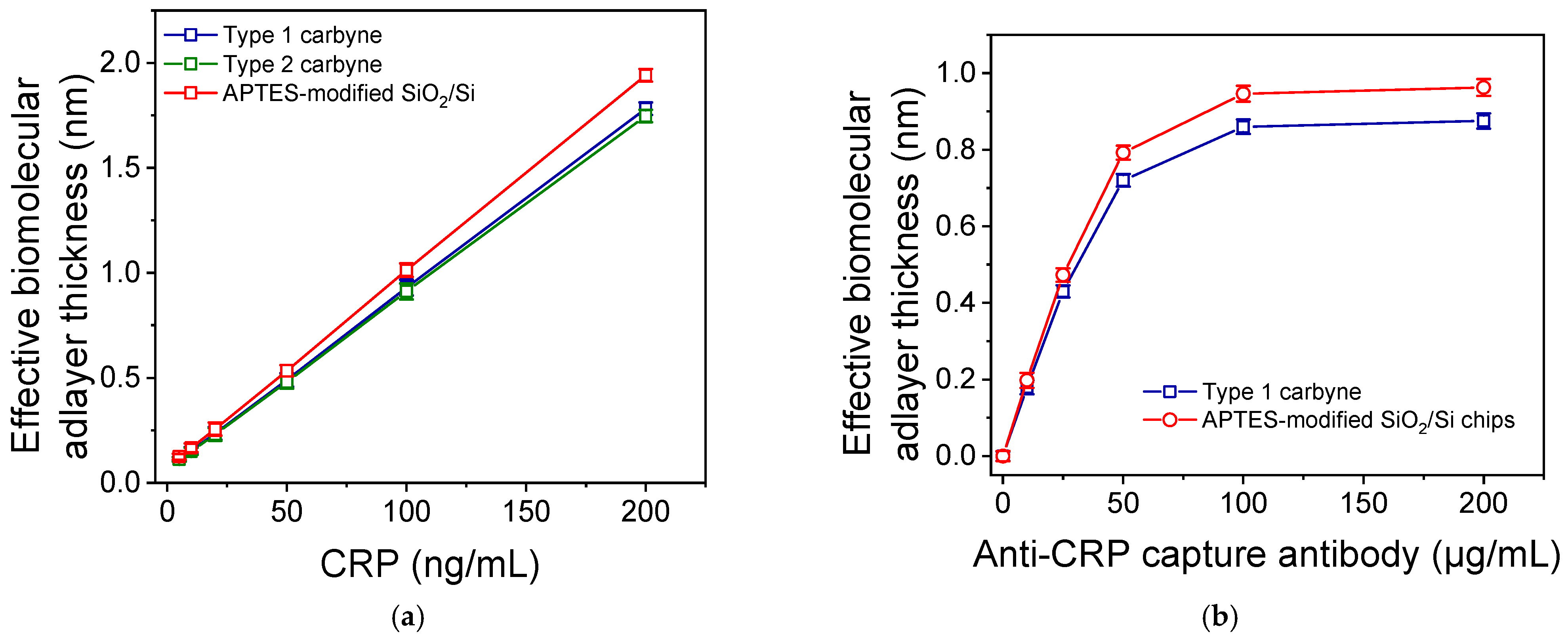
3.2. Biomolecule Immobilization onto Chips Modified with Carbyne-Enriched Carbon Layer
3.2.1. Covalent Bonding of Amine Derivative of Biotin
3.2.2. Physical Adsorption of Anti-CRP Antibody
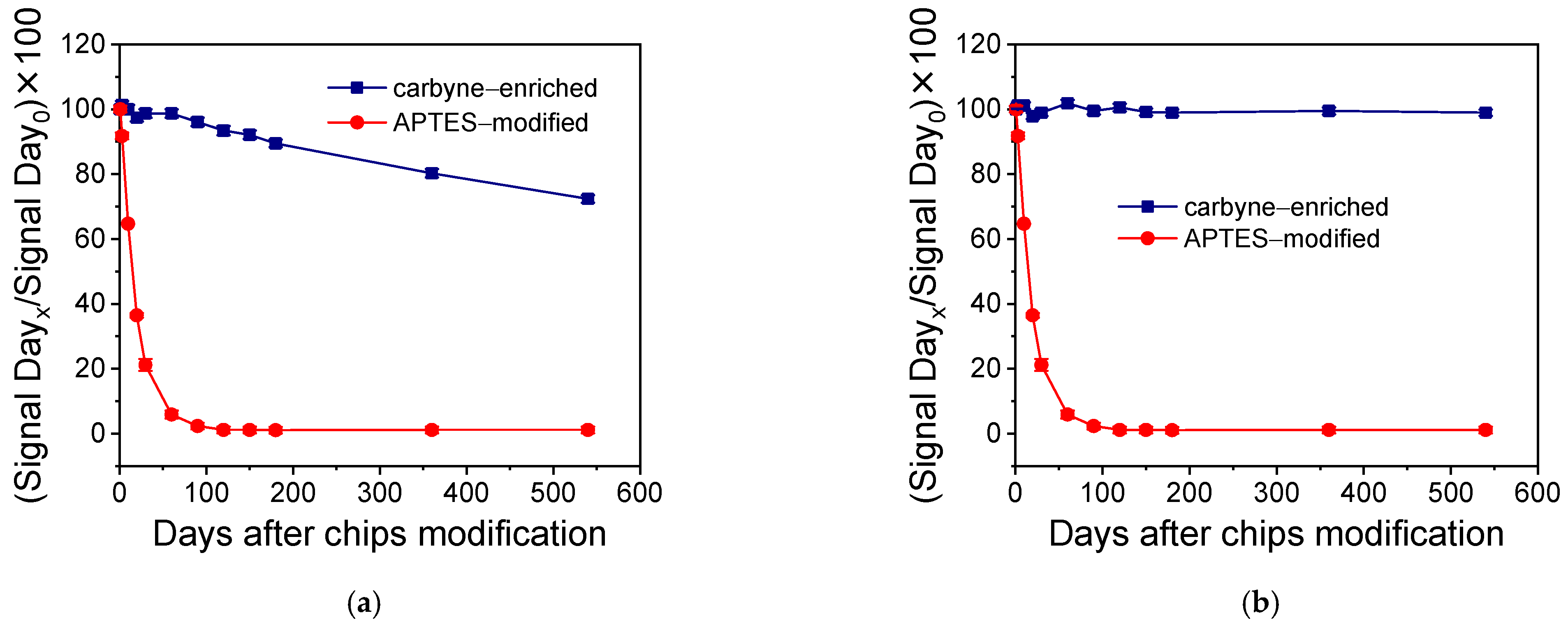
3.3. Stability of Biomolecule Binding Properties of Chips with Carbyne-Enriched Carbon Layers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oliverio, M.; Perotto, S.; Messina, G.C.; Lovato, L.; De Angelis, F. Chemical Functionalization of Plasmonic Surface Biosensors: A Tutorial Review on Issues, Strategies, and Costs. ACS Appl. Mater. Interfaces 2017, 9, 29394–29411. [Google Scholar] [CrossRef]
- Antoniou, M.; Tsounidi, D.; Petrou, P.S.; Beltsios, K.G.; Kakabakos, S.E. Functionalization of silicon dioxide and silicon nitride surfaces with aminosilanes for optical biosensing applications. Med Devices Sens. 2020, 3, e10072. [Google Scholar] [CrossRef]
- Udomsom, S.; Mankong, U.; Paengnakorn, P.; Theera-Umpon, N. Novel Rapid Protein Coating Technique for Silicon Photonic Biosensor to Improve Surface Morphology and Increase Bioreceptor Density. Coatings 2021, 11, 595. [Google Scholar] [CrossRef]
- Puumala, L.S.; Grist, S.M.; Morales, J.M.; Bickford, J.R.; Chrostowski, L.; Shekhar, S.; Cheung, K.C. Biofunctionalization of Multiplexed Silicon Photonic Biosensors. Biosensors 2022, 13, 53. [Google Scholar] [CrossRef]
- Joshi, P.; Mishra, R.; Narayan, R.J. Biosensing applications of carbon-based materials. Curr. Opin. Biomed. Eng. 2021, 18, 100274. [Google Scholar] [CrossRef]
- Ji, G.; Tian, J.; Xing, F.; Feng, Y. Optical Biosensor Based on Graphene and Its Derivatives for Detecting Biomolecules. Int. J. Mol. Sci. 2022, 23, 10838. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon Nanomaterials as Versatile Platforms for Biosensing Applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]
- Gupta, B.D.; Pathak, A.; Semwal, V. Carbon-Based Nanomaterials for Plasmonic Sensors: A Review. Sensors 2019, 19, 3536. [Google Scholar] [CrossRef]
- Su, D.; Li, H.; Yan, X.; Lin, Y.; Lu, G. Biosensors based on fluorescence carbon nanomaterials for detection of pesticides. TrAC Trends Anal. Chem. 2021, 134, 116126. [Google Scholar] [CrossRef]
