Surface Microstructure Engineering for Enhancing Li-Ion Diffusion and Structure Stability of Ni-Rich Cathode Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
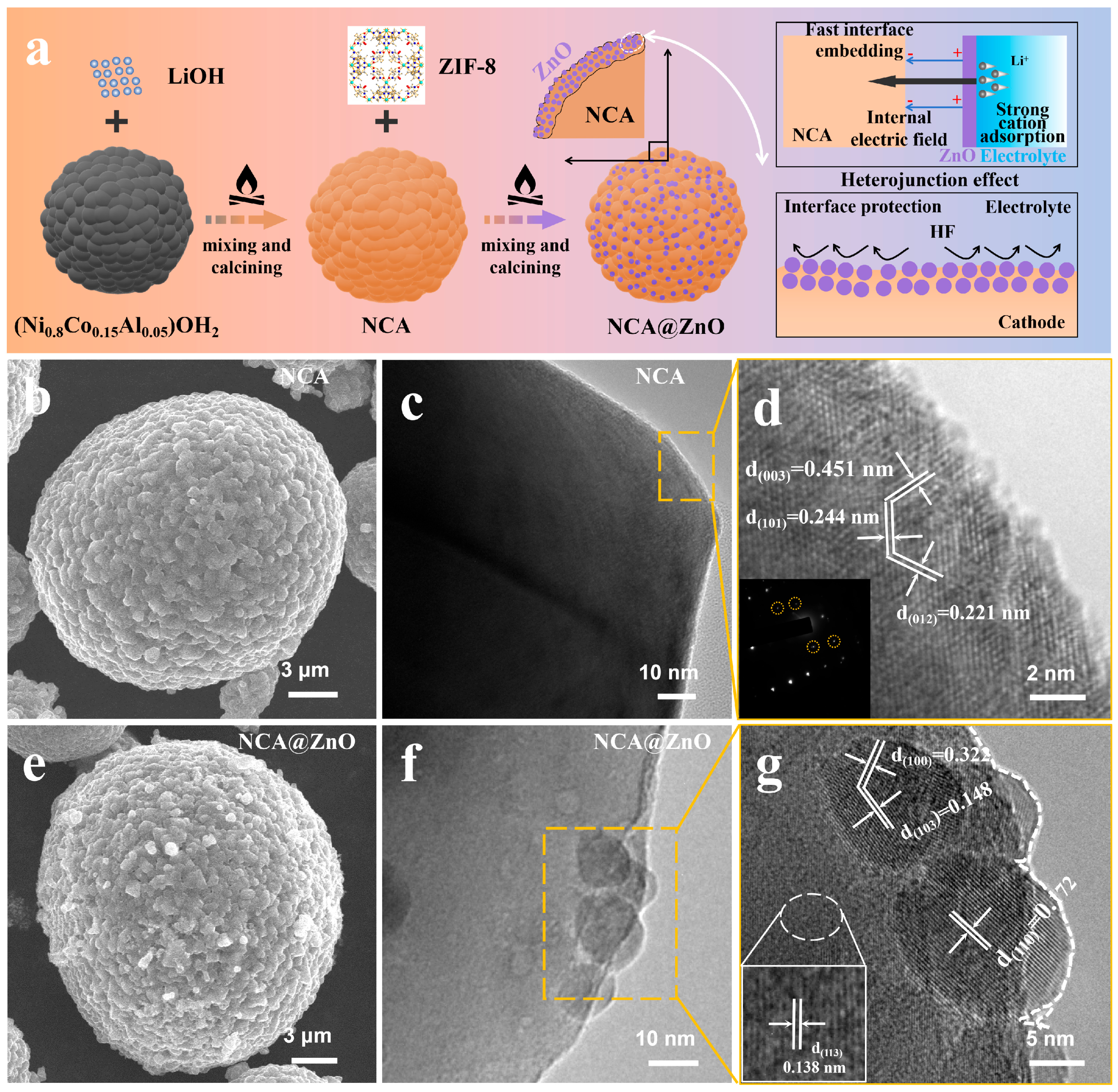
2.1.1. Synthesis of ZIF-8
2.1.2. Synthesis of NCA and NCA@ZnO
2.2. Characterizations
2.3. Electrochemical Tests
3. Results
3.1. Characterization of Materials
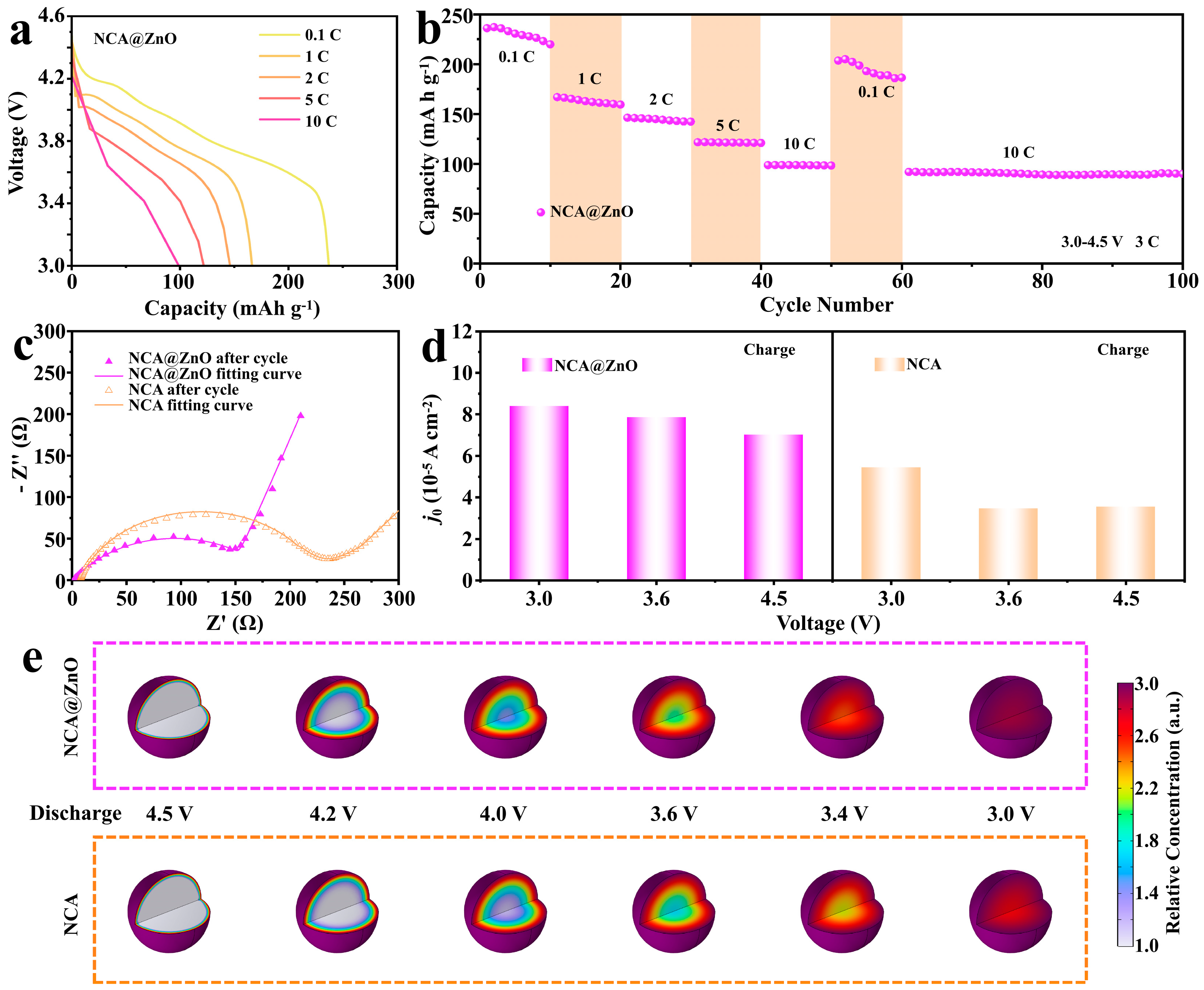
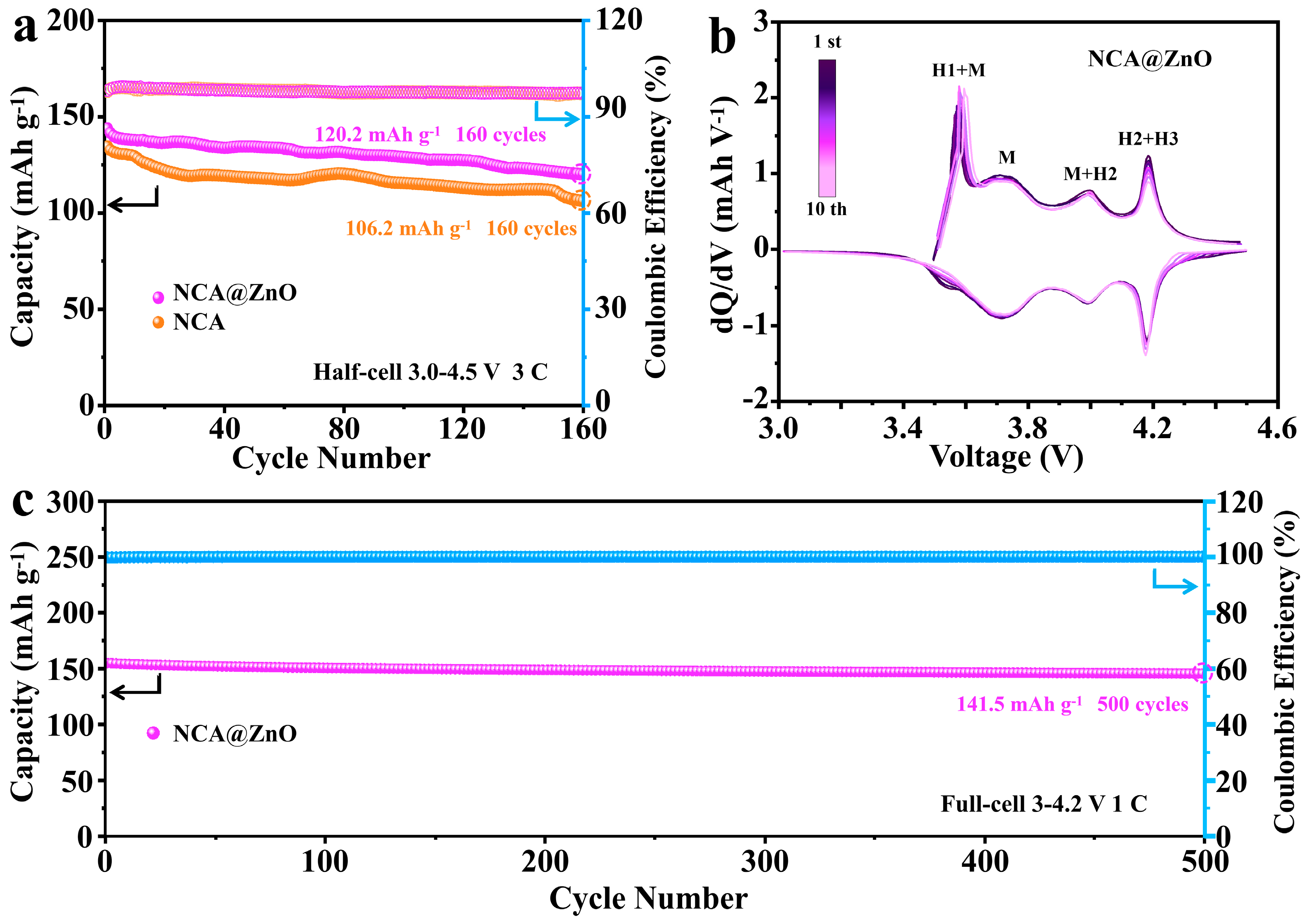
3.2. Electrochemical Properties
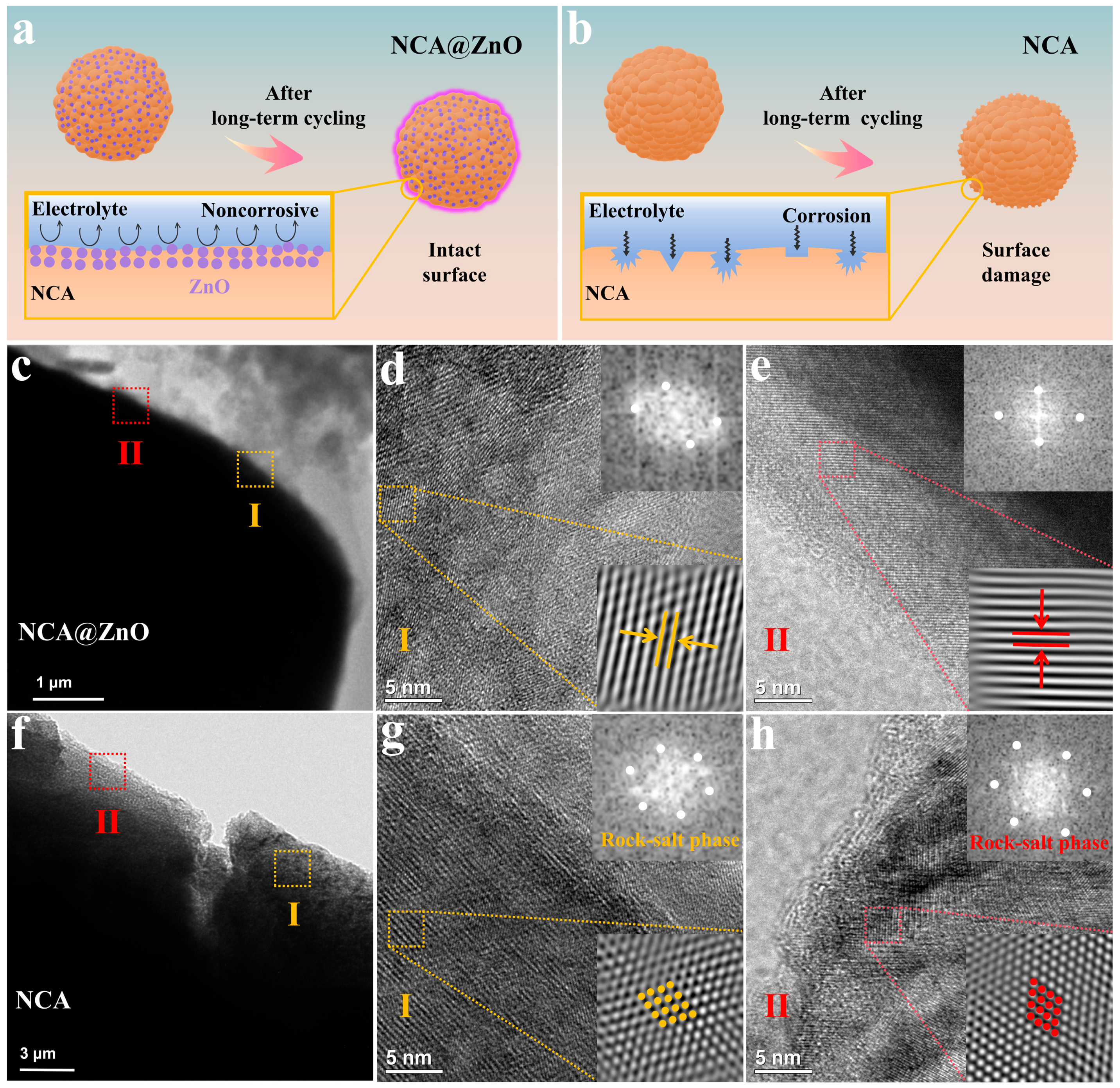
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryu, H.-H.; Sun, H.H.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Reducing Cobalt from Lithium-Ion Batteries for the Electric Vehicle Era. Energy Environ. Sci. 2021, 14, 844–852. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Wen, B.; Wolfman, M.; Choe, M.-J.; Pan, M.S.; Su, L.; Thornton, K.; Cabana, J.; Chiang, Y.-M. Single-Particle Measurements of Electrochemical Kinetics in Nmc and Nca Cathodes for Li-Ion Batteries. Energy Environ. Sci. 2018, 11, 860–871. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023, 6, 12450. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, Q.; Li, B.; Manthiram, A. An in-Depth Understanding of the Effect of Aluminum Doping in High-Nickel Cathodes for Lithium-Ion Batteries. Energy Storage Mater. 2021, 34, 229–240. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.; Manthiram, A. High-Nickel NMA: A Cobalt-Free Alternative to NMC and NCA Cathodes for Lithium-Ion Batteries. Adv. Mater. 2020, 32, 2002718. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Noh, M.; Oh, P.; Kim, Y.; Cho, J. A New High Power LiNi0.81Co0.1Al0.09O2 Cathode Material for Lithium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1301583. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Shin, W.; Jo, E.; Manthiram, A. Insights into the Microstructural Engineering of Cobalt-Free, High-Nickel Cathodes Based on Surface Energy for Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2204054. [Google Scholar] [CrossRef]
- Park, K.-J.; Hwang, J.-Y.; Ryu, H.-H.; Maglia, F.; Kim, S.-J.; Lamp, P.; Yoon, C.S.; Sun, Y.-K. Degradation Mechanism of Ni-Enriched NCA Cathode for Lithium Batteries: Are Microcracks Really Critical? ACS Energy Lett. 2019, 4, 1394–1400. [Google Scholar] [CrossRef]
- Park, K.J.; Choi, M.J.; Maglia, F.; Kim, S.J.; Kim, K.H.; Yoon, C.S.; Sun, Y.K. High-Capacity Concentration Gradient Li[Ni0.865Co0.120Al0.015]O2 Cathode for Lithium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703612. [Google Scholar] [CrossRef]
- Phan, T.T.T.; Nguyen, T.T.K.; Mac, T.K.; Trinh, M.T. Enhancing Stability and Emissions in Metal Halide Perovskite Nanocrystals through Mn2+ Doping. Nanomaterials 2025, 15, 847. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Ma, Y.; Anduaga-Quiros, K.; Briseno, G.; Ning, Y.; Chang, H.-J.; Ozkan, M.; Ozkan, C.S. Powering the Future: Unveiling the Potential of Na, K, and Mg Solid-State Batteries. Nanomaterials 2025, 15, 859. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiao, Z.; Liu, J.; He, X.; Wen, J.; Zhou, Y.; Cheng, L.; Zhang, B.; Liu, T.; Amine, K.; et al. Surface Atomic Rearrangement with High Cation Ordering for Ultra-Stable Single-Crystal Ni-Rich Co-Less Cathode Materials. Adv. Funct. Mater. 2024, 34, 2409956. [Google Scholar] [CrossRef]
