Immunomodulatory Effects of Gold Nanoparticles: Impacts on Immune Cells and Mechanisms of Action
Abstract
1. Introduction
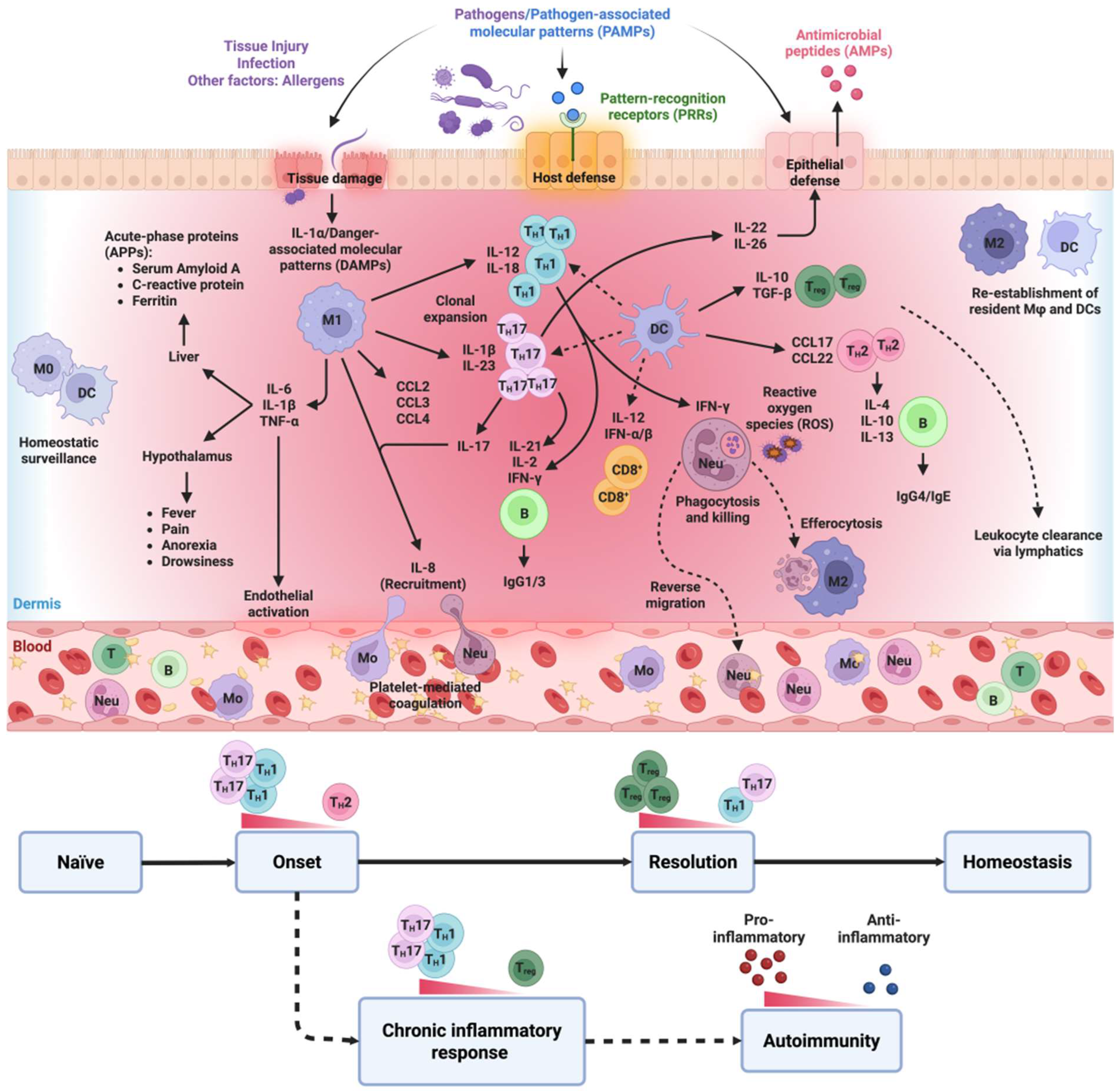
2. The Inflammatory Process
2.1. Acute Inflammatory Response
2.2. Chronic Inflammation
2.3. Role of the Immune System in Inflammatory Responses
3. Current Clinical Approaches to Inflammatory Diseases
Nanotechnology: Definition and Diverse Applications
4. Gold Nanoparticles (AuNPs): Characterization and Multifaceted Applications
5. Interactions Between AuNPs and Immune Cells: A Focus on Immunomodulation
5.1. Influence of Size and Dosage
5.2. Impact of Shape
5.3. Significance of Surface Functionalization
6. Anti-Inflammatory Effects of AuNPs on Innate Immune Cells
6.1. Neutrophils
6.2. Monocytes/Macrophages
6.3. Dendritic Cells
6.3.1. Impact of Surface Functionalization on DC Function
6.3.2. Influence of Size, Dosage, and Time of Exposure of AuNPs on DCs
6.4. Mast Cells
| Immune Cells | AuNPs Properties | Model/Cell Line | Mechanism of Action | Main Effect | Ref. | |
|---|---|---|---|---|---|---|
| Inflammatory | Anti-Inflammatory | |||||
| Neutrophils | 15–50 nm | Human PBMCs | - Trigger NETosis - ↓Inflammation | ✔ | [152] | |
| 1–10 nm | Human PBMCs and CD-1 mice | - ↑Proapoptotic effect via IL-8 and GRO-α - ↑IL-6 secretion after AuNP (−) vs. AuNP (+) exposure - ↑Neutrophil activation | ✔ (Positive charge) | ✔ (Negative charge) | [156] | |
| 20 and 70 nm | Human PBMCs | - ↑ER stress and trigger apoptosis | ✔ | [155] | ||
| Monocytes/Macrophages | 10 nm (serum albumin coated) | Splenic macrophages and TAMs | - ↑NADPH oxidase, ROS generation - ↓TNF-α and IL-10; increase IL-12 - Polarize TAMs (M2→M1) | ✔ | [182] | |
| 5, 15, 20, and 35 nm | C57BL6 mice/THP-1 | - ↓NF-κβ and IL-1β-driven inflammation - ↓TNF-α and HIF-1α | ✔ (small sizes) | [123] | ||
| nm | CCl4-injured rat liver/RAW 264.7 | - Modulating IL-10 secretion - ↓AST and ALT levels | ✔ | [124] | ||
| 50 nm | RAW 264.7 | - ↓LPS-triggered IL-17A, TNF-α, IL-1β - ↓ROS | ✔ | [122] | ||
| 10 and 15 nm | RAW 264.7 | - ↓NF-κβ and STAT1 activation in LPS-stimulated cells - ↓iNOS mRNA expression and NO production | ✔ | [183] | ||
| Up to 5 nm | Mice splenocytes | - 2.5 ppm—↓ IL-1β, IL-6, TNF-α; ↑ IL-2 in macrophages - 0.25 and 25 ppm—↑ IL-1β, IL-6, TNF-α; ↓ IL-2 | ✔ | ✔ | [125] | |
| 20 nm | RAW 264.7 | - ↓ NF-κβ activation by modulating the ERK1/2MAPK/Akt/tuberin-mTOR pathways - ↓IL-1β, CCL-8, CX3CL-1, CX3CL-10, ICAM, MMP-2/9, TNF-α expression (dose-dependent) | ✔ | [127] | ||
| 2.81, 5.52, and 38.05 nm | J774 A1 | - ↑IL-1β, IL-6, and TNF-α expression (small > large) | ✔ | [103] | ||
| 10–300 nm | RAW 264.7 | - ~On IL-6, IL-10, and TNF-α | ~ | ~ | [175] | |
| 30–40 nm | Rat hepatocytes and Kupffer cells | - ↑In IL-10 secretion - Controlled IL-6 secretion - ↓In TNF-α secretion | ✔ | [184] | ||
| 20 and 40 nm | RAW 264.7 | - ~On the induction of NF-κβ, IL-6 release, or ROS generation | ~ | ~ | [185] | |
| 60 nm | RAW 264.7 | - Not cytotoxic, nor elicit pro-inflammatory responses and oxidative stress - No additive or synergistic effects on LPS-induced TNF-α and IL-6 production - ~Intracellular ROS | ~ | ~ | [121] | |
| 20–50 nm | Primary human monocytes | - ↓Proportion of CD14+/CD16+ monocytes - Significantly ↓CD86 expression - ↓HLA-DR expression - ↓LPS-induced p40 subunit of IL-12 and IL-23 - ↓TNF-α cytokine production | ✔ | [186] | ||
| Up to 5 nm | Mice splenocytes | - <2.5 ppm: ↑IL-1β; ↓IL-2 and TNF-α - 2.5–5 ppm: No effect - 10 ppm: ↑IL-6 secretion; ↓IL-1β and TNF-α | ✔ | [126] | ||
| 5, 10, 15, 30, and 60 nm | RAW 264.7 | - ↓NF-κβ activation via TLR4 signal dampening - ↓IL-6 and TNF-α expression - ↓LPS-triggered iNOS expression, NO production | ✔ | [187] | ||
| 5, 13, 45 nm | RAW 264.7 | - ↓LPS-induced M1-related factors (CD86, iNOS, IL-6 and TNF- α) - ↑expression of M2-related factors (Arg1, CD206, IL-10, TGF-β) | ✔ | [114] | ||
| 4, 11, 19, 35, or 45 nm | RAW 264.7 | - ↓HMGB-1 and TLR9 binding and suppression of TLR-9 activation - ↓CpG-ODN induced TNF-α production | ✔ | [188] | ||
| 3, 11, 16, 30, and 40 nm | RAW 264.7; SV40; SVEC4-10; C3H10T1/2 | - 11 nm AuNPs ↓CCL5 secretion; 16 nm AuNPs increased CCL5 secretion - ↑TNF-α secretion - ~IL-10 secretion - ~IL-6 levels | ✔ (Small > large) | [189] | ||
| DCs | Unmodified AuNPs of 10 and 50 nm | Human monocyte-derived DCs | - 10 nm AuNPs ↓CD83, CD86, and IL-12p70 in LPS-stimulated cells | ✔ | [88] | |
| 2 and 12 nm | Human monocyte-derived DCs | - 2 nm: mild Immunosuppression - 12 nm: ↑inflammatory and cytotoxic response | ✔ (12 nm) | ✔ (2 nm) | [176] | |
| PVA-NH2 and PEG-CO2H AuNPs | Human monocyte-derived DCs | - No effect on cytokine secretion by human DCs | ✔ | [165] | ||
| 2 and 12 nm | Human monocyte-derived DCs, PBMCs | - 2 nm: ~DC maturation and lymphocyte proliferation and ↑tolerogenicity - 12 nm: ↑DC maturation | ✔ (12 nm) | ✔ (2 nm) | [166] | |
| PVA-coated AuNPs functionalized with DC-SIGN | 3D co-culture (epithelial cells, DCs, and macrophages) | - ↑DCs’ uptake efficiency - ~IL-10 | ~ | ~ | [170] | |
| 2–3 nm DC-SIGN-conjugated AuNPs (N-α-fucosyl-β-alanyl amide) | Human monocyte-derived DCs | - ↑DC-SIGN internalization - ~IL-10 | ~ | ~ | [168] | |
| Mast cells | 25.8 nm | RBL-2H3 | - Modulate IgE–FcεRI receptor cross-linking | ✔ | [178] | |
| Citrated-AuNPs | Murine peritoneal mast cells | - ↓Granule exocytosis | ✔ | [179,180] | ||
| Citrated-AuNPs | HMC-1 | - ↓ROS and TNF-α production - Modulate NF-κβ and MAPKs signaling pathways | ✔ | [181] | ||
7. Anti-Inflammatory Effects of AuNPs on Adaptive Immune Cells
7.1. T Lymphocytes
7.1.1. Impact of Surface Functionalization on T Cells
7.1.2. Effect of AuNP Size on T Cells
7.2. B Lymphocytes
8. Unraveling the Mechanisms Underlying Anti-Inflammatory Effects of AuNPs
8.1. Modulation of the NF-κB Pathway
8.2. Modulation of MAPK and PI3K Pathways
8.3. IL-1β Trapping
8.4. Interference with TLR Signaling
8.5. Other Possible Mechanisms
9. Therapeutic Applications of AuNPs in Inflammatory Diseases
| AuNPs Properties | Model/Cell Line | Mechanism of Actions | Main Effect | Ref. | |
|---|---|---|---|---|---|
| Inflammatory | Anti-Inflammatory | ||||
| 27.3 ± 0.5 nm Low (0.0785 μg/g/day), Medium (0.785 μg/g/day) High (7.85 μg/g/day) | HFD mice model | - ↓TLR4 - ↓TNF-α expression (low and medium) - ↓F4/80 (medium) | ✔ | [206,207] | |
| 6.3 nm | Murine model of atopic asthma | - ↓IL-1, IL-5 and IL-6 levels in BAL - ↓IL-4, IL-5, IL-6, IL-13, eotaxin-1, and eotaxin-2 in lung tissue - ↓Airway inflammatory infiltrates - ↓MDA levels | ✔ | [221] | |
| 20 nm | Rat model of sporadic Alzheimer’s dementia | - ↓In IL-1β and NF-κβ levels - Prevent STZ induced neuroinflammation and oxidative damage by ↑SOD, and GPX activity | ✔ | [222] | |
| 20 and 45 μm | Mice model for brain injury | - ↓Cerebral TNF-α levels, - ↑Oxidative DNA damage and pro-apoptotic markers (cleaved caspase-3, cytochrome c leakage) | ✔ | [223] | |
| 25 nm | Mouse model of EAE | - ↑IL-27 secretion (dose-dependent) - ↓CNS leukocyte infiltration and demyelinated foci - ↓IL-23 | ✔ | [224] | |
| 7.4 ± 2.8 nm | Swiss mice | - 49.3% ↓ in leukocyte migration - ↓IL-1β and TNF-α - ↓Peripheral analgesia and inflammation | ✔ | [195] | |
| 5, 20, and 50 nm | Mice | - 5 nm: delayed ↑in IL-1β and IL-6 mRNA expression (Day 7) in mouse brain - 20 and 50 nm: ~pro-inflammatory cytokines - ~TNF-α expression | ✔ (small sizes) | [113] | |
| 20 nm | SAECs | - ↑Serum amyloid A (SAA) and TLR2, and ↑NF-κβ activation | ✔ | [225] | |
| 15 nm | A549 | - ~mRNA expression of TNF-α, IL-8 and iNOS, or antioxidant (HO-1 and SOD2) markers - ~protein expression (IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, GM-CSF, INF-γ, and TNF-α) | ~ | ~ | [226] |
| 50 and 250 nm | Rat model of pulmonary inflammation | - 250 nm: ↑IL-6 and TNF-α - Agglomerated 50 nm: ↑TNF-α | ✔ Mild inflammatory reaction | [227] | |
| 16–25, 40 (400 μg/kg) | DNBS-colitis mice model | - ↓In the IL-6 and TNF-α levels - ↓CAT, GSH, and SOD levels in the colon | ✔ | [228] | |
| 13 nm | Rat CIA model | - ↓Leukocyte and macrophage infiltration - ↓IL-1β, VEGF, and TNF-α | ✔ | [229] | |
| 35 nm | Rat model of tendinous injury | - ↓IL-1β and TNF-α | ✔ | [117] | |
| 13 or 50 nm | CIA mice model | - 50 nm: ↓inflammatory infiltration (lymphocytes, leukocytes, macrophages) - 13 nm: ↓macrophage and lymphocyte infiltration | ✔ | [115] | |
| 10 nm | Rat Animal model of Tendinitis | - ↓IL-1β and TNF-α levels | ✔ | [230] | |
