Design Efficiency: A Critical Perspective on Testing Methods for Solar-Driven Photothermal Evaporation and Photocatalysis
Abstract
1. Introduction
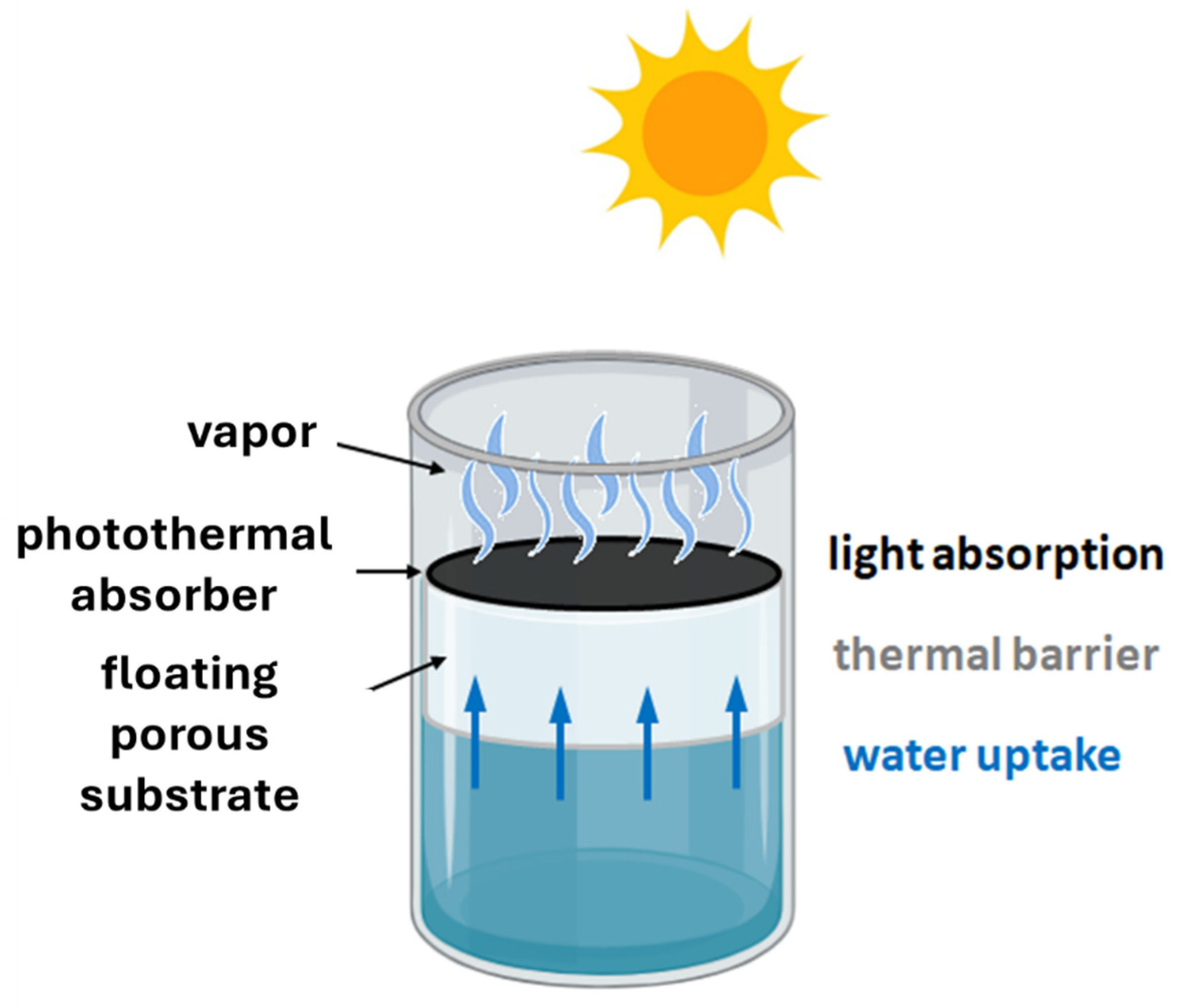
2. Materials for Photocatalysis and Photothermal Absorption
3. Solar Still Geometry and Cover Material
3.1. Solar Still Geometry
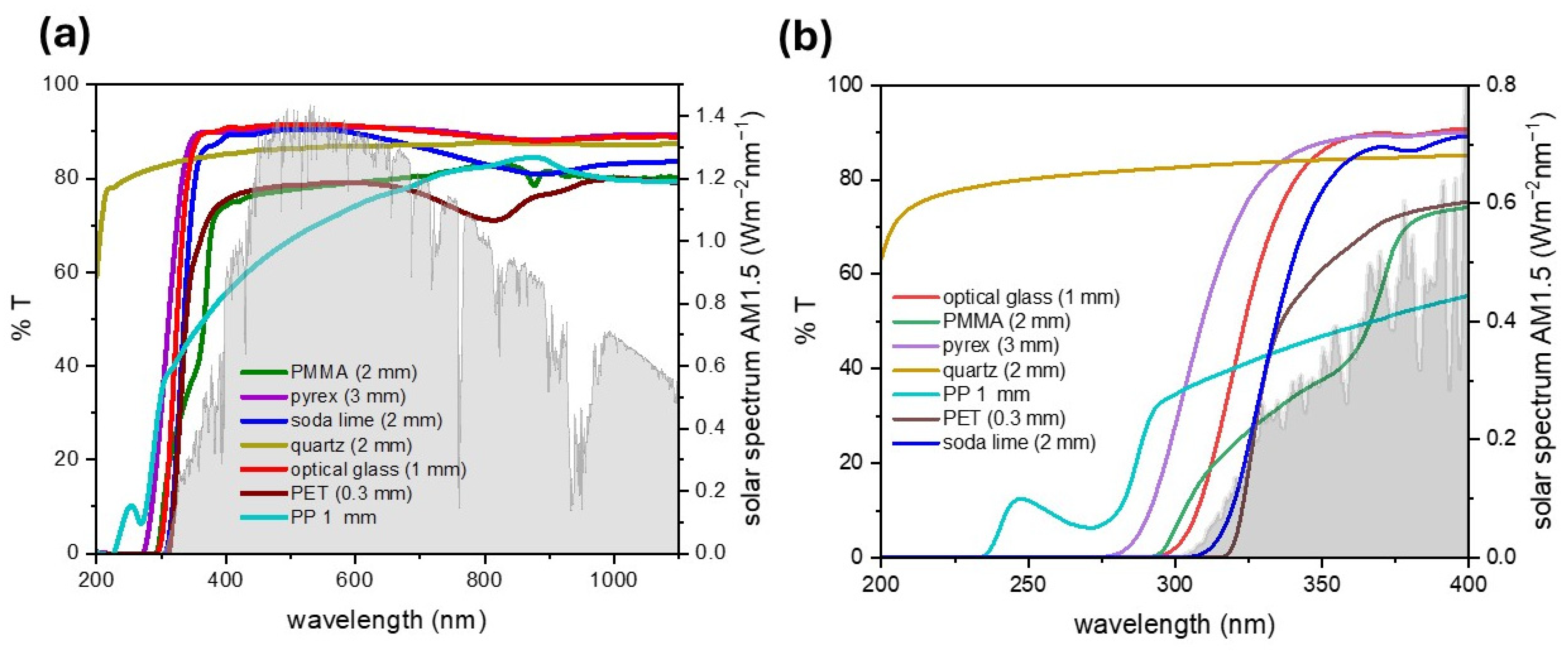
3.2. Optical Properties of the Cover Material
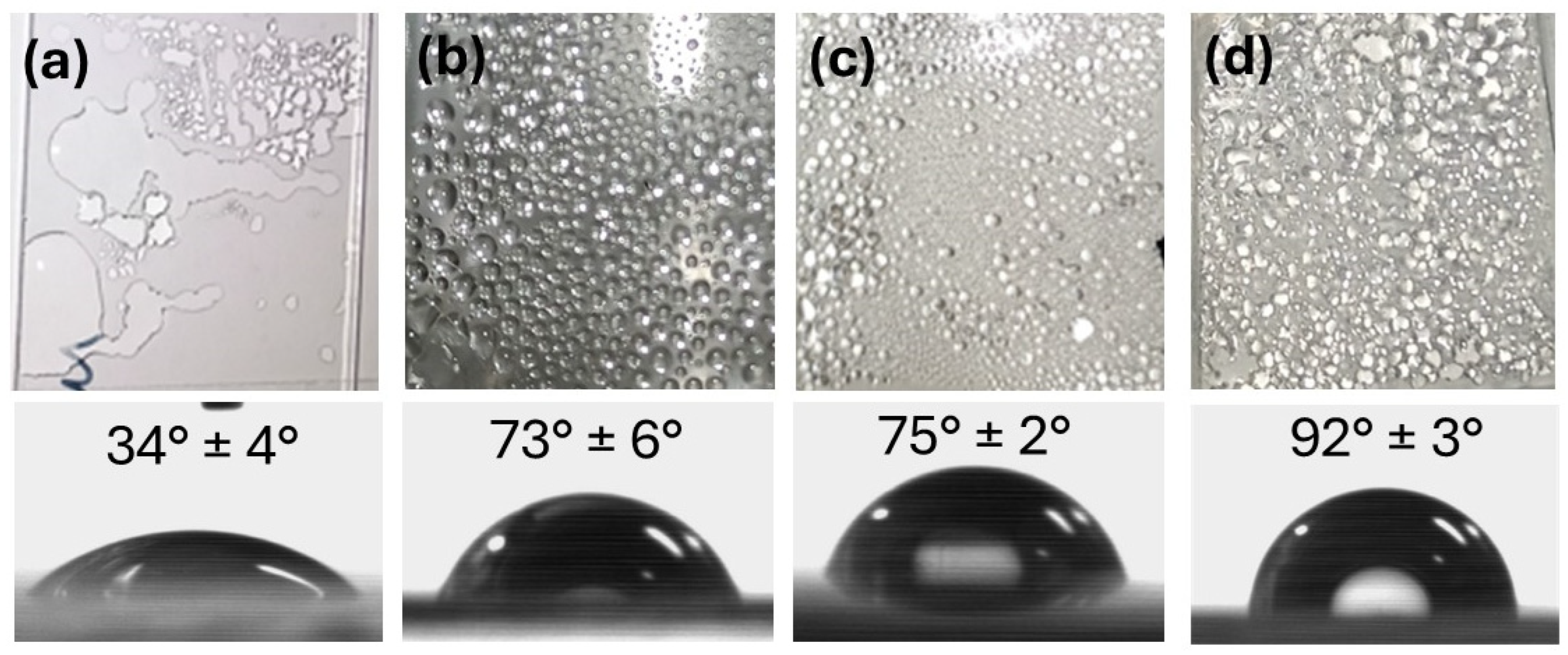
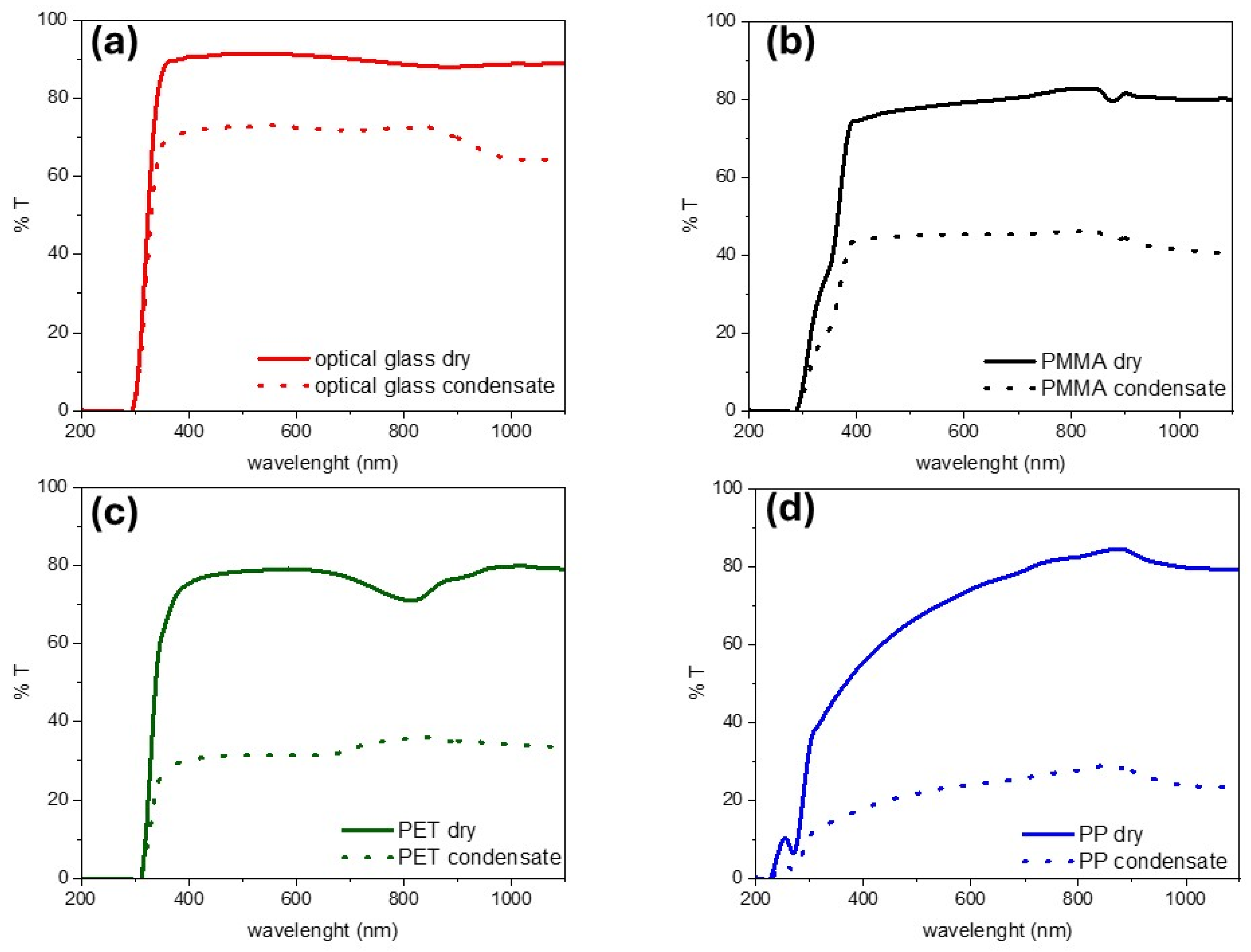
3.3. Wetting Properties of the Cover Material
4. Target Pollutants and Testing Protocols
5. Conclusions and Prospects
- (1)
- Prioritizing low-cost, widely available materials (e.g., optical glass over quartz);
- (2)
- Designing for manufacturability: While flat stills are cheaper and more versatile to fabricate, their lower efficiency highlights the need for cost-effective solutions to produce dome stills. While 3D printing offers design flexibility, currently available transparent printable materials present significant limitations in optical performance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of Food and Agriculture 2020: Overcoming Water Challenges in Agriculture; The State of Food and Agriculture (SOFA); FAO: Rome, Italy, 2020; ISBN 9789251334416. [Google Scholar]
- Kim, J.; Park, K.; Yang, D.R.; Hong, S. A Comprehensive Review of Energy Consumption of Seawater Reverse Osmosis Desalination Plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Mastoras, P.; Vakalis, S.; Fountoulakis, M.S.; Gatidou, G.; Katsianou, P.; Koulis, G.; Thomaidis, N.S.; Haralambopoulos, D.; Stasinakis, A.S. Evaluation of the Performance of a Pilot-Scale Solar Still for Olive Mill Wastewater Treatment. J. Clean. Prod. 2022, 365, 132695. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, F.; Zhang, P.; Yu, G. Solar Water Evaporation Toward Water Purification and Beyond. ACS Mater. Lett. 2021, 3, 1112–1129. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Y.; Luo, Y.; Wang, W.; Chen, X. Porous Evaporators with Special Wettability for Low-Grade Heat-Driven Water Desalination. J. Mater. Chem. A Mater. 2021, 9, 702–726. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhong, Y.; Leroy, A.; Xu, Z.; Zhao, L.; Wang, E.N. Highly Efficient and Salt Rejecting Solar Evaporation via a Wick-Free Confined Water Layer. Nat. Commun. 2022, 13, 849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhou, X.; Shi, Y.; Qian, X.; Alexander, M.; Zhao, X.; Mendez, S.; Yang, R.; Qu, L.; Yu, G. Highly Efficient Solar Vapour Generation via Hierarchically Nanostructured Gels. Nat. Nanotechnol. 2018, 13, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Yang, Y.; Bin, X.; Zhao, S.; Pan, C.; Nawaz, F.; Que, W. Recent Advanced Self-Propelling Salt-Blocking Technologies for Passive Solar-Driven Interfacial Evaporation Desalination Systems. Nano Energy 2021, 89, 106468. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Recent Progress in Solar-Driven Interfacial Water Evaporation: Advanced Designs and Applications. Nano Energy 2019, 57, 507–518. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, T.; Kim, J.; Peng, H.; Ye, M.; Huang, C.-H. Interfacial Solar Distillation for Freshwater Production: Fate of Volatile and Semivolatile Organic Contaminants. Environ. Sci. Technol. 2021, 55, 6248–6256. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C. Microbial Biofouling: Unsolved Problems, Insufficient Approaches, and Possible Solutions. In Biofilm Highlights; Flemming, H.-C., Wingender, J., Szewzyk, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 81–109. ISBN 978-3-642-19940-0. [Google Scholar]
- Zhang, P.; Wang, H.; Wang, J.; Ji, Z.; Qu, L. Boosting the Viable Water Harvesting in Solar Vapor Generation: From Interfacial Engineering to Devices Design. Adv. Mater. 2024, 36, 2303976. [Google Scholar] [CrossRef] [PubMed]