- Xia, S.; Huang, W.; Shen, X.; Liu, J.; Cheng, F.; Liu, J.-J.; Yang, X.; Guo, H. Rearrangement on Surface Structures by Boride to Enhanced Cycle Stability for LiNi0.80Co0.15Al0.05O2 Cathode in Lithium Ion Batteries. J. Energy Chem. 2020, 45, 110–118. [Google Scholar] [CrossRef]
- Xie, K.; Liu, X.; Xia, K.; Diao, L.; Lu, P.; Wang, M.; Fang, L.; Zou, Y.; Yang, D.; Zhang, X. Innovative Mn3−XO4−Y@NCA Design: Leveraging Mn/O Vacancies and Amorphous Architecture for Enhanced Sodium-Ion Storage. J. Energy Chem. 2024, 97, 747–756. [Google Scholar] [CrossRef]
- Crenna, E.; Gauch, M.; Widmer, R.; Wäger, P.; Hischier, R. Towards More Flexibility and Transparency in Life Cycle Inventories for Lithium-Ion Batteries. Resour. Conserv. Recycl. 2021, 170, 105619. [Google Scholar] [CrossRef]
- O’Malley, R.; Liu, L.; Depcik, C. Comparative Study of Various Cathodes for Lithium Ion Batteries Using an Enhanced Peukert Capacity Model. J. Power Sources 2018, 396, 621–631. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, F.-L.; Zhou, X.-A.; Wang, P.; Wang, J.; Ding, H.; Dong, H.; Liang, W.-B.; Zhang, N.-S.; Li, S.-Y. Which of the Nickel-Rich NCM and NCA Is Structurally Superior as a Cathode Material for Lithium-Ion Batteries? J. Mater. Chem. A 2021, 9, 13540–13551. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhang, R.; Lei, T.; Kisslinger, K.; Xin, H.L. Resolving Complex Intralayer Transition Motifs in High-Ni-Content Layered Cathode Materials for Lithium-Ion Batteries. Nat. Mater. 