| 25 nm | Experimental rat model of muscle overuse | - ↓IL-6 and TNF-α - ↓SOD and GPX activity - ↓O2− and NO2− levels | ✔ | [231] | |
| 21.3 ± 0.7 nm (12.5, 25, and 50 ppm) | Sepsis mouse model | - ↓IL-1β, IL-6, and TNF-α (6 h and 24 h) - ↓IL-10 secretion at 6 and 24 h. - ↓M1 polarization (↑CD86+ve ↓iNOS and Nur77) - ↑M2 polarization (↑CD206+ve ↑Arg1 and PPARγ) | ✔ | [161] | |
| 10 nm and 50 nm | Wistar rats | - Liver: 50 nm AuNPs ↑IL-1β, IL-6, and TNF-α gene expression on D1(decrease by D6) - Kidney: ~IL-1β expression - Systemic: 50 nm ↑IL-6 and TNF- α expression on D1 (decrease by D5) | ✔ (Transient inflammatory effects) | [157] | |
| 30 nm | Wistar rats | - Modulate TLR4-NF-κβ pathway - ↓Intraocular inflammation and oxidative damage | ✔ | [232] | |
| 20 nm | Wistar rats | - ↓IL-1β - ↓COX-2, iNOS, NF-κβ, and TNF-α mRNA expression | ✔ | [117] | |
| 25–50 nm | NHDF and NHEK | - ↓IL-6, IL-12, and TNF-α - ↓VEGF and bFGF | ✔ Anti-angiogenic activity | [233] | |
| 10–50 nm | Jurkat and U937 | - ↑TNF-α synthesis - ↓IL-6 and IL-12 | ✔ | [234] | |
| 5.5 nm | HUVEC and VEC | - ↓NF-κβ pathway - ↓TNF-α and ROS production - ↑Degradation of CAM proteins→ reduce monocyte adhesion to | ✔ | [235] | |
| Gold nanorods (50 × 15 nm) Stabilized with surface-coupled peptides | Human primary reticuloendothelial cells | - ↑CCL2, CCL3, and CCL4 (macrophages) PEG-OH capped particles ↑chemokine secretion (DCs) - ↑CXCL9 (Macrophages), and inhibit in DC - GLF modification ↑IL-1β, - RGD modification ↓IL-1β - ↑IL-6 (DCs only) - ↑TNF-α (macrophages) by peptide-bound AuNPs - ~TNF-α (DCs) | ✔ (AuNP-GLF) | ✔ (AuNP-RGD) | [148] |
10. Mechanisms of AuNP Cytotoxicity
10.1. AuNP Toxicity Is Mediated by ROS
10.2. Effect of AuNP Size on ROS Production
10.3. Protein Corona Significantly Modulates AuNP Cytotoxicity
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGIP | Amyloid growth inhibitory peptide |
| AgNPs | Silver nanoparticles |
| ALI | Acute lung injury |
| ALT | Alanine transaminase |
| APCs | Antigen-presenting cells |
| APPs | Acute-phase proteins |
| APR | Acute phase response |
| ARDS | Acute respiratory distress syndrome |
| Arg1 | Arginase 1 |
| AST | Aspartate transaminase |
| AuNPs | Gold nanoparticles |
| BBB | Blood–brain barrier |
| BMDCs | Bone-marrow-derived DCs |
| CIA | Collagen-induced arthritis |
| COX-2 | Cyclooxygenase-2 |
| CpG | Cytosine–phosphate–guanosine oligodeoxynucleotides |
| CTAB | Cetyltrimethylammonium bromide |
| DCs | Dendritic cells |
| ER-stress | Endoplasmic reticulum stress |
| GM-CSF | Granulocyte macrophage-colony stimulating factor |
| HFD | High-fat diet |
| HIF-1α | Hypoxia-inducible factor-1alpha |
| HMGB-1 | High mobility group box 1 |
| IBD | Inflammatory bowel disease |
| ICAM | Intercellular adhesion molecule |
| IFNs | Interferons |
| IκKβ | Ikappa B kinase-beta |
| IL | Interleukin |
| ILCs | innate lymphoid cells |
| IL-R | Interleukin receptor |
| iNOS | Inducible nitric oxide synthase |
| JAK | Janus kinase |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemotactic protein-1 |
| MDA | Malondialdehyde |
| MMP-2/9 | Matrix metalloproteinase-2/9 |
| MQs | Macrophages |
| MUC1 | Mucin 1 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NETs | Neutrophil extracellular traps |
| NF-κB | Nuclear factor-kappa B |
| NK | Natural killer |
| nm | Nanometers |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NPs | Nanoparticles |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OVA | Ovalbumin |
| PDDAC | Poly-diallyl dimethylammonium chloride |
| PEG | Polyethylene glycol |
| PEI | Polyethyleneimine |
| PMA | Phorbol myristate acetate |
| PRRs | Pattern recognition receptors |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| RA | Rheumatoid arthritis |
| ROS | Reactive oxygen species |
| SAP | Sweet arrow peptide |
| SLE | Systemic lupus erythematosus |
| SPR | Surface plasmon resonance |
| STAT | Signal transducer and activator of transcription |
| TA | Tannic acid |
| TAMs | Tumor-associated macrophages |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-alpha |
| TrxR | Thioredoxin reductase |
| UC | Ulcerative colitis |
| VLPs | Virus-like particles |
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Henson, P.M. Dampening inflammation. Nat. Immunol. 2005, 6, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Smith, T.; Affram, K.; Nottingham, E.L.; Han, B.; Amissah, F.; Krishnan, S.; Trevino, J.; Agyare, E. Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer. Sci. Rep. 2020, 10, 16989. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, R.; Raza, A.; Liaquat, A.; Shah, S.U.; Saeed, S.; Sarwar, U.; Hamza, M.; Chudhary, F.; Hussain, Z.; Butt, N.M. Nanoparticles as a Novel Tool to Inhibit Inflammatory Cytokines in Human Lymphocytes and Macrophages of Coronary Artery Disease. J. Pharm. Sci. 2022, 111, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Eifler, A.C.; Thaxton, C.S. Nanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case study. In Biomedical Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 325–338. [Google Scholar]
- Shukla, S.; Fletcher, S.; Chauhan, J.; Chalfant, V.; Riveros, C.; Mackeyev, Y.; Singh, P.K.; Krishnan, S.; Osumi, T.; Balaji, K.C. 3JC48-3 (methyl 4′-methyl-5-(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)-[1,1′-biphenyl]-3-carboxylate): A novel MYC/MAX dimerization inhibitor reduces prostate cancer growth. Cancer Gene Ther. 2022, 29, 1550–1557. [Google Scholar] [CrossRef]
- Taylor, M.L.; Wilson, R.E., Jr.; Amrhein, K.D.; Huang, X. Gold Nanorod-Assisted Photothermal Therapy and Improvement Strategies. Bioengineering 2022, 9, 200. [Google Scholar] [CrossRef]
- Sengupta, A.; Azharuddin, M.; Al-Otaibi, N.; Hinkula, J. Efficacy and Immune Response Elicited by Gold Nanoparticle- Based Nanovaccines against Infectious Diseases. Vaccines 2022, 10, 505. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Ruiz, N.A.L.; Del Angel, D.S.; Brizuela, N.O.; Peraza, A.V.; Olguin, H.J.; Soto, M.P.; Guzman, D.C. Inflammatory Process and Immune System in Major Depressive Disorder. Int. J. Neuropsychopharmacol. 2022, 25, 46–53. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Serbina, N.V.; Jia, T.; Hohl, T.M.; Pamer, E.G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008, 26, 421–452. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef]
- Caso, F.; Costa, L.; Nucera, V.; Barilaro, G.; Masala, I.F.; Talotta, R.; Caso, P.; Scarpa, R.; Sarzi-Puttini, P.; Atzeni, F. From autoinflammation to autoimmunity: Old and recent findings. Clin. Rheumatol. 2018, 37, 2305–2321. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Joosten, L.A.B.; Netea, M.G. The Potential Role of Trained Immunity in Autoimmune and Autoinflammatory Disorders. Front. Immunol. 2018, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Municio, C.; Criado, G. Therapies Targeting Trained Immune Cells in Inflammatory and Autoimmune Diseases. Front. Immunol. 2020, 11, 631743. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.C.; Rios, M.; Fernandez-Fierro, A.; Di Genaro, M.S.; Kalergis, A.M. Trained Immunity Contribution to Autoimmune and Inflammatory Disorders. Front. Immunol. 2022, 13, 868343. [Google Scholar] [CrossRef]
- Kumari, S.; Mukherjee, S.; Sinha, D.; Abdisalaam, S.; Krishnan, S.; Asaithamby, A. Immunomodulatory Effects of Radiotherapy. Int. J. Mol. Sci. 2020, 21, 8151. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Asadzadeh-Aghdaei, H.; Mashayekhi, K.; Koushki, K.; Azimzadeh, P.; Rostami-Nejad, M.; Amani, D.; Chaleshi, V.; Haftcheshmeh, S.M.; Sahebkar, A.; Zali, M.R. V617F-independent upregulation of JAK2 gene expression in patients with inflammatory bowel disease. J. Cell Biochem. 2019, 120, 15746–15755. [Google Scholar] [CrossRef]
- Hayter, S.M.; Cook, M.C. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun. Rev. 2012, 11, 754–765. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Henriquez-Olguin, C.; Altamirano, F.; Valladares, D.; Lopez, J.R.; Allen, P.D.; Jaimovich, E. Altered ROS production, NF-kappaB activation and interleukin-6 gene expression induced by electrical stimulation in dystrophic mdx skeletal muscle cells. Biochim. Biophys. Acta 2015, 1852, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Diagaradjane, P.; Deorukhkar, A.A.; Goins, B.; Bao, A.; Phillips, W.T.; Wang, Z.; Schwartz, J.; Krishnan, S. Integrin αvβ3-targeted gold nanoshells augment tumor vasculature-specific imaging and therapy. Int. J. Nanomed. 2011, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef]
