Nanocellulose-Based Carbon Aerogel Loaded with Composite Metal Oxides and Its Fenton Catalytic Oxidation Degradation of Phenol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
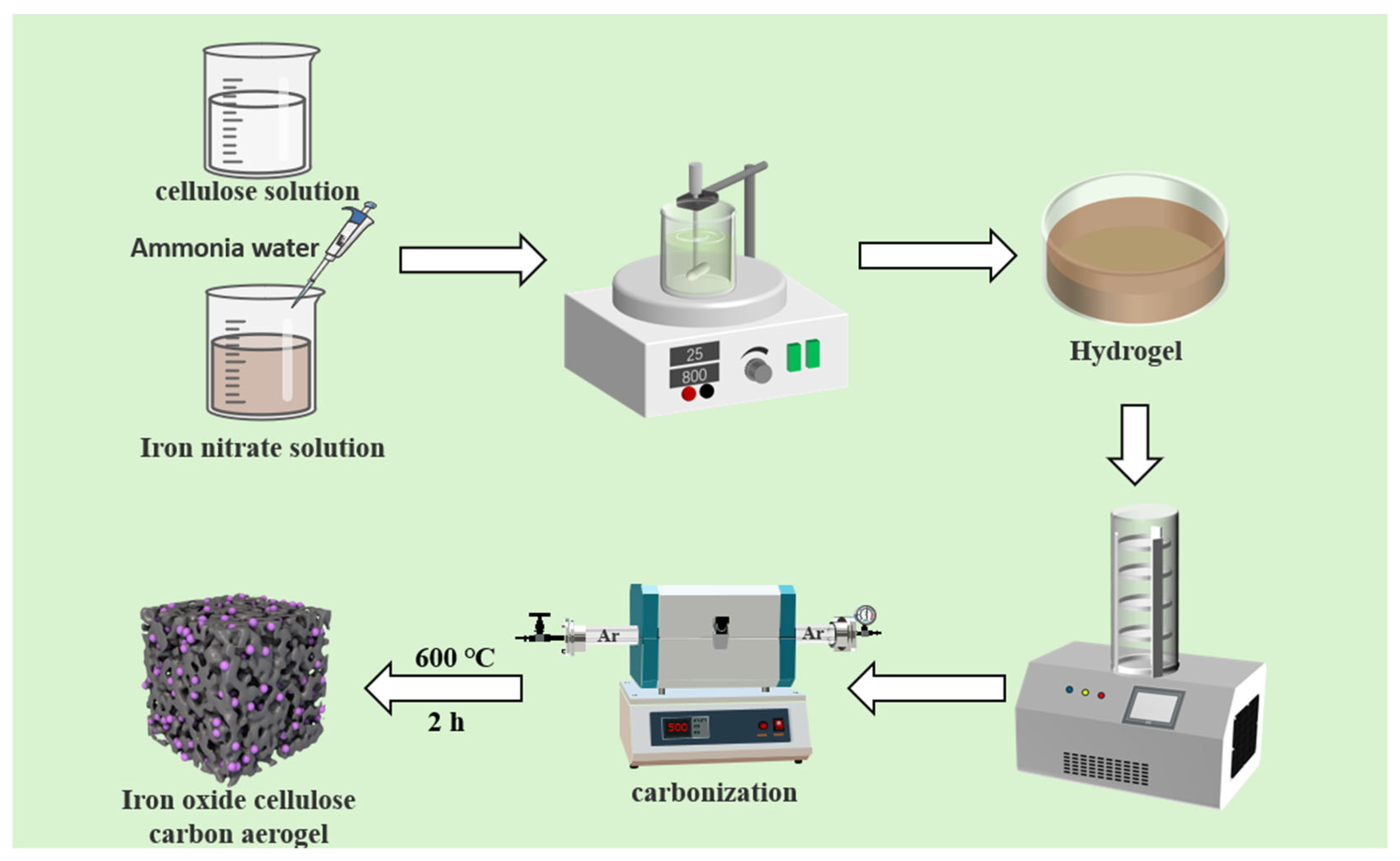
2.2. Preparation of Metal Oxide/Carbon Aerogel
2.3. Characterization
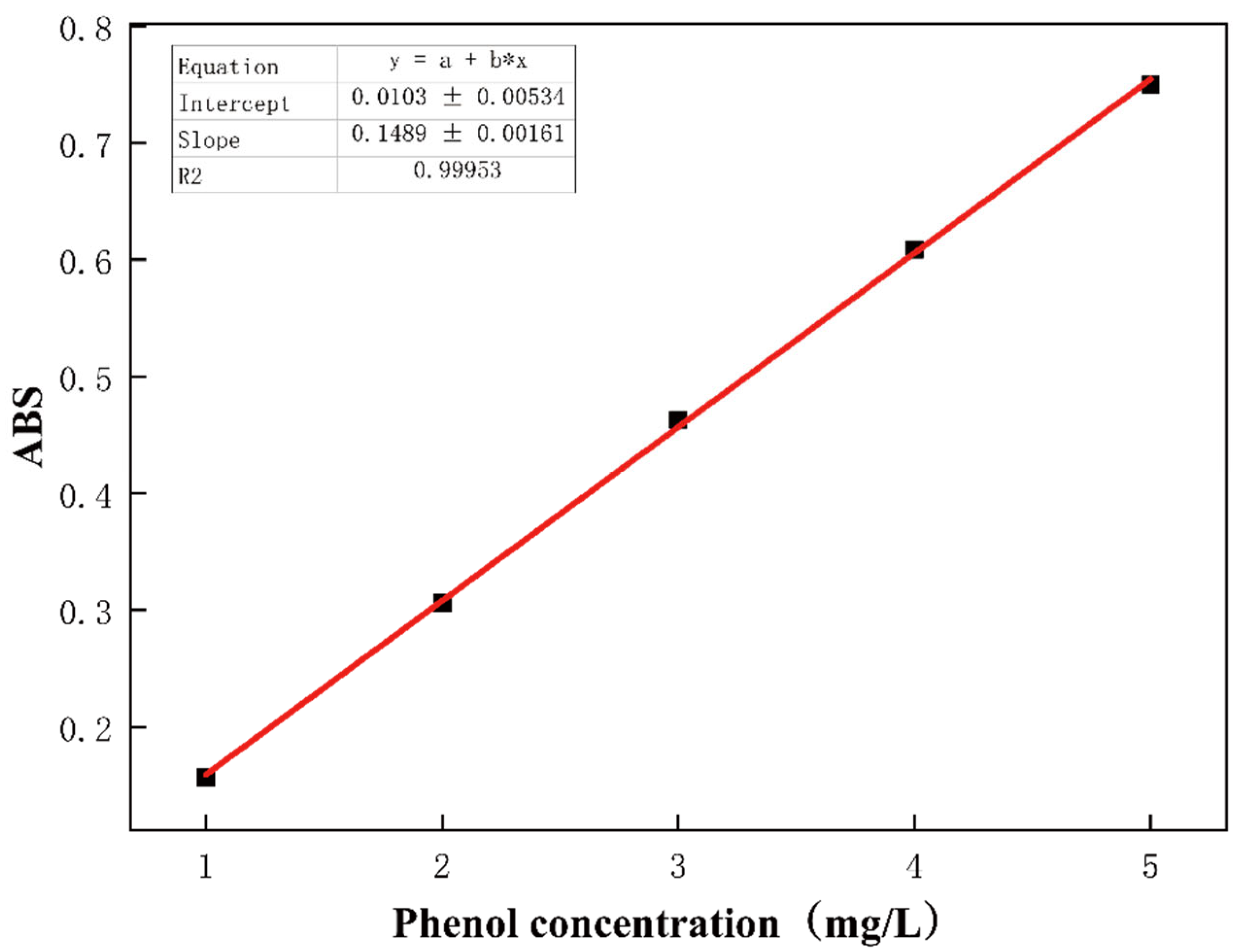
2.4. Adsorption of Phenol Aqueous
2.5. The Catalytic Degradation of Phenol with Metal Oxide/Carbon Aerogel Materials
2.6. Radical Inhibitors Experiment
2.7. Fukui Function Calculation
3. Results and Discussion
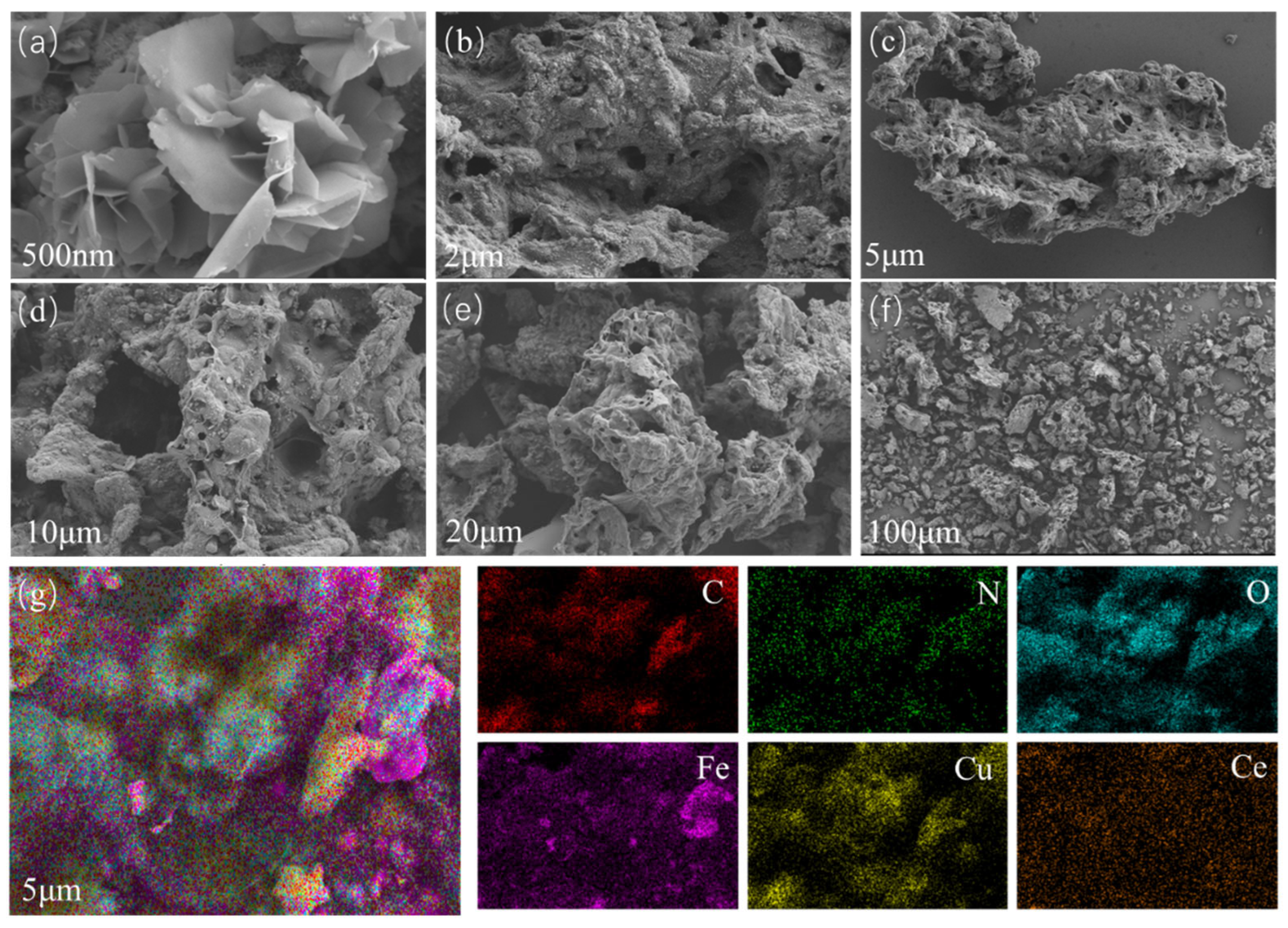
3.1. Morphology
3.2. Structure and Composition State
3.2.1. XRD Characterization
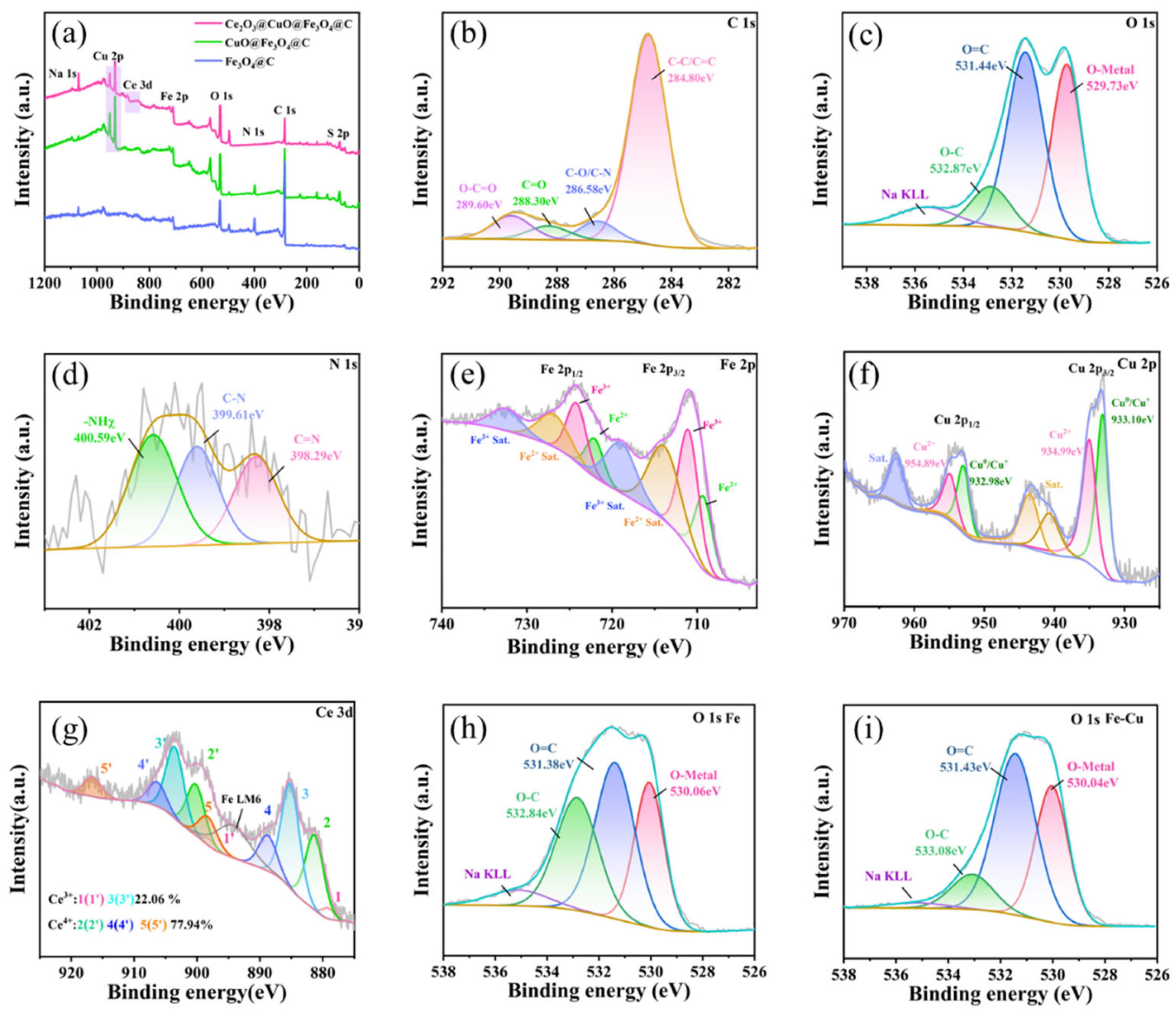
3.2.2. High-Resolution XPS Spectroscopic Analysis
3.2.3. Analysis of Specific Surface Area
3.2.4. VSM Test Analysis
3.3. The Adsorption and Catalytic Properties of Materials
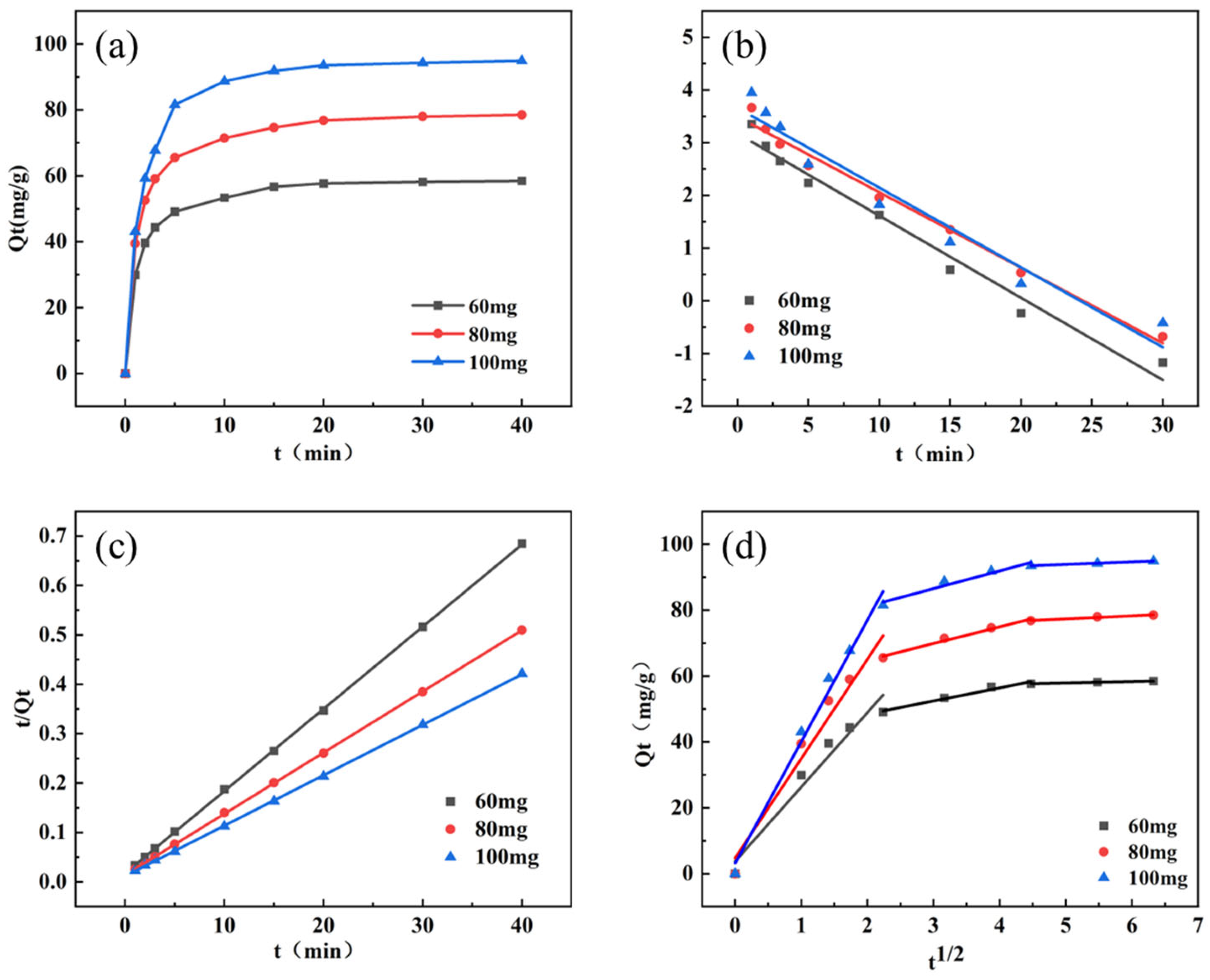
3.3.1. Adsorption Kinetics
3.3.2. Adsorption Thermodynamics
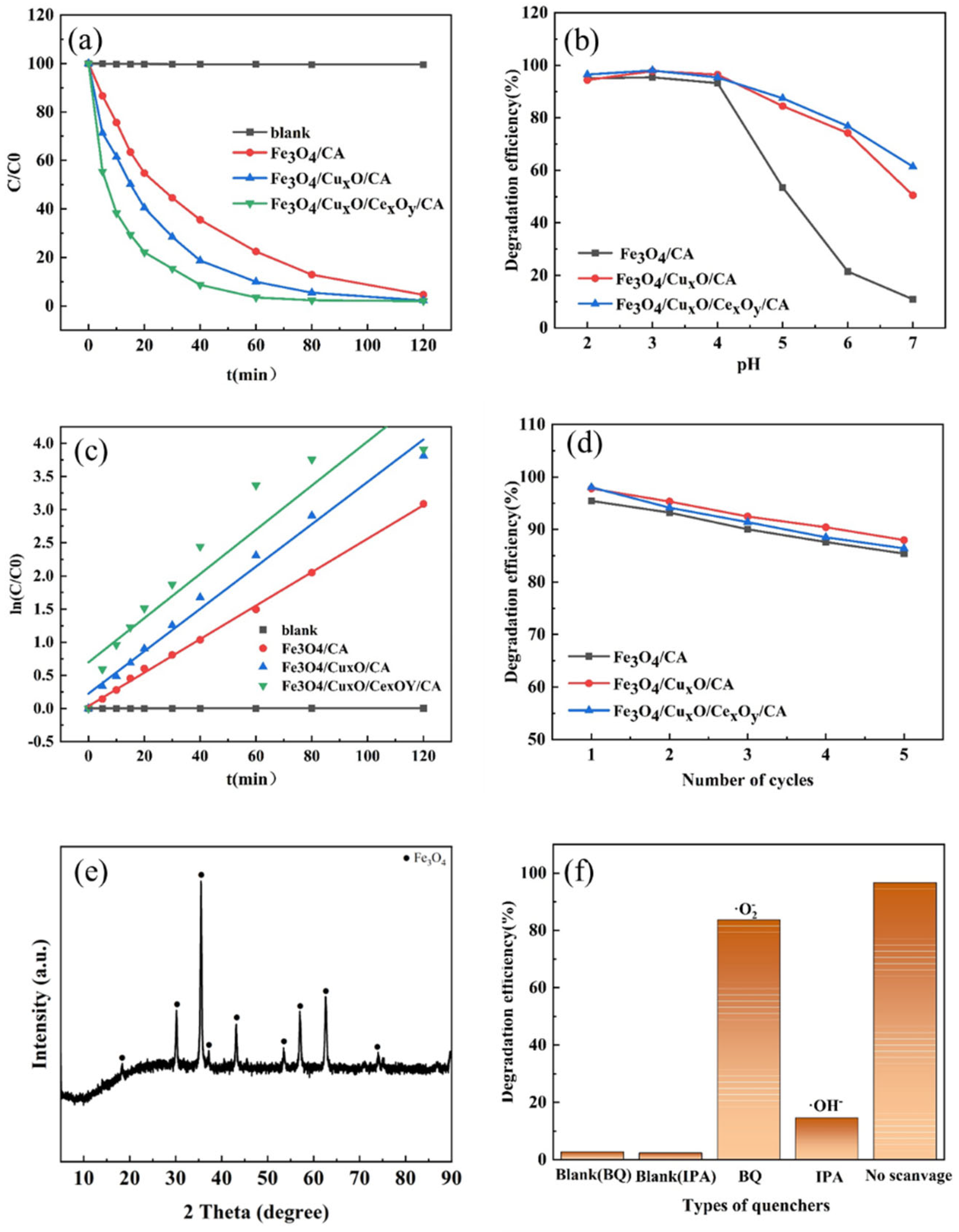
3.3.3. Catalytic Degradation with Metal Oxides/Carbon Aerogels
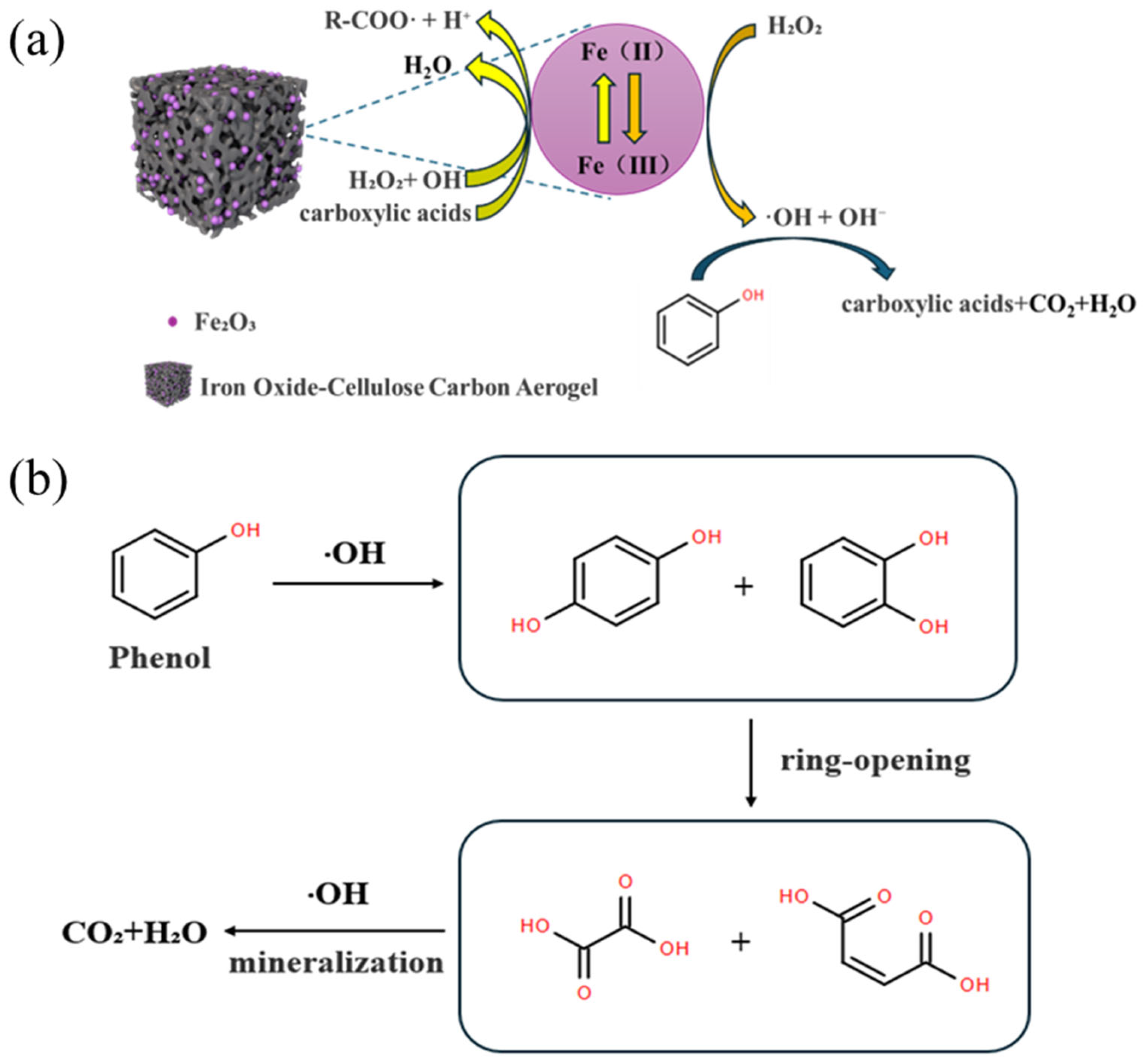
3.4. The Mechanism of Fenton Catalytic Oxidation Degradation of Phenol
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BQ | Benzoquinone |
| IPA | Isopropanol |
References
- Alinasab, M.; Navidjouy, N.; Alizadeh, S.; Rahimnejad, M. Bio-Electro-Fenton system assisted with metal-organic framework for degradation of bis-phenol S in wastewater as an emerging contaminant. Sci. Rep. 2025, 15, 6475. [Google Scholar] [CrossRef] [PubMed]
- da Silva Aires, F.I.; Dari, D.N.; Freitas, I.S.; da Silva, J.L.; de Matos Filho, J.R.; dos Santos, K.M.; de Castro Bizerra, V.; Sales, M.B.; de Souza Magalhães, F.L.; da Silva Sousa, P. Advanced and prospects in phenol wastewater treatment technologies: Unveiling opportunities and trends. Discov. Water 2024, 4, 20. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.H.; Fu, X.; Chen, J.Y. Steam-assisted synthesis of hectorite loaded with Fe2O3 and its catalytic Fenton degradation of phenol. Catalysts 2024, 14, 521. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Goh, P.S. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review of recent advances. Environ. Sci. Pollut. Res. 2025, 32, 14316–14350. [Google Scholar] [CrossRef]
- Lin, Y.J.; Dai, Y.Z.; Zhang, L.; Wu, Q. Efficient degradation of phenol in aqueous solution by Fe2+/H2O2/CaO2 system. Environ. Technol. Innov. 2022, 27, 102320. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Marques, C.C.; Neves, M.C.; Gomes, H.G.M.F.; Sarinho, L.; Tarelho, L.A.C.; Nunes, M.I. Catalytic performance of thermochemically treated sludge generated in the Fenton process for AOX degradation. J. Ind. Eng. Chem. 2025, 144, 337–345. [Google Scholar] [CrossRef]
