Single-Atom Catalysts for Hydrogen Evolution Reaction: The Role of Supports, Coordination Environments, and Synergistic Effects
Abstract
1. Introduction
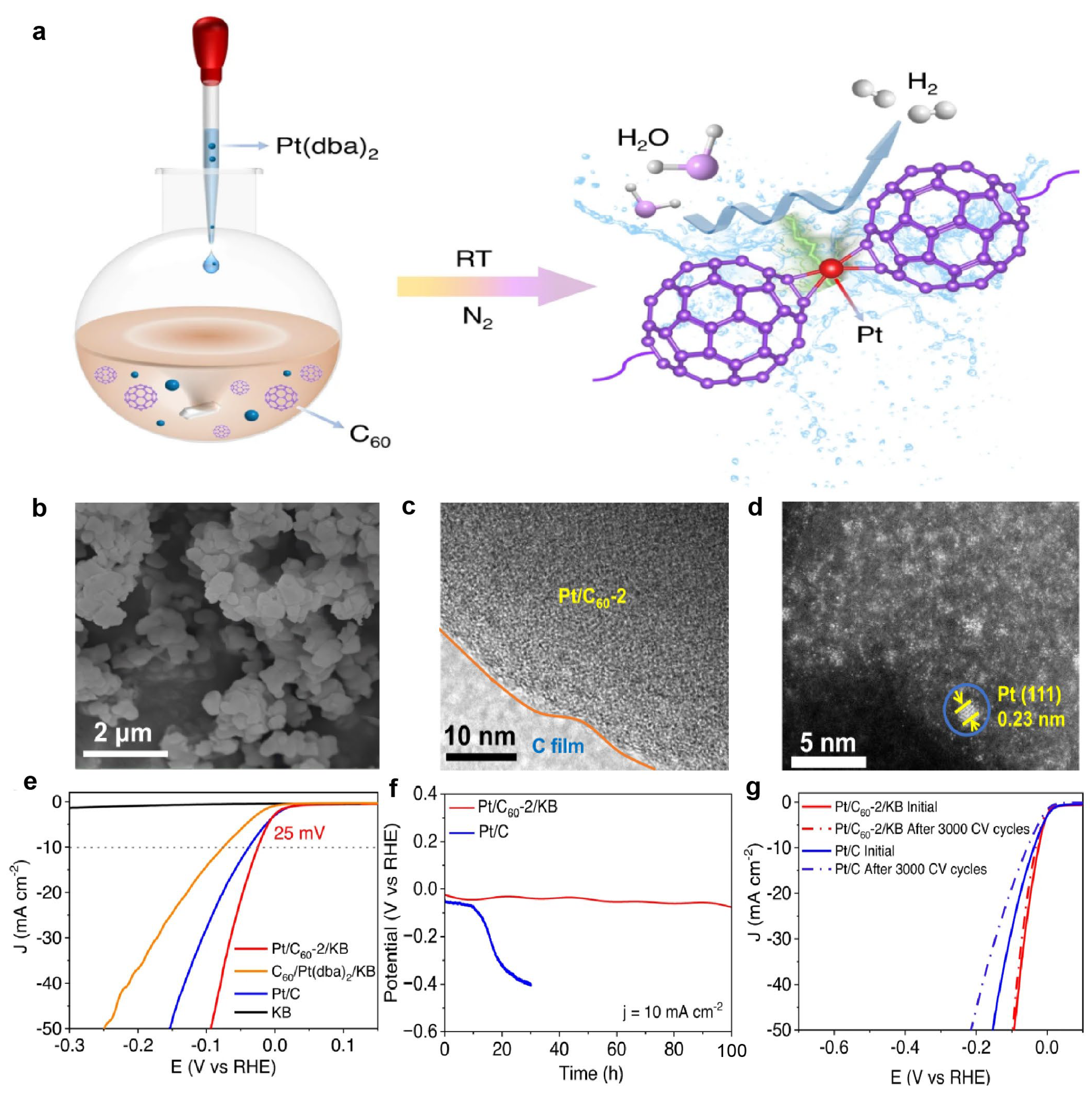
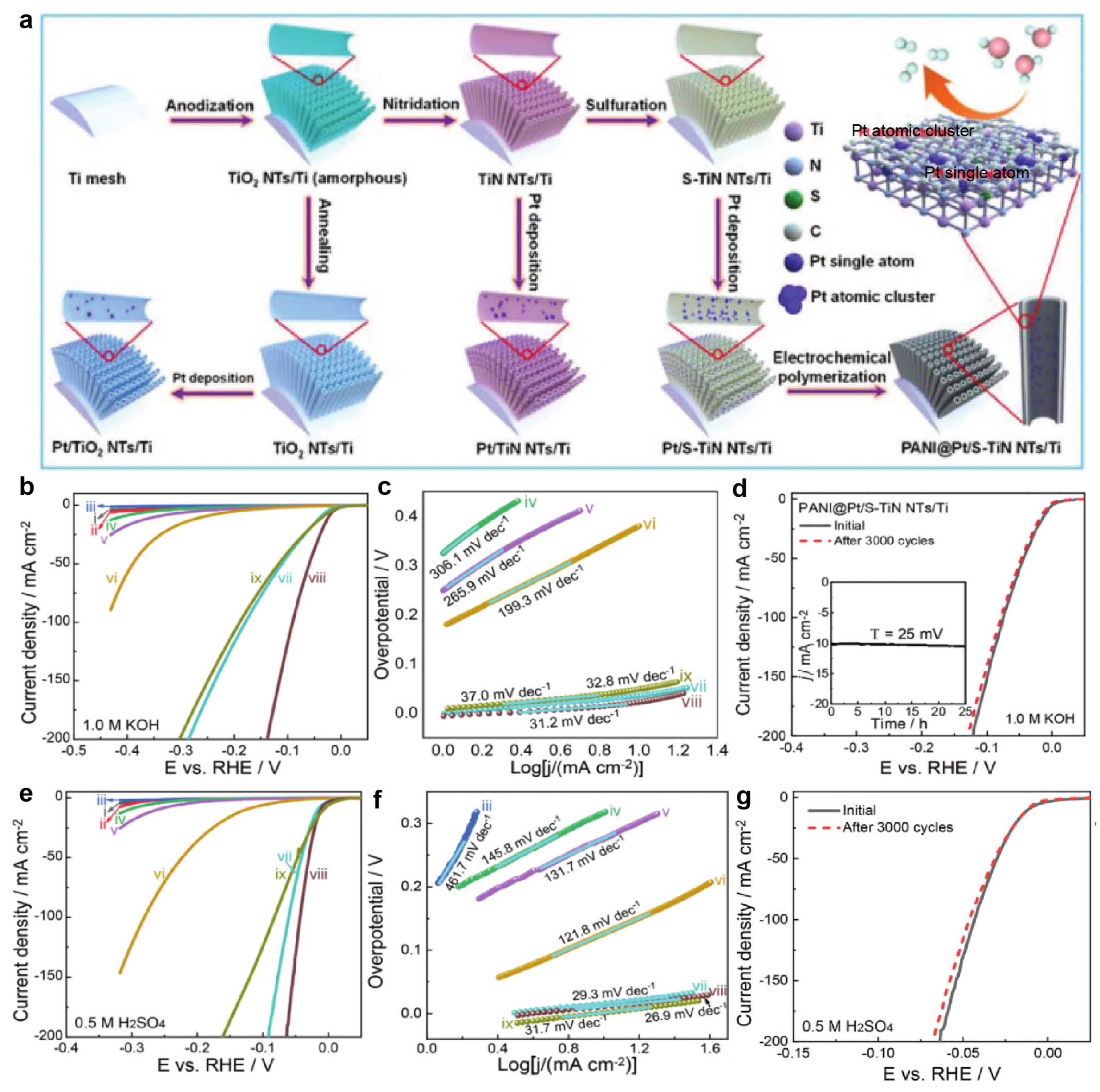
2. Pt-Based Catalysts
2.1. Supports
2.2. Coordination Environment
2.3. Synergistic Effect
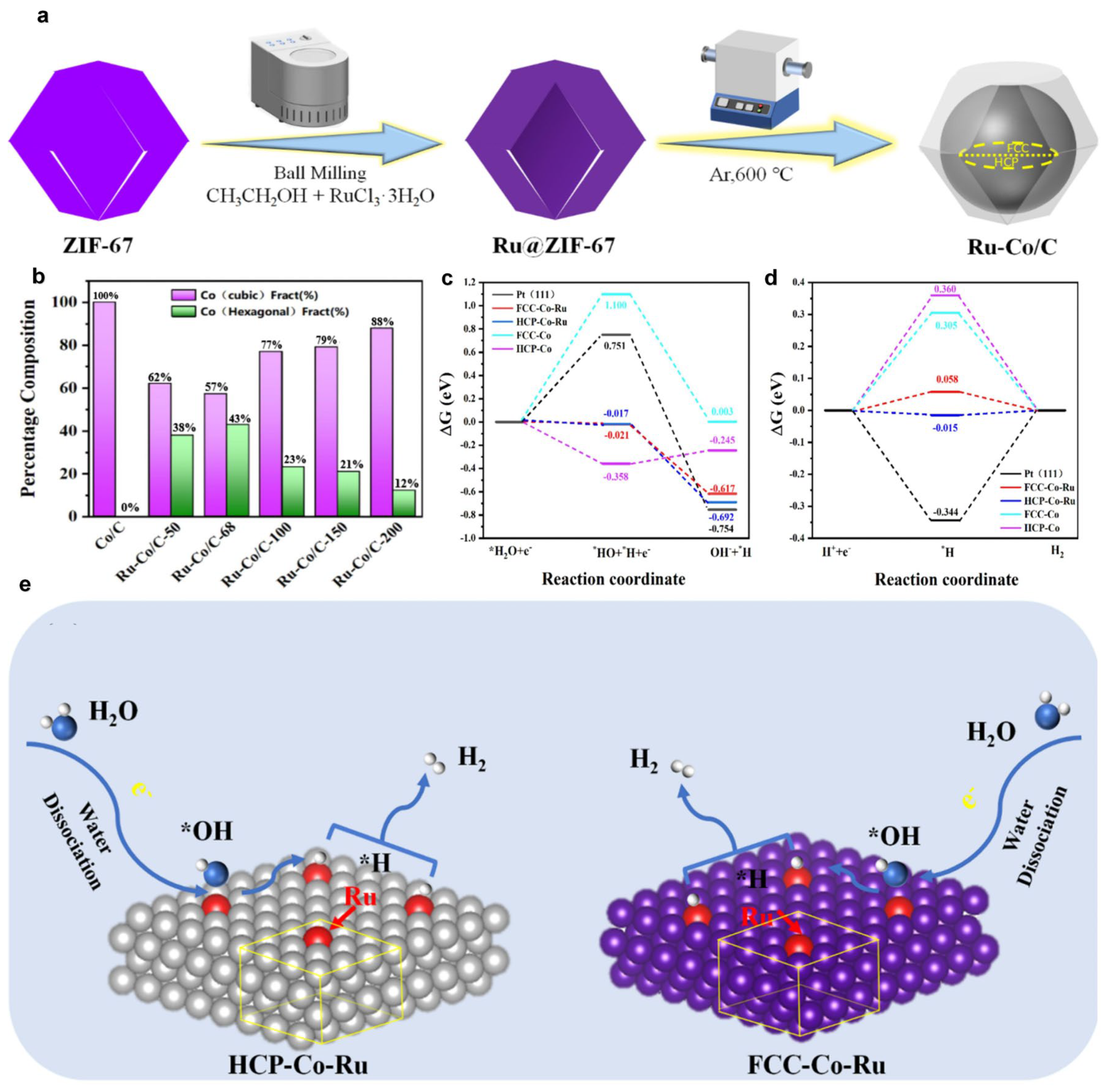
3. Co-Based Catalysts
3.1. Supports
3.2. Coordination Environment
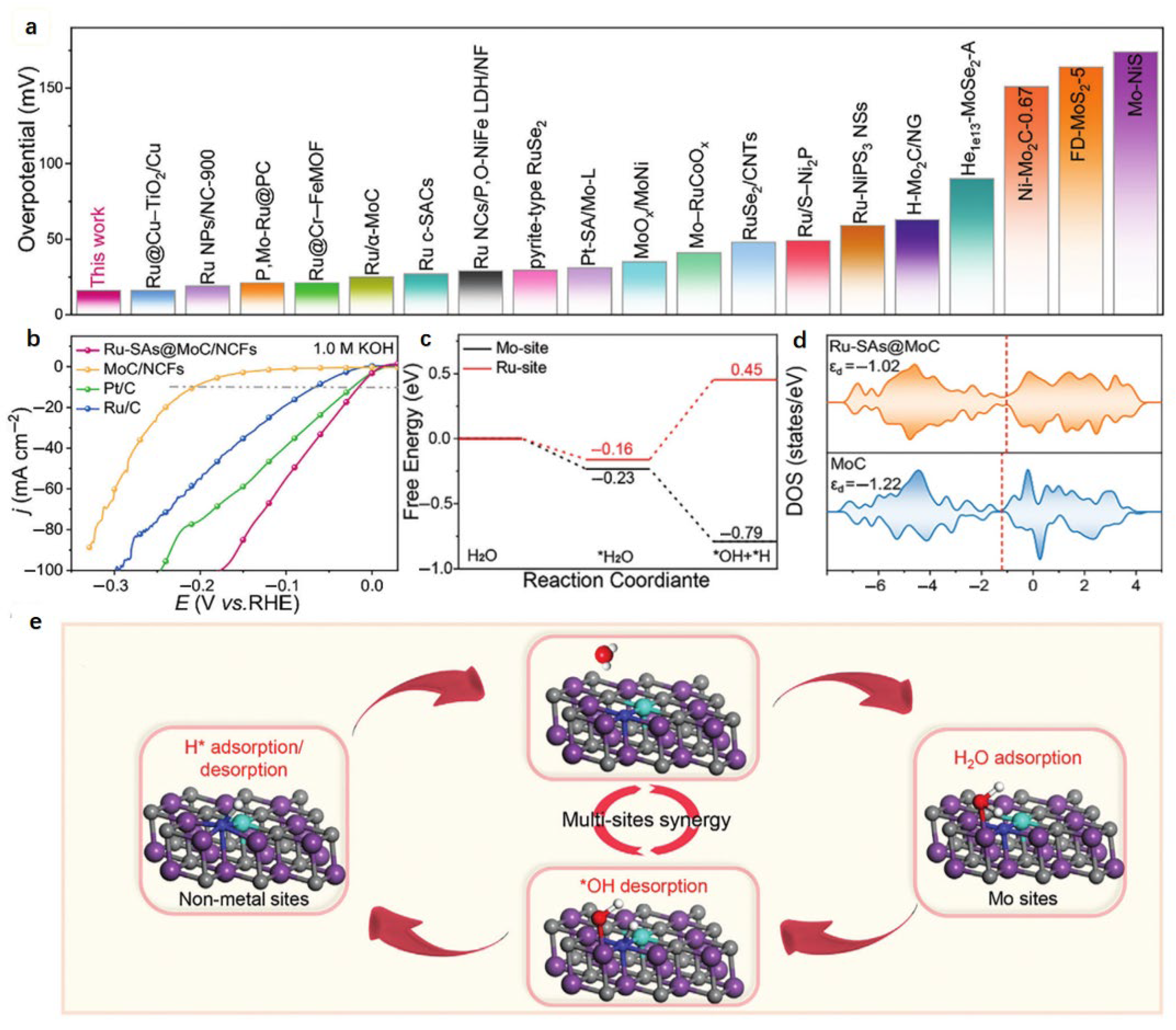
4. Ru-Based Catalysts
4.1. Synergistic Effect
4.2. Coordination Environment
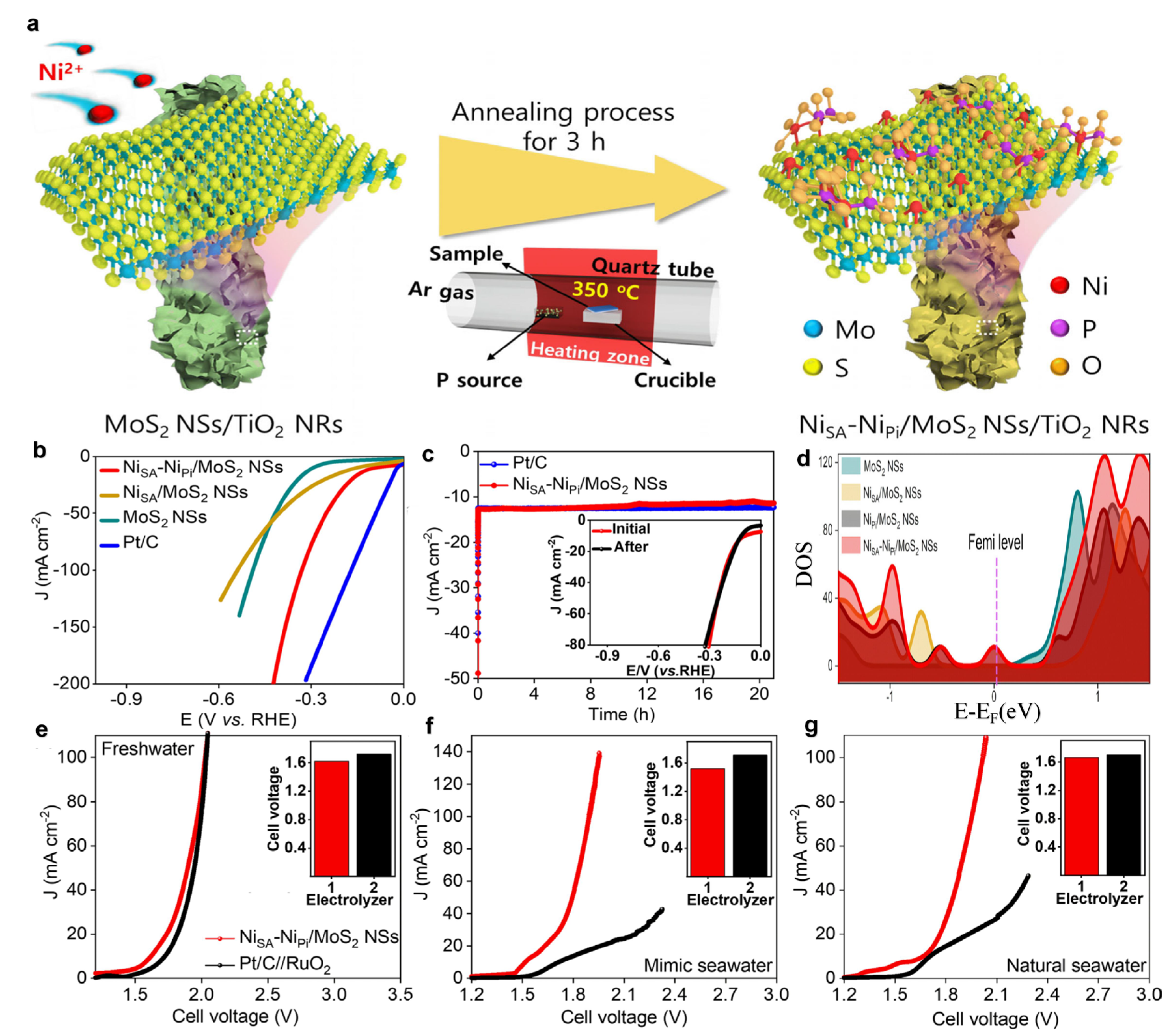
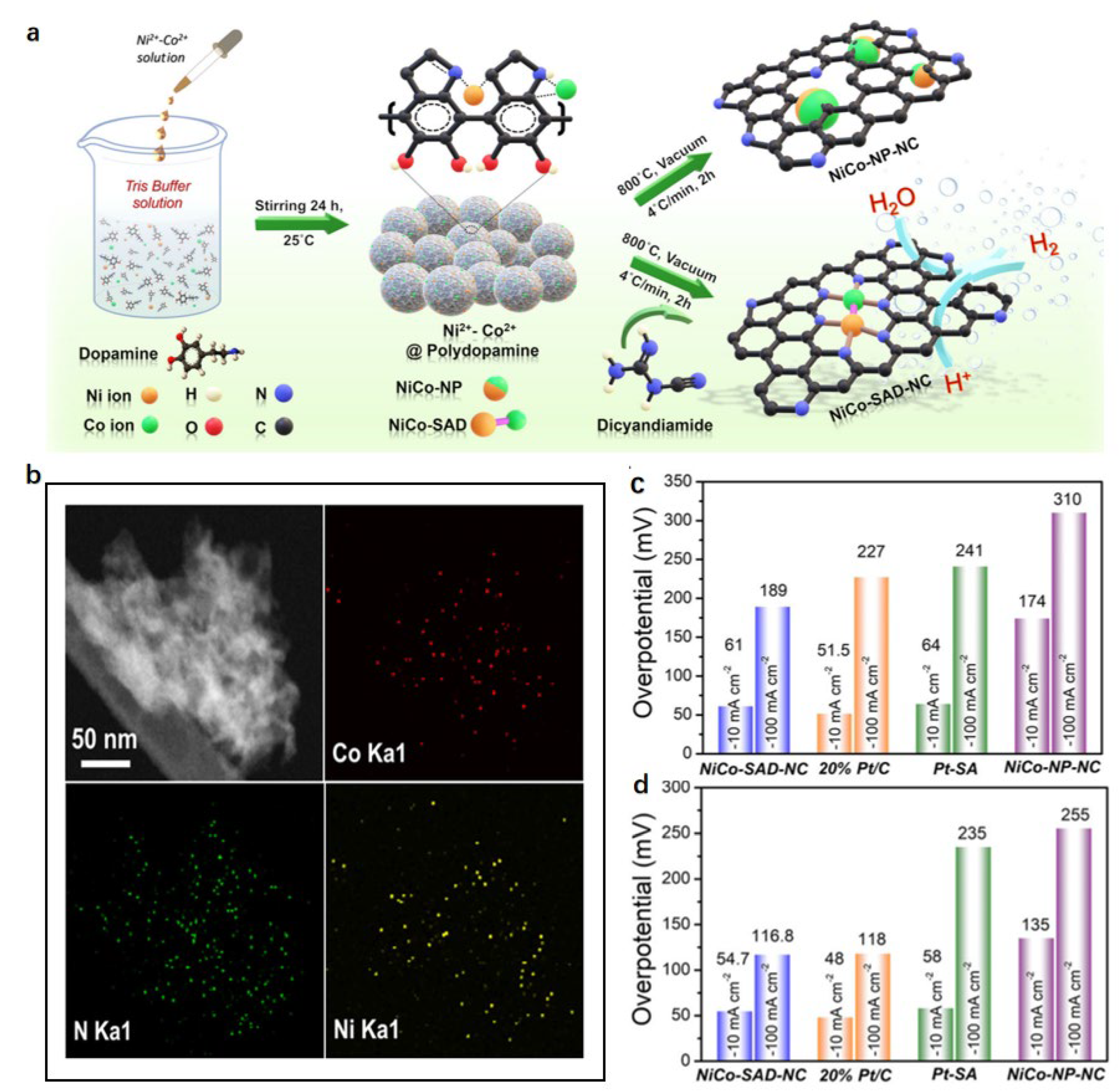
5. Ni-Based Catalysts
5.1. Supports
5.2. Synergistic Effect
6. Other Catalysts
7. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Li, F.; Liu, D.; Sun, K.; Yang, S.H.; Peng, F.Z.; Zhang, K.X.; Guo, G.D.; Si, Y. Towards a Future Hydrogen Supply Chain: A Review of Technologies and Challenges. Sustainability 2024, 16, 1890. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.M.; Ling, W. Policy optimization of hydrogen energy industry considering government policy preference in China. Sustain. Prod. Consum. 2022, 33, 890–902. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, R.Y.; Lv, Z.X.; Liu, J.; Zhou, H.J.; Xu, C.M. Green hydrogen: A promising way to the carbon-free society. Chin. J. Chem. Eng. 2022, 43, 2–13. [Google Scholar] [CrossRef]
- Choi, H.-S.; Hwang, G. Hydrogen Production Systems through Water Electrolysis. Membr. J. 2017, 27, 477–486. [Google Scholar] [CrossRef]
- Kim, H.; Choe, C.; Lee, A.; Lim, H. Application of green hydrogen with theoretical and empirical approaches of alkaline water electrolysis: Life cycle-based techno economic and environmental assessments of renewable urea synthesis. Int. J. Hydrog. Energy 2023, 48, 16148–16158. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.Y.; Jin, L.J.; Xu, H.; Du, Y.K. Advances in hydrogen production from electrocatalytic seawater splitting. Nanoscale 2021, 13, 7897–7912. [Google Scholar] [CrossRef]
- Franco, A.; Giovannini, C. Recent and Future Advances in Water Electrolysis for Green Hydrogen Generation: Critical Analysis and Perspectives. Sustainability 2023, 15, 16917. [Google Scholar] [CrossRef]
- Chang, S.H.; Rajuli, M.F. An overview of pure hydrogen production via electrolysis and hydrolysis. Int. J. Hydrog. Energy 2024, 84, 521–538. [Google Scholar] [CrossRef]
- Zhu, P.; Xiong, X.; Wang, D.S. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction. Nano Res. 2022, 15, 5792–5815. [Google Scholar] [CrossRef]
- Wang, X.S.; Zheng, Y.; Sheng, W.C.; Xu, Z.C.J.; Jaroniec, M.; Qiao, S.Z. Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater. Today 2020, 36, 125–138. [Google Scholar] [CrossRef]
- Zhai, W.F.; Ma, Y.Y.; Chen, D.; Ho, J.C.; Dai, Z.F.; Qu, Y.Q. Recent progress on the long-term stability of hydrogen evolution reaction electrocatalysts. Infomat 2022, 4, 12357. [Google Scholar] [CrossRef]
- Guan, J.Q.; Bai, X.; Tang, T.M. Recent progress and prospect of carbon-free single-site catalysts for the hydrogen and oxygen evolution reactions. Nano Res. 2022, 15, 818–837. [Google Scholar] [CrossRef]
- Liu, D.; Xu, G.Y.; Yang, H.; Wang, H.T.; Xia, B.Y. Rational Design of Transition Metal Phosphide-Based Electrocatalysts for Hydrogen Evolution. Adv. Funct. Mater. 2023, 33, 2208358. [Google Scholar] [CrossRef]
- Zhou, B.H.; Gao, R.J.; Zou, J.J.; Yang, H.M. Surface Design Strategy of Catalysts for Water Electrolysis. Small 2022, 18, 2202336. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Lai, Y.; Li, Z.; Zhou, Y. Fluffy ruthenium-Nickel-Molybdenum Oxide Foam Metal Self-Supporting Material Useful as HER Catalyst for Water Electrolysis to Produce Hydrogen, Comprises Metal Foam, Fibrous Molybdenum Oxide Grown on Framework, and Nano-Ruthenium and Nano-Nickel Metal Particles Dispersed in Molybdenum Oxide. Patent CN116314887-A, 16 March 2023. [Google Scholar]
- Wang, T.; Zhou, Q.Y.; Wang, X.J.; Zheng, J.; Li, X.G. MOF-derived surface modified Ni nanoparticles as an efficient catalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 16435–16439. [Google Scholar] [CrossRef]