2023, 22, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Märker, K.; Lee, J.; Mahadevegowda, A.; Reeves, P.J.; Day, S.J.; Groh, M.F.; Emge, S.P.; Ducati, C.; Layla Mehdi, B.; et al. Bulk Fatigue Induced by Surface Reconstruction in Layered Ni-Rich Cathodes for Li-Ion Batteries. Nat. Mater. 2020, 20, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Tang, Y.; Zheng, Q.; Zhang, H.; Yan, Y.; Xue, J.; Zhou, S.; Xu, J.; Yin, W.; Liao, H.G.; et al. Enabling the Strengthened Structural and Interfacial Stability of High-Nickel LiNi0.9Co0.05Mn0.05O2 Cathode by a Coating-Doping-Microstructure Regulation Three-in-One Strategy. Adv. Funct. Mater. 2024, 34, 2406086. [Google Scholar] [CrossRef]
- Zheng, C.; Xiao, Z.; Xian, K.; Wen, H.; Lu, N.; He, X.; Ye, L.; Du, K.; Zhang, B.; Ou, X.; et al. Reinforcing Ion Diffusion and Controlling Microcrack of Nickel-Rich Cobalt-Free Single-Crystalline Cathodes Via Interfacial Protection and Bulk Optimization. J. Colloid Interface Sci. 2025, 684, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.-B.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Comparative Study of Ni-Rich Layered Cathodes for Rechargeable Lithium Batteries: Li[Ni0.85Co0.11Al0.04]O2 and Li[Ni0.84Co0.06Mn0.09Al0.01]O2 with Two-Step Full Concentration Gradients. ACS Energy Lett. 2016, 1, 283–289. [Google Scholar] [CrossRef]
- Manthiram, A. A Reflection on Lithium-Ion Battery Cathode Chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.-T.; Maglia, F.; Park, K.-J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-Rich Layered Cathode Materials for Automotive Lithium-Ion Batteries: Achievements and Perspectives. ACS Energy Lett. 2016, 2, 196–223. [Google Scholar] [CrossRef]