- Raksha, C.H.; Yogeesh, M.P.; Shetty, N.S. Recent advances in the synthesis of polymer supported catalysts: A review. Discov. Appl. Sci. 2025, 7, 565. [Google Scholar] [CrossRef]
- Bouzayani, B.; Sanromán, M.Á. Polymer-supported heterogeneous Fenton catalysts for the environmental remediation of wastewater. Molecules 2024, 29, 2188. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Y. Ascorbic acid enhanced CuFe2O4-catalyzed heterogeneous photo-Fenton-like degradation of phenol. J. Environ. Chem. Eng. 2023, 11, 111009. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, D.; Yao, Z.; Jiang, Z. Revealing the enhancing mechanisms of Fe–Cu bimetallic catalysts for the Fenton-like degradation of phenol. Chemosphere 2022, 289, 133195. [Google Scholar] [CrossRef]
- Ke, P.; Zeng, D.; Wang, R.; Cui, J.; Li, X.; Fu, Y. Magnetic carbon microspheres as a reusable catalyst in heterogeneous Fenton system for the efficient degradation of phenol in wastewater. Colloids Surf. A 2022, 638, 128265. [Google Scholar] [CrossRef]
- Ren, J.X.; Chen, S.P.; Li, D.L.; Wang, M.L.; Zhu, J.L.; Zhong, G.J.; Huang, H.D.; Li, Z.M. Hierarchically porous cellulose-based carbon aerogels with N-doped skeletons and encapsulated iron-based catalysts for efficient tetracycline catalytic degradation. Int. J. Biol. Macromol. 2024, 261, 129829. [Google Scholar] [CrossRef]
- Stark, F.W.; Thue, P.S.; Missio, A.L.; Machado, F.M.; Delucis, R.d.A.; Andreazza, R. Cellulose-based aerogels for environmentally sustainable applications: A review of the production, modification, and sorption of environmental contaminants. Polymers 2025, 17, 236. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Q.; Fan, M.J.; Liu, X.; Chen, J.Y. One-pot synthesis of cellulose-based carbon aerogel loaded with TiO2 and g-C3N4 and its photocatalytic degradation of Rhodamine B. Nanomaterials 2024, 14, 1141. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Cai, S.Y.; Srinivasakannan, C.; Xue, G.; Wang, L.; Wang, Y.P.; Duan, X.H. In-situ growth of metal–organic framework on ionic cross-linking cellulose aerogel for adsorption and Fenton degradation of tetracycline. Sep. Purif. Technol. 2025, 366, 132707. [Google Scholar] [CrossRef]
- Xie, L.C.; Zhang, Z.C.; He, Y.C.; Jiang, Y. Preparation of polyvinyl alcohol-chitosan nanocellulose-biochar nanosilver composite hydrogel and its antibacterial property and dye removal capacity. Processes 2024, 12, 2277. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, J.W.; Xia, J.Y.; Zhao, G.T.; Yang, Y.D.; Zhang, Z.W.; Li, J.; Wei, F.; Song, W.G. Biomass cellulose-derived carbon aerogel supported magnetite-copper bimetallic heterogeneous Fenton-like catalyst towards the boosting redox cycle of ≡Fe(III)/≡Fe(II). Nanomaterials 2025, 15, 614. [Google Scholar] [CrossRef]