- Li, Q.Y.; Kong, D.D.; Yang, G.H.; Cai, Y.Z.; Pan, Q.C.; Zheng, F.H.; Ma, Z.L.; Wang, H.Q. Interface-tuned Mo-based nanospheres for efficient oxygen reduction and hydrogen evolution catalysis. Catal. Sci. Technol. 2020, 10, 6713–6722. [Google Scholar] [CrossRef]
- Ren, L.P.; Yang, D.; Yang, J.H. Ruthenium-manganese phosphide nanohybrid supported on graphene for efficient hydrogen evolution reaction in acid and alkaline conditions. Int. J. Hydrog. Energy 2022, 47, 13876–13886. [Google Scholar] [CrossRef]
- Jia, W.; Xu, X.; Wang, C.; Zhang, X.; Wu, Y. Bi-Functional Metal Organic Frame Nano Material Used as a Dual Electrocatalyst for HER and UOR, Where It Is Nickel-Rhodium-Manganese Metal Organic Framework/Nitrogen Fluoride Nano Material, Which Is Composed of Metal Organic Frame Material and Foam Nickel. Patent CN116905015-A, 4 July 2023. [Google Scholar]
- Wang, X.J.; Huang, Y.; Zhu, J.K.; Zhao, Z.Y.; Zhao, J.B.; Zhang, J.J. Cobalt vanadate nano/microrods as high-efficiency dual-functional electrocatalyst for hydrogen evolution and urea-assisted alkaline oxygen evolution reaction. Int. J. Hydrog. Energy 2024, 88, 78–85. [Google Scholar] [CrossRef]
- Qiao, B.T.; Wang, A.Q.; Yang, X.F.; Allard, L.F.; Jiang, Z.; Cui, Y.T.; Liu, J.Y.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.H.; Amiinu, I.S.; Cheng, R.L.; Wang, P.Y.; Zhang, C.T.; Mu, S.C.; Zhao, W.Y.; Su, F.M.; Zhang, G.X.; Liao, S.J.; et al. Single-Atom Catalysts for Electrochemical Hydrogen Evolution Reaction: Recent Advances and Future Perspectives. Nano-Micro Lett. 2020, 12, 21. [Google Scholar] [CrossRef]
- Zou, L.L.; Wei, Y.S.; Hou, C.C.; Li, C.X.; Xu, Q. Single-Atom Catalysts Derived from Metal-Organic Frameworks for Electrochemical Applications. Small 2021, 17, 2004809. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Shi, Q.R.; Feng, S.; Du, D.; Lin, Y.H. Single-Atom Catalysts for Electrochemical Water Splitting. Acs Energy Lett. 2018, 3, 1713–1721. [Google Scholar] [CrossRef]
- Wang, Y.X.; Su, H.Y.; He, Y.H.; Li, L.G.; Zhu, S.Q.; Shen, H.; Xie, P.F.; Fu, X.B.; Zhou, G.Y.; Feng, C.; et al. Advanced Electrocatalysts with Single-Metal-Atom Active Sites. Chem. Rev. 2020, 120, 12217–12314. [Google Scholar] [CrossRef]
- Li, D.; Chen, X.F.; Lv, Y.Z.; Zhang, G.Y.; Huang, Y.; Liu, W.; Li, Y.; Chen, R.S.; Nuckolls, C.; Ni, H.W. An effective hybrid electrocatalyst for the alkaline HER: Highly dispersed Pt sites immobilized by a functionalized NiRu-hydroxide. Appl. Catal. B-Environ. 2020, 269, 118824. [Google Scholar] [CrossRef]
- Mosallanezhad, A.; Wei, C.; Koudakan, P.A.; Fang, Y.Y.; Niu, S.W.; Bian, Z.A.; Liu, B.; Huang, T.; Pan, H.G.; Wang, G.M. Interfacial synergies between single-atomic Pt and CoS for enhancing hydrogen evolution reaction catalysis. Appl. Catal. B-Environ. Energy 2022, 315, 121534. [Google Scholar] [CrossRef]
- Wei, J.X.; Xiao, K.; Chen, Y.X.; Guo, X.P.; Huang, B.L.; Liu, Z.Q. In situ precise anchoring of Pt single atoms in spinel Mn3O4 for a highly efficient hydrogen evolution reaction. Energy Environ. Sci. 2022, 15, 4592–4600. [Google Scholar] [CrossRef]
- He, J.N.; Cui, Z.D.; Zhu, S.L.; Li, Z.Y.; Wu, S.L.; Zheng, L.R.; Gao, Z.H.; Liang, Y.Q. Insight into the electrochemical-cycling activation of Pt/molybdenum carbide toward synergistic hydrogen evolution catalysis. J. Catal. 2020, 384, 169–176. [Google Scholar] [CrossRef]
- Deng, J.; Li, H.B.; Xiao, J.P.; Tu, Y.C.; Deng, D.H.; Yang, H.X.; Tian, H.F.; Li, J.Q.; Ren, P.J.; Bao, X.H. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- Sheng, K.; Li, G.; Liu, M.; Gan, L.; Huang, S.Q.; Li, J. Hierarchical assembly of Pt single atoms and WC nanocrystals on porous carbon Boosting hydrogen evolution reactions. J. Colloid Interface Sci. 2025, 692, 137512. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.R.; Xiao, Y.Y.; Yin, Y.C.; Huang, W.M.; Huang, Z.C.; Zheng, Y.Z.; Mu, F.Y.; Huang, R.; Shi, G.Y.; et al. Electronic metal-support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nat. Commun. 2021, 12, 3021. [Google Scholar] [CrossRef]
