Preparation and Optimization of Mn2+-Activated Na2ZnGeO4 Phosphors: Insights into Precursor Selection and Microwave-Assisted Solid-State Synthesis
Abstract
1. Introduction
2. Materials and Methods
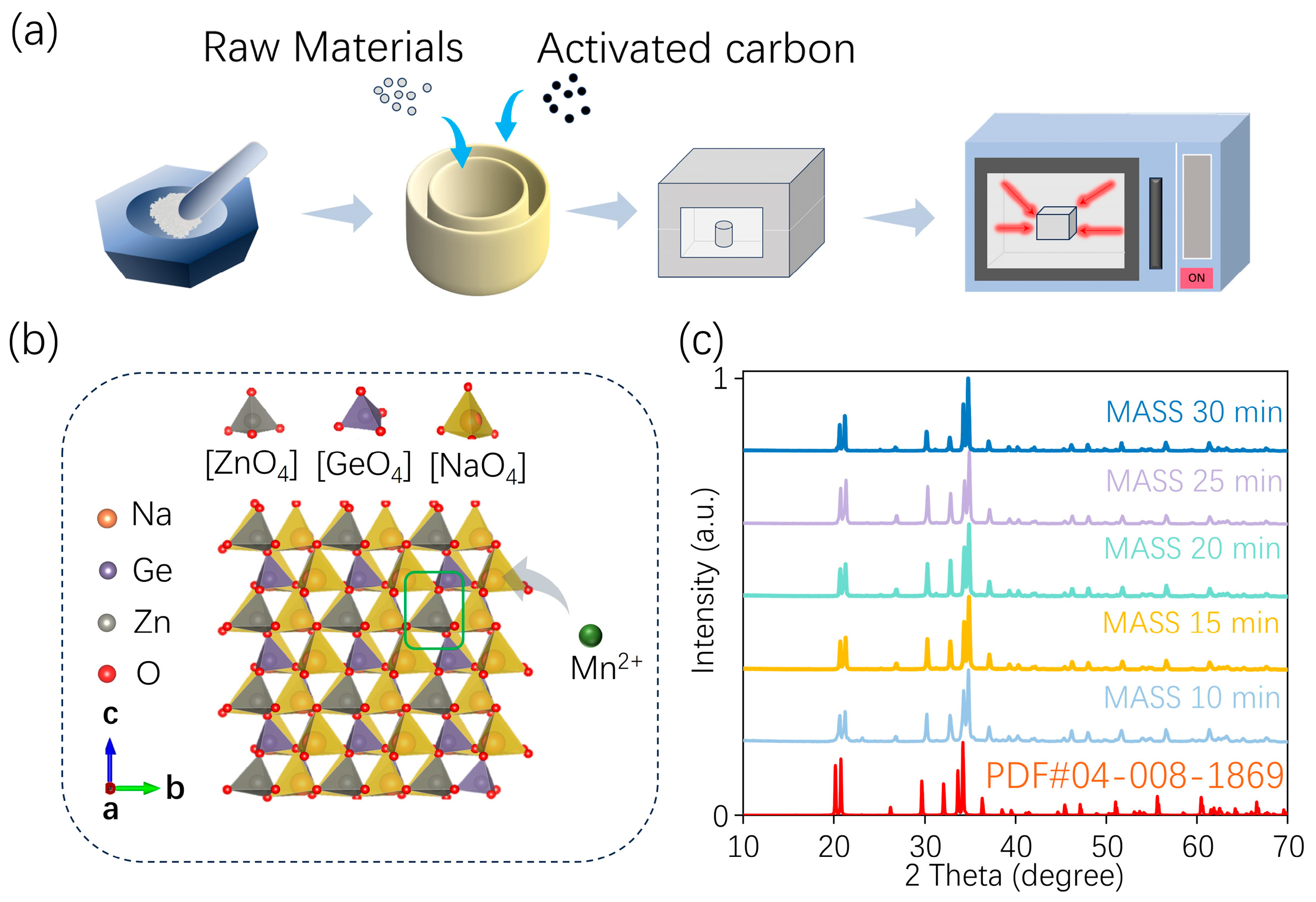
2.1. Materials Preparation
2.1.1. The MASS Samples
2.1.2. The SSR Samples
2.1.3. The SSR + MASS Samples
2.2. Structural and Morphological Characterization
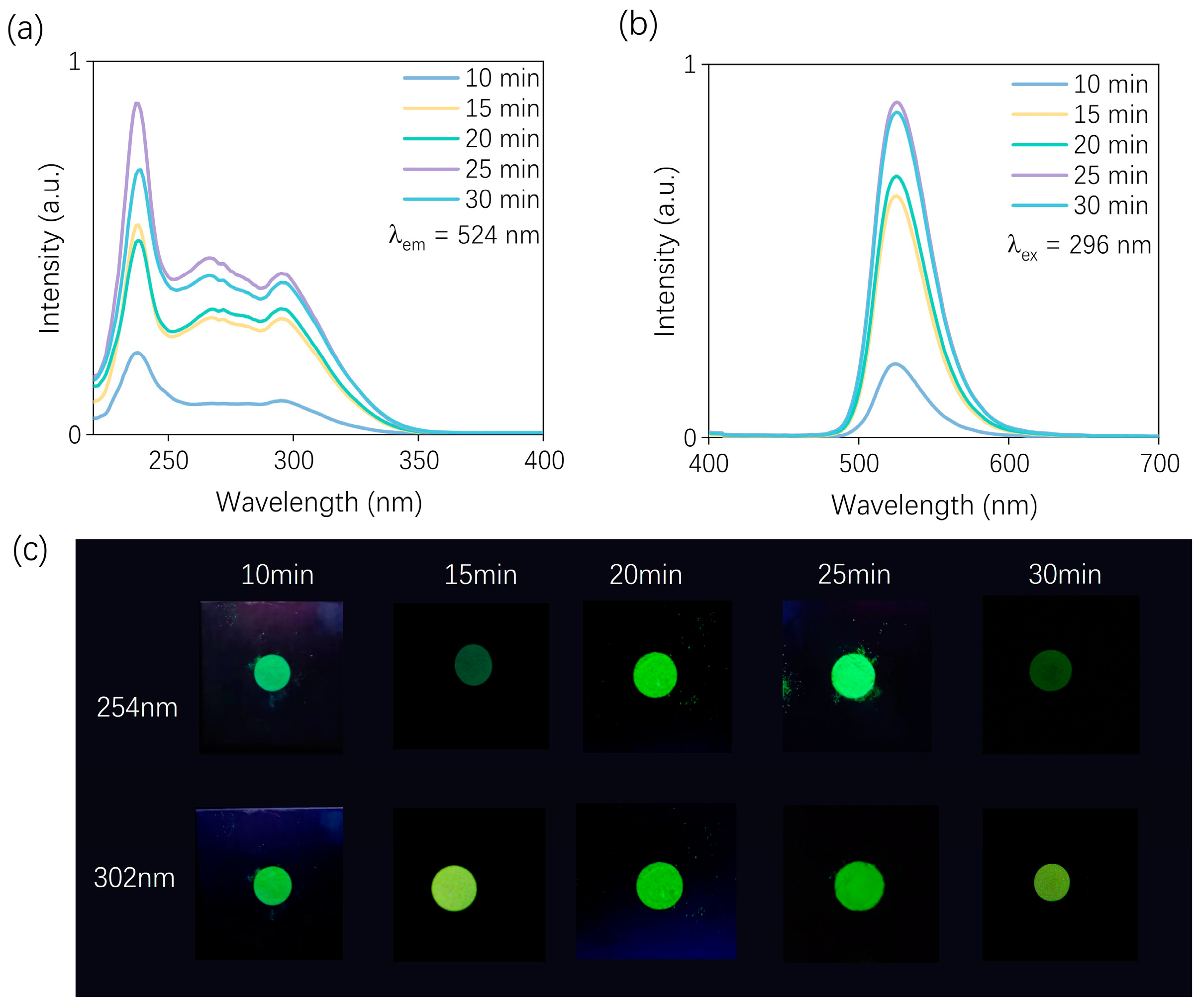
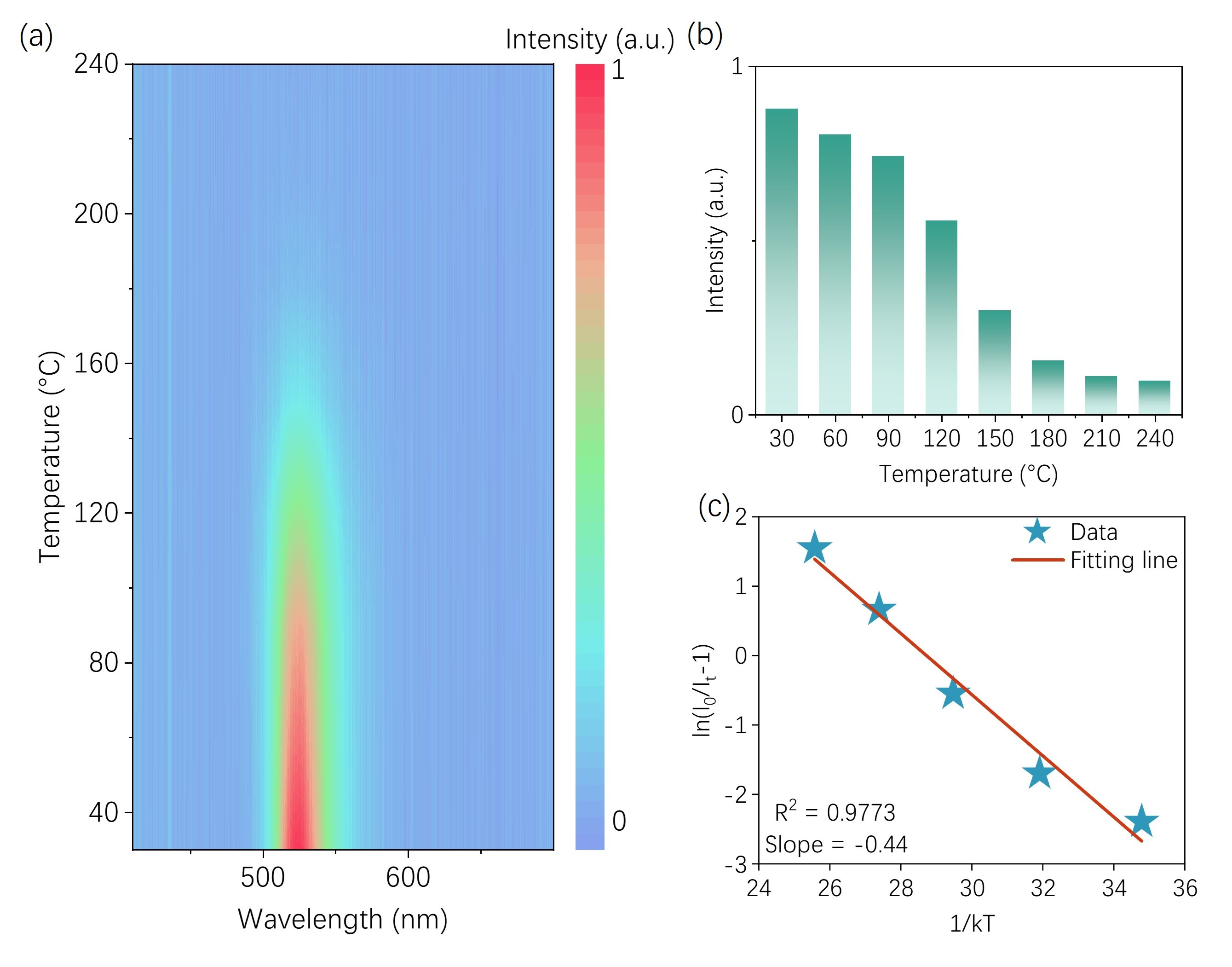
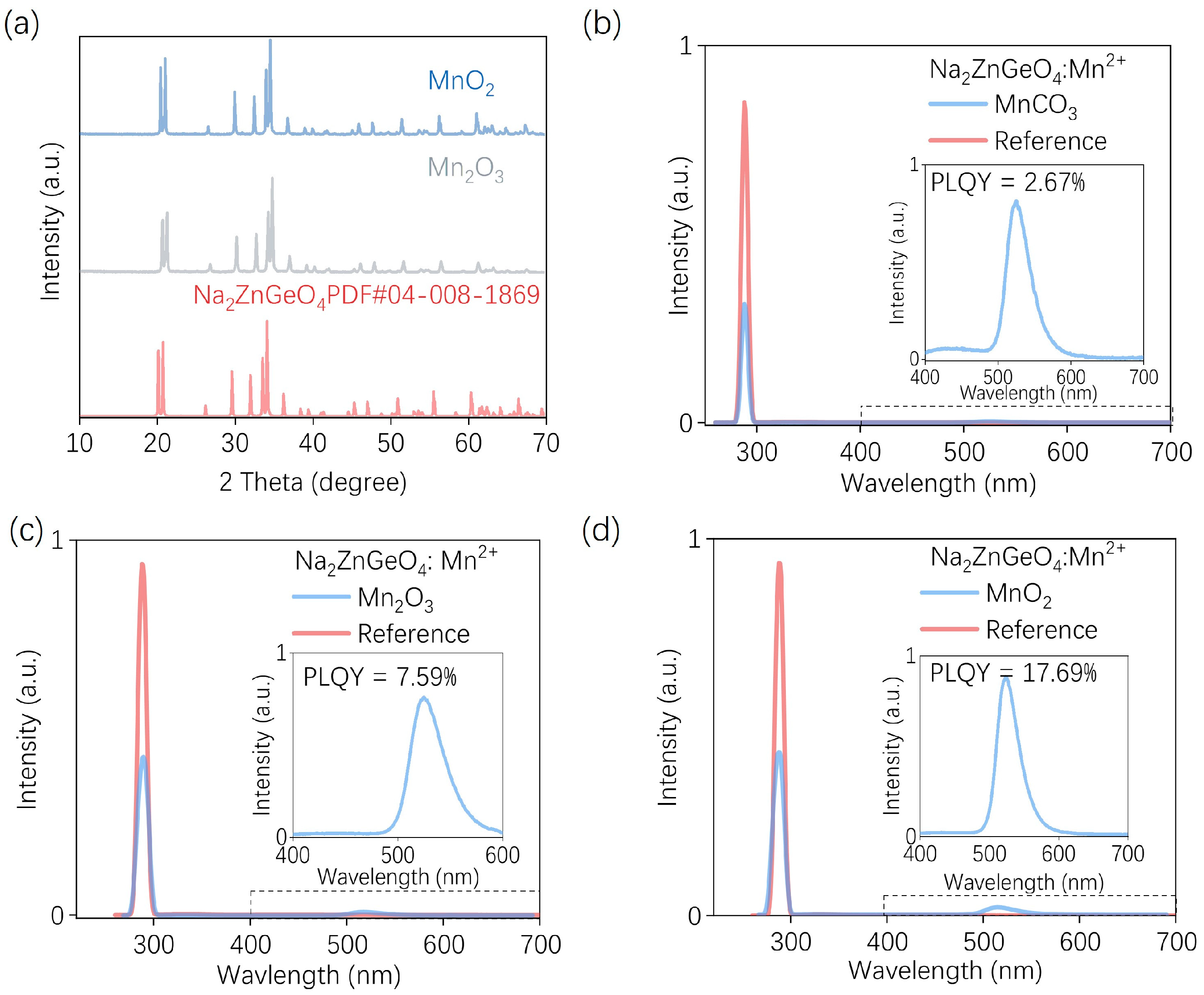
2.3. Luminescence Characterization
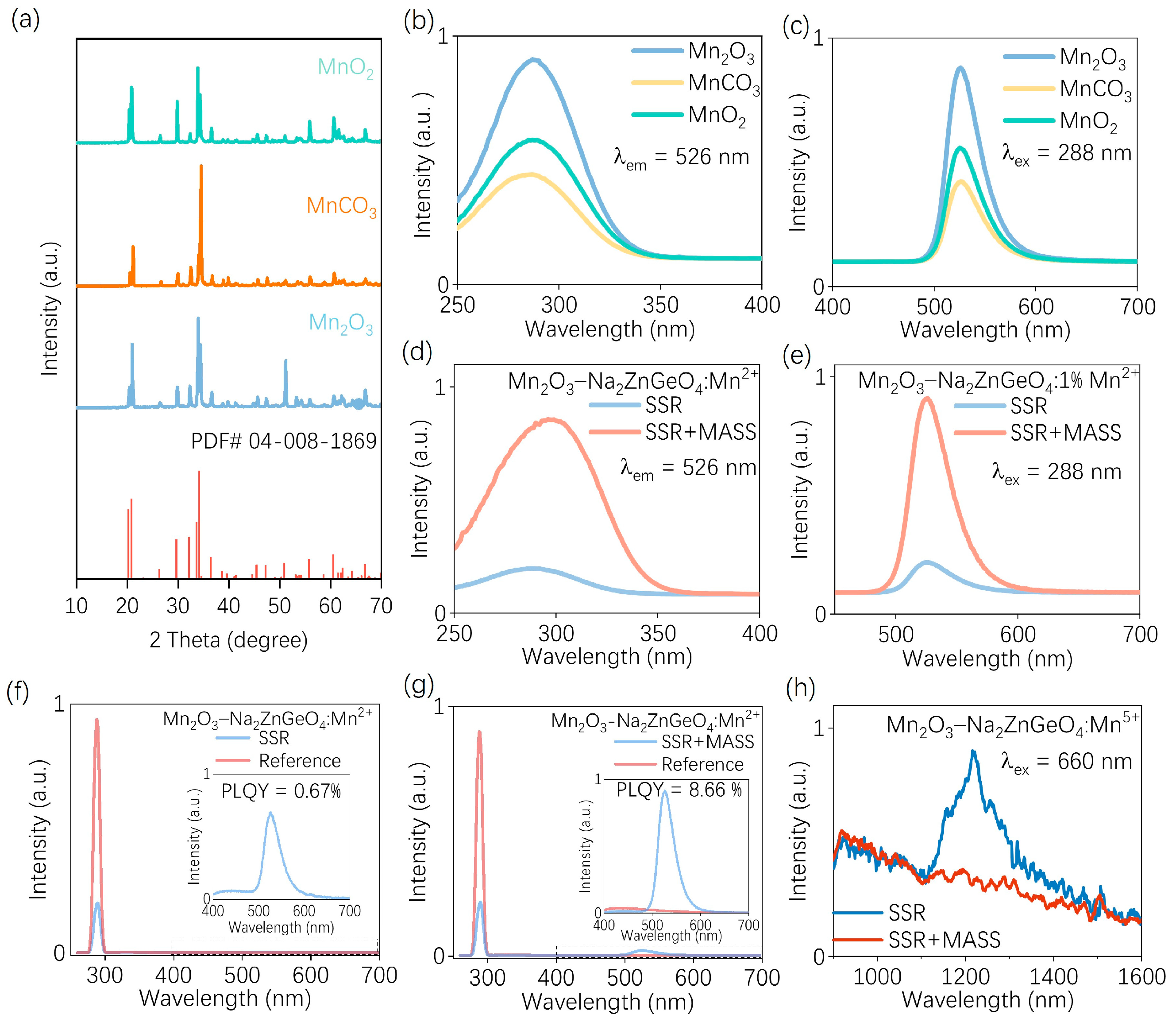
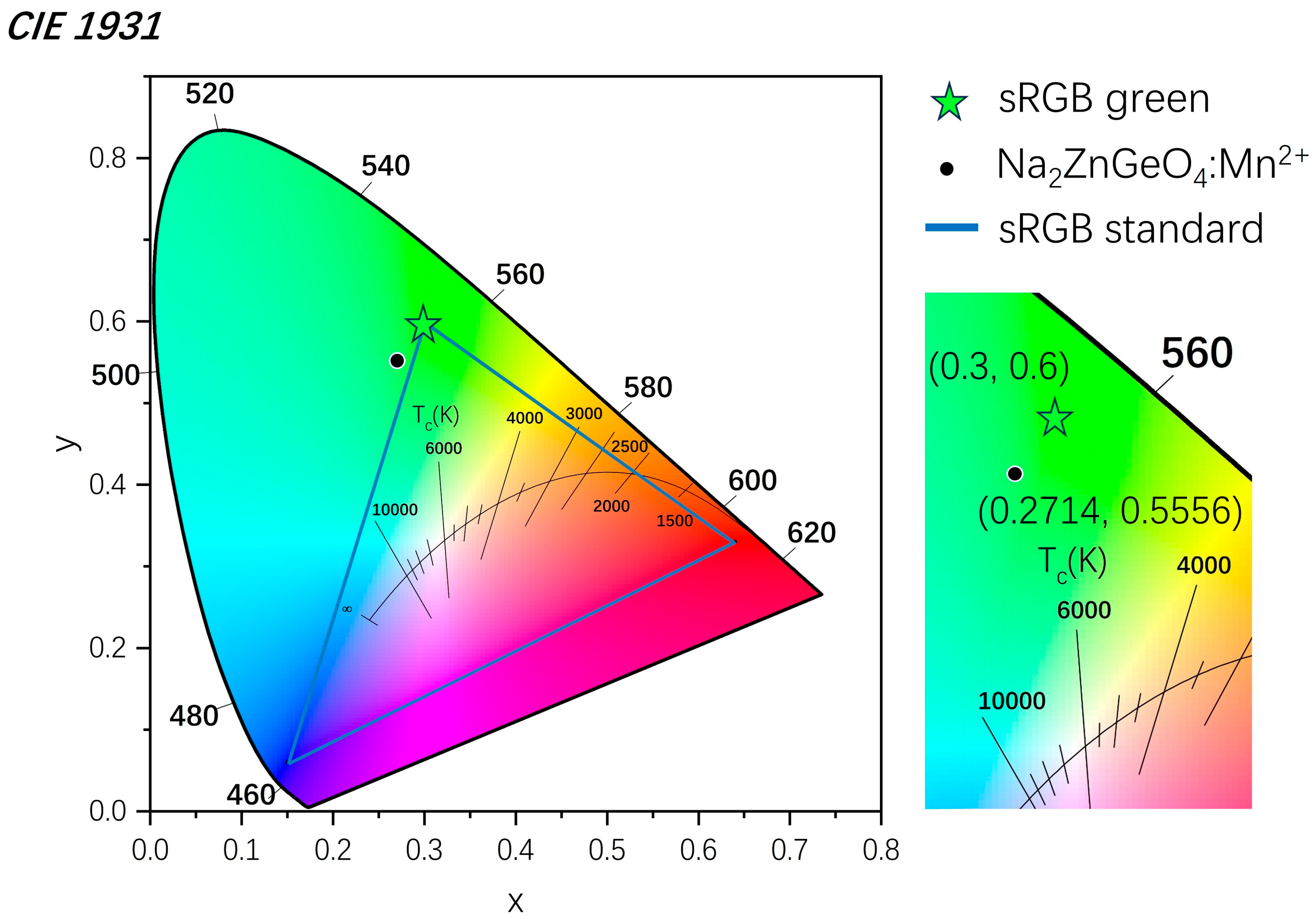
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, M.; Liao, H.; Ning, L.; Zhang, Q.; Liu, Q.; Xia, Z. Next-Generation Narrow-Band Green-Emitting RbLi(Li3SiO4)2:Eu2+ Phosphor for Backlight Display Application. Adv. Mater. 2018, 30, 1802489. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Zhou, G.; Huang, J.; Song, E.; Nag, A.; Xia, Z. Mn2+-Doped Metal Halide Perovskites: Structure, Photoluminescence, and Application. Laser Photonics Rev. 2020, 15, 2000334. [Google Scholar] [CrossRef]
- Träger, L.M.; Ekeya, J.I.; Liesenfeld, A.; Wieczorek, M.; Huppertz, H.; Suta, M. Mn2+-Activated Alkali Lithooxidosilicate Phosphors as Sustainable Alternative White-Light Emitters. Angew. Chem. Int. Ed. 2025, 64, e202504078. [Google Scholar] [CrossRef] [PubMed]
- Van der Heggen, D.; Joos, J.J.; Feng, A.; Fritz, V.; Delgado, T.; Gartmann, N.; Walfort, B.; Rytz, D.; Hagemann, H.; Poelman, D.; et al. Persistent Luminescence in Strontium Aluminate: A Roadmap to a Brighter Future. Adv. Funct. Mater. 2022, 32, 2208809. [Google Scholar] [CrossRef]
- Hirosaki, N.; Xie, R.J.; Kimoto, K.; Sekiguchi, T.; Yamamoto, Y.; Suehiro, T.; Mitomo, M. Characterization and properties of green-emitting—SiAlON: Eu2+ powder phosphors for white light-emitting diodes. Appl. Phys. Lett. 2006, 86, 211905. [Google Scholar] [CrossRef]