- Zhong, J.L.; Mao, X.F.; Wang, G.Y.; Li, H.; Li, J.F.; Qu, S.J.; Zhao, J.W. Synthesis of Cu/Mn/Ce polymetallic oxide catalysts and catalytic ozone treatment of wastewater. RSC Adv. 2024, 14, 35993–36004. [Google Scholar] [CrossRef]
- Zhu, J.L.; Wang, M.L.; Shi, S.C.; Ren, J.X.; Huang, H.D.; Lin, W.; Li, Z.M. In-situ constructing robust cellulose nanocomposite hydrogel network with well-dispersed dual catalysts for the efficient, stable and recyclable photo-Fenton degradation. Cellulose 2022, 29, 1929–1942. [Google Scholar] [CrossRef]
- Lopinski, G.P.; Kodra, O.; Kunc, F.; Kennedy, D.C.; Couillard, M.; Johnston, L.J. X-ray photoelectron spectroscopy of metal oxide nanoparticles: Chemical composition, oxidation state and functional group content. Nanoscale Adv. 2025, 7, 1671–1685. [Google Scholar] [CrossRef]
- Yang, F.; Hao, D.D.; Wu, M.M.; Fu, B.; Zhang, X.F. Amino-functionalized metal-organic framework-mediated cellulose aerogels for efficient Cr(VI) reduction. Polymers 2024, 16, 3162. [Google Scholar] [CrossRef] [PubMed]
- Camparotto, N.G.; Neves, T.d.F.; Mastelaro, V.R.; Prediger, P. Hydrophobization of aerogels based on chitosan, nanocellulose and tannic acid: Improvements on the aerogel features and the adsorption of contaminants in water. Environ. Res. 2023, 220, 115197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Chen, S.P.; Lin, W.; Huang, H.D.; Li, Z.M. Cellulose mineralization with in-situ synthesized amorphous titanium dioxide for enhanced adsorption and auto-accelerating photocatalysis on water pollutant. Chem. Eng. J. 2023, 456, 141036. [Google Scholar] [CrossRef]
- Zhu, R.L.; Zhu, Y.P.; Xian, H.Y.; Yan, L.X.; Fu, H.Y.; Zhu, G.Q.; Xi, Y.F.; Zhu, J.X.; He, H.P. CNTs/ferrihydrite as a highly efficient heterogeneous Fenton catalyst for the degradation of bisphenol A: The important role of CNTs in accelerating Fe(III)/Fe(II) cycling. Appl. Catal. B Environ. 2020, 270, 118891. [Google Scholar] [CrossRef]
- Li, G.Y.; Liu, W.F.; Gao, S.J.; Lu, H.Y.; Fu, D.J.; Wang, M.L.; Liu, X.G. MXene-based composite aerogels with bifunctional ferrous ions for the efficient degradation of phenol from wastewater. Chemosphere 2024, 358, 142151. [Google Scholar] [CrossRef]
- Li, J.W.; Wei, Y.F.; Liu, Q.; Guan, H.H.; Jiang, C.C.; Sun, X.H. Heterogeneous Fenton-like CuO-CoOx/SBA-15 catalyst for organic pollutant degradation: Synthesis, performance, and mechanism. Front. Chem. 2025, 13, 1552002. [Google Scholar] [CrossRef]
- Melnikova, N.; Malygina, D.; Korokin, V.; Al-Azzawi, H.; Zdorova, D.; Mokshin, E.; Liyaskina, E.; Kurgaeva, I.; Revin, V. Synthesis of cerium oxide nanoparticles in a bacterial nanocellulose matrix and the study of their oxidizing and reducing properties. Molecules 2023, 28, 2604. [Google Scholar] [CrossRef]