- Gong, L.; Zhu, J.W.; Xia, F.J.; Zhang, Y.H.; Shi, W.J.; Chen, L.; Yu, J.; Wu, J.S.; Mu, S.C. Marriage of Ultralow Platinum and Single-Atom MnN4 Moiety for Augmented ORR and HER Catalysis. Acs Catal. 2023, 13, 4012–4020. [Google Scholar] [CrossRef]
- Zhang, L.S.; Ma, Z.W.; Wu, Z.Z.; Liu, Y.X.; Bai, J.; Zhang, S.H.; Debroye, E.; Fan, W.; Lin, H.P.; Liu, T.X. Atomically Dispersed Platinum on Conjugated Polymers with Tailored Cation-π Interactions and Microstructures for Hydrogen Evolution Electrocatalysis. Adv. Funct. Mater. 2024, 34, 2404707. [Google Scholar] [CrossRef]
- Zeng, X.J.; Shui, J.L.; Liu, X.F.; Liu, Q.T.; Li, Y.C.; Shang, J.X.; Zheng, L.R.; Yu, R.H. Single-Atom to Single-Atom Grafting of Pt1 onto Fe-N4 Center: Pt1@Fe-N-C Multifunctional Electrocatalyst with Significantly Enhanced Properties. Adv. Energy Mater. 2018, 8, 1701345. [Google Scholar] [CrossRef]
- Liu, X.Y.; Song, X.T.; Jiang, G.M.; Tao, L.J.; Jin, Z.Y.; Li, F.K.; He, Y.Z.; Dong, F. Pt Single-Atom collaborate with Pt Atom-Clusters by an In-Situ confined strategy for accelerating electrocatalytic hydrogen evolution. Chem. Eng. J. 2024, 481, 148430. [Google Scholar] [CrossRef]
- Wang, C.; Chen, F.R.; Wang, Q.R.; Yang, X.F.; Zang, H.; Yu, N.; Geng, B.Y. Defect-stabilized platinum single atoms and clusters in bilayer nitrogen-doped porous carbon nanocages for synergistic catalysis of basic hydrogen evolution. Carbon 2023, 201, 278–284. [Google Scholar] [CrossRef]
- Liu, D.B.; Li, X.Y.; Chen, S.M.; Yang, H.; Wang, C.D.; Wu, C.Q.; Haleem, Y.A.; Duan, S.; Lu, J.L.; Ge, B.H.; et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 2019, 4, 512–518. [Google Scholar] [CrossRef]
- Luo, X.Y.; Yuan, P.; Xiao, H.M.; Li, S.W.; Luo, J.H.; Li, J.Y.; Lai, W.D.; Chen, Y.; Li, D. Effects of Intrinsic Defects in Pt-Based Carbon Supports on Alkaline Hydrogen Evolution Reaction. Acs Appl. Mater. Interfaces 2024, 16, 26044–26056. [Google Scholar] [CrossRef]
- Luo, X.Y.; Cao, B.K.; Fayaz, M.; Lai, W.D.; Du, B.D.; Zheng, H.; Xiong, X.H.; Chen, Y. Enhancing effects of edge-N in Pt-based carbon support on hydrogen evolution reaction. Surf. Interfaces 2025, 56, 105499. [Google Scholar] [CrossRef]
- Luo, X.Y.; Xiao, H.M.; Li, J.Y.; Yuan, P.; Du, B.D.; Zheng, H.; Li, D.; Chen, Y. Cyclic ether on Pt-based carbon support for enhanced alkaline hydrogen evolution. J. Electroanal. Chem. 2023, 939, 117476. [Google Scholar] [CrossRef]
- Yang, W.W.; Cheng, P.; Li, Z.; Lin, Y.X.; Li, M.Y.; Zi, J.Z.; Shi, H.H.; Li, G.S.; Lian, Z.C.; Li, H.X. Tuning the Cobalt-Platinum Alloy Regulating Single-Atom Platinum for Highly Efficient Hydrogen Evolution Reaction. Adv. Funct. Mater. 2022, 32, 2205920. [Google Scholar] [CrossRef]
- Zhang, R.L.; Li, Y.Z.; Zhou, X.; Yu, A.; Huang, Q.; Xu, T.T.; Zhu, L.T.; Peng, P.; Song, S.Y.; Echegoyen, L.; et al. Single-atomic platinum on fullerene C60 surfaces for accelerated alkaline hydrogen evolution. Nat. Commun. 2023, 14, 2460. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Nguyen, T.H.; Tran, D.T.; Dinh, V.; Lee, J.H.; Kim, N.H. Delaminated MBene sheets beyond usual 2D transition metal materials for securing Pt single atoms to boost hydrogen evolution. Energy Environ. Sci. 2023, 16, 4093–4104. [Google Scholar] [CrossRef]
- Ye, S.H.; Luo, F.Y.; Zhang, Q.L.; Zhang, P.Y.; Xu, T.T.; Wang, Q.; He, D.S.; Guo, L.C.; Zhang, Y.; He, C.X.; et al. Highly stable single Pt atomic sites anchored on aniline-stacked graphene for hydrogen evolution reaction. Energy Environ. Sci. 2019, 12, 1000–1007. [Google Scholar] [CrossRef]
- Ma, T.; Cao, H.; Li, S.; Cao, S.J.; Zhao, Z.Y.; Wu, Z.H.; Yan, R.; Yang, C.D.; Wang, Y.; van Aken, P.A.; et al. Crystalline Lattice-Confined Atomic Pt in Metal Carbides to Match Electronic Structures and Hydrogen Evolution Behaviors of Platinum. Adv. Mater. 2022, 34, 2206368. [Google Scholar] [CrossRef]
- Zhou, K.L.; Han, C.B.; Wang, Z.L.; Ke, X.X.; Wang, C.H.; Jin, Y.H.; Zhang, Q.Q.; Liu, J.B.; Wang, H.; Yan, H. Atomically Dispersed Platinum Modulated by Sulfide as an Efficient Electrocatalyst for Hydrogen Evolution Reaction. Adv. Sci. 2021, 8, 2100347. [Google Scholar] [CrossRef]