- Djellabi, R.; Noureen, L.; Dao, V.-D.; Meroni, D.; Falletta, E.; Dionysiou, D.D.; Bianchi, C.L. Recent Advances and Challenges of Emerging Solar-Driven Steam and the Contribution of Photocatalytic Effect. Chem. Eng. J. 2022, 431, 134024. [Google Scholar] [CrossRef]
- Liu, K.; Cao, M.; Fujishima, A.; Jiang, L. Bio-Inspired Titanium Dioxide Materials with Special Wettability and Their Applications. Chem. Rev. 2014, 114, 10044–10094. [Google Scholar] [CrossRef] [PubMed]
- Antonello, A.; Soliveri, G.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Photocatalytic Remediation of Indoor Pollution by Transparent TiO2 Films. Catal. Today 2014, 230, 35–40. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Esmail Ebrahim, S. Recent Advances in Nano-Semiconductors Photocatalysis for Degrading Organic Contaminants and Microbial Disinfection in Wastewater: A Comprehensive Review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100666. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Chen, P.; Li, F.; Hu, X.; Hua, T. Photocatalytic and Antifouling Properties of TiO2-Based Photocatalytic Membranes. Mater. Today Chem. 2022, 23, 100650. [Google Scholar] [CrossRef]
- An, N.; Ma, M.; Chen, Y.; Wang, Z.; Li, Q. Biomass Hydrogel Solar-Driven Multifunctional Evaporator for Desalination, VOC Removal, and Sterilization. ACS ES&T Eng. 2025, 5, 732–742. [Google Scholar] [CrossRef]
- Irshad, M.S.; Arshad, N.; Maqsood, G.; Asghar, M.S.; Wu, P.; Mushtaq, N.; Shah, M.A.K.Y.; Lin, L.; Li, X.; Ahmed, I.; et al. Interdisciplinary Hybrid Solar-Driven Evaporators: Theoretical Framework of Fundamental Mechanisms and Applications. Small 2025, 21, 2407280. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.S.; Arshad, N.; Maqsood, G.; Ahmed, I.; Shakoor, B.; Asghar, M.S.; Ghazanfar, U.; Lin, L.; Shah, M.A.K.Y.; Ahmed, I.; et al. Advancing Water Collection Efficiency in Hybrid Solar Evaporators: Key Factors, Strategic Innovations, and Synergistic Applications. Mater. Sci. Eng. R. Rep. 2025, 165, 101018. [Google Scholar] [CrossRef]
- Tessema, A.A.; Wu, C.-M.; Motora, K.G.; Lee, W.-H.; Peng, Y.-T. A Review on State of Art of Photothermal Nanomaterials for Interfacial Solar Water Evaporation and Their Applications. Desalination 2024, 591, 117998. [Google Scholar] [CrossRef]
- Xu, N.; Li, J.; Finnerty, C.; Song, Y.; Zhou, L.; Zhu, B.; Wang, P.; Mi, B.; Zhu, J. Going beyond Efficiency for Solar Evaporation. Nat. Water 2023, 1, 494–501. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Zhou, X.; Shi, W.; Yu, G. Materials for Solar-Powered Water Evaporation. Nat. Rev. Mater. 2020, 5, 388–401. [Google Scholar] [CrossRef]
- Shi, L.; Shi, Y.; Zhuo, S.; Zhang, C.; Aldrees, Y.; Aleid, S.; Wang, P. Multi-Functional 3D Honeycomb Ceramic Plate for Clean Water Production by Heterogeneous Photo-Fenton Reaction and Solar-Driven Water Evaporation. Nano Energy 2019, 60, 222–230. [Google Scholar] [CrossRef]
- Lv, B.; Li, S.; Wang, W.; Xu, Y.; Zhao, B.; Song, C.; Fan, X.; Liu, Y. A Lotus Leaf-Inspired Janus Dual-Functional Nanofiber Evaporator for Efficient Water Purification. J. Clean. Prod. 2024, 438, 140880. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, J.; Mu, X.; Qi, C.; Kang, M.; Zhang, Z.; Gao, J.; Liu, J.; Miao, L. Photo-Fenton catalyst embedded in photothermal aerogel for efficient solar interfacial water evaporation and purification. Green. Carbon. 2025, 3, 160–169. [Google Scholar] [CrossRef]
- Cui, L.; Wang, P.; Che, H.; Gao, X.; Chen, J.; Liu, B.; Ao, Y. Co Nanoparticles Modified N-Doped Carbon Nanosheets Array as a Novel Bifunctional Photothermal Membrane for Simultaneous Solar-Driven Interfacial Water Evaporation and Persulfate Mediating Water Purification. Appl. Catal. B 2023, 330, 122556. [Google Scholar] [CrossRef]