- Stramer, B.M.; Mori, R.; Martin, P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J. Investig. Dermatol. 2007, 127, 1009–1017. [Google Scholar] [CrossRef]
- Van Linthout, S.; Miteva, K.; Tschope, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef]
- Su, Y.; Gao, J.; Kaur, P.; Wang, Z. Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases. Pharmaceutics 2020, 12, 1222. [Google Scholar] [CrossRef]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef] [PubMed]
- Sobiepanek, A.; Kuryk, L.; Garofalo, M.; Kumar, S.; Baran, J.; Musolf, P.; Siebenhaar, F.; Fluhr, J.W.; Kobiela, T.; Plasenzotti, R.; et al. The Multifaceted Roles of Mast Cells in Immune Homeostasis, Infections and Cancers. Int. J. Mol. Sci. 2022, 23, 2249. [Google Scholar] [CrossRef]
- Hamad, M.A.; Krauel, K.; Schanze, N.; Gauchel, N.; Stachon, P.; Nuehrenberg, T.; Zurek, M.; Duerschmied, D. Platelet Subtypes in Inflammatory Settings. Front. Cardiovasc. Med. 2022, 9, 823549. [Google Scholar] [CrossRef]
- Kiss, A.L. Inflammation in Focus: The Beginning and the End. Pathol. Oncol. Res. POR 2021, 27, 1610136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Constructing Causal Cytokine Networks for Autoimmune and Inflammatory Diseases Target Identification. Biorxiv 2022, 2022, 478394. [Google Scholar]
- Kleiman, A.; Tuckermann, J.P. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: Lessons from conditional knockout mice. Mol. Cell. Endocrinol. 2007, 275, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E. Early management of severe sepsis: Concepts and controversies. Chest 2014, 145, 1407–1418. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Q.; Anrather, J.; Shi, F.D. Immune interventions in stroke. Nat. Rev. Neurol. 2015, 11, 524–535. [Google Scholar] [CrossRef]
- Mc Carthy, D.J.; Malhotra, M.; O’Mahony, A.M.; Cryan, J.F.; O’Driscoll, C.M. Nanoparticles and the blood-brain barrier: Advancing from in-vitro models towards therapeutic significance. Pharm. Res. 2015, 32, 1161–1185. [Google Scholar] [CrossRef]
- Jung, S.M.; Kim, W.U. Targeted Immunotherapy for Autoimmune Disease. Immune Netw. 2022, 22, e9. [Google Scholar] [CrossRef]
- Kroschinsky, F.; Stolzel, F.; von Bonin, S.; Beutel, G.; Kochanek, M.; Kiehl, M.; Schellongowski, P.; Intensive Care in Hematological and Oncological Patients (iCHOP) Collaborative Group. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care 2017, 21, 89. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Thomas, S.N.; Swartz, M.A. Materials engineering for immunomodulation. Nature 2009, 462, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, C.; Chen, Y.; Chen, T.; Han, T.; Xue, L.; Xu, B. Systemically Silencing Long Non-coding RNAs Maclpil With Short Interfering RNA Nanoparticles Alleviates Experimental Ischemic Stroke by Promoting Macrophage Apoptosis and Anti-inflammatory Activation. Front. Cardiovasc. Med. 2022, 9, 876087. [Google Scholar] [CrossRef]
- Muller, E.K.; Bialas, N.; Epple, M.; Hilger, I. Nanoparticles Carrying NF-kappaB p65-Specific siRNA Alleviate Colitis in Mice by Attenuating NF-kappaB-Related Protein Expression and Pro-Inflammatory Cellular Mediator Secretion. Pharmaceutics 2022, 14, 20419. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Pang, Y.; Zhou, H. The interaction between nanoparticles and immune system: Application in the treatment of inflammatory diseases. J. Nanobiotechnology 2022, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A. Emerging Nanotherapeutic Approaches to Overcome Drug Resistance in Cancers with Update on Clinical Trials. Pharmaceutics 2022, 14, 40866. [Google Scholar] [CrossRef] [PubMed]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nature reviews Drug discovery 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Koushki, K.; Keshavarz Shahbaz, S.; Keshavarz, M.; Bezsonov, E.E.; Sathyapalan, T.; Sahebkar, A. Gold Nanoparticles: Multifaceted Roles in the Management of Autoimmune Disorders. Biomolecules 2021, 11, 1289. [Google Scholar] [CrossRef]
- Perciani, C.T.; Liu, L.Y.; Wood, L.; MacParland, S.A. Enhancing Immunity with Nanomedicine: Employing Nanoparticles to Harness the Immune System. ACS Nano 2021, 15, 7–20. [Google Scholar] [CrossRef]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Costantino, L.; Italiani, P. Interaction of nanoparticles with immunocompetent cells: Nanosafety considerations. Nanomedicine 2012, 7, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, V.; Narat, M.; Drobne, D. Nanoparticle interaction with the immune system. Arh. Za Hig. Rada I Toksikol. 2015, 66, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Moyano, D.F.; Goldsmith, M.; Solfiell, D.J.; Landesman-Milo, D.; Miranda, O.R.; Peer, D.; Rotello, V.M. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012, 134, 3965–3967. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, Á.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Derry, P.J.; Aziz, K.; Singh, P.K.; Khoo, A.M.; Chadha, A.S.; Liopo, A.; Zubarev, E.R.; Krishnan, S. Gold nanotriangles: Scale up and X-ray radiosensitization effects in mice. Nanoscale 2017, 9, 5085–5093. [Google Scholar] [CrossRef] [PubMed]
- Norn, S.; Permin, H.; Kruse, P.R.; Kruse, E. History of gold–with danish contribution to tuberculosis and rheumatoid arthritis. Dan. Med. Arb. 2011, 39, 59–80. [Google Scholar]
- Guo, R.; Song, Y.; Wang, G.; Murray, R.W. Does core size matter in the kinetics of ligand exchanges of monolayer-protected Au clusters? J. Am. Chem. Soc. 2005, 127, 2752–2757. [Google Scholar] [CrossRef]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; López-Iglesias, C.; Teixidó, M. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef]
- Mackeyev, Y.; Raoof, M.; Cisneros, B.; Koshkina, N.; Berger, C.S.; Wilson, L.; Curley, S. Toward paclitaxel-[60] Fullerene immunoconjugates as a targeted prodrug against cancer. Nanosyst. Phys. Chem. Math. 2014, 5, 67–75. [Google Scholar]
- Pakravan, A.; Salehi, R.; Mahkam, M. Comparison study on the effect of gold nanoparticles shape in the forms of star, hallow, cage, rods, and Si-Au and Fe-Au core-shell on photothermal cancer treatment. Photodiagn. Photodyn. Ther. 2021, 33, 102144. [Google Scholar] [CrossRef]
- Koushki, K.; Varasteh, A.-R.; Shahbaz, S.K.; Sadeghi, M.; Mashayekhi, K.; Ayati, S.H.; Moghadam, M.; Sankian, M. Dc-specific aptamer decorated gold nanoparticles: A new attractive insight into the nanocarriers for allergy epicutaneous immunotherapy. Int. J. Pharm. 2020, 584, 119403. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef]
- Yang, X.; Venkatesulu, B.P.; Mahadevan, L.S.; Aliru, M.L.; Mackeyev, Y.; Singh, A.; Prasad, P.N.; Krishnan, S. Gold-Small Interfering RNA as Optically Responsive Nanostructures for Cancer Theranostics. J. Biomed. Nanotechnol. 2018, 14, 809–828. [Google Scholar] [CrossRef]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef]
- Schuemann, J.; Bagley, A.F.; Berbeco, R.; Bromma, K.; Butterworth, K.T.; Byrne, H.L.; Chithrani, B.D.; Cho, S.H.; Cook, J.R.; Favaudon, V.; et al. Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Phys. Med. Biol. 2020, 65, 21RM02. [Google Scholar] [CrossRef]
- Schuemann, J.; Berbeco, R.; Chithrani, D.B.; Cho, S.H.; Kumar, R.; McMahon, S.J.; Sridhar, S.; Krishnan, S. Roadmap to Clinical Use of Gold Nanoparticles for Radiation Sensitization. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 189–205. [Google Scholar] [CrossRef]
- Diagaradjane, P.; Shetty, A.; Wang, J.C.; Elliott, A.M.; Schwartz, J.; Shentu, S.; Park, H.C.; Deorukhkar, A.; Stafford, R.J.; Cho, S.H.; et al. Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: Characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy. Nano Lett. 2008, 8, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.E.; Brown, S.D.; Lamprou, D.A.; Graham, D.; Wheate, N.J. Cisplatin-tethered gold nanoparticles that exhibit enhanced reproducibility, drug loading, and stability: A step closer to pharmaceutical approval? Inorg. Chem. 2012, 51, 3490–3497. [Google Scholar] [CrossRef]
- Simpson, C.A.; Salleng, K.J.; Cliffel, D.E.; Feldheim, D.L. In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles. Nanomedicine 2013, 9, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Tom, R.T.; Suryanarayanan, V.; Reddy, P.G.; Baskaran, S.; Pradeep, T. Ciprofloxacin-protected gold nanoparticles. Langmuir 2004, 20, 1909–1914. [Google Scholar] [CrossRef]