- Yang, G. Synthesis, properties, and applications of carbyne nanocrystals. Mater. Sci. Eng. R: Rep. 2022, 151, 100692. [Google Scholar] [CrossRef]
- Liu, M.; Artyukhov, V.I.; Lee, H.; Xu, F.; Yakobson, B.I. Carbyne from First Principles: Chain of C Atoms, a Nanorod or a Nanorope. ACS Nano 2013, 7, 10075–10082. [Google Scholar] [CrossRef]
- Agwa, M.A.; Eltaher, M.A. Vibration of a carbyne nanomechanical mass sensor with surface effect. Appl. Phys. A 2016, 122, 335. [Google Scholar] [CrossRef]
- Ma, C.R.; Xiao, J.; Yang, G.W. Giant nonlinear optical responses of carbyne. J. Mater. Chem. C 2016, 4, 4692–4698. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Yang, G. Molecular Luminescence of White Carbon. Small 2017, 13, 1603495. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Z.; Lin, Z.; Wang, B.; Liu, P.; Yang, G. Visible-light-driven room-temperature gas sensor based on carbyne nanocrystals. Sens. Actuators B Chem. 2020, 316, 128200. [Google Scholar] [CrossRef]
- Aleksandrova, M.; Kolev, G.; Brigadin, A.; Lukin, A. Gas-Sensing Properties of a Carbyne-Enriched Nanocoating Deposited onto Surface Acoustic Wave Composite Substrates with Various Electrode Topologies. Crystals 2022, 12, 501. [Google Scholar] [CrossRef]
- Aleksandrova, M.; Ustova, B.; Tsanev, T.; Raptis, I.; Tserepi, A.; Gogolides, E.; Kolev, G. Microheater Topology for Advanced Gas Sensor Applications with Carbyne-Enriched Nanomaterials. Appl. Sci. 2024, 14, 1728. [Google Scholar] [CrossRef]
- Yang, F.; Chen, T.; Wu, X.; Chen, Y.; Yang, G. A hybrid gold-carbyne nanocrystals platform for light-induced crossover of redox enzyme-like activities. Chem. Eng. J. 2021, 408, 127244. [Google Scholar] [CrossRef]
- Chen, T.; Yang, F.; Wu, X.; Chen, Y.; Yang, G. A fluorescent and colorimetric probe of carbyne nanocrystals coated Au nanoparticles for selective and sensitive detection of ferrous ions. Carbon 2020, 167, 196–201. [Google Scholar] [CrossRef]
- Karachaliou, C.-E.; Koukouvinos, G.; Goustouridis, D.; Raptis, I.; Kakabakos, S.; Livaniou, E.; Petrou, P. Recent Developments in the Field of Optical Immunosensors Focusing on a Label-Free, White Light Reflectance Spectroscopy-Based Immunosensing Platform. Sensors 2022, 22, 5114. [Google Scholar] [CrossRef] [PubMed]