- Nam, G.W.; Park, N.Y.; Park, K.J.; Yang, J.; Liu, J.; Yoon, C.S.; Sun, Y.K. Capacity Fading of Ni-Rich NCA Cathodes: Effect of Microcracking Extent. ACS Energy Lett. 2019, 4, 2995–3001. [Google Scholar] [CrossRef]
- Su, M.; Chen, Y.; Song, Y.; Dou, A.; Wang, J.; Yan, G.; Zhou, Y.; Wang, Z.; Liu, Y. La4nilio8-Assistant Surface Reconstruction to Realize in-Situ Regeneration of the Degraded Nickel-Rich Cathodes. Chem. Eng. J. 2023, 477, 147202. [Google Scholar] [CrossRef]
- Zuo, J.; Wang, J.; Duan, R.; Bai, Y.; Xu, K.; Zhang, K.; Wang, J.; Zhang, K.; Yang, Z.; Yang, Z.; et al. Grain Binding Derived Reinforced Interfacial Mechanical Behavior of Ni-Rich Layered Cathode Materials. Nano Energy 2024, 121, 109214. [Google Scholar] [CrossRef]
- Yang, Y.; Dang, R.; Wu, K.; Li, Q.; Li, N.; Xiao, X.; Hu, Z. Semiconductor Material Zno-Coated P2-Type Na2/3Ni1/3Mn2/3O2 Cathode Materials for Sodium-Ion Batteries with Superior Electrochemical Performance. J. Phys. Chem. C 2020, 124, 1780–1787. [Google Scholar] [CrossRef]
- Ran, X.; Tao, J.; Chen, Z.; Yan, Z.; Yang, Y.; Li, J.; Lin, Y.; Huang, Z. Surface Heterostructure Induced by Tio2 Modification in Li-Rich Cathode Materials for Enhanced Electrochemical Performances. Electrochim. Acta 2020, 353, 135959. [Google Scholar] [CrossRef]
- Chang, W.; Choi, J.-W.; Im, J.-C.; Lee, J.K. Effects of Zno Coating on Electrochemical Performance and Thermal Stability of LiCoO2 as Cathode Material for Lithium-Ion Batteries. J. Power Sources 2010, 195, 320–326. [Google Scholar] [CrossRef]