- Huang, J.F.; Hsieh, W.J.; Chen, J.L. Carbon-Promoted Pt-Single Atoms Anchored on RuO2 Nanorods to Boost Electrochemical Hydrogen Evolution. Acs Appl. Mater. Interfaces 2024, 16, 27504–27510. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Denisov, N.; Kim, H.; Kim, J.; Will, J.; Spiecker, E.; Vaskevich, A.; Schmuki, P. Pt Single Atoms Loaded on Thin-Layer TiO2 Electrodes: Electrochemical and Photocatalytic Features. Small 2024, 20, 2404064. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zhao, Y.F.; Guo, X.; Chen, C.; Dong, C.L.; Liu, R.S.; Han, C.P.; Li, Y.D.; Gogotsi, Y.; Wang, G.X. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Li, C.; Chen, Z.; Yi, H.; Cao, Y.; Du, L.; Hu, Y.; Kong, F.; Campen, R.K.; Gao, Y.; Du, C.; et al. Polyvinylpyrrolidone-Coordinated Single-Site Platinum Catalyst Exhibits High Activity for Hydrogen Evolution Reaction. Angew. Chem.-Int. Ed. 2020, 59, 15902–15907. [Google Scholar] [CrossRef]
- Zeng, L.Y.; Zhao, Z.L.; Huang, Q.Z.; Zhou, C.H.; Chen, W.X.; Wang, K.; Li, M.G.; Lin, F.X.; Luo, H.; Gu, Y.; et al. Single-Atom Cr-N4 Sites with High Oxophilicity Interfaced with Pt Atomic Clusters for Practical Alkaline Hydrogen Evolution Catalysis. J. Am. Chem. Soc. 2023, 145, 21432–21441. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhang, Z.; Chen, C.L.; Wang, R.; Xie, M.G.; Wan, S.; Zhang, R.G.; Cong, L.C.; Lu, H.Y.; Han, Y.; et al. Single-atom platinum with asymmetric coordination environment on fully conjugated covalent organic framework for efficient electrocatalysis. Nat. Commun. 2024, 15, 2556. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.J.; Tang, Y.; Zhang, L.L.; Liu, X.Y.; Li, L.; Miao, S.; Su, D.S.; Wang, A.Q.; Li, J.; Zhang, T. Unraveling the coordination structure-performance relationship in Pt1/Fe2O3 single-atom catalyst. Nat. Commun. 2019, 10, 4500. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.P.; Wang, H.J.; Tang, S.F.; Lu, X.L.; Shu, M.; Si, R.; Lu, T.B. Engineering the Coordination Environment of Single-Atom Platinum Anchored on Graphdiyne for Optimizing Electrocatalytic Hydrogen Evolution. Angew. Chem. -Int. Ed. 2018, 57, 9382–9386. [Google Scholar] [CrossRef]
- Han, Y.; Duan, H.L.; Liu, W.; Zhou, C.H.; Wang, B.S.; Jiang, Q.Y.; Feng, S.H.; Yan, W.S.; Tan, T.; Zhang, R.F. Engineering the electronic structure of platinum single-atom sites via tailored porous carbon nanofibers for large-scale hydrogen production. Appl. Catal. B-Environ. Energy 2023, 335, 122898. [Google Scholar] [CrossRef]
- Lee, J.W.; Din, H.U.; Im, T.; Hwang, C.K.; Kim, J.M.; Lee, J.H.; Jeong, S. Coordination Tailoring of Pt Single-Atom Catalysts at Room Temperature and Their Exceptional Performance in Hydrogen Evolution Reaction. Carbon Energy 2025, 7, e720. [Google Scholar] [CrossRef]
- Ji, J.P.; Zhang, Y.P.; Tang, L.B.; Liu, C.Y.; Gao, X.H.; Sun, M.H.; Zheng, J.C.; Ling, M.; Liang, C.D.; Lin, Z. Platinum single-atom and cluster anchored on functionalized MWCNTs with ultrahigh mass efficiency for electrocatalytic hydrogen evolution. Nano Energy 2019, 63, 103849. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, H.; Qin, H.; Zhang, X.; Yang, H.; Cai, Q. Synergistic Electrocatalytic Material of Pt Single Atoms and Pt Atom Clusters Synthesized Using In-Situ Strategy Useful for Electro-Catalytic Hydrogen Evolution Reaction Under Acidic Condition, Comprises Composite Electrocatalytic Material PtSA-PtAC. Patent CN118756223-A, 18 June 2024. [Google Scholar]
- Wang, C.; Zang, H.; Liu, C.J.; Wang, J.H.; Kuai, L.; Geng, B.Y. Synergistic Acid Hydrogen Evolution of Neighboring Pt Single Atoms and Clusters: Understanding Their Superior Activity and Mechanism. Inorg. Chem. 2023, 62, 6856–6863. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhao, M.H.; Wu, J.T.; Wang, Y.N.; Zheng, L.F.; Gu, F.W.; Zou, J.J.; Gao, J.; Zhu, X.D. Construction of Pt Single-Atom and Cluster/FeOOH Synergistic Active Sites for Efficient Electrocatalytic Hydrogen Evolution Reaction. Acs Catal. 2024, 14, 7867–7876. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Yu, J.; Jing, Y.F.; Liu, J.Y.; Singh, M.; Lund, P.D.; Asghar, M.I. Self-assembled formation of platinum single atoms and nanoclusters stabilized on MXene as an efficient catalyst for boosting electrocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2024, 84, 502–510. [Google Scholar] [CrossRef]