- Ghosh, R.; Singh, L.C.; Shohet, J.M.; Gunaratne, P.H. A gold nanoparticle platform for the delivery of functional microRNAs into cancer cells. Biomaterials 2013, 34, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, S.; Herance, J.R.; Rojas, S.; Mena, J.F.; Gispert, J.D.; Acosta, G.A.; Albericio, F.; Kogan, M.J. Synthesis and in vivo evaluation of the biodistribution of a 18F-labeled conjugate gold-nanoparticle-peptide with potential biomedical application. Bioconjug. Chem. 2012, 23, 399–408. [Google Scholar] [CrossRef]
- Song, K.; Xu, P.; Meng, Y.; Geng, F.; Li, J.; Li, Z.; Xing, J.; Chen, J.; Kong, B. Smart gold nanoparticles enhance killing effect on cancer cells. Int. J. Oncol. 2013, 42, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Mackeyev, Y.; Venkatesulu, B.P.; Krishnan, S.; Singh, P.K. CXC chemokine receptor 4 (CXCR4) targeted gold nanoparticles potently enhance radiotherapy outcomes in breast cancer. Nanoscale 2021, 13, 19056–19065. [Google Scholar] [CrossRef]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.D.; Bhattarai, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishnan, S. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Rauta, P.R.; Mackeyev, Y.; Sanders, K.; Kim, J.B.K.; Gonzalez, V.V.; Zahra, Y.; Shohayeb, M.A.; Abousaida, B.; Vijay, G.V.; Tezcan, O.; et al. Pancreatic tumor microenvironmental acidosis and hypoxia transform gold nanorods into cell-penetrant particles for potent radiosensitization. Sci. Adv. 2022, 8, eabm9729. [Google Scholar] [CrossRef]
- Tomić, S.; Đokić, J.; Vasilijić, S.; Ogrinc, N.; Rudolf, R.; Pelicon, P.; Vučević, D.; Milosavljević, P.; Janković, S.; Anžel, I. Size-dependent effects of gold nanoparticles uptake on maturation and antitumor functions of human dendritic cells in vitro. PLoS ONE 2014, 9, e96584. [Google Scholar] [CrossRef]
- Qiu, T.A.; Bozich, J.S.; Lohse, S.E.; Vartanian, A.M.; Jacob, L.M.; Meyer, B.M.; Gunsolus, I.L.; Niemuth, N.J.; Murphy, C.J.; Haynes, C.L.; et al. Gene expression as an indicator of the molecular response and toxicity in the bacterium Shewanella oneidensis and the water flea Daphnia magna exposed to functionalized gold nanoparticles. Environ. Sci. Nano 2015, 2, 615–629. [Google Scholar] [CrossRef]
- Hashimoto, M.; Toshima, H.; Yonezawa, T.; Kawai, K.; Narushima, T.; Kaga, M.; Endo, K. Responses of RAW264. 7 macrophages to water-dispersible gold and silver nanoparticles stabilized by metal–carbon σ-bonds. J. Biomed. Mater. Res. Part A 2014, 102, 1838–1849. [Google Scholar] [CrossRef]
- Rizk, M.Z.; Ali, S.A.; Hamed, M.A.; El-Rigal, N.S.; Aly, H.F.; Salah, H.H. Toxicity of titanium dioxide nanoparticles: Effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomed. Pharmacother. 2017, 90, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Lasagna-Reeves, C.; Gonzalez-Romero, D.; Barria, M.A.; Olmedo, I.; Clos, A.; Sadagopa Ramanujam, V.M.; Urayama, A.; Vergara, L.; Kogan, M.J.; Soto, C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010, 393, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Bar-Ilan, O.; Albrecht, R.M.; Fako, V.E.; Furgeson, D.Y. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Schaffler, M.; Takenaka, S.; Moller, W.; Schmid, G.; Simon, U.; et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Cho, W.S.; Cho, M.; Jeong, J.; Choi, M.; Han, B.S.; Shin, H.S.; Hong, J.; Chung, B.H.; Jeong, J.; Cho, M.H. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2010, 245, 116–123. [Google Scholar] [CrossRef]
- Villiers, C.; Freitas, H.; Couderc, R.; Villiers, M.B.; Marche, P. Analysis of the toxicity of gold nano particles on the immune system: Effect on dendritic cell functions. J. Nanopart. Res. 2010, 12, 55–60. [Google Scholar] [CrossRef]
- Dey, A.K.; Gonon, A.; Pecheur, E.I.; Pezet, M.; Villiers, C.; Marche, P.N. Impact of Gold Nanoparticles on the Functions of Macrophages and Dendritic Cells. Cells 2021, 10, 96. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef]
- Yen, H.J.; Hsu, S.H.; Tsai, C.L. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef]
- Malaczewska, J. The splenocyte proliferative response and cytokine secretion in mice after 28-day oral administration of silver nanocolloid. Pol. J. Vet. Sci. 2014, 17, 27–35. [Google Scholar] [CrossRef]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Al Zaki, A.; Hui, J.Z.; Higbee, E.; Tsourkas, A. Biodistribution, Clearance, and Toxicology of Polymeric Micelles Loaded with 0.9 or 5 nm Gold Nanoparticles. J. Biomed. Nanotechnol. 2015, 11, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef]
- Wang, B.; He, X.; Zhang, Z.; Zhao, Y.; Feng, W. Metabolism of nanomaterials in vivo: Blood circulation and organ clearance. Acc. Chem. Res. 2013, 46, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Huh, M.S.; Sun, I.C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. In vivo targeted delivery of nanoparticles for theranosis. Acc. Chem. Res. 2011, 44, 1018–1028. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Ahn, S.; Lee, J.; Kim, J.Y.; Choi, M.; Gujrati, V.; Kim, H.; Kim, J.; Shin, E.C.; Jon, S. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Control Release 2017, 256, 56–67. [Google Scholar] [CrossRef]
- Abdelmegid, A.M.; Abdo, F.K.; Ahmed, F.E.; Kattaia, A.A.A. Therapeutic effect of gold nanoparticles on DSS-induced ulcerative colitis in mice with reference to interleukin-17 expression. Sci. Rep. 2019, 9, 10176. [Google Scholar] [CrossRef]
- Khan, H.A.; Alamery, S.; Ibrahim, K.E.; El-Nagar, D.M.; Al-Harbi, N.; Rusop, M.; Alrokayan, S.H. Size and time-dependent induction of proinflammatory cytokines expression in brains of mice treated with gold nanoparticles. Saudi J. Biol. Sci. 2019, 26, 625–631. [Google Scholar] [CrossRef]
- Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials 2019, 206, 115–132. [Google Scholar] [CrossRef]
- Kirdaite, G.; Leonaviciene, L.; Bradunaite, R.; Vasiliauskas, A.; Rudys, R.; Ramanaviciene, A.; Mackiewicz, Z. Antioxidant effects of gold nanoparticles on early stage of collagen-induced arthritis in rats. Res. Vet. Sci. 2019, 124, 32–37. [Google Scholar] [CrossRef]
- Rovais, M.R.A.; Alirezapour, B.; Moassesi, M.E.; Amiri, M.; Novin, F.B.; Maadi, E. Internalization capabilities of gold-198 nanoparticles: Comparative evaluation of effects of chitosan agent on cellular uptake into MCF-7. Appl. Radiat. Isot. 2018, 142, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, M.J. Nano-gold displayed anti-inflammatory property via NF-kB pathways by suppressing COX-2 activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Abdelhalim, M.; Alhomida, A.; Al Ayed, M. Transient increase in IL-1β, IL-6 and TNF-α gene expression in rat liver exposed to gold nanoparticles. Genet. Mol. Res. 2013, 12, 5851–5857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, Y.; Jia, J.; Gao, N.; Li, Y.; Zhang, R.; Jiang, G.; Yan, B. Perturbation of physiological systems by nanoparticles. Chem. Soc. Rev. 2014, 43, 3762–3809. [Google Scholar] [CrossRef] [PubMed]
- Arnida; Malugin, A.; Ghandehari, H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J. Appl. Toxicol. 2010, 30, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hitchins, V.M.; Schrand, A.M.; Hussain, S.M.; Goering, P.L. Uptake of gold nanoparticles in murine macrophage cells without cytotoxicity or production of pro-inflammatory mediators. Nanotoxicology 2011, 5, 284–295. [Google Scholar] [CrossRef]
- Kingston, M.; Pfau, J.C.; Gilmer, J.; Brey, R. Selective inhibitory effects of 50-nm gold nanoparticles on mouse macrophage and spleen cells. J. Immunotoxicol. 2016, 13, 198–208. [Google Scholar] [CrossRef]
- Sumbayev, V.V.; Yasinska, I.M.; Garcia, C.P.; Gilliland, D.; Lall, G.S.; Gibbs, B.F.; Bonsall, D.R.; Varani, L.; Rossi, F.; Calzolai, L. Gold nanoparticles downregulate interleukin-1β-induced pro-inflammatory responses. Small 2013, 9, 472–477. [Google Scholar] [CrossRef]
- Chen, Y.P.; Dai, Z.H.; Liu, P.C.; Chuu, J.J.; Lee, K.Y.; Lee, S.L.; Chen, Y.J. Effects of nanogold on the alleviation of carbon tetrachloride-induced hepatic injury in rats. Chin. J. Physiol. 2012, 55, 331–336. [Google Scholar] [CrossRef]
- Małaczewska, J. The splenocyte proliferative response and cytokine secretion in mice after oral administration of commercial gold nanocolloid. Pol. J. Vet. Sci. 2015, 18, 181–189. [Google Scholar] [CrossRef][Green Version]