- Chaw pattnayak, B.; Mohapatra, S. Photothermal–Photocatalytic CSG@ZFG Evaporator for Synergistic Salt Rejection and VOC Removal during Solar-Driven Water Distillation. Langmuir 2023, 39, 4651–4661. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Miao, H.; Chai, Y.; Yuan, R.; Liu, H. Highly Efficient Solar-Driven Photothermal–Photocatalytic System Based on Supramolecular Iron Phthalocyanine Organic Polymer/CoFe2O4/Polydopamine for Wastewater Purification. ACS Mater. Lett. 2024, 6, 132–139. [Google Scholar] [CrossRef]
- Yin, M.; Xiao, C.; Jin, Y.; He, Y.; Zhang, Y.; Chen, L. Bionic Solar-Driven Interfacial Evaporator for Synergistic Photothermal-Photocatalytic Activities and Salt Collection during Desalination. Chem. Eng. J. 2024, 499, 156282. [Google Scholar] [CrossRef]
- Su, L.; Liu, X.; Xia, W.; Wu, B.; Li, C.; Xu, B.; Yang, B.; Xia, R.; Zhou, J.; Qian, J.; et al. Simultaneous Photothermal and Photocatalytic MOF- Derived C/TiO2 Composites for High-Efficiency Solar Driven Purification of Sewage. J. Colloid. Interface Sci. 2023, 650, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kuate, L.J.N.; Zhang, H.; Hou, J.; Wen, H.; Lu, C.; Li, C.; Shi, W. Photothermally Enabled Black G-C3N4 Hydrogel with Integrated Solar-Driven Evaporation and Photo-Degradation for Efficient Water Purification. Sep. Purif. Technol. 2025, 355, 129751. [Google Scholar] [CrossRef]
- Kumar, D.; Awasthi, G.P.; Park, C.H.; Kim, C.S. Multifunctional Trimetallic Colloidal Plasmonic Nanohybrid: Highly Efficient Photocatalyst and Photothermal Agent. Adv. Mater. Interfaces 2018, 5, 1800331. [Google Scholar] [CrossRef]
- Huang, J.; He, Y.; Wang, L.; Huang, Y.; Jiang, B. Bifunctional Au@TiO2 Core–Shell Nanoparticle Films for Clean Water Generation by Photocatalysis and Solar Evaporation. Energy Convers. Manag. 2017, 132, 452–459. [Google Scholar] [CrossRef]
- Liu, Y.; Lou, J.; Ni, M.; Song, C.; Wu, J.; Dasgupta, N.P.; Tao, P.; Shang, W.; Deng, T. Bioinspired Bifunctional Membrane for Efficient Clean Water Generation. ACS Appl. Mater. Interfaces 2016, 8, 772–779. [Google Scholar] [CrossRef]
- Hao, D.; Yang, Y.; Xu, B.; Cai, Z. Bifunctional Fabric with Photothermal Effect and Photocatalysis for Highly Efficient Clean Water Generation. ACS Sustain. Chem. Eng. 2018, 6, 10789–10797. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, H.; Li, J.; Kang, L.; Wang, L.; Wu, J.; Guo, S. Simultaneous Evaporation and Decontamination of Water on a Novel Membrane under Simulated Solar Light Irradiation. Appl. Catal. B 2020, 267, 118695. [Google Scholar] [CrossRef]
- Li, Z.; Sun, L.; Liu, Y.; Zhu, L.; Yu, D.; Wang, Y.; Sun, Y.; Yu, M. SnSe@SnO2 Core–Shell Nanocomposite for Synchronous Photothermal–Photocatalytic Production of Clean Water. Environ. Sci. Nano 2019, 6, 1507–1515. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Liu, X. Synchronous Steam Generation and Photodegradation for Clean Water Generation Based on Localized Solar Energy Harvesting. Energy Convers. Manag. 2018, 173, 158–166. [Google Scholar] [CrossRef]
- Mo, H.; Wang, Y. A Bionic Solar-Driven Interfacial Evaporation System with a Photothermal-Photocatalytic Hydrogel for VOC Removal during Solar Distillation. Water Res. 2022, 226, 119276. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; An, L.; Liu, D.; Yao, J.; Qi, D.; Xu, H.; Song, C.; Cui, F.; Chen, X.; Ma, J.; et al. A Light-Permeable Solar Evaporator with Three-Dimensional Photocatalytic Sites to Boost Volatile-Organic-Compound Rejection for Water Purification. Environ. Sci. Technol. 2022, 56, 9797–9805. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yang, H.; Wu, S.; Gong, B.; Xu, C.; Yan, J.; Cen, K.; Bo, Z.; Ostrikov, K. (Ken) High-Performance Water Purification and Desalination by Solar-Driven Interfacial Evaporation and Photocatalytic VOC Decomposition Enabled by Hierarchical TiO-CuO Nanoarchitecture. Int. J. Energy Res. 2022, 46, 1313–1326. [Google Scholar] [CrossRef]
