Study on the Durability of Graphene Oxide–Nanosilica Hybrid-Modified Sticky Rice–Lime Paste
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of GO–NS
2.3. Production of Modified Sticky Rice–Lime Paste Specimens
2.4. Test Methods
2.4.1. Physical Property Tests
2.4.2. Durability Test
2.4.3. Characterisation Technique
3. Results and Discussions
3.1. Characterisation of GO–NS
3.2. Physical Property Analysis
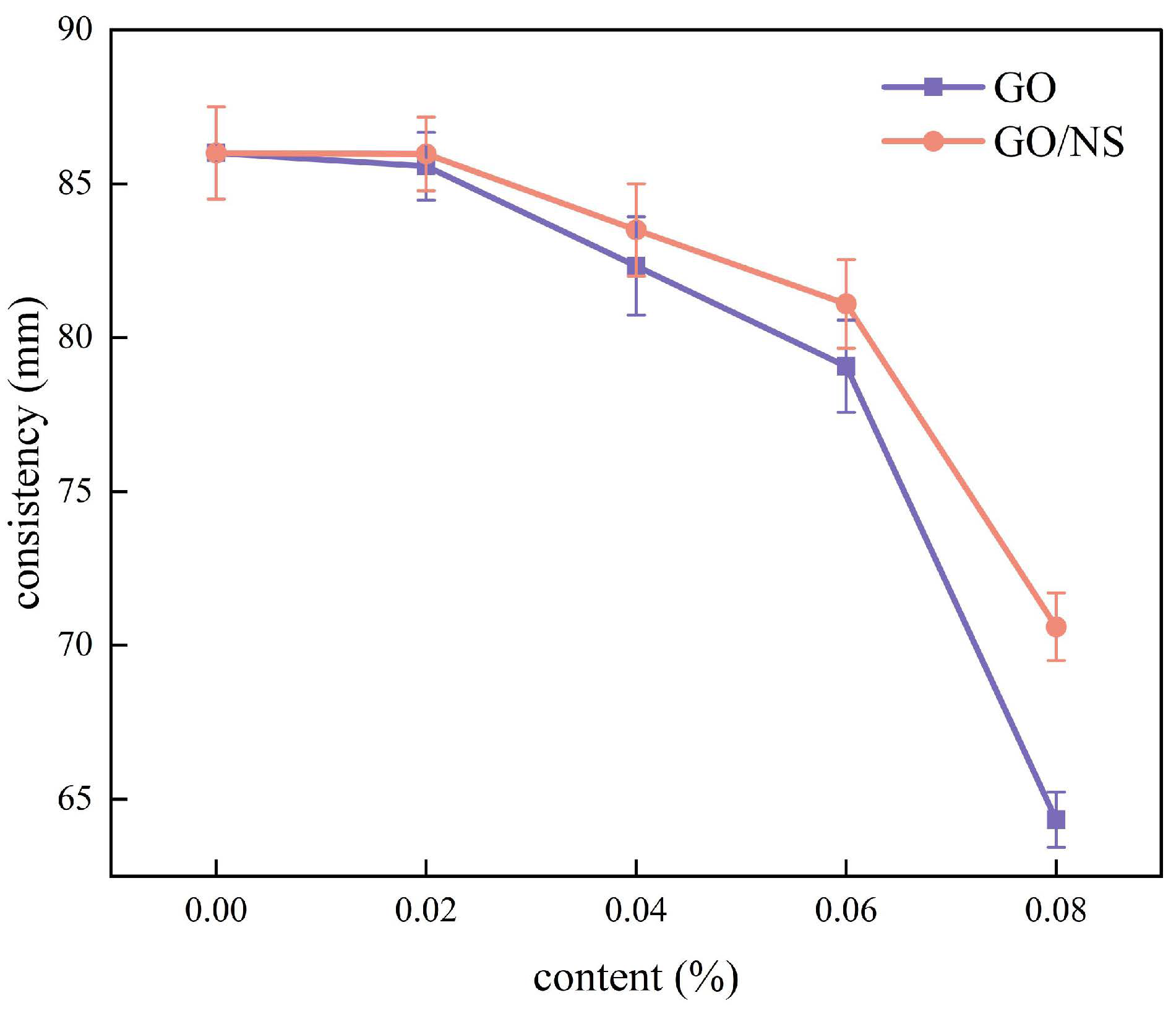
3.2.1. Consistency
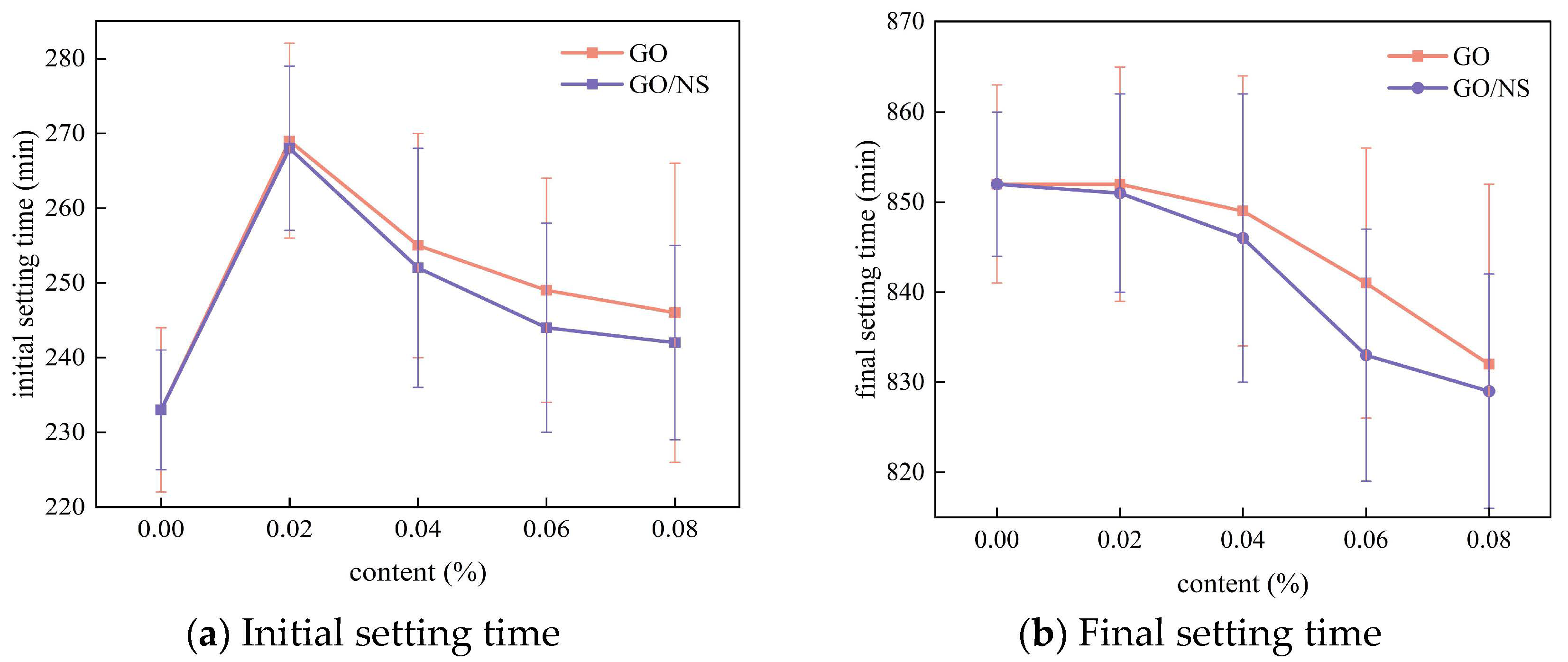