- Malaczewska, J. The in vitro effect of commercially available noble metal nanocolloids on the splenocyte proliferative response and cytokine production in mice. Pol. J. Vet. Sci. 2014, 17, 37–45. [Google Scholar] [CrossRef]
- Rizwan, H.; Mohanta, J.; Si, S.; Pal, A. Gold nanoparticles reduce high glucose-induced oxidative-nitrosative stress regulated inflammation and apoptosis via tuberin-mTOR/NF-κB pathways in macrophages. Int. J. Nanomed. 2017, 12, 5841–5862. [Google Scholar] [CrossRef] [PubMed]
- Arnida; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423. [Google Scholar] [CrossRef]
- Oh, N.; Kim, Y.; Kweon, H.S.; Oh, W.Y.; Park, J.H. Macrophage-Mediated Exocytosis of Elongated Nanoparticles Improves Hepatic Excretion and Cancer Phototherapy. ACS Appl. Mater. Interfaces 2018, 10, 28450–28457. [Google Scholar] [CrossRef]
- Golbek, T.W.; Harper, B.J.; Harper, S.L.; Baio, J.E. Shape-dependent gold nanoparticle interactions with a model cell membrane. Biointerphases 2022, 17, 061003. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Song, H.-M.; Wei, Q.; Wei, A. Differential response of macrophages to core–shell Fe3O4@Au nanoparticles and nanostars. Nanoscale 2012, 4, 7143–7148. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; Remy, S.; Vermeulen, J.P.; Hurkmans, E.G.; Kevenaar, K.; Bastús, N.G.; Pelaz, B.; Soliman, M.G.; Puntes, V.F.; Parak, W.J. Pathways related to NLRP3 inflammasome activation induced by gold nanorods. Int. J. Mol. Sci. 2022, 23, 5763. [Google Scholar] [CrossRef]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef]
- Bastús, N.G.; Sánchez-Tilló, E.; Pujals, S.; Farrera, C.; López, C.; Giralt, E.; Celada, A.; Lloberas, J.; Puntes, V. Homogeneous conjugation of peptides onto gold nanoparticles enhances macrophage response. ACS Nano 2009, 3, 1335–1344. [Google Scholar] [CrossRef]
- Staroverov, S.A.; Aksinenko, N.M.; Gabalov, K.P.; Vasilenko, O.A.; Vidyasheva, I.V.; Shchyogolev, S.Y.; Dykman, L.A. Effect of gold nanoparticles on the respiratory activity of peritoneal macrophages. Gold Bull. 2009, 42, 153–156. [Google Scholar] [CrossRef]
- Staroverov, S.A.; Vidyasheva, I.V.; Gabalov, K.P.; Vasilenko, O.A.; Laskavyi, V.N.; Dykman, L.A. Immunostimulatory effect of gold nanoparticles conjugated with transmissible gastroenteritis virus. Bull. Exp. Biol. Med. 2011, 151, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chen, N.; Li, J.; Yin, M.; Liang, L.; He, Y.; Song, H.; Fan, C.; Huang, Q. Polyvalent immunostimulatory nanoagents with self-assembled CpG oligonucleotide-conjugated gold nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 1202–1206. [Google Scholar] [CrossRef]
- Deng, Z.J.; Liang, M.; Monteiro, M.; Toth, I.; Minchin, R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nanotechnol. 2011, 6, 39–44. [Google Scholar] [CrossRef]
- Kanaras, A.G.; Kamounah, F.S.; Schaumburg, K.; Kiely, C.J.; Brust, M. Thioalkylated tetraethylene glycol: A new ligand for water soluble monolayer protected gold clusters. Chem. Commun. 2002, 20, 2294–2295. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Wang, L.; Hou, X.; Liu, W.; Chen, C. Interaction of gold nanoparticles with proteins and cells. Sci. Technol. Adv. Mater. 2015, 16, 034610. [Google Scholar] [CrossRef]
- Liu, X.; Huang, N.; Li, H.; Jin, Q.; Ji, J. Surface and size effects on cell interaction of gold nanoparticles with both phagocytic and nonphagocytic cells. Langmuir 2013, 29, 9138–9148. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.H. Surface chemistry of gold nanoparticles mediates their exocytosis in macrophages. ACS Nano 2014, 8, 6232–6241. [Google Scholar] [CrossRef] [PubMed]
- Srijampa, S.; Buddhisa, S.; Ngernpimai, S.; Leelayuwat, C.; Proungvitaya, S.; Chompoosor, A.; Tippayawat, P. Influence of Gold Nanoparticles with Different Surface Charges on Localization and Monocyte Behavior. Bioconjug. Chem. 2020, 31, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ayala-Orozco, C.; Rauta, P.R.; Krishnan, S. The application of nanotechnology in enhancing immunotherapy for cancer treatment: Current effects and perspective. Nanoscale 2019, 11, 17157–17178. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C. Influences of size and surface coating of gold nanoparticles on inflammatory activation of macrophages. Colloids Surf. B Biointerfaces 2017, 160, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T.; Matea, C.; Tabaran, F.; Iancu, C.; Orasan, R.; Mocan, L. In Vitro Administration of Gold Nanoparticles Functionalized with MUC-1 Protein Fragment Generates Anticancer Vaccine Response via Macrophage Activation and Polarization Mechanism. J. Cancer 2015, 6, 583–592. [Google Scholar] [CrossRef]
- Bartneck, M.; Keul, H.A.; Wambach, M.; Bornemann, J.; Gbureck, U.; Chatain, N.; Neuss, S.; Tacke, F.; Groll, J.; Zwadlo-Klarwasser, G. Effects of nanoparticle surface-coupled peptides, functional endgroups, and charge on intracellular distribution and functionality of human primary reticuloendothelial cells. Nanomedicine 2012, 8, 1282–1292. [Google Scholar] [CrossRef]
- Fromen, C.A.; Kelley, W.J.; Fish, M.B.; Adili, R.; Noble, J.; Hoenerhoff, M.J.; Holinstat, M.; Eniola-Adefeso, O. Neutrophil–particle interactions in blood circulation drive particle clearance and alter neutrophil responses in acute inflammation. ACS Nano 2017, 11, 10797–10807. [Google Scholar] [CrossRef] [PubMed]
- Bisso, P.W.; Gaglione, S.; Guimaraes, P.P.G.; Mitchell, M.J.; Langer, R. Nanomaterial Interactions with Human Neutrophils. ACS Biomater. Sci. Eng. 2018, 4, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Liz, R.; Simard, J.-C.; Leonardi, L.B.A.; Girard, D. Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. Int. Immunopharmacol. 2015, 28, 616–625. [Google Scholar] [CrossRef]
- Bartneck, M.; Keul, H.A.; Zwadlo-Klarwasser, G.; Groll, J. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett. 2010, 10, 59–63. [Google Scholar] [CrossRef]
- Hwang, T.L.; Aljuffali, I.A.; Hung, C.F.; Chen, C.H.; Fang, J.Y. The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs). Chem. Biol. Interact. 2015, 235, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, A.V.; Baranova, O.A.; Levin, A.D.; Solov’eva, E.; Fedin, A.I.; Kazarinov, K.D. Study of the influence of gold nanoparticles on activation of human blood neutrophils. Biofizika 2013, 58, 495–500. [Google Scholar]
- Noël, C.; Simard, J.-C.; Girard, D. Gold nanoparticles induce apoptosis, endoplasmic reticulum stress events and cleavage of cytoskeletal proteins in human neutrophils. Toxicol. In Vitro 2016, 31, 12–22. [Google Scholar] [CrossRef]
- Durocher, I.; Noel, C.; Lavastre, V.; Girard, D. Evaluation of the in vitro and in vivo proinflammatory activities of gold (+) and gold (-) nanoparticles. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2017, 66, 981–992. [Google Scholar] [CrossRef]
- Khan, H.A.; Abdelhalim, M.A.; Alhomida, A.S.; Al-Ayed, M.S. Effects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidney. BioMed Res. Int. 2013, 2013, 590730. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Hussein, A.A.; Meyer, M. The In Vitro Immunomodulatory Effects Of Gold Nanoparticles Synthesized From Hypoxis hemerocallidea Aqueous Extract And Hypoxoside On Macrophage And Natural Killer Cells. Int. J. Nanomed. 2019, 14, 9007–9018. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Sun, L.; Gao, W.; Xiong, Y.; Ma, A.; Liu, X.; Shen, L.; Li, Q.; Yang, H. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J. Nanobiotechnol. 2020, 18, 38. [Google Scholar] [CrossRef]
- Fujita, T.; Zysman, M.; Elgrabli, D.; Murayama, T.; Haruta, M.; Lanone, S.; Ishida, T.; Boczkowski, J. Anti-inflammatory effect of gold nanoparticles supported on metal oxides. Sci. Rep. 2021, 11, 23129. [Google Scholar] [CrossRef]
- Taratummarat, S.; Sangphech, N.; Vu, C.T.B.; Palaga, T.; Ondee, T.; Surawut, S.; Sereemaspun, A.; Ritprajak, P.; Leelahavanichkul, A. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiol. 2018, 18, 85. [Google Scholar] [CrossRef]
- MacParland, S.A.; Tsoi, K.M.; Ouyang, B.; Ma, X.Z.; Manuel, J.; Fawaz, A.; Ostrowski, M.A.; Alman, B.A.; Zilman, A.; Chan, W.C.; et al. Phenotype Determines Nanoparticle Uptake by Human Macrophages from Liver and Blood. ACS Nano 2017, 11, 2428–2443. [Google Scholar] [CrossRef]