- Guan, Z.L.; Wang, Y.D.; Wang, Z.; Hong, Y.; Liu, S.L.; Luo, H.W.; Liu, X.L.; Su, B.L. The synthesis, characteristics, and application of hierarchical porous materials in carbon dioxide reduction reactions. Catalysts 2024, 14, 936. [Google Scholar] [CrossRef]
- Zhao, J.X.; Yuan, X.S.; Wu, X.X.; Liu, L.; Guo, H.Y.; Xu, K.M.; Zhang, L.P.; Du, G.B. Preparation of nanocellulose-based aerogel and its research progress in wastewater treatment. Molecules 2023, 28, 3541. [Google Scholar] [CrossRef]
- Kuang, J.; Cai, T.M.; Dai, J.B.; Yao, L.H.; Liu, F.F.; Liu, Y.; Shu, J.C.; Fan, J.P.; Peng, H.L. High strength chitin/chitosan-based aerogel with 3D hierarchically macro-meso-microporous structure for high-efficiency adsorption of Cu(II) ions and Congo red. Int. J. Biol. Macromol. 2023, 230, 123238. [Google Scholar] [CrossRef]
- Mao, Y.B.; Gupta, S.K. Metal oxide nanomaterials: From fundamentals to applications. Nanomaterials 2022, 12, 4340. [Google Scholar] [CrossRef]
- Petrinic, I.; Stergar, J.; Bukšek, H.; Drofenik, M.; Gyergyek, S.; Hélix-Nielsen, C.; Ban, I. Superparamagnetic Fe3O4@CA nanoparticles and their potential as draw solution agents in forward osmosis. Nanomaterials 2021, 11, 2965. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Sharma, M.; Sahu, N.K. Assessing magnetic and inductive thermal properties of various surfactants functionalised Fe3O4 nanoparticles for hyperthermia. Sci. Rep. 2020, 10, 15045. [Google Scholar] [CrossRef] [PubMed]
- Alani, O.A.; Ari, H.A.; Alani, S.O.; Offiong, N.-A.O.; Feng, W. Visible-light-driven bio-templated magnetic copper oxide composite for heterogeneous photo-Fenton degradation of tetracycline. Water 2021, 13, 1918. [Google Scholar] [CrossRef]
- Sabzi Dizajyekan, B.; Jafari, A.; Vafaie-Sefti, M.; Saber, R.; Fakhroueian, Z. Preparation of stable colloidal dispersion of surface modified Fe3O4 nanoparticles for magnetic heating applications. Sci. Rep. 2024, 14, 1296. [Google Scholar] [CrossRef]
- Liu, M.Y.; Ye, Y.Y.; Ye, J.M.; Gao, T.; Wang, D.H.; Chen, G.; Song, Z.J. Recent advances of magnetite (Fe3O4)-based magnetic materials in catalytic applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Xu, J.C.; Su, H.; Kumar, R.; Luo, S.S.; Nie, Z.Y.; Wang, A.; Du, F.; Li, R.; Smidman, M.; Yuan, H.Q. Ce-site dilution in the ferromagnetic kondo lattice CeRh6Ge4. Chin. Phys. Lett. 2021, 38, 087101. [Google Scholar] [CrossRef]
- Horsley, E.; Rao, X.; Yi, S.B.; Kim, Y.J. Magnetic dilution of a honeycomb lattice XY magnet CoTiO3. J. Phys. Condens. Mat. 2022, 34, 135803. [Google Scholar] [CrossRef]