- Yan, S.; Song, H.; Li, Y.; Yang, J.; Jia, X.; Wang, S.; Yang, X. Integrated Reduced Graphene Oxide/Polypyrrole Hybrid Aerogels for Simultaneous Photocatalytic Decontamination and Water Evaporation. Appl. Catal. B 2022, 301, 120820. [Google Scholar] [CrossRef]
- Wu, J.-L.; Xu, L.; Han, S.-J.; Labiadh, L.; Fu, M.-L.; Yuan, B. Manganese Oxide Hydrogel for Efficient Removal of Volatile Organic Compound in the Photothermal Water Purification. Solar RRL 2024, 8, 2300971. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Xu, L.; Liu, C.-H.; Han, S.-J.; Fu, M.-L.; Yuan, B. MXene/CdS Photothermal–Photocatalytic Hydrogels for Efficient Solar Water Evaporation and Synergistic Degradation of VOC. J. Mater. Chem. A Mater. 2024, 12, 10991–11003. [Google Scholar] [CrossRef]
- Deng, J.; Xiao, S.; Wang, B.; Li, Q.; Li, G.; Zhang, D.; Li, H. Self-Suspended Photothermal Microreactor for Water Desalination and Integrated Volatile Organic Compound Removal. ACS Appl. Mater. Interfaces 2020, 12, 51537–51545. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yang, X.; Sun, X.; Yuan, Y. Synchronizing Efficient Purification of VOCs in Durable Solar Water Evaporation over a Highly Stable Cu/W18O49@Graphene Material. Nano Lett. 2024, 24, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Qi, D.; Han, Y.; Xu, Y.; Xu, H.; You, S.; Wang, W.; Wang, C.; Wei, Y.; Ma, J. Volatile-Organic-Compound-Intercepting Solar Distillation Enabled by a Photothermal/Photocatalytic Nanofibrous Membrane with Dual-Scale Pores. Environ. Sci. Technol. 2020, 54, 9025–9033. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and Applications of Photo-Thermal Catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; Duan, X.; Sun, H.; Wang, S. Photothermal Catalysis: From Fundamentals to Practical Applications. Mater. Today 2023, 68, 234–253. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Hu, Y.; Jin, G.; Jiang, B.; Huang, Y. Photothermal-Conversion-Enhanced Photocatalytic Activity of Flower-like CuS Superparticles under Solar Light Irradiation. Solar Energy 2018, 170, 586–593. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Fan, D.; Chen, Z.; Yang, X. Coupling Solar-Driven Photothermal Effect into Photocatalysis for Sustainable Water Treatment. J. Hazard. Mater. 2022, 423, 127128. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Q.; Tan, C.F.; Lu, W.; Zeng, K.; Ho, G.W. Spectrum Tailored Defective 2D Semiconductor Nanosheets Aerogel for Full-Spectrum-Driven Photothermal Water Evaporation and Photochemical Degradation. Adv. Funct. Mater. 2020, 30, 2004460. [Google Scholar] [CrossRef]
- Stucchi, M.; Meroni, D.; Safran, G.; Villa, A.; Bianchi, C.L.; Prati, L. Noble Metal Promoted TiO2 from Silver-Waste Valorisation: Synergism between Ag and Au. Catalysts 2022, 12, 235. [Google Scholar] [CrossRef]
- Bhattacharyya, A. Solar Stills for Desalination of Water in Rural Households. Int. J. Environ. Sustain. 2013, 2, 21–30. [Google Scholar] [CrossRef]
- Al-Mezeini, S.S.S.; Siddiqui, M.A.; Shariq, M.; Althagafi, T.M.; Ahmed, I.A.; Asif, M.; Alsufyani, S.J.; Algarni, S.A.; Ahamed, M.B.N.; Elamin, K.M.A. Design and Experimental Studies on a Single Slope Solar Still for Water Desalination. Water 2023, 15, 704. [Google Scholar] [CrossRef]
- Kabeel, A.E.; Abdelgaied, M.; Almulla, N. Performances of Pyramid-Shaped Solar Still with Different Glass Cover Angles: Experimental Study. In Proceedings of the 2016 7th International Renewable Energy Congress (IREC), Hammamet, Tunisia, 22–24 March 2016; IEEE: New York, NY, USA, 2016; pp. 1–6. [Google Scholar]
- Nayi, K.H.; Modi, K.V. Pyramid Solar Still: A Comprehensive Review. Renew. Sustain. Energy Rev. 2018, 81, 136–148. [Google Scholar] [CrossRef]
- Patel, S.K.; Modi, K.V. Techniques to Improve the Performance of Enhanced Condensation Area Solar Still: A Critical Review. J. Clean. Prod. 2020, 268, 122260. [Google Scholar] [CrossRef]