- Blott, E.J.; Griffiths, G.M. Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 2002, 3, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.C.; Robson, S.; Quintana, F.J. Regulation of the T Cell Response by CD39. Trends Immunol. 2016, 37, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Fytianos, K.; Rodriguez-Lorenzo, L.; Clift, M.J.; Blank, F.; Vanhecke, D.; Von Garnier, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Uptake efficiency of surface modified gold nanoparticles does not correlate with functional changes and cytokine secretion in human dendritic cells in vitro. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Le Guével, X.; Palomares, F.; Torres, M.J.; Blanca, M.; Fernandez, T.D.; Mayorga, C. Nanoparticle size influences the proliferative responses of lymphocyte subpopulations. RSC Adv. 2015, 5, 85305–85309. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.; Fytianos, K.; Blank, F.; Von Garnier, C.; Rothen-Rutishauser, B.; Petri-Fink, A. Fluorescence-encoded gold nanoparticles: Library design and modulation of cellular uptake into dendritic cells. Small 2014, 10, 1341–1350. [Google Scholar] [CrossRef]
- Arosio, D.; Chiodo, F.; Reina, J.J.; Marelli, M.; Penades, S.; van Kooyk, Y.; Garcia-Vallejo, J.J.; Bernardi, A. Effective targeting of DC-SIGN by alpha-fucosylamide functionalized gold nanoparticles. Bioconjug. Chem. 2014, 25, 2244–2251. [Google Scholar] [CrossRef]
- Sadeghi, M.; Koushki, K.; Mashayekhi, K.; Ayati, S.H.; Shahbaz, S.K.; Moghadam, M.; Sankian, M. DC-targeted gold nanoparticles as an efficient and biocompatible carrier for modulating allergic responses in sublingual immunotherapy. Int. Immunopharmacol. 2020, 86, 106690. [Google Scholar] [CrossRef]
- Fytianos, K.; Chortarea, S.; Rodriguez-Lorenzo, L.; Blank, F.; von Garnier, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Aerosol Delivery of Functionalized Gold Nanoparticles Target and Activate Dendritic Cells in a 3D Lung Cellular Model. ACS Nano 2017, 11, 375–383. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Du, J.; Li, Y.; Zhou, Y.; Fu, Q.; Zhang, J.; Wang, X.; Zhan, L. Different-Sized Gold Nanoparticle Activator/Antigen Increases Dendritic Cells Accumulation in Liver-Draining Lymph Nodes and CD8+ T Cell Responses. ACS Nano 2016, 10, 2678–2692. [Google Scholar] [CrossRef]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef]
- Ye, F.; Vallhov, H.; Qin, J.; Daskalaki, E.; Sugunan, A.; Toprak, M.; Fornara, A.; Gabrielsson, S.; Scheynius, A.; Muhammed, M. Synthesis of high aspect ratio gold nanorods and their effects on human antigen presenting dendritic cells. Int. J. Nanotechnol. 2011, 8, 631–652. [Google Scholar] [CrossRef]
- Oh, E.; Delehanty, J.B.; Sapsford, K.E.; Susumu, K.; Goswami, R.; Blanco-Canosa, J.B.; Dawson, P.E.; Granek, J.; Shoff, M.; Zhang, Q. Cellular uptake and fate of PEGylated gold nanoparticles is dependent on both cell-penetration peptides and particle size. ACS Nano 2011, 5, 6434–6448. [Google Scholar] [CrossRef] [PubMed]
- Bancos, S.; Stevens, D.L.; Tyner, K.M. Effect of silica and gold nanoparticles on macrophage proliferation, activation markers, cytokine production, and phagocytosis in vitro. Int. J. Nanomed. 2015, 10, 183–206. [Google Scholar] [CrossRef]
- Fernández, T.D.; Pearson, J.R.; Leal, M.P.; Torres, M.J.; Blanca, M.; Mayorga, C.; Le Guével, X. Intracellular accumulation and immunological properties of fluorescent gold nanoclusters in human dendritic cells. Biomaterials 2015, 43, 1–12. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Huang, Y.F.; Liu, H.; Xiong, X.; Chen, Y.; Tan, W. Nanoparticle-mediated IgE-receptor aggregation and signaling in RBL mast cells. J. Am. Chem. Soc. 2009, 131, 17328–17334. [Google Scholar] [CrossRef]
- Marquis, B.J.; Maurer-Jones, M.A.; Braun, K.L.; Haynes, C.L. Amperometric assessment of functional changes in nanoparticle-exposed immune cells: Varying Au nanoparticle exposure time and concentration. Analyst 2009, 134, 2293–2300. [Google Scholar] [CrossRef]
- Marquis, B.J.; McFarland, A.D.; Braun, K.L.; Haynes, C.L. Dynamic measurement of altered chemical messenger secretion after cellular uptake of nanoparticles using carbon-fiber microelectrode amperometry. Anal. Chem. 2008, 80, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Calleja, R.A.; Rodríguez-Cortés, O.; Flores-Mejía, R.; Muñoz-Diosdado, A. Gold nanoparticles: Uptake in human mast cells and effect on cell viability, inflammatory mediators, and proliferation. Mol. Cell. Toxicol. 2021, 17, 439–452. [Google Scholar] [CrossRef]
- Pal, R.; Chakraborty, B.; Nath, A.; Singh, L.M.; Ali, M.; Rahman, D.S.; Ghosh, S.K.; Basu, A.; Bhattacharya, S.; Baral, R.; et al. Noble metal nanoparticle-induced oxidative stress modulates tumor associated macrophages (TAMs) from an M2 to M1 phenotype: An in vitro approach. Int. Immunopharmacol. 2016, 38, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.S.; Kim, W.J.; Kim, J.J.; Kim, T.J.; Ye, S.K.; Song, M.D.; Kang, H.; Kim, D.W.; Moon, W.K.; Lee, K.H. Gold nanoparticles attenuate LPS-induced NO production through the inhibition of NF-κB and IFN-β/STAT1 pathways in RAW264. 7 cells. Nitric Oxide 2010, 23, 214–219. [Google Scholar] [CrossRef]
- Kesarkar, R.; Sangar, V.; Oza, G.; Sawant, T.; Kothari, S.; Sharon, M.; Chowdhary, A. Synthesis, Characterization and Hepatoprotective Activity of Neem Gold Nanoparticles for Improved Efficacy and Sustained Drug Release Profile of Azidothymidine. Int. J. Pharma. Sci. Rev. Res. 2014, 26, 117–122. [Google Scholar]
- Nishanth, R.P.; Jyotsna, R.G.; Schlager, J.J.; Hussain, S.M.; Reddanna, P. Inflammatory responses of RAW 264.7 macrophages upon exposure to nanoparticles: Role of ROS-NFκB signaling pathway. Nanotoxicology 2011, 5, 502–516. [Google Scholar] [CrossRef]
- Rudolf, R.; Majerič, P.; Tomić, S.; Shariq, M.; Ferčec, U.; Budič, B.; Friedrich, B.; Vučević, D.; Čolić, M. Morphology, Aggregation Properties, Cytocompatibility, and Anti-Inflammatory Potential of Citrate-Stabilized AuNPs Prepared by Modular Ultrasonic Spray Pyrolysis. J. Nanomater. 2017, 2017, 9365012. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, X.; Boudreau, M.D.; Feng, G.; Miao, Y.; Dong, S.; Wu, H.; Zeng, M.; Yin, J.-J. Orally administered gold nanoparticles protect against colitis by attenuating Toll-like receptor 4-and reactive oxygen/nitrogen species-mediated inflammatory responses but could induce gut dysbiosis in mice. J. Nanobiotechnol. 2018, 16, 86. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Lu, S.L.; Hu, C.W.; Yeh, C.S.; Lee, G.B.; Lei, H.Y. Size-dependent attenuation of TLR9 signaling by gold nanoparticles in macrophages. J. Immunol. 2012, 188, 68–76. [Google Scholar] [CrossRef]
- Mulens-Arias, V.; Balfourier, A.; Nicolás-Boluda, A.; Carn, F.; Gazeau, F. Disturbance of adhesomes by gold nanoparticles reveals a size-and cell type-bias. Biomater. Sci. 2019, 7, 389–408. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Liu, Y.; Wu, X.; Ji, Y.; Zhao, Y.; et al. Surface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012, 12, 2003–2012. [Google Scholar] [CrossRef]
- Vang, K.B.; Safina, I.; Darrigues, E.; Nedosekin, D.; Nima, Z.A.; Majeed, W.; Watanabe, F.; Kannarpady, G.; Kore, R.A.; Casciano, D.; et al. Modifying Dendritic Cell Activation with Plasmonic Nano Vectors. Sci. Rep. 2017, 7, 5513. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.; Korotchenko, E.; Scheiblhofer, S.; Weiss, R.; Schneider, M. Functionalized multifunctional nanovaccine for targeting dendritic cells and modulation of immune response. Int. J. Pharm. 2021, 593, 120123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chiu, Y.C.; Tostanoski, L.H.; Jewell, C.M. Polyelectrolyte Multilayers Assembled Entirely from Immune Signals on Gold Nanoparticle Templates Promote Antigen-Specific T Cell Response. ACS Nano 2015, 9, 6465–6477. [Google Scholar] [CrossRef] [PubMed]
- Hočevar, S.; Milošević, A.; Rodriguez-Lorenzo, L.; Ackermann-Hirschi, L.; Mottas, I.; Petri-Fink, A.; Rothen-Rutishauser, B.; Bourquin, C.; Clift, M.J.D. Polymer-Coated Gold Nanospheres Do Not Impair the Innate Immune Function of Human B Lymphocytes in Vitro. ACS Nano 2019, 13, 6790–6800. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Júnior, R.F.; de Araújo, A.A.; Pessoa, J.B.; Neto, F.P.F.; da Silva, G.R.; Oliveira, A.L.C.L.; de Carvalho, T.G.; Silva, H.F.; Eugênio, M.; Sant’Anna, C. Anti-inflammatory, analgesic and anti-tumor properties of gold nanoparticles. Pharmacol. Rep. 2017, 69, 119–129. [Google Scholar] [CrossRef]
- Dohnert, M.B.; Venancio, M.; Possato, J.C.; Zeferino, R.C.; Dohnert, L.H.; Zugno, A.I.; De Souza, C.T.; Paula, M.M.; Luciano, T.F. Gold nanoparticles and diclofenac diethylammonium administered by iontophoresis reduce inflammatory cytokines expression in Achilles tendinitis. Int. J. Nanomed. 2012, 7, 1651–1657. [Google Scholar] [CrossRef]