- Strobel, P.; Schmiechen, S.; Siegert, M.; Tücks, A.; Schmidt, P.J.; Schnick, W. Narrow-Band Green Emitting Nitridolithoalumosilicate Ba[Li2(Al2Si2)N6]:Eu2+ with Framework Topology whj for LED/LCD-Backlighting Applications. Chem. Mater. 2015, 27, 6109–6115. [Google Scholar] [CrossRef]
- Takeda, T.; Hirosaki, N.; Funahshi, S.; Xie, R.-J. Narrow-Band Green-Emitting Phosphor Ba2LiSi7AlN12:Eu2+ with High Thermal Stability Discovered by a Single Particle Diagnosis Approach. Chem. Mater. 2015, 27, 5892–5898. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Hirosaki, N.; Xie, R.J. Color Conversion Materials for High-Brightness Laser-Driven Solid-State Lighting. Laser Photonics Rev. 2018, 12, 1800173. [Google Scholar] [CrossRef]
- Smet, P.F.; Botterman, J.; Van den Eeckhout, K.; Korthout, K.; Poelman, D. Persistent luminescence in nitride and oxynitride phosphors: A review. Opt. Mater. 2014, 36, 1913–1919. [Google Scholar] [CrossRef]
- You, S.; Li, S.; Zheng, P.; Zhou, T.; Wang, L.; Liu, L.; Horisaki, N.; Xu, F.; Xie, R.J. A Thermally Robust La3Si6N11:Ce-in-Glass Film for High-Brightness Blue-Laser-Driven Solid State Lighting. Laser Photonics Rev. 2018, 13, 1800216. [Google Scholar] [CrossRef]
- Zhuang, Y.; Lv, Y.; Wang, L.; Chen, W.; Zhou, T.-L.; Takeda, T.; Hirosaki, N.; Xie, R.-J. Trap Depth Engineering of SrSi2O2N2:Ln2+,Ln3+ (Ln2+ = Yb, Eu; Ln3+ = Dy, Ho, Er) Persistent Luminescence Materials for Information Storage Applications. ACS Appl. Mater. Interfaces 2018, 10, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wang, L.; Lv, Y.; Zhou, T.L.; Xie, R.J. Optical Data Storage and Multicolor Emission Readout on Flexible Films Using Deep-Trap Persistent Luminescence Materials. Adv. Funct. Mater. 2017, 28, 1705769. [Google Scholar] [CrossRef]
- Erasmus, L.J.B.; Smet, P.F.; Kroon, R.E.; Poelman, D.; Terblans, J.J.; Joos, J.J.; Van der Heggen, D.; Swart, H.C. Making Eu2+- and Sm2+-Doped Borates Fit for Solar Energy Applications. ACS Photonics 2023, 10, 609–622. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, X.; Zhao, H.-R.; Wu, D.; Chen, M.-X.; Zheng, T.; Zhang, R.; Sun, L.-D.; Yan, C.-H. Tracing the origin of near-infrared emissions emanating from manganese (II). Light Sci. Appl. 2025, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, K.; Van Deun, R.; Poelman, D.; Lin, H. Near-Infrared Persistent Luminescence and Trap Reshuffling in Mn4+ Doped Alkali-Earth Metal Tungstates. Adv. Opt. Mater. 2021, 10, 2101714. [Google Scholar] [CrossRef]