- Tsounidi, D.; Tsaousis, V.; Xenos, N.; Kroupis, C.; Moutsatsou, P.; Christianidis, V.; Goustouridis, D.; Raptis, I.; Kakabakos, S.; Petrou, P. Simultaneous determination of procalcitonin and interleukin-6 in human serum samples with a point-of-care biosensing device. Talanta 2023, 258, 124403. [Google Scholar] [CrossRef]
- Tsounidi, D.; Koukouvinos, G.; Petrou, P.; Misiakos, K.; Zisis, G.; Goustouridis, D.; Raptis, I.; Kakabakos, S.E. Rapid and sensitive label-free determination of aflatoxin M1 levels in milk through a White Light Reflectance Spectroscopy immunosensor. Sensors Actuators B: Chem. 2019, 282, 104–111. [Google Scholar] [CrossRef]
- Gajos, K.; Sanocka, K.; Wytrwał, M.; Dąbczyński, P.; Budkowski, A. pH-dependent orientation of physisorbed and chemisorbed antibodies on silicon determined with TOF-SIMS and its effect on in-flow capture assay monitored with WLRS sensor. Appl. Surf. Sci. 2024, 656, 159644. [Google Scholar] [CrossRef]
- Gajos, K.; Orzech, A.; Sanocka, K.; Petrou, P.; Budkowski, A. Covalent and Non-covalent In-Flow Biofunctionalization for Capture Assays on Silicon Chips: White Light Reflectance Spectroscopy Immunosensor Combined with TOF-SIMS Resolves Immobilization Stability and Binding Stoichiometry. Langmuir 2023, 39, 10216–10229. [Google Scholar] [CrossRef]
- Pan, B.; Xiao, J.; Li, J.; Liu, P.; Wang, C.; Yang, G. Carbyne with finite length: The one-dimensional sp carbon. Sci. Adv. 2015, 1, e1500857. [Google Scholar] [CrossRef]
- Hu, A.; Griesing, S.; Rybachuk, M.; Lu, Q.-B.; Duley, W.W. Nanobuckling and x-ray photoelectron spectra of carbyne-rich tetrahedral carbon films deposited by femtosecond laser ablation at cryogenic temperatures. J. Appl. Phys. 2007, 102, 074311. [Google Scholar] [CrossRef]
- Hu, A.; Rybachuk, M.; Lu, Q.-B.; Duley, W.W. Direct synthesis of sp-bonded carbon chains on graphite surface by femtosecond laser irradiation. Appl. Phys. Lett. 2007, 91, 131906. [Google Scholar] [CrossRef]
- Drozdov, M.; Ieshkin, A.; Streletskiy, O.; Nishchak, O.Y.; Belykh, S.; Tolstoguzov, A. TOF-SIMS for carbon hybridization state analysis. Carbon 2022, 186, 83–90. [Google Scholar] [CrossRef]