- Yang, J.P.; Merin, J.P.; Nakano, T.; Kato, T.; Kitade, Y.; Okamoto, T. Inhibition of the DNA-binding activity of NF-kappa B by gold compounds in vitro. FEBS Lett. 1995, 361, 89–96. [Google Scholar] [CrossRef]
- Yamashita, M.; Ashino, S.; Oshima, Y.; Kawamura, S.; Ohuchi, K.; Takayanagi, M. Inhibition of TPA-induced NF-κB nuclear translocation and production of NO and PGE2 by the anti-rheumatic gold compounds. J. Pharm. Pharmacol. 2003, 55, 245–251. [Google Scholar] [CrossRef]
- Sakurai, A.; Yuasa, K.; Shoji, Y.; Himeno, S.; Tsujimoto, M.; Kunimoto, M.; Imura, N.; Hara, S. Overexpression of thioredoxin reductase 1 regulates NF-κB activation. J. Cell. Physiol. 2004, 198, 22–30. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, M.Y.; Park, S.J.; Choi, J.S.; Oh, M.K.; Kim, I.S. Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3. Immunology 2007, 122, 607–614. [Google Scholar] [CrossRef]
- Jeon, K.-I.; Byun, M.-S.; Jue, D.-M. Gold compound auranofin inhibits IκB kinase (IKK) by modifying Cys-179 of IKKβ subunit. Exp. Mol. Med. 2003, 35, 61–66. [Google Scholar] [CrossRef]
- Hwang, D.; Jang, B.C.; Yu, G.; Boudreau, M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide: Mediation through both mitogen-activated protein kinase and NF-kB signaling pathways in macrophages. Biochem. Pharmacol. 1997, 54, 87–96. [Google Scholar] [CrossRef]
- de Carvalho, T.G.; Garcia, V.B.; de Araújo, A.A.; da Silva Gasparotto, L.H.; Silva, H.; Guerra, G.C.B.; de Castro Miguel, E.; de Carvalho Leitão, R.F.; da Silva Costa, D.V.; Cruz, L.J.; et al. Spherical neutral gold nanoparticles improve anti-inflammatory response, oxidative stress and fibrosis in alcohol-methamphetamine-induced liver injury in rats. Int. J. Pharm. 2018, 548, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yuan, Q.; Cai, P.; Gao, L.; Zhao, L.; Liu, M.; Yao, Y.; Chai, Z.; Gao, X. Au Clusters Treat Rheumatoid Arthritis with Uniquely Reversing Cartilage/Bone Destruction. Adv. Sci. 2019, 6, 1801671. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, Y.; Xiong, Y.; Sun, L.; Wang, L.; Wang, K.; Lu, H.Y.; Bao, A.; Turvey, S.E.; Li, Q.; et al. Size-dependent anti-inflammatory activity of a peptide-gold nanoparticle hybrid in vitro and in a mouse model of acute lung injury. Acta Biomater. 2019, 85, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ng, J.P.; Bishop, D.P.; Milthorpe, B.K.; Valenzuela, S.M. Gold nanoparticles as cell regulators: Beneficial effects of gold nanoparticles on the metabolic profile of mice with pre-existing obesity. J. Nanobiotechnol. 2018, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ng, J.P.M.; Tan, Y.; McGrath, K.; Bishop, D.P.; Oliver, B.; Chan, Y.L.; Cortie, M.B.; Milthorpe, B.K.; Valenzuela, S.M. Gold nanoparticles improve metabolic profile of mice fed a high-fat diet. J. Nanobiotechnol. 2018, 16, 11. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Forestier, J. Rheumatoid arthritis and its treatment by gold salts. Lancet 1934, 224, 646–648. [Google Scholar] [CrossRef]
- Kiely, P.D.; Helbert, M.R.; Miles, J.; Oliveira, D.B. Immunosuppressant effect of gold on IgG subclasses and IgE; evidence for sparing of Th2 responses. Clin. Exp. Immunol. 2000, 120, 369–374. [Google Scholar] [CrossRef]
- Lockie, L.M.; Smith, D.M. Forty-seven years experience with gold therapy in 1019 rheumatoid arthritis patients. Semin. Arthritis Rheum. 1985, 14, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Eichler, P.; Lubenow, N.; Kiefel, V. Drug-inducedand Drug-dependent Immune Thrombocytopenias. Rev. Clin. Exp. Hematol. 2001, 5, 166–200. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Chikanza, I.C.; Vaughan, R.W.; Welsh, K.I.; Panayi, G.S. Gold induced nephropathy in rheumatoid arthritis and HLA class II genes. Ann. Rheum. Dis. 1993, 52, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, M.; Gonzalez-Dominguez, J.; Mataran, L.; Garcia-Perez, S.; Salvatierra, D. Association of HLA-DR5 with mucocutaneous lesions in patients with rheumatoid arthritis receiving gold sodium thiomalate. J. Rheumatol. 1994, 21, 41–43. [Google Scholar] [PubMed]
- Evans, D.T.; Knapp, L.A.; Jing, P.; Mitchen, J.L.; Dykhuizen, M.; Montefiori, D.C.; Pauza, C.D.; Watkins, D.I. Rapid and slow progressors differ by a single MHC class I haplotype in a family of MHC-defined rhesus macaques infected with SIV. Immunol. Lett. 1999, 66, 53–59. [Google Scholar] [CrossRef]
- Merchant, B. Gold, the noble metal and the paradoxes of its toxicology. Biologicals 1998, 26, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.L.; Whitehouse, M.W.; Tiekink, E.R.; Bushell, G.R. Colloidal metallic gold is not bio-inert. Inflammopharmacology 2008, 16, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zou, J. Gold (III)-induced oxidation of glycine: Relevance to the toxic sideeffects of gold drugs. J. Inorg. Biochem. 1999, 74, 352. [Google Scholar]
- Eisler, R. Chrysotherapy: A synoptic review. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2003, 52, 487–501. [Google Scholar] [CrossRef]
- Barreto, E.; Serra, M.F.; Dos Santos, R.V.; Dos Santos, C.E.; Hickmann, J.; Cotias, A.C.; Pao, C.R.; Trindade, S.G.; Schimidt, V.; Giacomelli, C.; et al. Local Administration of Gold Nanoparticles Prevents Pivotal Pathological Changes in Murine Models of Atopic Asthma. J. Biomed. Nanotechnol. 2015, 11, 1038–1050. [Google Scholar] [CrossRef]
- Muller, A.P.; Ferreira, G.K.; Pires, A.J.; de Bem Silveira, G.; de Souza, D.L.; Brandolfi, J.A.; de Souza, C.T.; Paula, M.M.S.; Silveira, P.C.L. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of Alzheimer’s type. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 476–483. [Google Scholar] [CrossRef]
- Pedersen, M.O.; Larsen, A.; Pedersen, D.S.; Stoltenberg, M.; Penkowa, M. Metallic gold reduces TNFalpha expression, oxidative DNA damage and pro-apoptotic signals after experimental brain injury. Brain Res. 2009, 1271, 103–113. [Google Scholar] [CrossRef]
- Aghaie, T.; Jazayeri, M.H.; Avan, A.; Anissian, A.; Salari, A.A. Gold nanoparticles and polyethylene glycol alleviate clinical symptoms and alter cytokine secretion in a mouse model of experimental autoimmune encephalomyelitis. IUBMB Life 2019, 71, 1313–1321. [Google Scholar] [CrossRef]
- Ng, C.T.; Yip, G.W.C.; Chen, E.S.; Poh, W.Y.R.; Bay, B.H.; Yung, L.Y.L. Gold nanoparticles induce serum amyloid A 1–Toll-like receptor 2 mediated NF-kB signaling in lung cells in vitro. Chem. Biol. Interact. 2018, 289, 81–89. [Google Scholar] [CrossRef]
- Brandenberger, C.; Rothen-Rutishauser, B.; Mühlfeld, C.; Schmid, O.; Ferron, G.A.; Maier, K.L.; Gehr, P.; Lenz, A.-G. Effects and uptake of gold nanoparticles deposited at the air–liquid interface of a human epithelial airway model. Toxicol. Appl. Pharmacol. 2010, 242, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Gosens, I.; Post, J.A.; de la Fonteyne, L.J.; Jansen, E.H.; Geus, J.W.; Cassee, F.R.; de Jong, W.H. Impact of agglomeration state of nano-and submicron sized gold particles on pulmonary inflammation. Part. Fibre Toxicol. 2010, 7, 37. [Google Scholar] [CrossRef]
- Hussein, R.M.; Saleh, H. Promising therapeutic effect of gold nanoparticles against dinitrobenzene sulfonic acid-induced colitis in rats. Nanomed. 2018, 13, 1657–1679. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Shiau, A.L.; Chen, S.Y.; Chen, Y.H.; Cheng, P.C.; Chang, M.Y.; Chen, D.H.; Chou, C.H.; Wang, C.R.; Wu, C.L. Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. 2007, 56, 544–554. [Google Scholar] [CrossRef]
- Dohnert, M.B.; Ferreira, G.K.; Silveira, P.C.; Zanoni, E.T.; Dohnert, L.H.; de Souza, C.T.; Paula, M.M. Inflammatory cytokines content in Achilles tendinopathy after phonophoresis treatment combined with gold nanoparticles and diclophenac diethylammonium in rats. Inflammation 2015, 38, 1044–1049. [Google Scholar] [CrossRef]
- Zortéa, D.; Silveira, P.C.; Souza, P.S.; Fidelis, G.S.; Paganini, C.S.; Pozzi, B.G.; Tuon, T.; De Souza, C.T.; Paula, M.M.; Pinho, R.A. Effects of phonophoresis and gold nanoparticles in experimental model of muscle overuse: Role of oxidative stress. Ultrasound Med. Biol. 2015, 41, 151–162. [Google Scholar] [CrossRef]