- Yan, J.; Xi, Z.C.; Cong, L.L.; Lv, K.; Xin, R.Y.; Cao, B.; Liu, B.C.; He, J.L.; Zhang, J. Synergy of Platinum Single Atoms and Platinum Atomic Clusters on Sulfur-Doped Titanium Nitride Nanotubes for Enhanced Hydrogen Evolution Reaction. Small 2022, 18, 2205603. [Google Scholar] [CrossRef]
- Yu, J.Q.; Yan, Y.; Lin, Y.M.; Liu, H.Z.; Li, Y.T.; Xie, S.H.; Sun, S.M.; Liu, F.D.; Zhang, Z.G.; Li, W.Z.; et al. Improved high-current-density hydrogen evolution reaction kinetics on single-atom Co embedded in an order pore-structured nitrogen assembly carbon support. Nanoscale Horiz. 2024, 9, 2326–2333. [Google Scholar] [CrossRef]
- Wang, M.M.; Sun, K.A.; Mi, W.L.; Feng, C.; Guan, Z.K.; Liu, Y.Q.; Pan, Y. Interfacial Water Activation by Single-Atom Co-N3 Sites Coupled with Encapsulated Co Nanocrystals for Accelerating Electrocatalytic Hydrogen Evolution. Acs Catal. 2022, 12, 10771–10780. [Google Scholar] [CrossRef]
- Lv, S.S.; Zhou, Y.; Yang, W.J.; Song, M.M.; Li, F.; Feng, M.M.; Chen, C.; Chen, Z. The Defects of Single Atom Co1-N-C Regulate the H-Binding Energy to Achieve Divergent Hydrogen Evolution or Transfer Hydrogenation with HCOOH. Adv. Funct. Mater. 2025, 35, 2423864. [Google Scholar] [CrossRef]
- Jiang, K.; Siahrostami, S.; Zheng, T.T.; Hu, Y.F.; Hwang, S.; Stavitski, E.; Peng, Y.D.; Dynes, J.; Gangisetty, M.; Su, D.; et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 2018, 11, 893–903. [Google Scholar] [CrossRef]
- Shah, K.; Dai, R.Y.; Mateen, M.; Hassan, Z.; Zhuang, Z.W.; Liu, C.H.; Israr, M.; Cheong, W.C.; Hu, B.T.; Tu, R.Y.; et al. Cobalt Single Atom Incorporated in Ruthenium Oxide Sphere: A Robust Bifunctional Electrocatalyst for HER and OER. Angew. Chem. -Int. Ed. 2022, 61, 2114951. [Google Scholar] [CrossRef]
- Wan, J.W.; Zhao, Z.H.; Shang, H.S.; Peng, B.; Chen, W.X.; Pei, J.J.; Zheng, L.R.; Dong, J.C.; Cao, R.; Sarangi, R.; et al. In Situ Phosphatizing of Triphenylphosphine Encapsulated within Metal-Organic Frameworks to Design Atomic Co1-P1N3 Interfacial Structure for Promoting Catalytic Performance. J. Am. Chem. Soc. 2020, 142, 8431–8439. [Google Scholar] [CrossRef]
- Li, T.; Ren, S.Y.; Zhang, C.; Qiao, L.X.; Wu, J.; He, P.; Lin, J.; Liu, Y.S.; Fu, Z.G.; Zhu, Q.Z.; et al. Cobalt single atom anchored on N-doped carbon nanoboxes as typical single-atom catalysts (SACs) for boosting the overall water splitting. Chem. Eng. J. 2023, 458, 141435. [Google Scholar] [CrossRef]
- Kim, K.; Kim, C.; Bak, S.M.; Nam, C.Y.; Moon, J.H. Amorphous-crystalline transition-driven synthesis of Co single-atom catalysts on MoO3 for enhanced hydrogen evolution in acidic and alkaline media. Chem. Eng. J. 2024, 488, 150976. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, M.M.; Feng, C. A doping-adsorption-pyrolysis strategy for constructing atomically dispersed cobalt sites anchored on a N-doped carbon framework as an efficient bifunctional electrocatalyst for hydrogen evolution and oxygen reduction. Rsc Adv. 2022, 12, 20578–20582. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.H.; Jia, Z.Q.; Li, R.M.; Yu, J. Co Single Atoms Anchored in N and P Co-doped Porous Carbon Fibers for Efficient Water Splitting. Chem. -Asian J. 2023, 18, 2300393. [Google Scholar] [CrossRef]
- Huang, K.; Wei, Z.X.; Liu, J.B.; Gong, Z.C.; Liu, J.J.; Yan, M.N.; He, G.C.; Gong, H.S.; Hu, Y.F.; He, Y.M.; et al. Engineering the Morphology and Microenvironment of a Graphene-Supported Co-N-C Single-Atom Electrocatalyst for Enhanced Hydrogen Evolution. Small 2022, 18, 2201139. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.Y.; Lang, Z.L.; Yin, L.Y.; Feng, K.; Xia, Y.J.; Tan, H.Q.; Zhu, H.T.; Zhong, J.; Kang, Z.H.; Li, Y.G. Pt-O bond as an active site superior to Pt0 in hydrogen evolution reaction. Nat. Commun. 2020, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, S.C.; He, P.N.; Shuai, Y.; Leng, H.F.; Hong, W.L.; Liu, Y. Ruthenium-doped dual-phase cobalt catalysts for enhanced electrocatalytic hydrogen evolution. Appl. Surf. Sci. 2025, 679, 161297. [Google Scholar] [CrossRef]