- Zatsepin, A.F.; Buntov, E.A.; Zatsepin, D.A.; Boqizoda, D.A.; Guseva, M.B.; Vyatkina, S.P.; Kas’yAnova, A.V. Plasma Synthesis and XPS Attestation of Thin-Film Carbon Coatings with Predetermined sp-Hybridization. Phys. At. Nucl. 2018, 81, 1660–1663. [Google Scholar] [CrossRef]
- Piedade, A.P.; Cangueiro, L. Influence of Carbyne Content on the Mechanical Performance of Nanothick Amorphous Carbon Coatings. Nanomaterials 2020, 10, 780. [Google Scholar] [CrossRef]
- Ansar, W.; Ghosh, S. C-reactive protein and the biology of disease. Immunol. Res. 2013, 56, 131–142. [Google Scholar] [CrossRef]
- Landry, A.; Docherty, P.; Ouellette, S.; Cartier, L.J. Causes and outcomes of markedly elevated C-reactive protein levels. Can. Fam. Physician 2017, 63, e316–e323. [Google Scholar] [PubMed] [PubMed Central]
- Grondman, I.; Pirvu, A.; Riza, A.; Ioana, M.; Netea, M.G. Biomarkers of inflammation and the etiology of sepsis. Biochem. Soc. Trans. 2020, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sypabekova, M.; Hagemann, A.; Rho, D.; Kim, S. Review: 3-Aminopropyltriethoxysilane (APTES) Deposition Methods on Oxide Surfaces in Solution and Vapor Phases for Biosensing Applications. Biosensors 2022, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ravagnan, L.; Siviero, F.; Lenardi, C.; Piseri, P.; Barborini, E.; Milani, P.; Casari, C.S.; Bassi, A.L.; Bottani, C.E. Cluster-Beam Deposition and in situ Characterization of Carbyne-Rich Carbon Films. Phys. Rev. Lett. 2002, 89, 285506. [Google Scholar] [CrossRef]
- Casari, C.S.; Bassi, A.L.; Ravagnan, L.; Siviero, F.; Lenardi, C.; Piseri, P.; Bongiorno, G.; Bottani, C.E.; Milani, P. Chemical and thermal stability of carbyne-like structures in cluster-assembled carbon films. Phys. Rev. B 2004, 69, 075422. [Google Scholar] [CrossRef]
- Barborini, E.; Piseri, P.; Bassi, A.L.; Ferrari, A.; Bottani, C.; Milani, P. Synthesis of carbon films with controlled nanostructure by separation of neutral clusters in supersonic beams. Chem. Phys. Lett. 1999, 300, 633–638. [Google Scholar] [CrossRef]
- Swain, B.P.; Dusane, R.O. Multiphase structure of hydrogen diluted a-SiC:H deposited by HWCVD. Mater. Chem. Phys. 2006, 99, 240–246. [Google Scholar] [CrossRef]