- Allahkarami, E.; Dehghan Monfared, A.; Silva, L.F.O.; Dotto, G.L. Lead ferrite-activated carbon magnetic composite for efficient removal of phenol from aqueous solutions: Synthesis, characterization, and adsorption studies. Sci. Rep. 2022, 12, 10718. [Google Scholar] [CrossRef]
- Si, R.R.; Pu, J.W.; Luo, H.G.; Wu, C.J.; Duan, G.G. Nanocellulose-based adsorbents for heavy metal ion. Polymers 2022, 14, 5479. [Google Scholar] [CrossRef]
- Wang, B.X.; Xu, B.B.; Chen, G.F.; Wang, C.Z.; Liu, Y.; Bai, Y.; Li, M.G.; Peng, L.G. Preparation of polypyrrole/montmorillonite/polypropylene composite membranes and investigation of their adsorption performance for methyl orange and Pb2+. Polymers 2025, 17, 1158. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.Y.; Wang, S.J.; Lv, H.F.; Wang, Z.X.; Han, X.W.; Zhou, Z.J.; Pu, J.W. Porous cellulose nanofiber (CNF)-based aerogel with the loading of zeolitic imidazolate frameworks-8 (ZIF-8) for Cu (II) removal from wastewater. BioResources 2022, 17, 2615–2631. [Google Scholar] [CrossRef]
- Hasani, N.; Selimi, T.; Mele, A.; Thaçi, V.; Halili, J.; Berisha, A.; Sadiku, M. Theoretical, equilibrium, kinetics and thermodynamic investigations of methylene blue adsorption onto lignite coal. Molecules 2022, 27, 1856. [Google Scholar] [CrossRef]
- Liu, P.P.; Zheng, C.L.; Yao, Z.; Zhang, F. A biomimetic lignocellulose aerogel-based membrane for efficient phenol extraction from water. Gels 2024, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Ehiomogue, P.; Ahuchaogu, I.I.; Ahaneku, I.E. Review of adsorption isotherms models. Acta Tech. Corviniensis-Bull. Eng. 2021, 14, 87–96. [Google Scholar]
- Dal, M.C. Modeling of the linear equations of langmuir isotherm in the adsorption of Cd (II) Ion with siirt kurtalan koçpinar clay. Türk Doğa ve Fen Dergisi 2024, 13, 67–72. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, C.G.; Park, S.J. Adsorption of phenol on kenaf-derived biochar: Studies on physicochemical and adsorption characteristics and mechanism. Biomass Convers. Biorefin. 2024, 14, 9621–9638. [Google Scholar] [CrossRef]
- Dehmani, Y.; Sellaoui, L.; Alghamdi, Y.; Lainé, J.; Badawi, M.; Amhoud, A.; Bonilla-Petriciolet, A.; Lamhasni, T.; Abouarnadasse, S. Kinetic, thermodynamic and mechanism study of the adsorption of phenol on Moroccan clay. J. Mol. Liq. 2020, 312, 113383. [Google Scholar] [CrossRef]
- Zhang, J.C.; Qin, L.; Yang, Y.Z.; Liu, X.G. Porous carbon nanospheres aerogel based molecularly imprinted polymer for efficient phenol adsorption and removal from wastewater. Sep. Purif. Technol. 2021, 274, 119029. [Google Scholar] [CrossRef]