- Du, J.; Poelman, D.; Lin, H. Modulating trap distribution of persistent phosphors upon simple microwave-assisted solid-state reactions. Chem. Eng. J. 2022, 431, 133706. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Z.; Hu, W.; Delaey, M.; Yin, L.; Jiang, Y.; Lei, S.; Vrielinck, H.; Poelman, D. Dual-Emitting Mn4+/Mn5+ in Ba6Na2Nb2P2O17 phosphors for enhanced NIR-I and NIR-II spectroscopy applications. Chem. Eng. J. 2025, 508, 161131. [Google Scholar] [CrossRef]
- Joos, J.J.; Lejaeghere, K.; Korthout, K.; Feng, A.; Poelman, D.; Smet, P.F. Charge transfer induced energy storage in CaZnOS:Mn—Insight from experimental and computational spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 9075–9085. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cai, Y.; Dou, K.; Chang, J.; Li, W.; Wang, S.; Sun, M.; Huang, B.; Liu, X.; Qiu, J.; et al. Dynamic multicolor emissions of multimodal phosphors by Mn2+ trace doping in self-activated CaGa4O7. Nat. Commun. 2024, 15, 3209. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, R.; Sijbom, H.F.; Joos, J.J.; Korthout, K.; Poelman, D.; Detavernier, C.; Smet, P.F. Red Mn4+-Doped Fluoride Phosphors: Why Purity Matters. ACS Appl. Mater. Interfaces 2018, 10, 18845–18856. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Zhang, J. White-light emission in a single-phase Ca9.3Mg0.7K(PO4)7:Eu2+,Tb3+,Mn2+ phosphor for light-emitting diodes. J. Lumin. 2018, 194, 334–340. [Google Scholar] [CrossRef]