- Popescu, A.C.; Stan, G.E.; Duta, L.; Nita, C.; Popescu, C.; Surdu, V.-A.; Husanu, M.-A.; Bita, B.; Ghisleni, R.; Himcinschi, C.; et al. The Role of Ambient Gas and Pressure on the Structuring of Hard Diamond-Like Carbon Films Synthesized by Pulsed Laser Deposition. Materials 2015, 8, 3284–3305. [Google Scholar] [CrossRef]
- Irmer, G.; Dorner-Reisel, A. Micro-Raman Studies on DLC coatings. Adv. Eng. Mater. 2005, 7, 694–705. [Google Scholar] [CrossRef]
- Milenov, T.; Dikovska, A.; Avdeev, G.; Avramova, I.; Kirilov, K.; Karashanova, D.; Terziyska, P.; Georgieva, B.; Arnaudov, B.; Kolev, S.; et al. Pulsed laser deposition of thin carbon films on SiO2/Si substrates. Appl. Surf. Sci. 2019, 480, 323–329. [Google Scholar] [CrossRef]
- Casari, C.S.; Milani, A. Carbyne: From the elusive allotrope to stable carbon atom wires. MRS Commun. 2018, 8, 207–219. [Google Scholar] [CrossRef]
- Streletskiy, O.; Zavidovskiy, I.; Nischak, O.; Pavlikov, A. Multiphonon replicas in Raman spectra and conductivity properties of carbon films with different concentrations of sp1-bonds. Thin Solid Films 2018, 671, 31–35. [Google Scholar] [CrossRef]
- Chepyala, R.; Panda, S. Tunable surface free energies of functionalized molecular layers on Si surfaces for microfluidic immunosensor applications. Appl. Surf. Sci. 2013, 271, 77–85. [Google Scholar] [CrossRef]
- Chen, J.S.; Lau, S.P.; Tay, B.K.; Chen, G.Y.; Sun, Z.; Tan, Y.Y.; Tan, G.; Chai, J.W. Surface energy of amorphous carbon films containing iron. J. Appl. Phys. 2001, 89, 7814–7819. [Google Scholar] [CrossRef]
- Ostrovskaya, L.; Perevertailo, V.; Ralchenko, V.; Dementjev, A.; Loginova, O. Wettability and surface energy of oxidized and hydrogen plasma-treated diamond films. Diam. Relat. Mater. 2002, 11, 845–850. [Google Scholar] [CrossRef]
- Beucher, L.; Schlebrowski, T.; Rohe, K.; Wehner, S.; Fischer, C. Surface treatment of biopolymer films Polylactic acid and Polyhydroxybutyrat with angular changing oxygen plasma–More than just gradual purification. Surfaces Interfaces 2022, 30, 101856. [Google Scholar] [CrossRef]