3.2.2. Setting Time
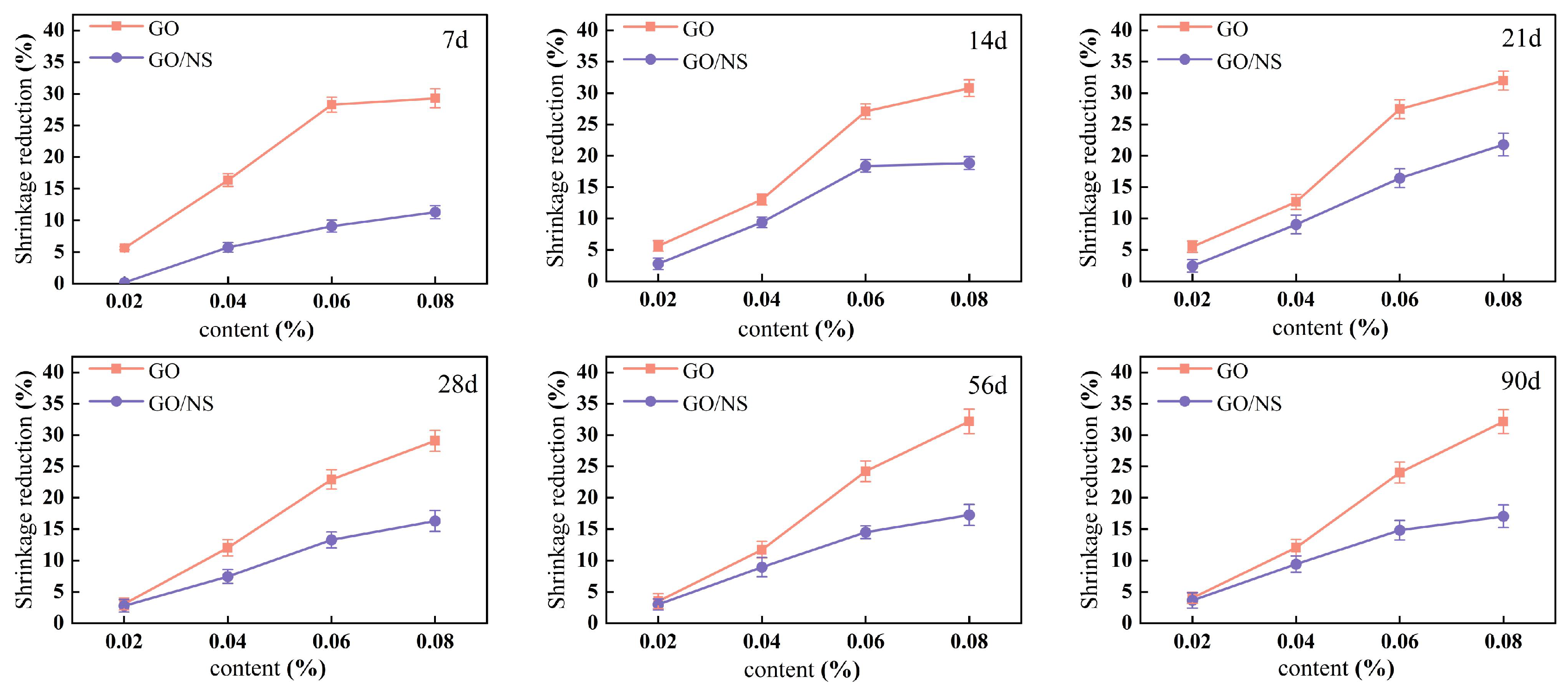
3.2.3. Shrinkage Rate
3.3. Durability Analysis
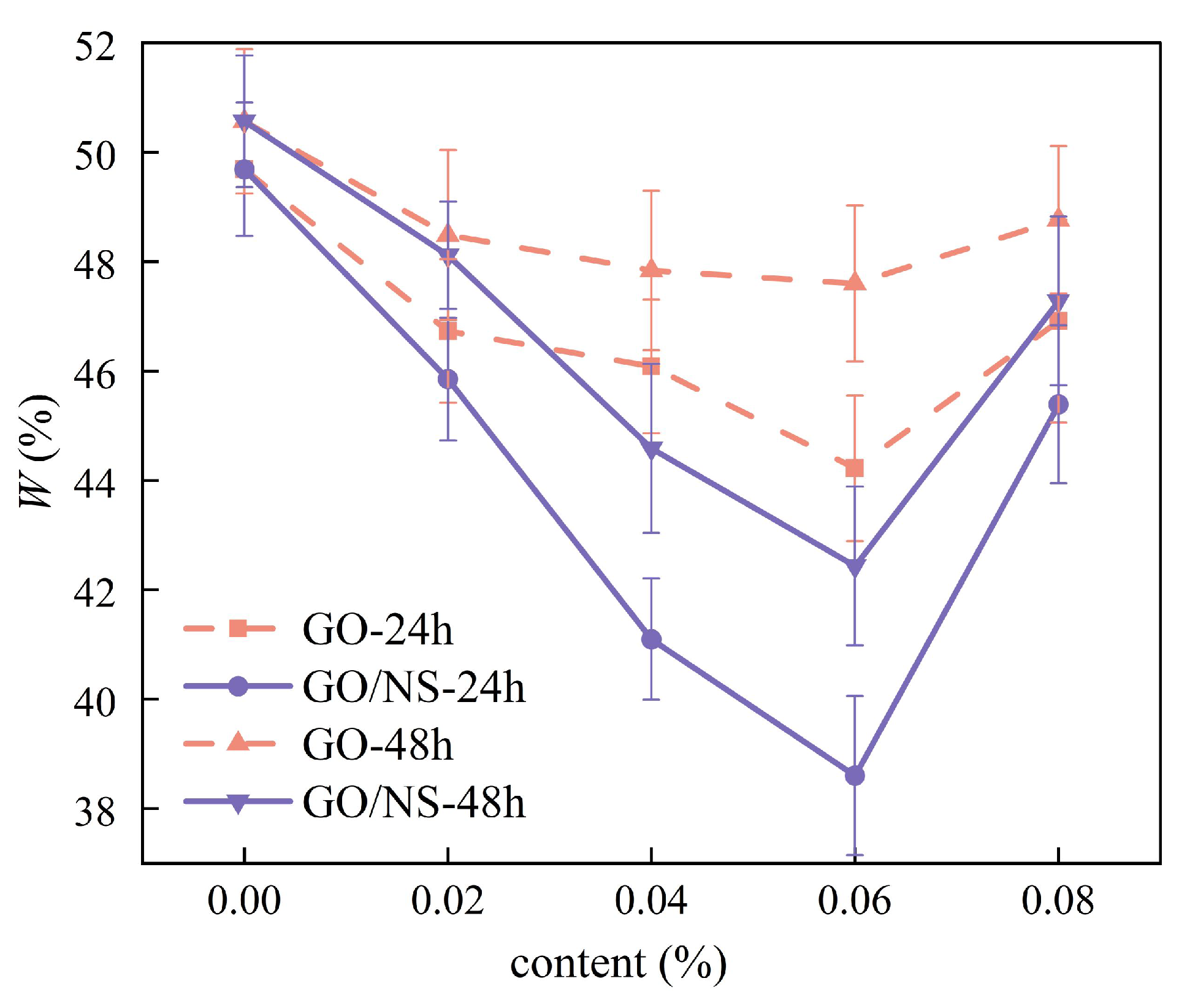
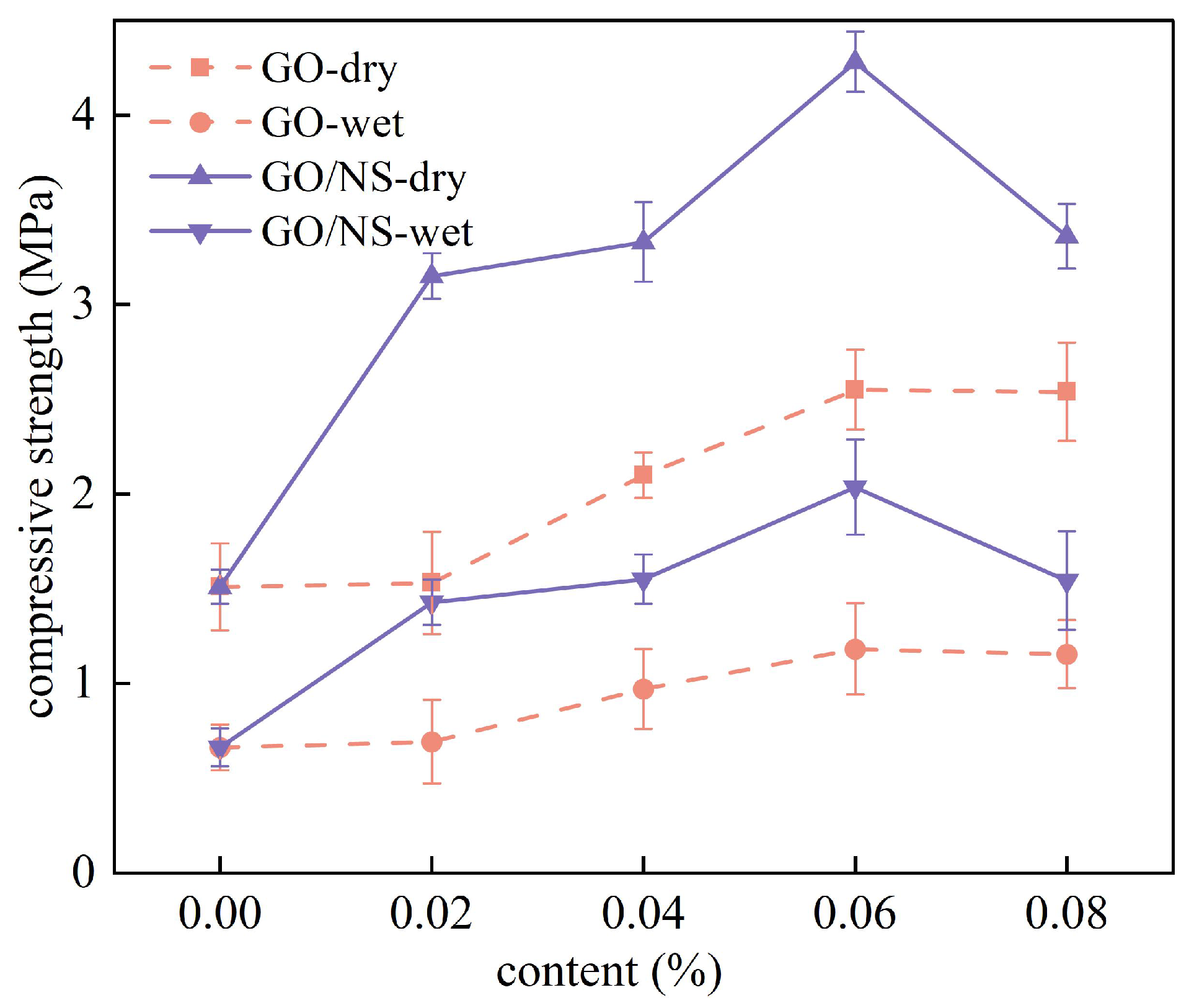
3.3.1. Water Resistance
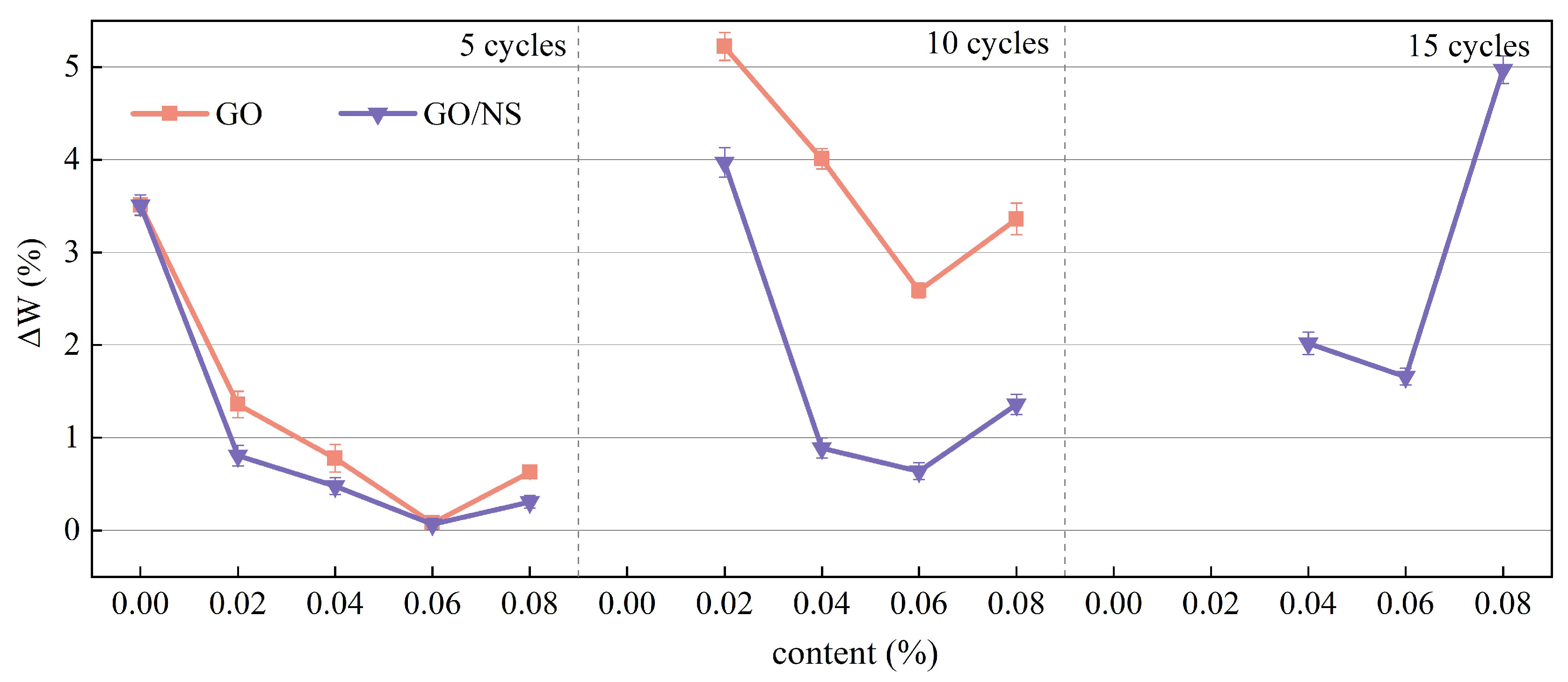
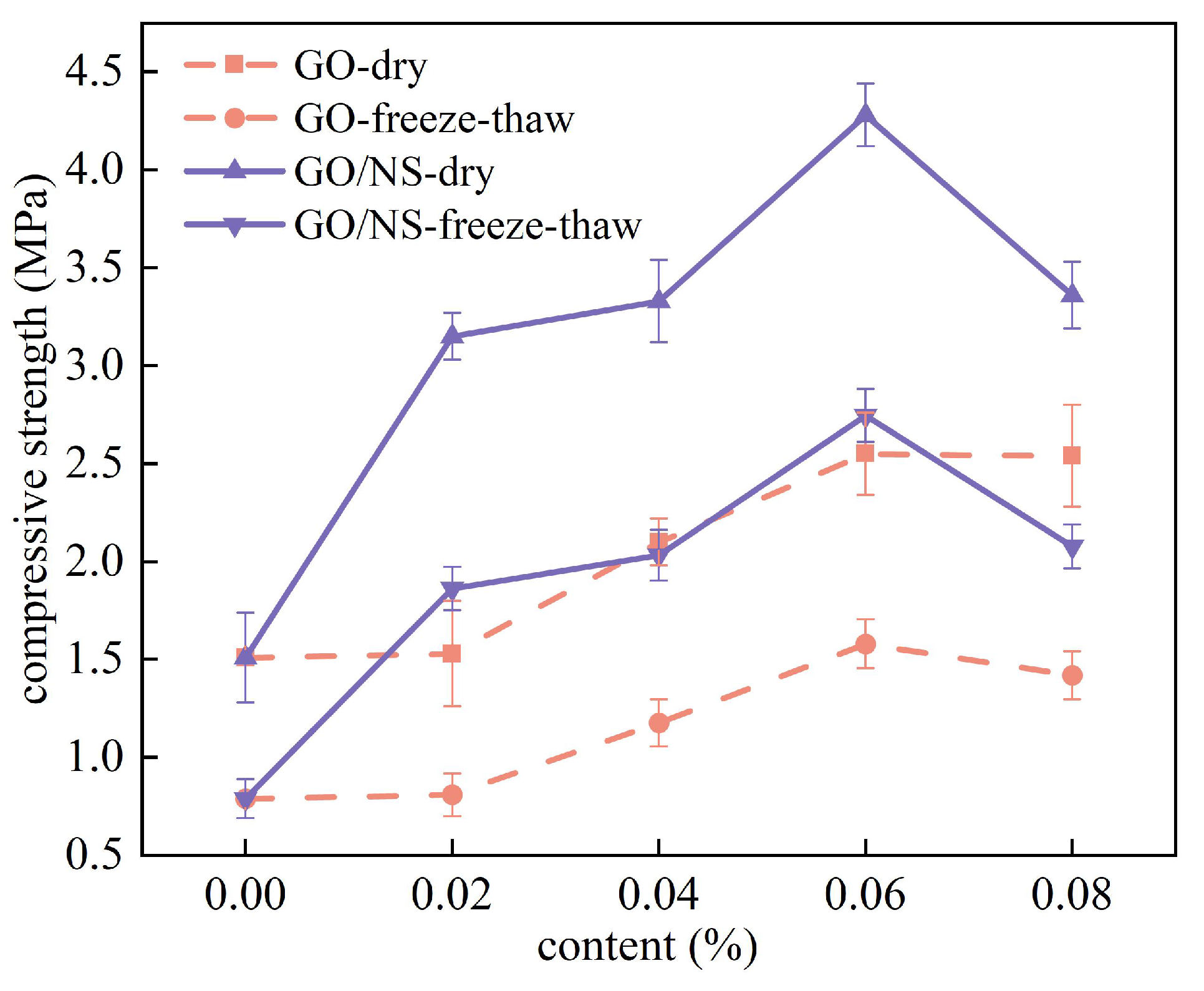
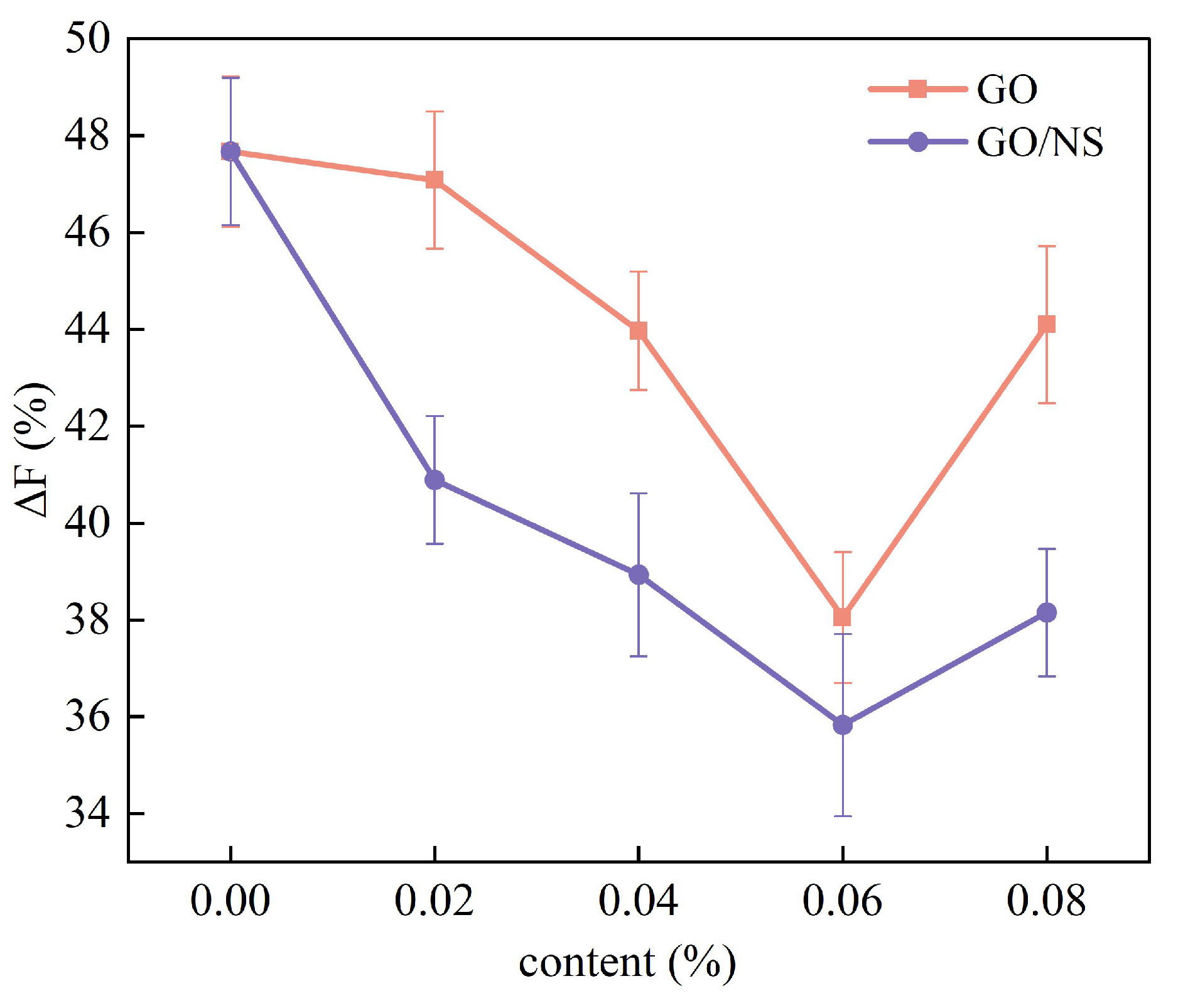
3.3.2. Freeze–Thaw Cycle Resistance
- (1)
- Mass loss
- (2)
- Strength loss
3.4. Characterisation of GO–NS-Modified Sticky Rice–Lime Paste