- Cao, L.L.; Luo, Q.Q.; Liu, W.; Lin, Y.K.; Liu, X.K.; Cao, Y.J.; Zhang, W.; Wu, Y.; Yang, J.L.; Yao, T.; et al. Identification of single-atom active sites in carbon-based cobalt catalysts during electrocatalytic hydrogen evolution. Nat. Catal. 2019, 2, 134–141. [Google Scholar] [CrossRef]
- Song, Q.W.; Gong, Z.C.; Liu, J.B.; Huang, K.; Ye, G.L.; Niu, S.W.; Fei, H.L. Boosting the Hydrogen Evolution Activity of a Low-Coordinated Co―N―C Catalyst via Vacancy Defect-Mediated Alteration of the Intermediate Adsorption Configuration. Adv. Sci. 2025, 12, 2415665. [Google Scholar] [CrossRef]
- Wang, L.Q.; Ma, M.Y.; Zhang, C.C.; Chang, H.H.; Zhang, Y.; Li, L.L.; Chen, H.Y.; Peng, S.J. Manipulating the Microenvironment of Single Atoms by Switching Support Crystallinity for Industrial Hydrogen Evolution. Angew. Chem. -Int. Ed. 2024, 63, 2317220. [Google Scholar] [CrossRef]
- Liu, S.C.; Shen, L.Y.; Tang, Y.J.; Guo, C.Y.; Ciucci, F.; Tang, Z.H. Alkaline hydrogen evolution reaction catalyzed by atomically dispersed ruthenium on phosphorus-nitrogen co-doped porous carbon. Int. J. Hydrog. Energy 2024, 82, 102–110. [Google Scholar] [CrossRef]
- Li, F.; Wu, Q.K.; Yuan, W.J.; Chen, Z. Ruthenium-based single atom catalysts: Synthesis and application in the electrocatalytic hydrogen evolution reaction. Dalton Trans. 2024, 53, 12022–12033. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Cao, Y.Y.; Ji, J.Y.; Li, Z.C.; Niu, Z.; Gu, H.W.; Braunstein, P.; Lang, J.P. Electronic engineering of Co-Ru diatomic sites and Ru nanoparticles for synergistic promotion of hydrogen evolution. Nano Res. 2024, 17, 3714–3723. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.H.; Zhang, Y.C.; Jin, X.; Li, J.M.; Xi, X.K.; Deng, Y.; Jiao, S.H.; Lei, Z.W.; Li, X.Y.; et al. Ensemble Effect of Ruthenium Single-Atom and Nanoparticle Catalysts for Efficient Hydrogen Evolution in Neutral Media. Acs Appl. Mater. Interfaces 2023, 15, 14240–14249. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, Q.; Han, C.; Xing, Z.C.; Yang, X.R. Single-atom ruthenium based catalyst for enhanced hydrogen evolution. Appl. Catal. B-Environ. Energy 2019, 249, 91–97. [Google Scholar] [CrossRef]
- Ma, R.P.; Wang, X.; Yang, X.L.; Li, Y.; Liu, C.P.; Ge, J.J.; Xing, W. Identification of active sites and synergistic effect in multicomponent carbon-based Ru catalysts during electrocatalytic hydrogen evolution. Nano Res. 2023, 16, 166–173. [Google Scholar] [CrossRef]
- Yao, H.X.; Wang, X.K.; Li, K.; Li, C.; Zhang, C.H.; Zhou, J.; Cao, Z.W.; Wang, H.L.; Gu, M.; Huang, M.H.; et al. Strong electronic coupling between ruthenium single atoms and ultrafine nanoclusters enables economical and effective hydrogen production. Appl. Catal. B-Environ. Energy 2022, 312, 121378. [Google Scholar] [CrossRef]
- Zhang, J.M.; Xu, X.P.; Yang, L.; Cheng, D.J.; Cao, D.P. Single-Atom Ru Doping Induced Phase Transition of MoS2 and S Vacancy for Hydrogen Evolution Reaction. Small Methods 2019, 3, 1900653. [Google Scholar] [CrossRef]
- Yin, J.W.; Lu, T.Y.; Li, J.; Liu, J.Y.; Lin, Y.Z.; Sun, D.M.; Xu, L.; Zhao, Q.; Pang, H.; Zhang, S.T.; et al. Synergistic Alkaline Hydrogen Evolution Catalysis over MoC Triggered by Doping Single Ru Atoms. Adv. Funct. Mater. 2025, 35, 2417034. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Xu, K.Y.; Sun, N.; Zhuang, Y.L.; Wang, L.L.; Yan, D.F.; Weng, B. Bimetallic Single-Atom Catalysts for Electrocatalytic and Photocatalytic Hydrogen Production. Catalysts 2023, 13, 1409. [Google Scholar] [CrossRef]
- Ge, J.M.; Zhang, D.B.; Qin, Y.; Dou, T.; Jiang, M.H.; Zhang, F.Z.; Lei, X.D. Dual-metallic single Ru and Ni atoms decoration of MoS2 for high-efficiency hydrogen production. Appl. Catal. B-Environ. Energy 2021, 298, 120557. [Google Scholar] [CrossRef]