- Yu, S.; Gong, X.; Xiang, R.; Han, K.; Li, N.; Gao, K.; Zhang, P.; Zhang, Y.; Li, F.; Hou, G. Luminescence characteristics of SrZn2(PO4)2:Mn2+ and its anti-counterfeiting application. Opt. Mater. 2025, 161, 116788. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, Y.; Liu, S.; Li, H.; Chen, J. Narrow-Band Green-Emitting Sr2MgAl22O36:Mn2+ Phosphors with Superior Thermal Stability and Wide Color Gamut for Backlighting Display Applications. Adv. Opt. Mater. 2019, 7, 1801419. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, Y.; Fu, Y.; Ju, G.; Mu, Z.; Chen, R.; Lin, J.; Wang, Z.; Ballato, J. Preparation, Design, and Characterization of the Novel Long Persistent Phosphors: Na2ZnGeO4 and Na2ZnGeO4:Mn2+. J. Am. Ceram. Soc. 2015, 98, 1555–1561. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.; Liu, Y.; Shi, Q.; Wang, W.; Zhai, Y. Green and red photoluminescence from ZnAl2O4:Mn phosphors prepared by sol–gel method. J. Lumin. 2012, 132, 1529–1531. [Google Scholar] [CrossRef]
- Vien, L.T.T.; Tu, N.; Viet, D.X.; Anh, D.D.; Nguyen, D.H.; Huy, P.T. Mn2+- doped Zn2SnO4 green phosphor for WLED applications. J. Lumin. 2020, 227, 117522. [Google Scholar] [CrossRef]
- Su, Z.; Wang, Y.; Seto, T. Synthesis of efficient submicron γ-AlON Mn2+, Mg2+ phosphors for mini-LEDs by a coprecipitation precursor method. J. Mater. Chem. 2024, 12, 5587–5595. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Wang, X.; Lin, H.; Suchocki, A. Facile construction of mechanoluminescence heterojunctions facilitating personalized anti-counterfeiting E-signature devices. Chem. Eng. J. 2025, 512, 162658. [Google Scholar] [CrossRef]
- Carvalho, J.M.; Heggen, D.V.; Martin, L.; Smet, P.F. Microwave-assisted synthesis followed by a reduction step: Making persistent phosphors with a large storage capacity. Dalton Trans. 2020, 49, 4518–4527. [Google Scholar] [CrossRef] [PubMed]
- Al-Obeidi, F.; Austin, R.E.; Okonya, J.F.; Bond, D.R.S. Microwave-Assisted Solid-phase Synthesis (MASS): Parallel and Combinatorial Chemical Library Synthesis. Mini Rev. Med. Chem. 2003, 3, 460. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, T.; Mizushina, H.; Uematsu, K.; Matsushita, N.; Yoshimura, M.; Toda, K.; Sato, M. Microwave synthesis technique for long phosphorescence phosphor SrAl2O4:Eu2+, Dy3+ using carbon reduction. Mater. Sci. Eng. B 2010, 173, 109–112. [Google Scholar] [CrossRef]