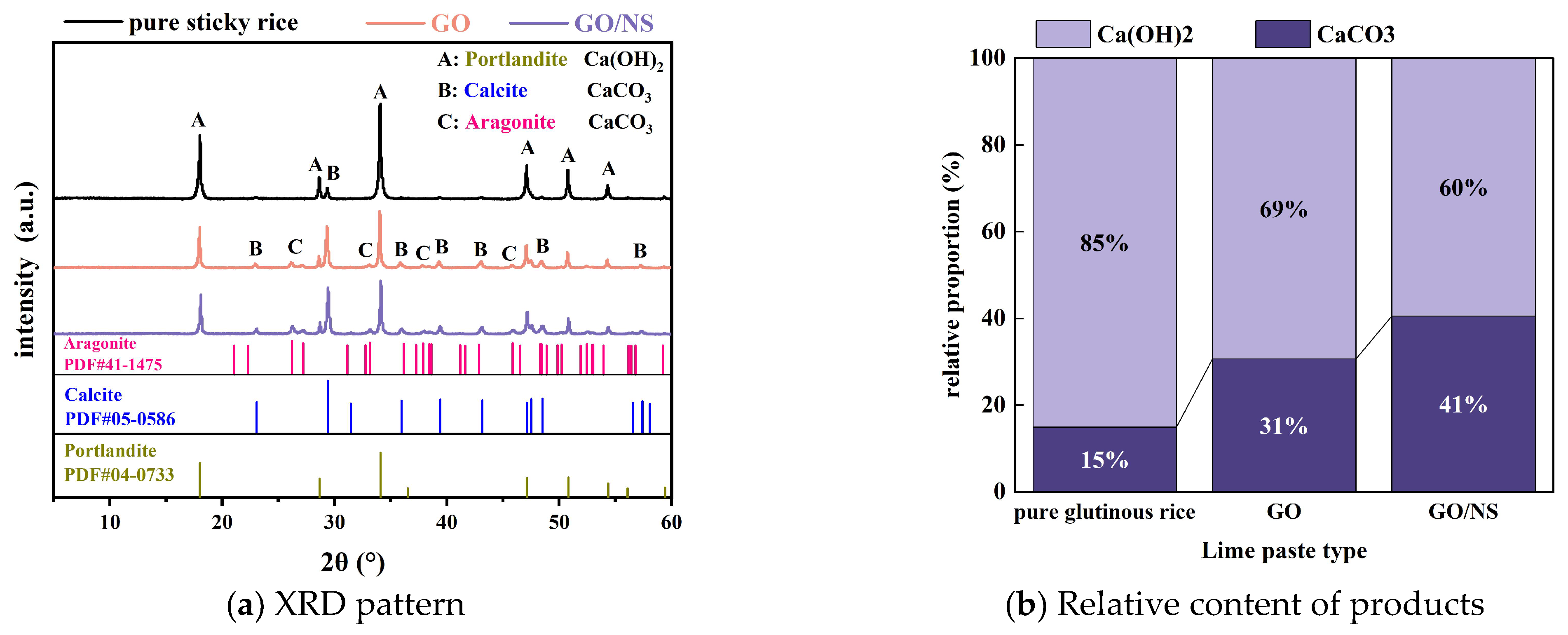
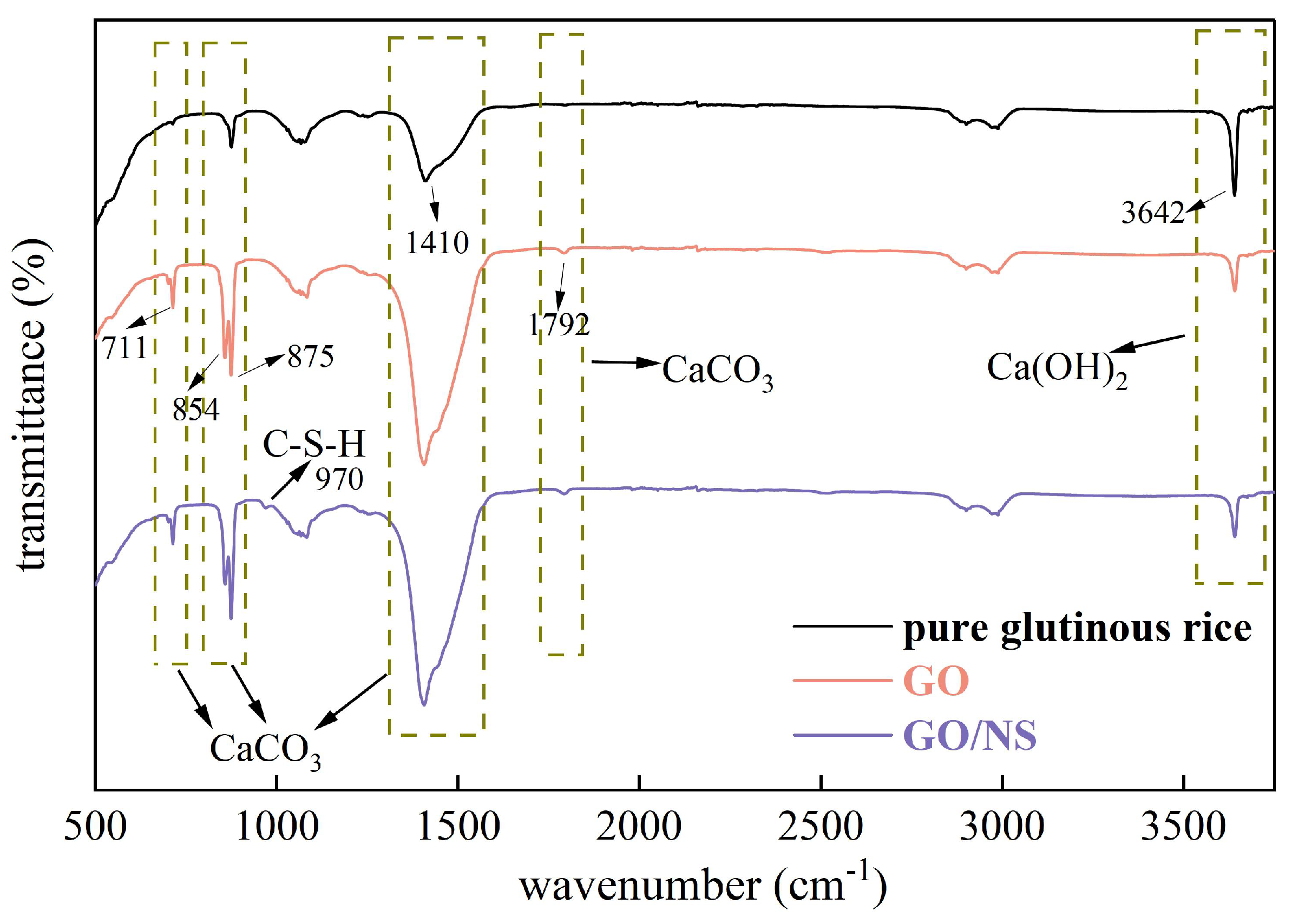
3.4.1. XRD and FTIR
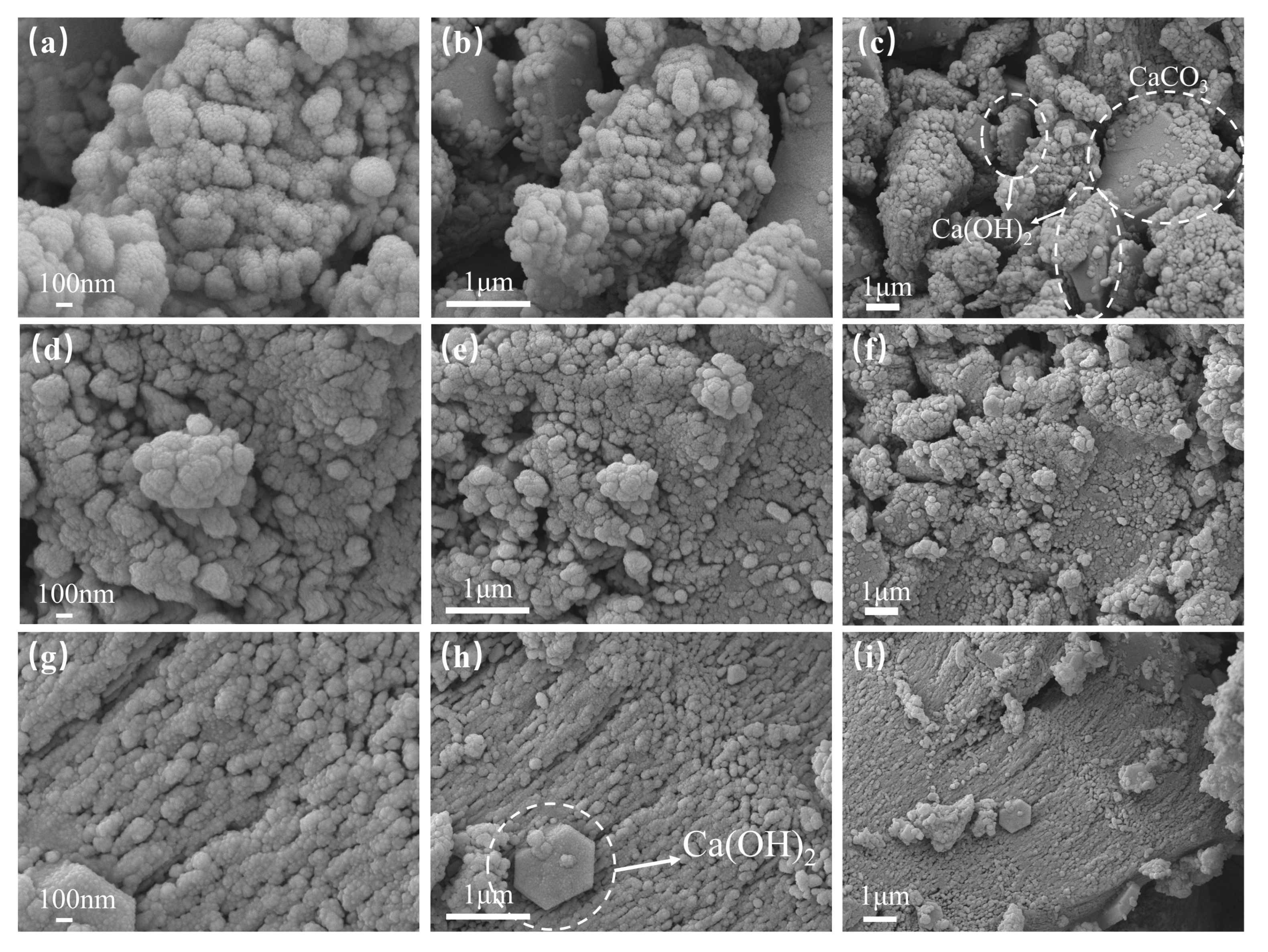
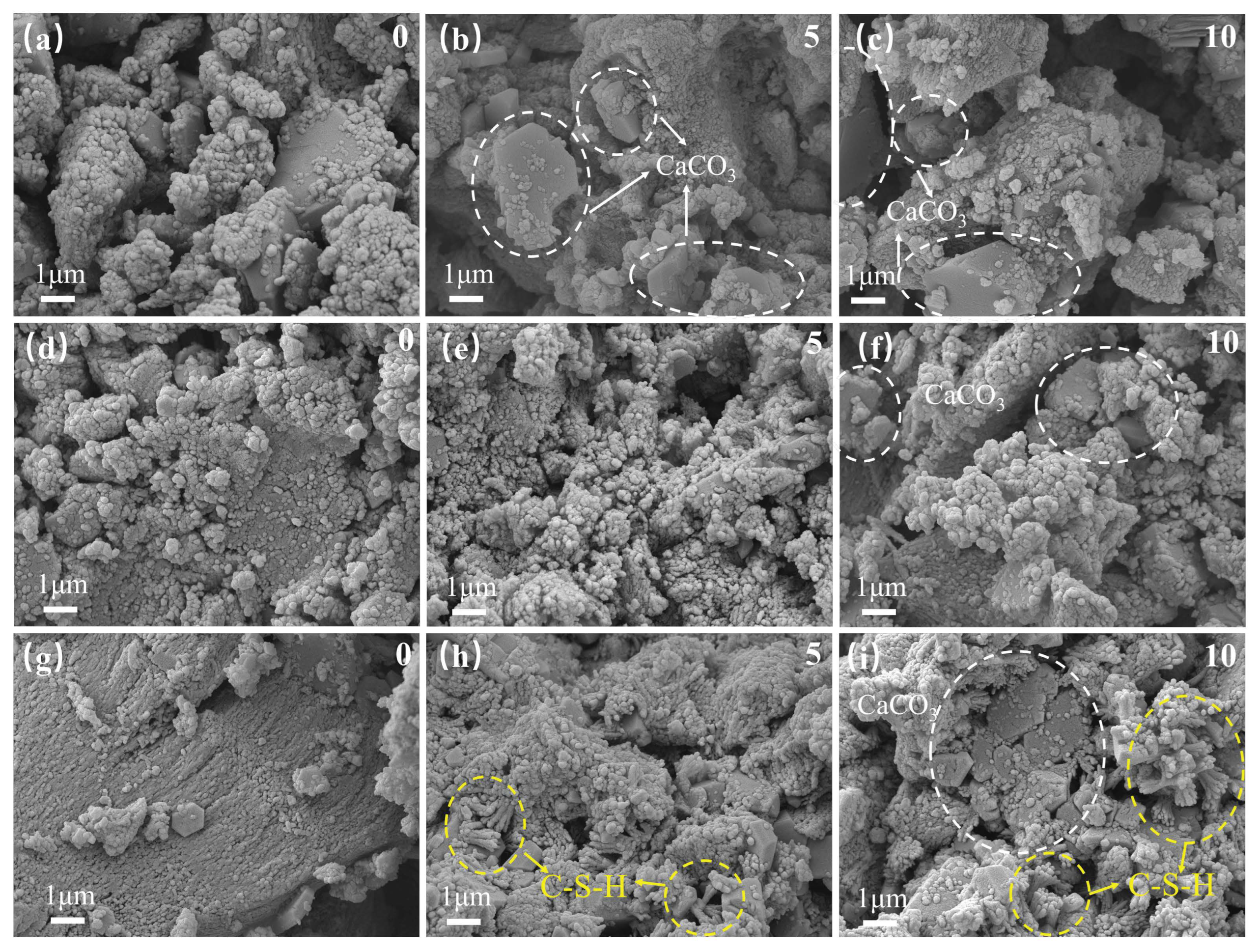
3.4.2. SEM
- (1)
- Microstructure of hardened sticky rice–lime paste
- (2)
- Microstructure after freeze–thaw cycles
3.5. The Relationship Between Macro and Micro
4. Conclusions
- (1).
- The characterisation of the surface morphology and chemical structure of GO–NS hybrids shows that NS can be successfully attached to the surface of GO, which can increase the interlayer spacing of GO to reduce the stacking effect caused by interlayer van der Waals forces and ultimately give full play to the template role of GO.
- (2).
- GO–NS reduces the fluidity of the sticky rice–lime paste, and is able to significantly prolong the initial setting time and shorten the final setting time. It also reduces the shrinkage of the sticky rice–lime paste, but its ability to reduce shrinkage is slightly weaker than that of GO due to the chemical shrinkage caused by C–S–H in the hardening product of GO–NS.
- (3).
- GO–NS significantly improves the compressive strength of sticky rice–lime paste and reduces water absorption as well as the strength loss after water absorption. Moreover, it can increase the number of freeze–thaw cycles and reduce the mass loss and strength loss after freeze–thaw cycles. When the optimum content of GO–NS is 0.06%, the durability of sticky rice–lime paste can be significantly improved.