- Lai, Y.-Q.; Xu, M.; Zhang, Z.-A.; Gao, C.-H.; Wang, P.; Yu, Z.-Y. Optimized Structure Stability and Electrochemical Performance of LiNi0.8Co0.15Al0.05O2 by Sputtering Nanoscale Zno Film. J. Power Sources 2016, 309, 20–26. [Google Scholar] [CrossRef]
- Seenivasan, M.; Jeyakumar, J.; Wu, Y.-S.; Pham, Q.-T.; Chern, C.-S.; Hwang, B.-J.; Yang, C.-C. Bifunctional Coating Layer on Ni-Rich Cathode Materials to Enhance Electrochemical Performance and Thermal Stability in Lithium-Ion Batteries. Compos. Part B Eng. 2022, 242, 110083. [Google Scholar] [CrossRef]
- Zhang, B.; Wen, H.; Xian, K.; Lu, N.; Zheng, C.; Xiao, Z.; He, X.; Ye, L.; Wang, J.; Ming, L.; et al. Synergistically Regulating the Structural Tolerance of Co-Free Ni-Rich Cathode Materials at High-Rate through Multi-Heteroatoms Substitution. J. Colloid Interface Sci. 2025, 682, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Hawley, W.B.; Meyer, H.M.; Li, J. Enabling Aqueous Processing for LiNi0.80Co0.15Al0.05O2 (NCA)-Based Lithium-Ion Battery Cathodes Using Polyacrylic Acid. Electrochim. Acta 2021, 380, 138203. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, N.-Y.; Seo, J.H.; Yu, Y.-S.; Sharma, M.; Mücke, R.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. A Highly Stabilized Ni-Rich NCA Cathode for High-Energy Lithium-Ion Batteries. Mater. Today 2020, 36, 73–82. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Z.; Huang, Y.; Zhang, M.; Liu, C.; Zhang, D.; Cao, G. Artificial Interface Stabilized LiNi0.80Co0.15Al0.05O2@Polysiloxane Cathode for Stable Cycling Lithium-Ion Batteries. J. Power Sources 2020, 471, 228480. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, L.; Zhang, Y.; Tan, Q.; Li, J. An Overview of Global Power Lithium-Ion Batteries and Associated Critical Metal Recycling. J. Hazard. Mater. 2022, 425, 127900. [Google Scholar] [CrossRef] [PubMed]
- Purwanto, A.; Yudha, C.S.; Ubaidillah, U.; Widiyandari, H.; Ogi, T.; Haerudin, H. NCA Cathode Material: Synthesis Methods and Performance Enhancement Efforts. Mater. Res. Express 2018, 5, 122001. [Google Scholar] [CrossRef]
- Vorfolomeeva, A.A.; Zaguzina, A.A.; Maksimovskiy, E.A.; Gusel’nikov, A.V.; Plyusnin, P.E.; Okotrub, A.V.; Bulusheva, L.G. Molybdenum Disulfide and Reduced Graphene Oxide Hybrids as Anodes for Low-Temperature Lithium- and Sodium-Ion Batteries. Nanomaterials 2025, 15, 824. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kinoshita, M.; Nakura, K. Capacity Fade of LiNi(1−X−Y)CoxAlyO2 Cathode for Lithium-Ion Batteries During Accelerated Calendar and Cycle Life Test. I. Comparison Analysis between LiNi(1−X−Y)CoxAlyO2 and LiCoO2 Cathodes in Cylindrical Lithium-Ion Cells During Long Term Storage Test. J. Power Sources 2014, 247, 412–422. [Google Scholar] [CrossRef]
- Chakraborty, A.; Kunnikuruvan, S.; Kumar, S.; Markovsky, B.; Aurbach, D.; Dixit, M.; Major, D.T. Layered Cathode Materials for Lithium-Ion Batteries: Review of Computational Studies on LiNi1−X−YCoxMnyO2 and LiNi1−X−YCoxAlyO2. Chem. Mater. 2020, 32, 915–952. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, L.; Zhang, Z.; Wang, Z.; Dorrell, D.D. Degradation Characteristics Investigation for Lithium-Ion Cells with Nca Cathode During Overcharging. Appl. Energy 2022, 327, 120026. [Google Scholar] [CrossRef]