- Du, J.; Zhang, J.; Wang, T.; Zhou, P.; Cao, L.; Liu, Q.; Lin, H. Brighten strontium aluminate long-persistence materials via optimizing defect energy level distribution. Mater. Today Phys. 2023, 38, 101229. [Google Scholar] [CrossRef]
- Wang, X.; Du, J.; Lin, H. Facilitating Near-Infrared Persistent Luminescence in Cr3+-Doped Gadolinium Gallium Garnets. Small Methods 2023, 8, 2301001. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.M.; Norrbo, I.; Ando, R.A.; Brito, H.F.; Fantini, M.C.A.; Lastusaari, M. Fast, low-cost preparation of hackmanite minerals with reversible photochromic behavior using a microwave-assisted structure-conversion method. Chem. Comm. 2018, 54, 7326–7329. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Poelman, D. Facile Synthesis of Mn4+-Activated Double Perovskite Germanate Phosphors with Near-Infrared Persistent Luminescence. Nanomaterials 2019, 9, 1759. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chen, Y.; Zhao, Y.; Pang, H.; Zhang, Q.; Tian, J.; Zhang, C. Preparation and properties of Li2ZnGeO4:Mn2+ green light emitting materials. New J. Chem. 2025, 49, 11900–11905. [Google Scholar] [CrossRef]
- Zhang, J.C.; Gao, N.; Li, L.; Wang, S.; Shi, X.; Sun, M.; Yan, X.; He, H.W.; Ning, X.; Huang, B.; et al. Discovering and Dissecting Mechanically Excited Luminescence of Mn2+ Activators via Matrix Microstructure Evolution. Adv. Funct. Mater. 2021, 31, 2100221. [Google Scholar] [CrossRef]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern Microwave Methods in Solid-State Inorganic Materials Chemistry: From Fundamentals to Manufacturing. Chem. Rev. 2013, 114, 1170–1206. [Google Scholar] [CrossRef] [PubMed]
- Miranda de Carvalho, J.; Pedroso, C.C.S.; Saula, M.S.d.N.; Felinto, M.C.F.C.; Brito, H.F.d. Microwave-Assisted Preparation of Luminescent Inorganic Materials: A Fast Route to Light Conversion and Storage Phosphors. Molecules 2021, 26, 2882. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, X.; Du, P.; Feng, C.; Niu, P.; Xu, D.; Wang, L.; Gao, W.; Song, A. Tetracoordinated Manganese(II) Halide with Multimode Thermochromic Luminescence for Anticounterfeiting and Encryption. Inorg. Chem. 2025, 64, 11529–11538. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, Y.; Qiu, L.; Molokeev, M.S.; Yang, H.; Liu, H.; Gao, R.; Guan, M.; Tu, D.; Li, G. Mn–Mn Magnetic Coupling Interaction-Induced Red Emission in a Tetra-Coordinated Lattice. Chem. Mater. 2024, 36, 5574–5586. [Google Scholar] [CrossRef]
- Zheng, W.; Li, X.; Liu, N.; Yan, S.; Wang, X.; Zhang, X.; Liu, Y.; Liang, Y.; Zhang, Y.; Liu, H. Solution-Grown Chloride Perovskite Crystal of Red Afterglow. Angew. Chem. Int. Ed. 2021, 60, 24450–24455. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Han, L.; Xiao, Z.; Song, J.; Li, T.; Liu, J.; Liu, T.; Huang, D.; You, W.; Han, X.; et al. Near-Unity and Near-Zero-Thermal-Quenching Luminescent GAGG–Al2O3:Cr3+ Ceramic via Containerless Solidification and Glass Crystallization Methods for NIR Spectroscopy Application. Laser Photonics Rev. 2025, 19, 2500165. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zhang, C.; Xu, J.; Liu, G.; Jiang, J.; Jiang, H. Ba9Lu2Si6O24:Ce3+: An Efficient Green Phosphor with High Thermal and Radiation Stability for Solid-State Lighting. Adv. Opt. Mater. 2015, 3, 1096–1101. [Google Scholar] [CrossRef]
- Wang, H.; Mei, L.; Su, K.; Liu, J.; Wang, Q.; Wu, Z.; Guo, Q.; Liao, L. Structure Modulation and Charge Transfer in Self-Reduction Phosphors: A Review. Laser Photonics Rev. 2024, 19, 2400665. [Google Scholar] [CrossRef]
- Wu, L.; Bai, Y.; Chen, H.; Zheng, L.; Yi, H.; Wu, L.; Hu, Z.; Zhang, X.; Kong, Y.; Zhang, Y.; et al. Unique Self-Reduction of Transitional Metal Ion in a Borate with Planar [BO3]3− Groups. Adv. Opt. Mater. 2023, 11, 2300515. [Google Scholar] [CrossRef]