- Tao, Z.H.; Lv, N.; Zhao, H.Y.; Luo, X.; Li, Z.L.; Yu, J.; Chen, L.; Liu, X.P.; Mu, S.C. Dual active site-mediated Ir single-atom-doped RuO2 catalysts for highly efficient and stable water splitting. Chem. Sci. 2024, 15, 16796–16803. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Yu, Y.H.; Li, J.; Chen, Q.R.; Du, Y.L.; Rao, P.; Li, R.S.; Jia, C.M.; Kang, Z.Y.; Deng, P.L.; et al. Engineering Ruthenium-Based Electrocatalysts for Effective Hydrogen Evolution Reaction. Nano-Micro Lett. 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xue, X.X.; Jiao, M.G.; Liu, J.H.; Wu, T.; Ma, X.D.; Lu, D.; Zhang, R.; Zhang, S.J.; Shao, G.L.; et al. Coordination engineering of single-atom ruthenium in 2D MoS2 for enhanced hydrogen evolution. Chem. Sci. 2024, 15, 16281–16290. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Q.; Ding, S.Q.; Liu, D.B.; Li, D.D.; Chen, S.M.; Wang, H.J.; Qi, Z.M.; Ge, B.H.; Song, L. A Unique Ru-N4-P Coordinated Structure Synergistically Waking Up the Nonmetal P Active Site for Hydrogen Production. Research 2020, 2020, 5860712. [Google Scholar] [CrossRef]
- Rong, C.L.; Shen, X.J.; Wang, Y.; Thomsen, L.; Zhao, T.W.; Li, Y.B.; Lu, X.Y.; Amal, R.; Zhao, C. Electronic Structure Engineering of Single-Atom Ru Sites via Co-N4 Sites for Bifunctional pH-Universal Water Splitting. Adv. Mater. 2022, 34, 2110103. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.Z.; Li, Y.S.; Hu, X.D.; Sun, S.Y.; Yan, B.; Hu, H.Y.; Zhang, X.; Wang, Z.L.; Guo, L. Application of MoS2-FeS functional carrier loaded Ni single-atom catalysts on HER: First principle. Nanotechnology 2022, 33, 275401. [Google Scholar] [CrossRef]
- Pattengale, B.; Huang, Y.C.; Yan, X.X.; Yang, S.Z.; Younan, S.; Hu, W.H.; Li, Z.D.; Lee, S.; Pan, X.Q.; Gu, J.; et al. Dynamic evolution and reversibility of single-atom Ni(II) active site in 1T-MoS2 electrocatalysts for hydrogen evolution. Nat. Commun. 2020, 11, 4114. [Google Scholar] [CrossRef]
- Zang, W.J.; Sun, T.; Yang, T.; Xi, S.B.; Waqar, M.; Kou, Z.K.; Lyu, Z.Y.; Feng, Y.P.; Wang, J.; Pennycook, S.J. Efficient Hydrogen Evolution of Oxidized Ni-N3 Defective Sites for Alkaline Freshwater and Seawater Electrolysis. Adv. Mater. 2021, 33, 2003846. [Google Scholar] [CrossRef]
- Park, J.W.; Park, G.; Kim, M.; Han, M.; Jang, J.; Yamauchi, Y.; Yuliarto, B.; Krüger, P.; Kim, J.; Park, N.; et al. Ni-single atom decorated mesoporous carbon electrocatalysts for hydrogen evolution reaction. Chem. Eng. J. 2023, 468, 143733. [Google Scholar] [CrossRef]
- Li, M.; Zhu, H.Y.; Yuan, Q.; Li, T.Y.; Wang, M.M.; Zhang, P.; Zhao, Y.L.; Qin, D.L.; Guo, W.Y.; Liu, B.; et al. Proximity Electronic Effect of Ni/Co Diatomic Sites for Synergistic Promotion of Electrocatalytic Oxygen Reduction and Hydrogen Evolution. Adv. Funct. Mater. 2023, 33, 2210867. [Google Scholar] [CrossRef]
- Liu, H.H.; Liu, Q.Q.; Shao, Y.F.; Wang, R.R.; Cheng, M.; Hu, J.; Wei, T.; Liu, B.; Jiang, H.F.; Qi, L.; et al. Single-Atom Nickel Encapsulated in Nanosheet-Coiled rGO-CTAB-MoS2 Nanoflowers for High-Efficiency and Long-Term Hydrogen Evolution in Acidic Medium. Adv. Funct. Mater. 2025, 2425826. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.X.; Zhou, Y.W.; Meng, Z.D.; Luo, L.; Liu, S.Q. Single-atom nickel anchored on surface of molybdenum disulfide for efficient hydrogen evolution. J. Electroanal. Chem. 2021, 894, 115359. [Google Scholar] [CrossRef]
- Qiu, H.J.; Ito, Y.; Cong, W.T.; Tan, Y.W.; Liu, P.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M.W. Nanoporous Graphene with Single-Atom Nickel Dopants: An Efficient and Stable Catalyst for Electrochemical Hydrogen Production. Angew. Chem. -Int. Ed. 2015, 54, 14031–14035. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.L.; Liu, P.F.; Yan, X.C.; Gu, L.; Yang, Z.Z.; Yang, H.G.; Qiu, S.L.; Yao, X.D. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat. Commun. 2016, 7, 10667. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.L.; Dong, S.; He, D.S.; Lawrence, M.J.; Han, S.B.; Cai, C.; Xiang, S.H.; Rodriguez, P.; Xiang, B.; et al. Design of active nickel single-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction. Nano Energy 2018, 53, 458–467. [Google Scholar] [CrossRef]