- Tabrizi, F.F.; Sharak, A.Z. Experimental Study of an Integrated Basin Solar Still with a Sandy Heat Reservoir. Desalination 2010, 253, 195–199. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Alshutal, F.S.; Ibrahim, G. Impact of Different Configurations on Solar Still Productivity. J. Adv. Sci. Eng. Res. 2014, 3, 118–126. [Google Scholar]
- Kabeel, A.E.; El Hadi Attia, M.; Abdelgaied, M.; Essa, F.A.; Aly Aboud, M.F. Comparative Performance of Spherical, Hemispherical, and Single-Sloped Solar Distillers. Desalination Water Treat. 2024, 317, 100051. [Google Scholar] [CrossRef]
- Yadav, S.; Sudhakar, K. Different Domestic Designs of Solar Stills: A Review. Renew. Sustain. Energy Rev. 2015, 47, 718–731. [Google Scholar] [CrossRef]
- Gross, A.; Stangl, F.; Hönes, K.; Sift, M.; Hessling, M. Improved Drinking Water Disinfection with UVC-LEDs for Escherichia Coli and Bacillus Subtilis Utilizing Quartz Tubes as Light Guide. Water 2015, 2015, 4605–4621. [Google Scholar] [CrossRef]
- Zvorykin, V.; Arlantsev, S.; Gainutdinov, R.V.; Kholin, I.; Levchenko, A.; Mogilenetz, N.; Molchanov, A.; Oreshkin, V.; Rogulev, M.; Sagitov, S.; et al. Quests for Inertial Fusion Energy Conducted at GARPUN KrF Laser Facility. J. Phys. Conf. Ser. 2008, 112, 032055. [Google Scholar] [CrossRef]
- Supriyono, S.; Surahman, H.; Krisnandi, Y.; Gunlazuardi, J. Preparation and Characterization of Transparent Conductive SnO2-F Thin Film Deposited by Spray Pyrolysis: Relationship between Loading Level and Some Physical Properties. Procedia Environ. Sci. 2015, 28, 242–251. [Google Scholar] [CrossRef]
- Nieto, D. Single-Pulse Laser Ablation Threshold of Borosilicate, Fused Silica, Sapphire, and Soda-Lime Glass for Pulse Widths of 500 Fs, 10 Ps, 20 Ns. Appl. Opt. 2015, 54, 8602–8606. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.; Ali, M.; Schuch, R.; Mohamed, T. Experimental Investigations of Nonlinear Optical Properties of Soda-Lime Glasses and Theoretical Study of Self-Compression of Fs Laser Pulses. Opt. Laser Technol. 2019, 116, 276–283. [Google Scholar] [CrossRef]
- Blume, R.; Drummond, C. Modeling and Optimization of Solar-Control Glasses. J. Am. Ceram. Soc. 2002, 85, 1070–1076. [Google Scholar] [CrossRef]
- Abrisa Technologies. Abrisa Technologies Glass Materials. Available online: https://abrisatechnologies.com/ (accessed on 19 November 2024).
- Glassglobal. Glassglobal Reports. Available online: https://www.glassglobal.com/ (accessed on 19 November 2024).
- Mohelnikova, J. 7—Nanocoatings for Architectural Glass. In Nanocoatings and Ultra-Thin Films; Makhlouf, A.S.H., Tiginyanu, I., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 182–202. ISBN 978-1-84569-812-6. [Google Scholar]
- Zhu, Y.; Shi, J.; Huang, Q.; Fang, Y.; Wang, L.; Xu, G. A Superhydrophobic Solar Selective Absorber Used in a Flat Plate Solar Collector. RSC Adv. 2017, 7, 34125–34130. [Google Scholar] [CrossRef]
- Belardi, W.; Sazio, P.J. Borosilicate Based Hollow-Core Optical Fibers. Fibers 2019, 7, 73. [Google Scholar] [CrossRef]
- Hans Warlimont, W.M. Springer Handbook of Materials Data, 2nd ed.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-69743-7. [Google Scholar]
- Tang, T.; Yuan, Y.; Yalikun, Y.; Hosokawa, Y.; Li, M.; Tanaka, Y. Glass Based Micro Total Analysis Systems: Materials, Fabrication Methods, and Applications. Sens. Actuators B Chem. 2021, 339, 129859. [Google Scholar] [CrossRef]
- Branco, G.; Basile, E.; Morrone, R.; Cardoso, A.; Folgar, F. Ballistic Armoring of Passenger Cars on the Assembly Line Adds Quality and Passengers Comfort by Using Advanced and Light Weight Composite Materials; SAE International: Warrendale, PA, USA, 2004; Volume 113, pp. 650–659. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Imam, N.G. Photophysical Parameters of Functional Transparent Polymethyl-Methacrylate/Double-Walled Carbon Nanotubes Nanocomposite Sheet Under UV-Irradiation. J. Inorg. Organomet. Polym. Mater. 2016, 26, 780–787. [Google Scholar] [CrossRef]