- Mädler, S.; Bich, C.; Touboul, D.; Zenobi, R. Chemical cross-linking with NHS esters: A systematic study on amino acid reactivities. J. Mass Spectrom. 2009, 44, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, A.; Muñoz, J.; Martínez-Domingo, C.; Mas-Torrent, M. Magnetic carbon gate electrodes for the development of electrolyte-gated organic field effect transistor bio-sensing platforms. J. Mater. Chem. C 2023, 11, 11912–11919. [Google Scholar] [CrossRef]
- Lin, W.-Z.; Chen, Y.-H.; Liang, C.-K.; Liu, C.-C.; Hou, S.-Y. A competitive immunoassay for biotin detection using magnetic beads and gold nanoparticle probes. Food Chem. 2019, 271, 440–444. [Google Scholar] [CrossRef]
- Suganuma, M.; Kubo, T.; Ishiki, K.; Tanaka, K.; Suto, K.; Ejima, D.; Toyota, M.; Tsumoto, K.; Sato, T.; Nishikawa, Y. Mirror-image streptavidin with specific binding to L-biotin, the unnatural enantiomer. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsounidi, D.; Petrou, P.; Aleksandrova, M.; Tsanev, T.; Tserepi, A.; Gogolides, E.; Bernasik, A.; Awsiuk, K.; Janiszewska, N.; Budkowski, A.; et al. Carbyne-Enriched Carbon Coatings on Silicon Chips as Biosensing Surfaces with Stable-over-Time Biomolecule Binding Capacity. Nanomaterials 2025, 15, 1384. https://doi.org/10.3390/nano15181384
Tsounidi D, Petrou P, Aleksandrova M, Tsanev T, Tserepi A, Gogolides E, Bernasik A, Awsiuk K, Janiszewska N, Budkowski A, et al. Carbyne-Enriched Carbon Coatings on Silicon Chips as Biosensing Surfaces with Stable-over-Time Biomolecule Binding Capacity. Nanomaterials. 2025; 15(18):1384. https://doi.org/10.3390/nano15181384
Chicago/Turabian StyleTsounidi, Dimitra, Panagiota Petrou, Mariya Aleksandrova, Tsvetozar Tsanev, Angeliki Tserepi, Evangelos Gogolides, Andrzej Bernasik, Kamil Awsiuk, Natalia Janiszewska, Andrzej Budkowski, and et al. 2025. "Carbyne-Enriched Carbon Coatings on Silicon Chips as Biosensing Surfaces with Stable-over-Time Biomolecule Binding Capacity" Nanomaterials 15, no. 18: 1384. https://doi.org/10.3390/nano15181384
APA StyleTsounidi, D., Petrou, P., Aleksandrova, M., Tsanev, T., Tserepi, A., Gogolides, E., Bernasik, A., Awsiuk, K., Janiszewska, N., Budkowski, A., & Raptis, I. (2025). Carbyne-Enriched Carbon Coatings on Silicon Chips as Biosensing Surfaces with Stable-over-Time Biomolecule Binding Capacity. Nanomaterials, 15(18), 1384. https://doi.org/10.3390/nano15181384