- Wu, L.; Sun, S.; Bai, Y.; Xia, Z.; Wu, L.; Chen, H.; Zheng, L.; Yi, H.; Sun, T.; Kong, Y.; et al. Defect-Induced Self-Reduction and Anti-Thermal Quenching in NaZn(PO3)3:Mn2+ Red Phosphor. Adv. Opt. Mater. 2021, 9, 2100870. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Z.; Wu, L.; Kong, Y.; Zhang, Y.; Xu, J. Self-Reduction-Related Defects, Long Afterglow, and Mechanoluminescence in Centrosymmetric Li2ZnGeO4:Mn2+. Inorg. Chem. 2021, 60, 18432–18441. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, S.; Sun, L.; Liang, J.; Devakumar, B.; Huang, X. Achieving full-visible-spectrum LED lighting via employing an efficient Ce3+-activated cyan phosphor. Mater. Today Energy 2020, 17, 100448. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Q.; Devakumar, B.; Liang, J.; Sun, L.; Huang, X. Novel highly efficient and thermally stable Ca2GdTaO6:Eu3+ red-emitting phosphors with high color purity for UV/blue-excited WLEDs. J. Alloys Compd. 2019, 804, 93–99. [Google Scholar] [CrossRef]
- Wang, X.; Du, J.; Hreniak, D.; Stręk, W.; Jiang, K.; Lin, H. Construction of an Fe–Ni energy bridge for NIR-II luminescence enhancement and anti-thermal quenching via microwave-induced defect engineering. Mater. Horiz. 2025, 12, 5277–5286. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, J.; Fan, J.; Zhang, J.; Huang, J.; Chen, P.; Zhou, L.; Zhang, X. Intense NIR-II luminescence through stabilization of tetrahedral (Mn5+O4) lumophore in apatites with high external quantum efficiency. Acta Mater. 2024, 273, 119981. [Google Scholar] [CrossRef]
- Kuzman, S.; Dramićanin, T.; Popov, A.I.; Brik, M.G.; Dramićanin, M.D. Pigments and Near-Infrared Phosphors Based on Mn5+. Nanomaterials 2025, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Song, E.; Zhang, Q. Selective site occupancy engineering enabling Mn5+ activated highly efficient near infrared-II emission in Ba3BPO7:Mn5+. Appl. Phys. Lett. 2024, 124, 091102. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Z.; Zhou, X.; Delaey, M.; Wang, M.; Fu, R.; Lei, S.; Vrielinck, H.; Poelman, D. Achieving High Quantum Efficiency in Mn5+ Activated Phosphors for NIR-II Deep Bioimaging Application. Laser Photonics Rev. 2024, 18, 2400781. [Google Scholar] [CrossRef]