- (4).
- The characterisation analysis revealed that GO–NS provided more nucleation sites for the hardening of sticky rice–lime paste due to its superior templating role. GO–NS is able to induce more Ca(OH)2 to produce more CaCO3, and at the same time, is able to generate C–S–H due to the volcanic ash effect of NS. After the freeze–thaw cycle, with the dissolution of the sticky rice paste and Ca(OH)2, the CaCO3 crystal structure and the unique C–S–H structure of GO–NS can be observed in the micro-morphology. Therefore, with the synergy of the dual products, the hardened GO–NS-modified sticky rice–lime paste obtained higher compactness and showed better durability.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zhang, B. Why Ancient Chinese People Like to Use Organic–Inorganic Composite Mortars?—Application History and Reasons of Organic–Inorganic Mortars in Ancient Chinese Buildings. J. Archaeol. Method Theory 2019, 26, 502–536. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, B.; Liang, X. A case study and mechanism investigation of typical mortars used on ancient architecture in China. Thermochim. Acta 2008, 473, 1–6. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Anagnostopoulou, S. Composite materials in ancient structures. Cem. Concr. Compos. 2004, 27, 295–300. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, H.; Fang, S.; Li, J.; Zheng, Y.; Zhang, B. Textual and Experimental Studies on The Compositions of Traditional Chinese Organic–Inorganic Mortars. Archaeometry 2014, 56, 100–115. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, B.; Ma, Q. Study of Sticky Rice−Lime Mortar Technology for the Restoration of Historical Masonry Construction. Acc. Chem. Res. 2010, 43, 936–944. [Google Scholar] [CrossRef]
- Zhai, K.; Zhu, H.; Luo, L.; Zhang, B.; Zhu, L.; Zhang, Q.; Zhao, P. Exploration of the rules for the use of organic additives in the mortar of the Forbidden city. J. Cult. Herit. 2024, 70, 71–79. [Google Scholar] [CrossRef]
- Tian, P.; Yang, W.; Lu, J.; Wu, X.; Wang, Z. Study on the shear properties of masonry pagoda mortar and its influencing factors. Case Stud. Constr. Mater. 2024, 20, e02981. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, H.; Wang, H.; Fang, S.; Zhang, B.; Yang, F. An experimental study on application of sticky rice–lime mortar in conservation of the stone tower in the Xiangji Temple. Constr. Build. Mater. 2012, 28, 624–632. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreira Pinto, A.P.; Gomes, A. Natural hydraulic lime versus cement for blended lime mortars for restoration works. Constr. Build. Mater. 2015, 94, 346–360. [Google Scholar] [CrossRef]
- Groot, C.; Veiga, R.; Papayianni, I.; Van Hees, R.; Secco, M.; Alvarez, J.I.; Faria, P.; Stefanidou, M. RILEM TC 277-LHS report: Lime-based mortars for restoration–a review on long-term durability aspects and experience from practice. Mater. Struct. 2022, 55, 245. [Google Scholar] [CrossRef]
- Ventolà, L.; Vendrell, M.; Giraldez, P.; Merino, L. Traditional organic additives improve lime mortars: New old materials for restoration and building natural stone fabrics. Constr. Build. Mater. 2011, 25, 3313–3318. [Google Scholar] [CrossRef]
- Wang, S.; Meng, Z.; Zhao, Q.; Zhao, T.; Jin, J.; Lu, Z. Experimental study on the characteristics and resistance against the coupling effect of freeze-thaw and chloride salt erosion of sticky rice-lime composites modified with metakaolin and hemp fibers. Constr. Build. Mater. 2024, 416, 135240. [Google Scholar] [CrossRef]
- Faria, P.; Duarte, P.; Barbosa, D.; Ferreira, I. New composite of natural hydraulic lime mortar with graphene oxide. Constr. Build. Mater. 2017, 156, 1150–1157. [Google Scholar] [CrossRef]
- Theodoridou, M.; Charalambous, E.; Maravelaki-Kalaitzaki, P.; Ioannou, I. Amelioration of crushed brick-lime composites using nano-additives. Cem. Concr. Compos. 2016, 68, 77–87. [Google Scholar] [CrossRef]
- Duran, A.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Long-term mechanical resistance and durability of air lime mortars with large additions of nanosilica. Constr. Build. Mater. 2014, 58, 147–158. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Shen, L.; Bao, N. Revisiting the Oxidation of Graphite: Reaction Mechanism, Chemical Stability, and Structure Self-Regulation. ACS Omega 2020, 5, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ge, Y.; Cai, C.S.; Zhang, Y.; Liu, Z. Mechanical properties and microstructure of graphene oxide cement-based composites. Constr. Build. Mater. 2019, 194, 102–109. [Google Scholar] [CrossRef]
- Zhu, X.H.; Kang, X.J.; Yang, K.; Yang, C.H. Effect of graphene oxide on the mechanical properties and the formation of layered double hydroxides (LDHs) in alkali-activated slag cement. Constr. Build. Mater. 2017, 132, 290–295. [Google Scholar] [CrossRef]
- Shamsaei, E.; de Souza, F.B.; Yao, X.; Benhelal, E.; Akbari, A.; Duan, W. Graphene-based nanosheets for stronger and more durable concrete: A review. Constr. Build. Mater. 2018, 183, 642–660. [Google Scholar] [CrossRef]
- Long, W.-J.; Wei, J.-J.; Xing, F.; Khayat, K.H. Enhanced dynamic mechanical properties of cement paste modified with graphene oxide nanosheets and its reinforcing mechanism. Cem. Concr. Compos. 2018, 93, 127–139. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Papers Modified by Divalent Ions—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Chuah, S.; Li, W.; Chen, S.J.; Sanjayan, J.G.; Duan, W.H. Investigation on dispersion of graphene oxide in cement composite using different surfactant treatments. Constr. Build. Mater. 2018, 161, 519–527. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Ge, C.; Li, Q.; Guo, L.; Shu, X.; Liu, J. Mechanical behavior and toughening mechanism of polycarboxylate superplasticizer modified graphene oxide reinforced cement composites. Compos. Pt. B-Eng. 2017, 113, 308–316. [Google Scholar] [CrossRef]
- Lu, Z.; Hanif, A.; Ning, C.; Shao, H.; Yin, R.; Li, Z. Steric stabilization of graphene oxide in alkaline cementitious solutions: Mechanical enhancement of cement composite. Mater. Des. 2017, 127, 154–161. [Google Scholar] [CrossRef]
- José, D.; Radek, Š.; Petra, M.; Beatriz, M.; Dita, F.; Zuzana, S. Impact of nanosilica on lime restoration mortars properties. J. Cult. Herit. 2022, 55, 210–220. [Google Scholar] [CrossRef]
- Miao, X.; Xing, Y.; Zheng, H.; Liu, Q.; Hu, M.; Guo, J. Effects of Hybrid Graphene Oxide-Nanosilica on Calcium Silicate Hydrate in the Simulation Environment and Cement. ACS Omega 2023, 8, 22975–22983. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Fernández, J.M.; Navarro-Blasco, I.; Duran, A.; Sirera, R. Microstructural consequences of nanosilica addition on aerial lime binding materials: Influence of different drying conditions. Mater. Charact. 2013, 80, 36–49. [Google Scholar] [CrossRef]
- Lin, J.; Shamsaei, E.; Basquiroto de Souza, F.; Sagoe-Crentsil, K.; Duan, W.H. Dispersion of graphene oxide–silica nanohybrids in alkaline environment for improving ordinary Portland cement composites. Cem. Concr. Compos. 2020, 106, 103488. [Google Scholar] [CrossRef]
- Lu, Z.; Hou, D.; Hanif, A.; Hao, W.; Li, Z.; Sun, G. Comparative evaluation on the dispersion and stability of graphene oxide in water and cement pore solution by incorporating silica fume. Cem. Concr. Compos. 2018, 94, 33–42. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, L.; Xu, N.; Jin, M.; Jiang, S. Enhancement of mechanical and electrical properties of graphene/cement composite due to improved dispersion of graphene by addition of silica fume. Constr. Build. Mater. 2018, 164, 433–441. [Google Scholar] [CrossRef]
- Kou, L.; Gao, C. Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 2011, 3, 519–528. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, S.; Wu, H.; Zhang, X.; Tao, Q.; Song, L.; Song, Y.; Guo, X. Deep research about the mechanisms of graphene oxide (GO) aggregation in alkaline cement pore solution. Constr. Build. Mater. 2020, 247, 118446. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, G.; Zhan, D.; Wang, Y.; Zheng, H. Influence of the molecular structure of a polycarboxylate superplasticiser on the dispersion of graphene oxide in cement pore solutions and cement-based composites. Constr. Build. Mater. 2021, 272, 121969. [Google Scholar] [CrossRef]
- JGJ/T 70-2009; Standard for Test Method of Basic Properties of Construction Mortar. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2009.
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. National Standardization Administration: Beijing, China, 2011.