- Huo, L.X.; Jin, C.Q.; Tang, J.L.; Xu, X.H.; Jiang, K.; Shang, L.Y.; Li, Y.W.; Zhang, J.Z.; Zhu, L.Q.; Chu, J.H.; et al. Ultrathin NiPt Single-Atom Alloy for Synergistically Accelerating Alkaline Hydrogen Evolution. Acs Appl. Energy Mater. 2022, 5, 15136–15145. [Google Scholar] [CrossRef]
- Jo, Y.; Choi, M.; Kim, M.; Yoo, J.S.; Choi, W.; Lee, D. Promotion of alkaline hydrogen production via Ni-doping of atomically precise Ag nanoclusters. Bull. Korean Chem. Soc. 2021, 42, 1672–1677. [Google Scholar] [CrossRef]
- Gao, D.; Guo, J.N.; Cui, X.; Yang, L.; Yang, Y.; He, H.C.; Xiao, P.; Zhang, Y.H. Three-Dimensional Dendritic Structures of NiCoMo as Efficient Electrocatalysts for the Hydrogen Evolution Reaction. Acs Appl. Mater. Interfaces 2017, 9, 22420–22431. [Google Scholar] [CrossRef]
- Kim, M.S.; Tran, D.T.; Nguyen, T.H.; Dinh, V.A.; Kim, N.H.; Lee, J.H. Ni Single Atoms and Ni Phosphate Clusters Synergistically Triggered Surface-Functionalized MoS2 Nanosheets for High-performance Freshwater and Seawater Electrolysis. Energy Environ. Mater. 2022, 5, 1340–1349. [Google Scholar] [CrossRef]
- Kumar, A.; Bui, V.Q.; Lee, J.; Wang, L.L.; Jadhav, A.R.; Liu, X.H.; Shao, X.D.; Liu, Y.; Yu, J.M.; Hwang, Y.; et al. Moving beyond bimetallic-alloy to single-atom dimer atomic-interface for all-pH hydrogen evolution. Nat. Commun. 2021, 12, 6766. [Google Scholar] [CrossRef]
- Feng, T.; Wang, F.; Xu, Y.J.; Chang, M.J.; Jin, X.J.; Zhou, Y.L.; Piao, J.H.; Lei, J.F. CoP/Ni2P heteronanoparticles integrated with atomic Co/Ni dual sites for enhanced electrocatalytic performance toward hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 8431–8443. [Google Scholar] [CrossRef]
- Li, M.N.; Yu, J.; Liu, Q.; Liu, J.Y.; Chen, R.R.; Zhu, J.H.; Li, R.M.; Wang, J. Atomically Dispersed Fe-N5 Sites Anchored in Porous N-Doped Carbon Nanofibers for Effective Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2022, 10, 13505–13513. [Google Scholar] [CrossRef]
- Liu, M.M.; Fan, X.F.; Cui, X.Q.; Singh, D.J.; Zheng, W.T. Different surfaces of copper with single-atom doping for hydrogen evolution reaction in alkaline conditions. Mol. Catal. 2025, 570, 114699. [Google Scholar] [CrossRef]
- Li, W.X.; Yin, D.S.; Li, P.; Zhao, X.H.; Hao, S.C. Iridium single-atoms anchored on a TiO2 support as an efficient catalyst for the hydrogen evolution reaction. Phys. Chem. Chem. Phys. 2024, 26, 19822–19830. [Google Scholar] [CrossRef]
- Wang, T.T.; Zhao, Q.D.; Fu, Y.Y.; Lei, C.J.; Yang, B.; Li, Z.J.; Lei, L.C.; Wu, G.; Hou, Y. Carbon-Rich Nonprecious Metal Single Atom Electrocatalysts for CO2 Reduction and Hydrogen Evolution. Small Methods 2019, 3, 1900210. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Z.G.; Zhang, Y.W. Synergizing Cu dimers and N atoms in graphene towards an active catalyst for hydrogen evolution reaction. Nanoscale Adv. 2021, 3, 5332–5338. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Zhang, Y.; Liu, L.; Jiao, M.; Ye, C. Single-Atom Catalysts for Hydrogen Evolution Reaction: The Role of Supports, Coordination Environments, and Synergistic Effects. Nanomaterials 2025, 15, 1175. https://doi.org/10.3390/nano15151175
Liang Z, Zhang Y, Liu L, Jiao M, Ye C. Single-Atom Catalysts for Hydrogen Evolution Reaction: The Role of Supports, Coordination Environments, and Synergistic Effects. Nanomaterials. 2025; 15(15):1175. https://doi.org/10.3390/nano15151175
Chicago/Turabian StyleLiang, Zhuoying, Yu Zhang, Linli Liu, Miaolun Jiao, and Chenliang Ye. 2025. "Single-Atom Catalysts for Hydrogen Evolution Reaction: The Role of Supports, Coordination Environments, and Synergistic Effects" Nanomaterials 15, no. 15: 1175. https://doi.org/10.3390/nano15151175
APA StyleLiang, Z., Zhang, Y., Liu, L., Jiao, M., & Ye, C. (2025). Single-Atom Catalysts for Hydrogen Evolution Reaction: The Role of Supports, Coordination Environments, and Synergistic Effects. Nanomaterials, 15(15), 1175. https://doi.org/10.3390/nano15151175