- Prajzler, V.; Klapuch, J.; Lyutakov, O.; Huttel, I.; Špirková, J.; Nekvindová, P.; Jeřábek, V. Design, Fabrication and Properties of Rib Poly (Methylmethacrylimide) Optical Waveguides. J. Radioeng 2011, 20, 479–485. [Google Scholar]
- Steeneken, S.F.; Buma, A.G.J.; Gieskes, W.W.C. Changes in Transmission Characteristics of Polymethylacrylate and Cellulose (III) Acetate during Exposure to Ultraviolet Light. Photochem. Photobiol. 1995, 61, 276–280. [Google Scholar] [CrossRef]
- Maji, P.; Choudhary, R.B.; Majhi, M. Structural, Optical and Dielectric Properties of ZrO2 Reinforced Polymeric Nanocomposite Films of Polymethylmethacrylate (PMMA). Optik 2016, 127, 4848–4853. [Google Scholar] [CrossRef]
- Prajzler, V.; Nekvindová, P.; Hyps, P.; Lyutakov, O.; Jeřábek, V. Flexible Polymer Planar Optical Waveguides. Radioengineering 2014, 23, 776–782. [Google Scholar]
- Wang, Z.; Zhang, C.; Chen, D.; Tang, S.; Zhang, J.; Wang, Y.; Han, G.; Xu, S.; Hao, Y. Flexible ITO-Free Organic Solar Cells Based on MoO3/Ag Anodes Anodes. IEEE Photonics J. 2015, 7, 8400109. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mun, C.; Ma, J.; Park, S.-G.; Lee, S.; Kim, C.S. Simple Fabrication of Transparent, Colorless, and Self-Disinfecting Polyethylene Terephthalate Film via Cold Plasma Treatment. Nanomaterials 2020, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Righini, G.C.; Krzak, J.; Lukowiak, A.; Macrelli, G.; Varas, S.; Ferrari, M. From Flexible Electronics to Flexible Photonics: A Brief Overview. Opt Mater. 2021, 115, 111011. [Google Scholar] [CrossRef]
- Szczurek, A.; Tran, T.N.L.; Kubacki, J.; Gąsiorek, A.; Startek, K.; Mazur-Nowacka, A.; Dell’Anna, R.; Armellini, C.; Varas, S.; Carlotto, A.; et al. Polyethylene Terephthalate (PET) Optical Properties Deterioration Induced by Temperature and Protective Effect of Organically Modified SiO2–TiO2 Coating. Mater. Chem. Phys. 2023, 306, 128016. [Google Scholar] [CrossRef]
- Mustapha, S.; Lease, J.; Eksiler, K.; Sim, S.T.; Ariffin, H.; Andou, Y. Facile Preparation of Cellulose Fiber Reinforced Polypropylene Using Hybrid Filler Method. Polymers 2022, 14, 1630. [Google Scholar] [CrossRef] [PubMed]
- Tone, A.M.; Herranz Solana, N.; Khan, M.R.; Borriello, A.; Torrieri, E.; Sánchez Reig, C.; Monedero Prieto, F.M. Study on the Properties of PLA-and PP-Based Films for Food Applications Incorporating Orange Peel Extract from Agricultural by-Products. Polymers 2024, 16, 1245. [Google Scholar] [CrossRef]
- Safavi, S.M.; Masoumi, H.; Mirian, S.S.; Tabrizchi, M. Sorting of Polypropylene Resins by Color in MSW Using Visible Reflectance Spectroscopy. Waste Manag. 2010, 30, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- ASTM, A.G. 173-03; Standard Tables for Reference Solar Spectral Irradiance at Air Mass 1.5: Direct Normal and Hemispherical on 37 Tilted Surface. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- Presciutti, A.; Asdrubali, F.; Marrocchi, A.; Broggi, A.; Pizzoli, G.; Damiani, A. Sun Simulators: Development of an Innovative Low Cost Film Filter. Sustainability 2014, 6, 6830–6846. [Google Scholar] [CrossRef]
- Wei, L.; Wang, P.; Chen, X.; Chen, Z. Water Vapor Condensation Behavior on Different Wetting Surfaces via Molecular Dynamics Simulation. Surf. Interfaces 2024, 52, 104981. [Google Scholar] [CrossRef]
- He, Z.; Lan, X.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Effect of Surface Wettability on Transparency in Different Water Conditions. J. Coat. Technol. Res. 2013, 10, 641–647. [Google Scholar] [CrossRef]