- Pereira, D.V.; Petronilho, F.; Pereira, H.R.; Vuolo, F.; Mina, F.; Possato, J.C.; Vitto, M.F.; de Souza, D.R.; da Silva, L.; da Silva Paula, M.M.; et al. Effects of gold nanoparticles on endotoxin-induced uveitis in rats. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8036–8041. [Google Scholar] [CrossRef] [PubMed]
- Pivodová, V.; Franková, J.; Galandáková, A.; Ulrichová, J. In vitro AuNPs’ cytotoxicity and their effect on wound healing. Nanobiomedicine 2015, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Parnsamut, C.; Brimson, S. Effects of silver nanoparticles and gold nanoparticles on IL-2, IL-6, and TNF-alpha production via MAPK pathway in leukemic cell lines. Genet. Mol. Res. 2015, 14, 3650–3668. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-H.; Chung, C.-H.; Chen, B.-H.; Hung, C.-F.; Inbaraj, B.S.; Ma, M.-C.; Chen, H.-M.; Tsou, C.-J.; Wu, P.-H.; Wu, W.-B. Gold nanoparticles compromise TNF-α-induced endothelial cell adhesion molecule expression through NF-κB and protein degradation pathways and reduce neointima formation in a rat carotid balloon injury model. J. Biomed. Nanotechnol. 2016, 12, 2185–2201. [Google Scholar] [CrossRef]
- Warheit, D.B.; Sayes, C.M.; Reed, K.L.; Swain, K.A. Health effects related to nanoparticle exposures: Environmental, health and safety considerations for assessing hazards and risks. Pharmacol. Ther. 2008, 120, 35–42. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Chompoosor, A.; Saha, K.; Ghosh, P.S.; Macarthy, D.J.; Miranda, O.R.; Zhu, Z.-J.; Arcaro, K.F.; Rotello, V.M. The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage by cationic gold nanoparticles. Small 2010, 6, 2246. [Google Scholar] [CrossRef]
- Khaing Oo, M.K.; Yang, Y.; Hu, Y.; Gomez, M.; Du, H.; Wang, H. Gold nanoparticle-enhanced and size-dependent generation of reactive oxygen species from protoporphyrin IX. ACS Nano 2012, 6, 1939–1947. [Google Scholar] [CrossRef]
- Cardinal, J.; Klune, J.R.; Chory, E.; Jeyabalan, G.; Kanzius, J.S.; Nalesnik, M.; Geller, D.A. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery 2008, 144, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Maddah, A.; Danesh, H.; Ziamajidi, N.; Khosravi, H.; Abbasalipourkabir, R. Oxidative Stress Induction by Gold Nanoparticles in HCT-116 Colon Cancer Cells. Compr. Health Biomed. Stud. 2023, 2, e145183. [Google Scholar] [CrossRef]
- Li, J.J.; Hartono, D.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y.L. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Johnston, H.; Gubbins, E.; Stone, V. Cytotoxicity and cytokine release in rat hepatocytes, C3A cells and macrophages exposed to gold nanoparticles—Effect of biological dispersion media or corona. J. Biomed. Nanotechnol. 2014, 10, 3416–3429. [Google Scholar] [CrossRef]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Sen, G.T.; Ozkemahli, G.; Shahbazi, R.; Erkekoglu, P.; Ulubayram, K.; Kocer-Gumusel, B. The Effects of Polymer Coating of Gold Nanoparticles on Oxidative Stress and DNA Damage. Int. J. Toxicol. 2020, 39, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Torres, A.C.; Lorenzo-Anota, H.Y.; García-Juárez, M.G.; Zarate-Triviño, D.G.; Rodríguez-Padilla, C. Chitosan gold nanoparticles induce different ROS-dependent cell death modalities in leukemic cells. Int. J. Nanomed. 2019, 14, 7173–7190. [Google Scholar] [CrossRef] [PubMed]
- Lingabathula, H.; Yellu, N. Cytotoxicity, oxidative stress, and inflammation in human Hep G2 liver epithelial cells following exposure to gold nanorods. Toxicol. Mech. Methods 2016, 26, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.; Morales, P.; Ávalos, A.; Haza, A.I. Oxidative stress contributes to gold nanoparticle-induced cytotoxicity in human tumor cells. Toxicol. Mech. Methods 2014, 24, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Riviere, J.E.; Monteiro-Riviere, N.A. Protein corona modulation of hepatocyte uptake and molecular mechanisms of gold nanoparticle toxicity. Nanotoxicology 2017, 11, 64–75. [Google Scholar] [CrossRef]
- Park, S.; Ha, M.K.; Lee, Y.; Song, J.; Yoon, T.H. Effects of Immune Cell Heterogeneity and Protein Corona on the Cellular Association and Cytotoxicity of Gold Nanoparticles: A Single-Cell-Based, High-Dimensional Mass Cytometry Study. ACS Nanosci. Au 2023, 3, 323–334. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Zheng, J.; Clogston, J.D.; Ayub, N.; Aggarwal, P.; Neun, B.W.; Hall, J.B.; McNeil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomedicine 2009, 5, 106–117. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Neun, B.W.; Man, S.; Ye, X.; Hansen, M.; Patri, A.K.; Crist, R.M.; McNeil, S.E. Protein corona composition does not accurately predict hematocompatibility of colloidal gold nanoparticles. Nanomedicine 2014, 10, 1453–1463. [Google Scholar] [CrossRef]




| Immune Cells | AuNPs Properties | Model/Cell Line | Mechanism of Action | Main Effect | Ref. | |
|---|---|---|---|---|---|---|
| Inflammatory | Anti-Inflammatory | |||||
| T cells | PDDAC-, CTAB-, and PEI-modified Au nanorods (60 × 15 nm) | BALB/c mice | - ↑DC maturation - ↑APC co-stimulator molecules - ↑T CD4+ and CD8+ proliferation - ↑Th2 responses | ✔ | [190] | |
| 50 and 10 nm | DCs and T cells (PBMCs) | - ↑DC activation - 50 nm—↑Th1 and Th17 differentiation and cytokine secretion - 10 nm AuNP—↓IL-12p70, ↑Th2 differentiation and ↑IL-10 | ✔ (50 nm) | ✔ (10 nm) | [88] | |
| Dextran-modified AuNPs (~210–305 nm) | Splenocytes (OT-I and OT-II mice); BMDCs | - ↑Antigen presentation (BMDCs) - ↑MHC-I, MHC-II - ↑CD40, CD80, CD86 - ↑CTL and Th1 responses | ✔ | [192] | ||
| Polyelectrolyte-coated AuNPs (~100–200 nm) | Splenic CD11c + DCs (B6 mice); Splenocytes (OT-I and OT-II mice) | - ↑DCs activation and antigen presentation - ↑CD86, CD80, CD40, TLR3 signaling - ↑Antigen-specific T CD8 response | ✔ | [193] | ||
| 2 and 12 nm | PBMCs, human monocyte-derived DCs | - 12 nm: ↑NK cell proliferation, IL-12 and IFN-γ cytokines, Th1 and CTLs responses - 2 nm: ↑Uptake; mild immunosuppression | ✔ (12 nm) | ✔ (2 nm) | [176] | |
| 2 and 12 nm | PBMCs; human monocyte-derived DCs | - 12 nm: Th1 cell-mediated immunity, inflammatory NK cells - 2 nm: ~DC maturation | ✔ (12 nm) | ✔ (2 nm) | [166] | |
| AuNP-Allergen (15 nm) | BALB/c mice | - ↑IFN-γ and TGF-β - ↑Th1-Treg polarization - ↓IL-4 | ✔ | [169] | ||
| AuNP-Allergen (15 nm) | BALB/c mice | - ↑IFN-γ, IL-10, and TGF-β - ↑Th1-Treg polarization - ↓Th2-Th17 polarization - ↓IL-4 and IL-17A secretion | ✔ | [71] | ||
| 97.01 ± 7.29 nm | BMDCs (C57BL/6 mice); CD4+ (OT-II mice); J774.1A | - ↑IL-6, MCP-1, ROS, and TNF-α and antigen presentation capacity (MQs and DCs) - ↑Mitochondrial respiration and glycolysis (MQs) - ↑Th1, Th2, and Th17 responses | ✔ | [100] | ||
| B cells | PDDAC-, CTAB-, and PEI-modified Au nanorods (60 × 15 nm) | BALB/c mice | - ↑IgG2a production | ✔ | [190] | |
| PEG- and PEG/PVA-AuNPs | B lymphocytes (human Buffy coats) | - Uncoated nanospheres and nanorods AuNPs ↓IL-6 secretion | ✔ | [194] | ||
| AuNP-Allergen (15 nm) | BALB/c mice | - allergen-IgE B cell secretory IgG B cells | ✔ | [169] | ||
| AuNP-Allergen (15 nm) | BALB/c mice | - Change allergen-IgE B cell responses to secretory IgG B cells | ✔ | [71] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koushki, K.; Biswal, P.; Vijay, G.V.; Sadeghi, M.; Dehnavi, S.; Tra, N.T.; Samala, S.K.; Yousefi Taba, M.; Vasan, A.B.; Han, E.; et al. Immunomodulatory Effects of Gold Nanoparticles: Impacts on Immune Cells and Mechanisms of Action. Nanomaterials 2025, 15, 1201. https://doi.org/10.3390/nano15151201
Koushki K, Biswal P, Vijay GV, Sadeghi M, Dehnavi S, Tra NT, Samala SK, Yousefi Taba M, Vasan AB, Han E, et al. Immunomodulatory Effects of Gold Nanoparticles: Impacts on Immune Cells and Mechanisms of Action. Nanomaterials. 2025; 15(15):1201. https://doi.org/10.3390/nano15151201
Chicago/Turabian StyleKoushki, Khadijeh, Prapannajeet Biswal, Geraldine Vidhya Vijay, Mahvash Sadeghi, Sajad Dehnavi, Ngoc Tuyet Tra, Sai Kumar Samala, Mahdieh Yousefi Taba, Arjun Balaji Vasan, Emily Han, and et al. 2025. "Immunomodulatory Effects of Gold Nanoparticles: Impacts on Immune Cells and Mechanisms of Action" Nanomaterials 15, no. 15: 1201. https://doi.org/10.3390/nano15151201
APA StyleKoushki, K., Biswal, P., Vijay, G. V., Sadeghi, M., Dehnavi, S., Tra, N. T., Samala, S. K., Yousefi Taba, M., Vasan, A. B., Han, E., Mackeyev, Y., & Krishnan, S. (2025). Immunomodulatory Effects of Gold Nanoparticles: Impacts on Immune Cells and Mechanisms of Action. Nanomaterials, 15(15), 1201. https://doi.org/10.3390/nano15151201