- Salvestrini, S.; Fenti, A.; Chianese, S.; Iovino, P.; Musmarra, D. Diclofenac sorption from synthetic water: Kinetic and thermodynamic analysis. J. Environ. Chem. Eng. 2020, 8, 104105. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, isotherms, and thermodynamic modeling of the adsorption of phosphates from model wastewater using recycled brick waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Xu, Y.L.; Peng, Q.; Yu, Y.L.; Li, Z.F.; Liu, Y.M.; Qin, Z.J.; Fan, X.F. Facile electrophoretically deposited of iron/carbon nanotube-based electro-Fenton cathode for high-performance organics degradation. J. Environ. Chem. Eng. 2025, 13, 115127. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Galal, A.M.; Abd El-Monaem, E.M.; Al Harby, N.; Batouti, M.E. Enhanced Fenton degradation of tetracycline over cerium-doped MIL88-A/g-C3N4: Catalytic performance and mechanism. Nanomaterials 2024, 14, 1282. [Google Scholar] [CrossRef]
- Peera, S.G.; Kim, S.W. Rare earth Ce/CeO2 electrocatalysts: Role of high electronic spin state of Ce and Ce3+/Ce4+ redox couple on oxygen reduction reaction. Nanomaterials 2025, 15, 600. [Google Scholar] [CrossRef]
- Wei, J.H.; Yuan, M.; Wang, S.T.; Wang, X.H.; An, N.; Lv, G.P.; Wu, L.N. Recent advances in metal organic frameworks for the catalytic degradation of organic pollutants. Collagen Leather 2023, 5, 33. [Google Scholar] [CrossRef]
- Zhang, H.N.; Tian, L.H.; Zhang, Z.Q.; Han, J.P.; Wu, Z.G.; Wei, Z.Q.; Wang, S.F.; Cao, Y.; Zhang, S.; Zhang, Y. Effective degradation of phenol by in-situ photocatalytic-Fenton-like technology with BiVO4/Bi2WO6/Ti3C2 QDs. Surf. Interf. 2024, 55, 105315. [Google Scholar] [CrossRef]
- Yang, Z.C.; Yin, Y.Y.; Liang, M.Y.; Fu, W.Y.; Zhang, J.H.; Liu, F.Z.; Zhang, W.; Pan, B.C. Incidental iron oxide nanoclusters drive confined Fenton-like detoxification of solid wastes towards sustainable resource recovery. Nat. Commun. 2025, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.Z.; Yin, H.Q.; Wang, R.J.; Wang, J.N.; Li, A.M. Novel Fenton-like catalyst HKUST-1(Cu)/MoS2-3-C with non-equilibrium-state surface for selective degradation of phenolic contaminants: Synergistic effects of σ-Cu-ligand and ≡Mo–OOSO3− complex. Water 2024, 16, 121. [Google Scholar] [CrossRef]
- Gu, S.J.; Cui, J.Y.; Liu, F.Q.; Chen, J.Y. Biochar loaded with cobalt ferrate activated persulfate to degrade naphthalene. RSC Adv. 2023, 13, 5283–5292. [Google Scholar] [CrossRef]
- Xin, L.; Hu, J.W.; Xiang, Y.Q.; Li, C.F.; Fu, L.Y.; Li, Q.H.; Wei, X.H. Carbon-based nanocomposites as Fenton-like catalysts in wastewater treatment applications: A review. Materials 2021, 14, 2643. [Google Scholar] [CrossRef]