- Hu, M.; Guo, J.; Li, P.; Chen, D.; Xu, Y.; Feng, Y.; Yu, Y.; Zhang, H. Effect of characteristics of chemical combined of graphene oxide-nanosilica nanocomposite fillers on properties of cement-based materials. Constr. Build. Mater. 2019, 225, 745–753. [Google Scholar] [CrossRef]
- Yang, T.; Ma, X.; Zhang, B.; Zhang, H. Investigations into the function of sticky rice on the microstructures of hydrated lime putties. Constr. Build. Mater. 2016, 102, 105–112. [Google Scholar] [CrossRef]
- Zhao, P.; Duan, H.; Zhang, Y.; Shen, Y.; Li, X.; Shen, H.; Zhu, W.; Liu, G.; Pang, B. Study on the difference of composition between traditional and modern lime used in the restoration of Qufu San Kong Ancient Architectural. Case Stud. Constr. Mater. 2024, 21, e04055. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Y.; Xu, X.; Pan, H.; Tang, R. Transformation of amorphous calcium carbonate into aragonite. J. Cryst. Growth 2012, 343, 62–67. [Google Scholar] [CrossRef]
- Higl, J.; Hinder, D.; Rathgeber, C.; Ramming, B.; Lindén, M. Detailed in situ ATR-FTIR spectroscopy study of the early stages of C-S-H formation during hydration of monoclinic C3S. Cem. Concr. Res. 2021, 142, 106367. [Google Scholar] [CrossRef]
- Solanki, A.; Sharma, U.; Singh, L.P.; Karade, S.R. Mineralogical and microstructural studies of C–S–H exposed to sulphate attack. Eur. J. Environ. Civ. Eng. 2023, 27, 3489–3506. [Google Scholar] [CrossRef]
- Xu, S.; Liu, J.; Xue, Y.; Wu, T.; Zhang, Z. Appropriate conditions for preparing few-layered graphene oxide and reduced graphene oxide. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 40–46. [Google Scholar] [CrossRef]
- Thomas, J.J.; Jennings, H.M.; Allen, A.J. Relationships between Composition and Density of Tobermorite, Jennite, and Nanoscale CaO−SiO2−H2O. J. Phys. Chem. C 2010, 114, 7594–7601. [Google Scholar] [CrossRef]

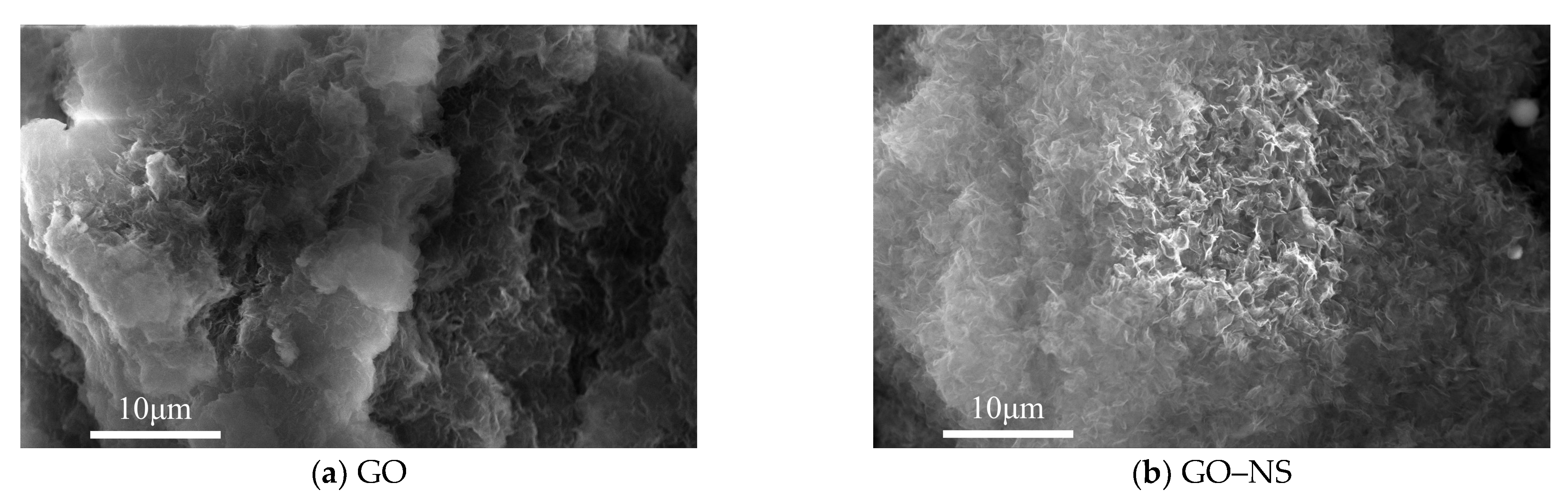
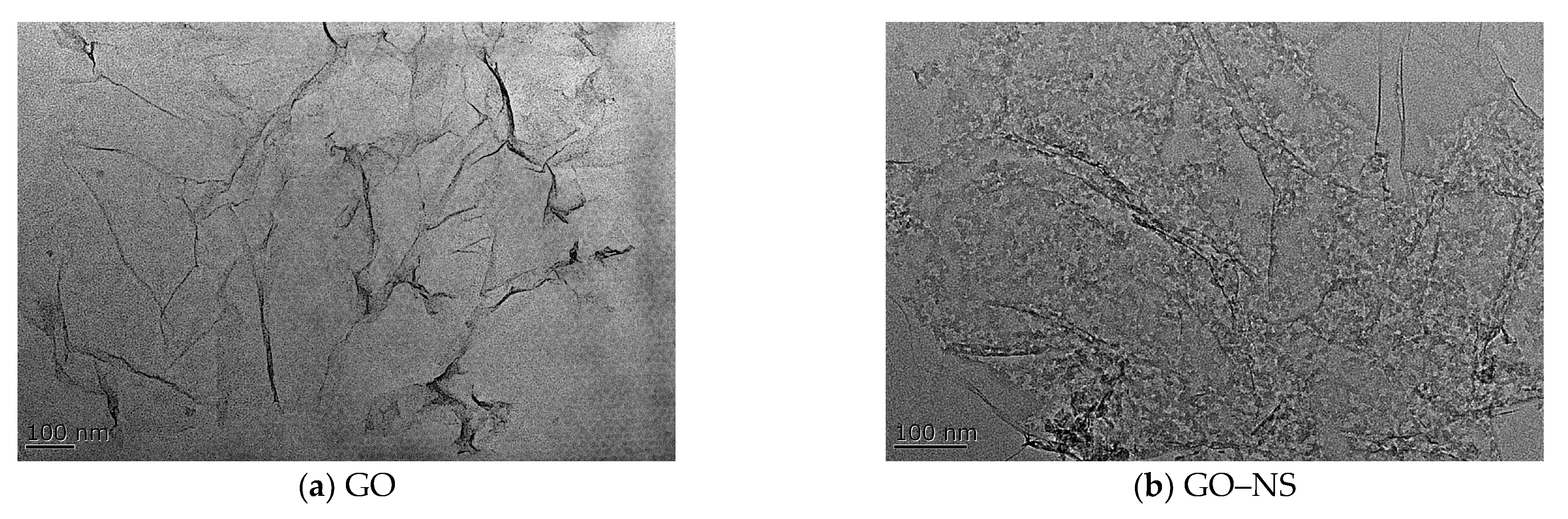
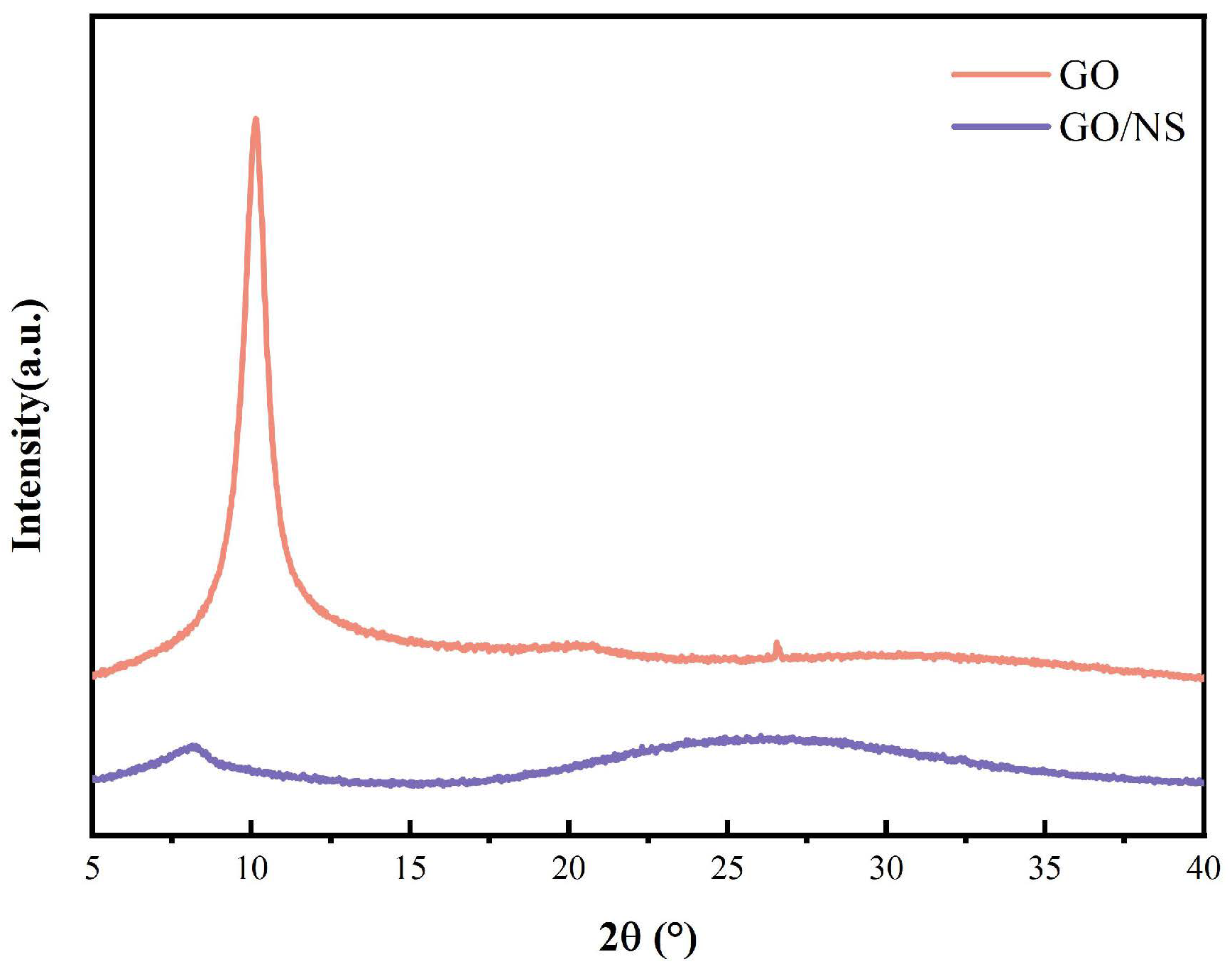
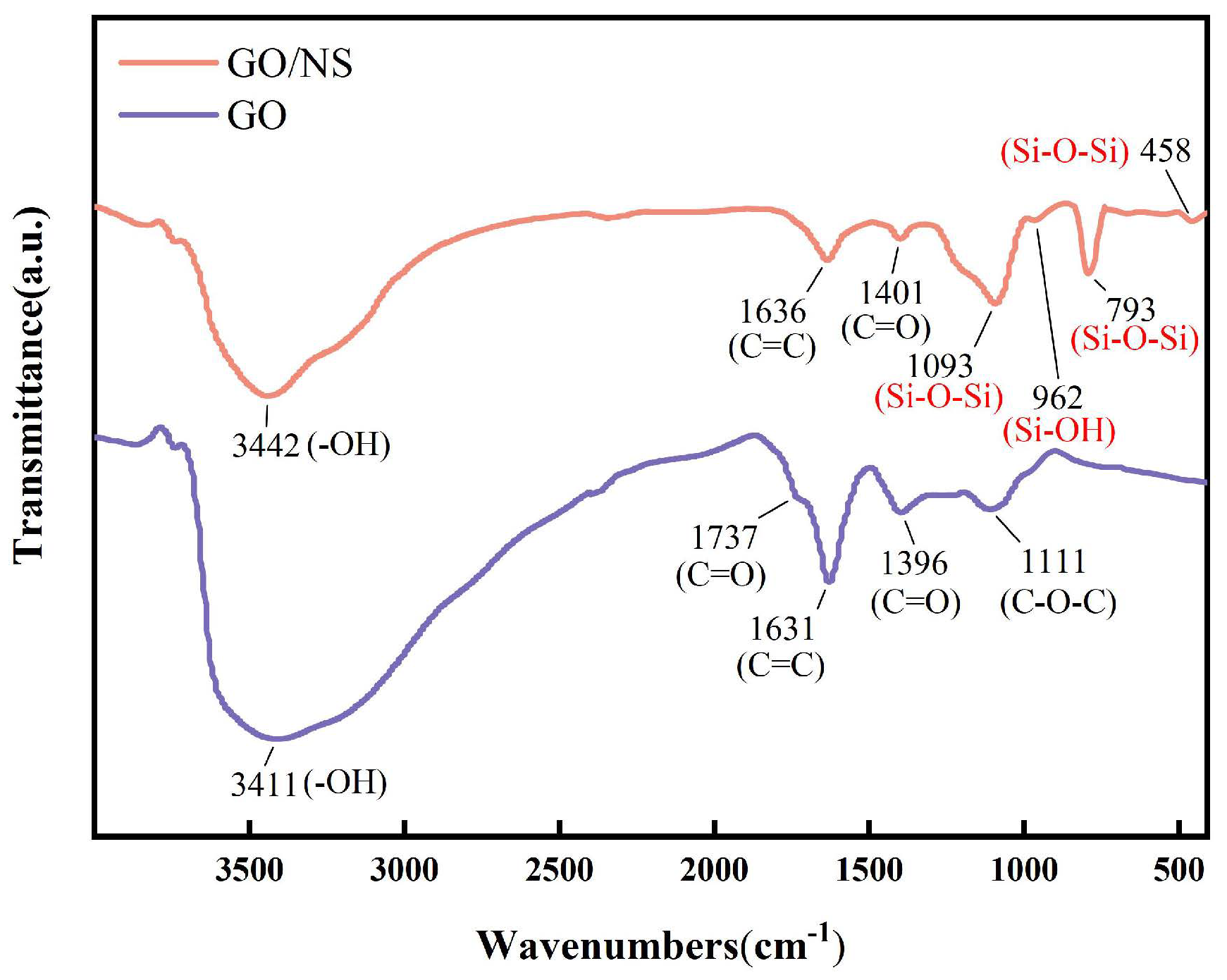


| Appearances | Layer Diameter/um | Purity/% | Carbon Content/% | Oxygen Content/% | Sulphur Content/% |
|---|---|---|---|---|---|
| Brown powder | 0.2~5.0 | >95 | <45 | >54 | <1 |
| Instruments | Type | Parameters | Manufacturer |
|---|---|---|---|
| Ultrasonic disperser | F-100SD | Frequency: 40 KHz, power: 600 W | Shenzhen Fuyang Technology Group Co., Ltd. (Shenzhen, China) |