- Gao, D.; Wang, P.; Gao, F.; Nguyen, W.; Chen, W. Tuning Multicolor Emission of Manganese-Activated Gallogermanate Nanophosphors by Regulating Mn Ions Occupying Sites for Multiple Anti-Counterfeiting Application. Nanomaterials 2022, 12, 2029. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Q.; Song, E.H.; Ye, S.; Zhou, B.; Zhang, Q.Y. Anomalous spontaneous-reduction of Mn7+/Mn4+to Mn2+and luminescence properties in Zn2GeO4:Mn. J. Mater. Chem. 2017, 5, 3343–3351. [Google Scholar] [CrossRef]
- Li, S.; Hu, W.; Brik, M.G.; Lian, S.; Qiu, Z. Achieving highly thermostable red emission in singly Mn2+-doped BaXP2O7 (X = Mg/Zn) via self-reduction. Inorg. Chem. Front. 2022, 9, 3224–3232. [Google Scholar] [CrossRef]
- Wu, S.; Hu, S.; Liu, Q.; Zhang, S.; Jiang, D.; Zhang, G.; Le, Y.; Xiao, Y.; Xiao, B.; Xiong, P.; et al. Self-reduction of Mn4+ to Mn2+: NaY9Si6O26:Mn2+ red phosphors with excellent thermal stability for NUV LEDs. J. Mater. Chem. 2023, 11, 3865–3874. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wei, S.; Zhang, J.; Du, J.; Li, Y.; Chen, K.; Lin, H. Preparation and Optimization of Mn2+-Activated Na2ZnGeO4 Phosphors: Insights into Precursor Selection and Microwave-Assisted Solid-State Synthesis. Nanomaterials 2025, 15, 1117. https://doi.org/10.3390/nano15141117
Wang X, Wei S, Zhang J, Du J, Li Y, Chen K, Lin H. Preparation and Optimization of Mn2+-Activated Na2ZnGeO4 Phosphors: Insights into Precursor Selection and Microwave-Assisted Solid-State Synthesis. Nanomaterials. 2025; 15(14):1117. https://doi.org/10.3390/nano15141117
Chicago/Turabian StyleWang, Xiaomeng, Siyi Wei, Jiaping Zhang, Jiaren Du, Yukun Li, Ke Chen, and Hengwei Lin. 2025. "Preparation and Optimization of Mn2+-Activated Na2ZnGeO4 Phosphors: Insights into Precursor Selection and Microwave-Assisted Solid-State Synthesis" Nanomaterials 15, no. 14: 1117. https://doi.org/10.3390/nano15141117
APA StyleWang, X., Wei, S., Zhang, J., Du, J., Li, Y., Chen, K., & Lin, H. (2025). Preparation and Optimization of Mn2+-Activated Na2ZnGeO4 Phosphors: Insights into Precursor Selection and Microwave-Assisted Solid-State Synthesis. Nanomaterials, 15(14), 1117. https://doi.org/10.3390/nano15141117