- Zhang, B.; Wen, H.; Xian, K.; Lu, N.; Zheng, C.; Xiao, Z.; He, X.; Ye, L.; Wang, J.; Ming, L.; et al. Stabilizing Ultrahigh-Nickel Cobalt-Free Cathode Materials by Using Tri-Element Doping Engineering. Mater. Today Energy 2025, 48, 101787. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, H.; Zhao, S.; Xu, R.; Zhou, L.; Li, Y.; Peng, Y.; Rao, X.; Tao, Y.; Ou, X. Surface Microstructure Engineering for Enhancing Li-Ion Diffusion and Structure Stability of Ni-Rich Cathode Materials. Nanomaterials 2025, 15, 1144. https://doi.org/10.3390/nano15151144
Zhuo H, Zhao S, Xu R, Zhou L, Li Y, Peng Y, Rao X, Tao Y, Ou X. Surface Microstructure Engineering for Enhancing Li-Ion Diffusion and Structure Stability of Ni-Rich Cathode Materials. Nanomaterials. 2025; 15(15):1144. https://doi.org/10.3390/nano15151144
Chicago/Turabian StyleZhuo, Huanming, Shuangshuang Zhao, Ruijie Xu, Lu Zhou, Ye Li, Yuehuan Peng, Xuelong Rao, Yuqiang Tao, and Xing Ou. 2025. "Surface Microstructure Engineering for Enhancing Li-Ion Diffusion and Structure Stability of Ni-Rich Cathode Materials" Nanomaterials 15, no. 15: 1144. https://doi.org/10.3390/nano15151144
APA StyleZhuo, H., Zhao, S., Xu, R., Zhou, L., Li, Y., Peng, Y., Rao, X., Tao, Y., & Ou, X. (2025). Surface Microstructure Engineering for Enhancing Li-Ion Diffusion and Structure Stability of Ni-Rich Cathode Materials. Nanomaterials, 15(15), 1144. https://doi.org/10.3390/nano15151144