- Maino, G.; Meroni, D.; Pifferi, V.; Falciola, L.; Soliveri, G.; Cappelletti, G.; Ardizzone, S. Electrochemically Assisted Deposition of Transparent, Mechanically Robust TiO2 Films for Advanced Applications. J. Nanoparticle Res. 2013, 15, 2087. [Google Scholar] [CrossRef]
- Lei, J.; Guo, Z. A fog-collecting surface mimicking the Namib beetle: Its water collection efficiency and influencing factors. Nanoscale 2020, 12, 6921–6936. [Google Scholar] [CrossRef] [PubMed]
- Amalia, F.R.; Ohtani, B.; Kowalska, E. Spectrophotometric Analysis: Challenge for a Reliable Evaluation of Photocatalytic Activity under Visible Light Irradiation. J. Photochem. Photobiol. C Photochem. Rev. 2025, 64, 100701. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Falletta, E.; Pifferi, V.; Falciola, L.; Cappelletti, G.; Ardizzone, S. Emerging Pollutant Mixture Mineralization by TiO2 Photocatalysts. The Role of the Water Medium. Photochem. Photobiol. Sci. 2017, 16, 60–66. [Google Scholar] [CrossRef] [PubMed]



| Organic Pollutants | Photothermal Component | Photocatalyst | Support | Solar Still Design | Tested Concentration (mg L−1) | VOC Removal | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Photothermal (No Evaporator)—RD | Only Photocatalysis—Cfinal/C0 in Treated Liquid | Photothermal + Photocatalytic—RD | |||||||
| phenol | calcined Saccharum spontaneous | ZnFe2O4 | polyethylene foam | single solar still | 10 | 90 | - | 5 | [28] |
| 4-nitrophenol | 100 | - | 4 | ||||||
| nitrobenzene | 90 | - | 10 | ||||||
| thionine | iron phthalocyanine organic polymer | CoFe2O4 and polydopamine | melamine foam | beaker/single solar still | - | - | 0 | - | [29] |
| methylene blue | - | 0 | - | ||||||
| rhodamine B | - | 0 | - | ||||||
| phenol | 1 | - | - | 1 | |||||
| N,N-dimethylformamide | - | - | 10 | ||||||
| aniline | - | - | 7 | ||||||
| methylene blue | polyimide/MXene | polypyrrole/BiVO4 | thermal insulation material (cotton) | hemispherical still | 20 | - | 21 | - | [30] |
| rhodamine B | - | 29 | - | ||||||
| Congo red | - | 23 | - | ||||||
| malachite green | - | 19 | - | ||||||
| methyl orange | MOF-derived C/TiO2 | expanded polyethylene foam | beaker | 10 | - | - | - | [31] | |
| rhodamine B | - | 12 | - | ||||||
| tetracycline | black g-C3N4/chitosan | Hydrogel and polyethylene foam | double-surface solar still | 100 | - | 17 | - | [32] | |
| methyl orange | Au nanoparticles@TiO2@Pt | - | Pyrex glass, open reactor | 10 | - | 2 | - | [33] | |
| methyl red | - | 2 | - | ||||||
| methylene blue | - | 0 | - | ||||||
| rhodamine B | Au@TiO2 | microporous membrane | beaker | 20 | - | 46 | - | [34] | |
| rhodamine B | Au@TiO2 | anodized aluminium oxide membrane | beaker (type of cover not shown) | 20 | - | 40 | 0 | [35] | |
| methyl orange | TiO2–polydopamine@polypyrrole | Cotton | beaker | 10 | - | 4 | - | [36] | |
| rhodamine B | MoO3−x/ BiOCl/CNTs | cellulose acetate membrane | beaker | 5 | - | - | 0 | [37] | |
| toluene | not shown | 10 | 52 | - | 0 | ||||
| methyl orange | SnSe@SnO2 | - | not shown | 10 | - | 0 | 0 | [38] | |
| rhodamine B | Au@hierarchical ZnO | mixed cellulose ester membrane | beaker | 15 | - | 30 | 0 | [39] | |
| phenol | TiO2/Ti3C2/C3N4/polyvinyl alcohol | Hydrogel | single solar still | 10 | 117 * | 0 | 15 * | [40] | |
| phenol | BiOBr0.85I0.15 | Melamine sponge | dome-shaped still | 10 | 78 | 10 | 0 | [41] | |
| phenol | carbonized carboxymethyl chitosan/alginate hydrogel crosslinked by Cu2+ | polyethylene foam | beaker in quartz close container | 10 | - | - | 3 | [18] | |
| p-chlorophenol | - | - | ca. 5 | ||||||
| p-methylphenol | - | - | ca. 5 | ||||||
| rhodamine B | TiO2-CuO | Cu foam | dome-shaped still | 10 | - | - | 13 | [42] | |
| phenol | 164 | - | 20 | ||||||
| phenol | graphene/polypyrrole aerogels | polystyrene foam | dome-shaped still | 20 | 148 | 17 | 7 | [43] | |
| ciprofloxacin | 10 | - | 7 | 1 | |||||
| methyl orange | - | 5 | 1 | ||||||