- Ye, F.; Jin, X.; Chen, Z. Biomass-derived in-situ carbonized ferrous sulfide for Fenton oxidation of phenol in wastewater: Mechanisms, pathways, and applications. J. Colloid Interface Sci. 2025, 691, 137391. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Lee, J.; Xing, L. A review of application mechanism and research progress of Fe/montmorillonite-based catalysts in heterogeneous Fenton reactions. J. Environ. Chem. Eng. 2024, 12, 112152. [Google Scholar] [CrossRef]
- Yang, R.S.; Liu, Q.; Liu, Z.; Wang, J.X.; Zhang, A.N.; Liu, Y.J. Insights into the Fenton-like degradation efficiency and mechanism of phenolic pollutants by Cu-doped sludge-based biochar catalyst. Chem. Eng. Sci. 2025, 317, 122110. [Google Scholar] [CrossRef]
- Tsuneda, T. Fenton reaction mechanism generating no OH radicals in Nafion membrane decomposition. Sci. Rep. 2020, 10, 18144. [Google Scholar] [CrossRef]
- Iii, R.C.; Lujan, B.; Martinez, A.; Manasi, R.; DeBow, J.D.; Kou, K.G.M. A Fenton approach to aromatic radical cations and diarylmethane synthesis. J. Org. Chem. 2023, 88, 15060–15066. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, Y.Q.; Wang, C.; Si, Y.; Wang, Y.J.; Xia, W.Q.; Liu, T.; Cao, X.; Guo, Z.Y.; Chen, J.J. Nanoconfinement steers nonradical pathway transition in single atom Fenton-like catalysis for improving oxidant utilization. Nat. Commun. 2024, 15, 5314. [Google Scholar] [CrossRef]
- Gonfa, M.T.; Shen, S.; Chen, L.; Yin, S.F. Selective hydroxylation of benzene via enhanced generation and utilization of hydroxyl radicals with CuZnSbO photocatalyst. Colloid. Surf. A 2025, 706, 135746. [Google Scholar] [CrossRef]
- Walling, S.A.; Um, W.; Corkhill, C.L.; Hyatt, N.C. Fenton and Fenton-like wet oxidation for degradation and destruction of organic radioactive wastes. npj Mater. Degrad. 2021, 5, 50. [Google Scholar] [CrossRef]
- Cheng, P.; Mailhot, G.; Sarakha, M.; Voyard, G.; Scheres Firak, D.; Schaefer, T.; Herrmann, H.; Brigante, M. The effect of amino acids on the Fenton and photo-Fenton reactions in cloud water: Unraveling the dual role of glutamic acid. EGUsphere 2025, 2025, 1–31. [Google Scholar] [CrossRef]
- Wang, X.P.; Liu, W.; Qin, J.Y.; Lei, L.C. Improvement of H2O2 utilization by the persistent heterogeneous Fenton reaction with the Fe3O4-zeolite-cyclodextrin composite. Ind. Eng. Chem. Res. 2020, 59, 2192–2202. [Google Scholar] [CrossRef]
- Pourshaban-Mazandarani, M.; Ahmadian, M.; Nasiri, A.; Poormohammadi, A. CuCoFe2O4@ AC magnetic nanocomposite as a novel heterogeneous Fenton-like nanocatalyst for Ciprofloxacin degradation from aqueous solutions. Appl. Water Sci. 2023, 13, 179. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, J.J.; Wang, L.L.; Wang, C.; Chen, L.; Chen, X.Q.; Qian, J.S.; Pan, B.C. Nanoconfinement-triggered oligomerization pathway for efficient removal of phenolic pollutants via a Fenton-like reaction. Nat. Commun. 2024, 15, 917. [Google Scholar] [CrossRef]