| High-speed centrifuge | TG16 | Maximum RPM: 16,000 r/min Maximum Relative Centrifugal Force: 17,800× g | Hunan Changsha Yingtai Instrument Co., Ltd. (Changsha, China) |
| Vacuum drying oven | DZF6020 | Vacuum: 133 Pa, temperature control: RT + 10~200 °C | Tianjin Zhongjia Instrument Co., Ltd. (Tianjin, China) |
| Precision electronic balance | CN-LQC1003 | Precision: 0.001 g | Kunshan Youkewei Electronic Technology Co., Ltd. (Kunshan, China) |
| Paste Type | Sticky Rice Content/% | Water–Lime Ratio | PEC/% | GO Content/% | GO–NS Content/% |
|---|---|---|---|---|---|
| Pure sticky rice–lime paste | 5 | 0.8 | 1 | — | — |
| GO-0.02 | 0.02 | — | |||
| GO-0.04 | 0.04 | — | |||
| GO-0.06 | 0.06 | — | |||
| GO-0.08 | 0.08 | — | |||
| GO–NS-0.02 | — | 0.02 | |||
| GO–NS-0.04 | — | 0.04 | |||
| GO–NS-0.06 | — | 0.06 | |||
| GO–NS-0.08 | — | 0.08 |
| Lime Paste Type | Max. Number of Freeze–Thaw Cycles | Description of Typical Damage Patterns | Typical Damage Patterns |
|---|---|---|---|
| Pure sticky rice–lime paste | 7 | Corner flaking |  |
| GO-0.02 | 10 | Severe peeling |  |
| GO-0.04 | 13 | ||
| GO-0.06 | 14 | ||
| GO-0.08 | 12 | Peeling, missing blocks |  |
| GO–NS-0.02 | 13 | Cracks |  |
| GO–NS-0.04 | 16 | Seriously missing blocks |  |
| GO–NS-0.06 | 18 | ||
| GO–NS-0.08 | 16 | Missing corner blocks, cracks |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Cheng, D.; Fu, Y.; Yan, X.; Wang, L.; Ren, H. Study on the Durability of Graphene Oxide–Nanosilica Hybrid-Modified Sticky Rice–Lime Paste. Nanomaterials 2025, 15, 1194. https://doi.org/10.3390/nano15151194
Li K, Cheng D, Fu Y, Yan X, Wang L, Ren H. Study on the Durability of Graphene Oxide–Nanosilica Hybrid-Modified Sticky Rice–Lime Paste. Nanomaterials. 2025; 15(15):1194. https://doi.org/10.3390/nano15151194
Chicago/Turabian StyleLi, Ke, Donghui Cheng, Yingqi Fu, Xuwen Yan, Li Wang, and Haisheng Ren. 2025. "Study on the Durability of Graphene Oxide–Nanosilica Hybrid-Modified Sticky Rice–Lime Paste" Nanomaterials 15, no. 15: 1194. https://doi.org/10.3390/nano15151194
APA StyleLi, K., Cheng, D., Fu, Y., Yan, X., Wang, L., & Ren, H. (2025). Study on the Durability of Graphene Oxide–Nanosilica Hybrid-Modified Sticky Rice–Lime Paste. Nanomaterials, 15(15), 1194. https://doi.org/10.3390/nano15151194