| rhodamine B | - | 4 | 0 | ||||||
| mixture | 20 | - | - | 10 | |||||
| phenol | β-MnO2/porous hydrogel | polyurethane sponge | N.A. | 20 | 50 | 28 | 1 | [44] | |
| methylene blue | 1 | 73 | 1 | ||||||
| methyl orange | 1 | 71 | 1 | ||||||
| rhodamine B | Ti3C2 MXene/CdS | polystyrene (PS) foam | double-jacketed beaker | 10 | - | 0 | - | [45] | |
| phenol | Petri dish and inverted beaker | 116 | - | 15 | |||||
| metronidazole | 31.5 | - | 0 | ||||||
| phenol | TiO2 | porous clustered carbon array foams | reactor with horizontal cover | 5 | 200 | - | 20 | [46] | |
| quinol | 120 | - | 40 | ||||||
| aniline | 210 | - | 40 | ||||||
| phenol | Cu/W18O49@graphene | polydimethylsiloxane (PDMS) sponge | dome-shaped still with quartz cover | 10 | 120 | - | 1 | [47] | |
| phenol | m-TiO2−x nanofibrous membrane | polystyrene foam | dome-shaped still with quartz cover | 10 | 105 | - | 4.5 | [48] | |
| UV | Visible | IR | References | |
|---|---|---|---|---|
| Quartz | up to 90% transmittance across most of the UV spectrum, including UV-C | up to 92–95% transmittance | 90–95% transmittance till 2500 nm and up to 4000 nm in some types | [1,64,65,66] |
| Soda–Lime Glass (Standard Window Glass) | 40–75% UV-A transmittance; 1–10% transmittance of UV-B and UV-C | ca. 85–90% transmittance | good transmittance in 750–2500 nm, it blocks >2500 nm | [64,67,68,69] |
| Low-Iron Glass (Optical or Extra-Clear Glass) | higher UV-A transmittance than soda–lime glass but still significantly limits UV-B and UV-C | ca. 91–93% transmittance | similar to soda–lime glass but with slightly more transmission in 750–1400 nm range | [70,71,72] |
| Borosilicate Glass (Pyrex) | 75–90% transmittance in UV-A; higher transmittance in the UV-B range than optical glass; blocks UV-C | ca. 85–90% transmittance | high transmittance in 750–1500 nm range, it blocks >2000 nm | [73,74,75,76] |
| Laminated Glass | <1% transmittance across the UV spectrum | 70–88% transmittance | 20–50%, depending on glass thickness and type of interlayer used | [77] |
| Poly(methyl methacrylate) (PMMA) | up to 75% transmittance in the 300–400 nm range | up to 90% transmittance | good transmittance in 700–2500 nm but with strong absorption bands in mid-IR due to C–H and C=O vibrational modes | [78,79,80,81,82] |
| Polyethylene Terephthalate (PET) | moderate transmittance >360 nm | ca. 85% transmittance (amorphous form) ca. 80% transmittance (semi-crystalline form) | moderate transmittance in NIR, poor transmittance in mid- and far-IR | [83,84,85,86] |
| Polypropylene (PP) | 10–30% transmittance in the UVA range | semi-transparent | moderate transmittance in near-IR but with strong overtone absorption peaks | [87,88,89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, H.; Diamanti, M.V.; Lughi, V.; Rossi, S.; Meroni, D. Design Efficiency: A Critical Perspective on Testing Methods for Solar-Driven Photothermal Evaporation and Photocatalysis. Nanomaterials 2025, 15, 1121. https://doi.org/10.3390/nano15141121
Hamza H, Diamanti MV, Lughi V, Rossi S, Meroni D. Design Efficiency: A Critical Perspective on Testing Methods for Solar-Driven Photothermal Evaporation and Photocatalysis. Nanomaterials. 2025; 15(14):1121. https://doi.org/10.3390/nano15141121
Chicago/Turabian StyleHamza, Hady, Maria Vittoria Diamanti, Vanni Lughi, Sergio Rossi, and Daniela Meroni. 2025. "Design Efficiency: A Critical Perspective on Testing Methods for Solar-Driven Photothermal Evaporation and Photocatalysis" Nanomaterials 15, no. 14: 1121. https://doi.org/10.3390/nano15141121
APA StyleHamza, H., Diamanti, M. V., Lughi, V., Rossi, S., & Meroni, D. (2025). Design Efficiency: A Critical Perspective on Testing Methods for Solar-Driven Photothermal Evaporation and Photocatalysis. Nanomaterials, 15(14), 1121. https://doi.org/10.3390/nano15141121