- Peng, L.J.; Duan, X.G.; Shang, Y.N.; Gao, B.Y.; Xu, X. Engineered carbon supported single iron atom sites and iron clusters from Fe-rich Enteromorpha for Fenton-like reactions via nonradical pathways. Appl. Catal. B Environ. 2021, 287, 119963. [Google Scholar] [CrossRef]
- Ma, J.Q.; Xu, L.L.; Shen, C.S.; Hu, C.; Liu, W.P.; Wen, Y.Z. Fe-N-graphene wrapped Al2O3/Pentlandite from microalgae: High Fenton catalytic efficiency from enhanced Fe3+ reduction. Environ. Sci. Technol. 2018, 52, 3608–3614. [Google Scholar] [CrossRef]
- Zhang, N.; Yi, Y.; Lian, J.; Fang, Z. Effects of Ce doping on the Fenton-like reactivity of Cu-based catalyst to the fluconazole. Chem. Eng. J. 2020, 395, 124897. [Google Scholar] [CrossRef]




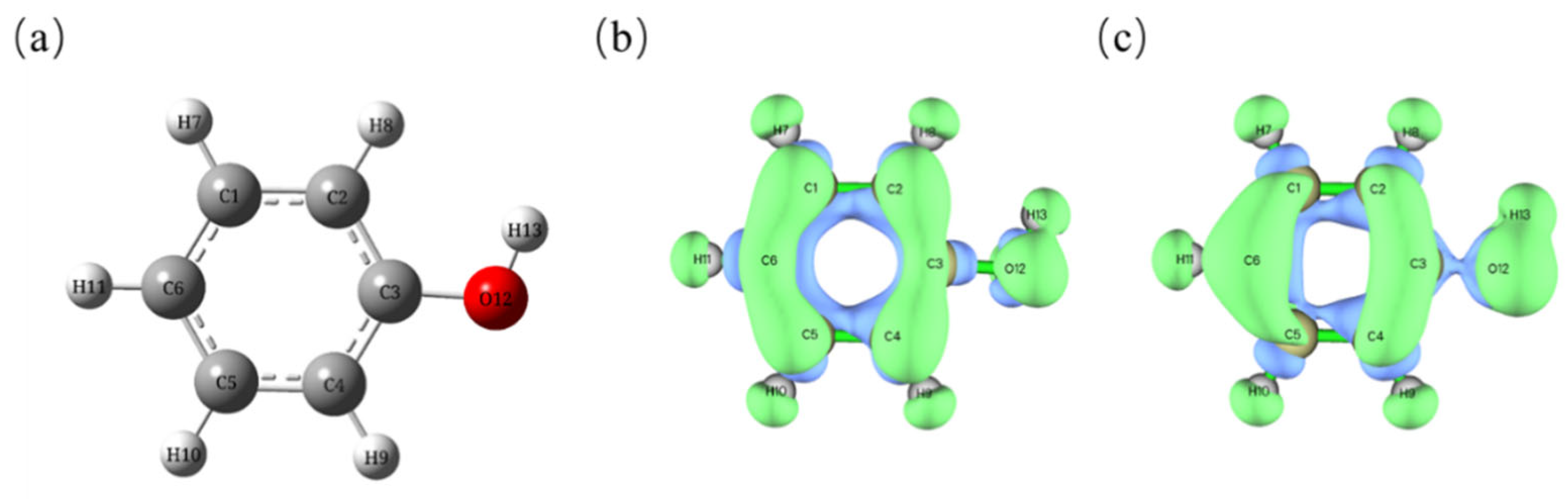

| Atom | q(N) | q(N + 1) | q(N − 1) | f− | f+ | f0 | Δf |
|---|---|---|---|---|---|---|---|
| 1(C) | −0.0392 | −0.1684 | 0.0357 | 0.075 | 0.1292 | 0.1021 | 0.0542 |
| 2(C) | −0.0709 | −0.1917 | 0.0116 | 0.0825 | 0.1207 | 0.1016 | 0.0382 |
| 3(C) | 0.0738 | 0.0212 | 0.1736 | 0.0998 | 0.0526 | 0.0762 | −0.0472 |
| 4(C) | −0.0578 | −0.1832 | 0.0333 | 0.0911 | 0.1254 | 0.1083 | 0.0343 |
| 5(C) | −0.0367 | −0.1632 | 0.0295 | 0.0661 | 0.1265 | 0.0963 | 0.0604 |
| 6(C) | −0.0565 | −0.1176 | 0.0875 | 0.144 | 0.0611 | 0.1026 | −0.083 |
| 7(H) | 0.0417 | −0.0254 | 0.0888 | 0.0471 | 0.0671 | 0.0571 | 0.02 |
| 8(H) | 0.0359 | −0.0278 | 0.0828 | 0.0469 | 0.0637 | 0.0553 | 0.0168 |
| 9(H) | 0.0445 | −0.0208 | 0.0928 | 0.0483 | 0.0653 | 0.0568 | 0.0169 |
| 10(H) | 0.0423 | −0.0238 | 0.0879 | 0.0456 | 0.0661 | 0.0558 | 0.0205 |
| 11(H) | 0.0386 | −0.0077 | 0.0973 | 0.0587 | 0.0463 | 0.0525 | −0.0124 |
| 12(O) | −0.1886 | −0.2335 | −0.0451 | 0.1435 | 0.0448 | 0.0942 | −0.0987 |
| 13(H) | 0.1728 | 0.1418 | 0.2241 | 0.0512 | 0.0311 | 0.0412 | −0.0202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Chen, J. Nanocellulose-Based Carbon Aerogel Loaded with Composite Metal Oxides and Its Fenton Catalytic Oxidation Degradation of Phenol. Nanomaterials 2025, 15, 1292. https://doi.org/10.3390/nano15161292
Gao Y, Chen J. Nanocellulose-Based Carbon Aerogel Loaded with Composite Metal Oxides and Its Fenton Catalytic Oxidation Degradation of Phenol. Nanomaterials. 2025; 15(16):1292. https://doi.org/10.3390/nano15161292
Chicago/Turabian StyleGao, Yunpeng, and Jinyang Chen. 2025. "Nanocellulose-Based Carbon Aerogel Loaded with Composite Metal Oxides and Its Fenton Catalytic Oxidation Degradation of Phenol" Nanomaterials 15, no. 16: 1292. https://doi.org/10.3390/nano15161292
APA StyleGao, Y., & Chen, J. (2025). Nanocellulose-Based Carbon Aerogel Loaded with Composite Metal Oxides and Its Fenton Catalytic Oxidation Degradation of Phenol. Nanomaterials, 15(16), 1292. https://doi.org/10.3390/nano15161292







