Elucidating the Role of Graphene Oxide Surface Architecture and Properties in Loess Soil Remediation Efficacy
Abstract
1. Introduction
2. Experimental Materials and Details
2.1. Materials Preparation
2.2. Macroscopic Testing
2.2.1. Physical Properties of the Specimen
2.2.2. Lanzhou Loess Disintegration Test
2.2.3. Lanzhou Loess SWCC Test
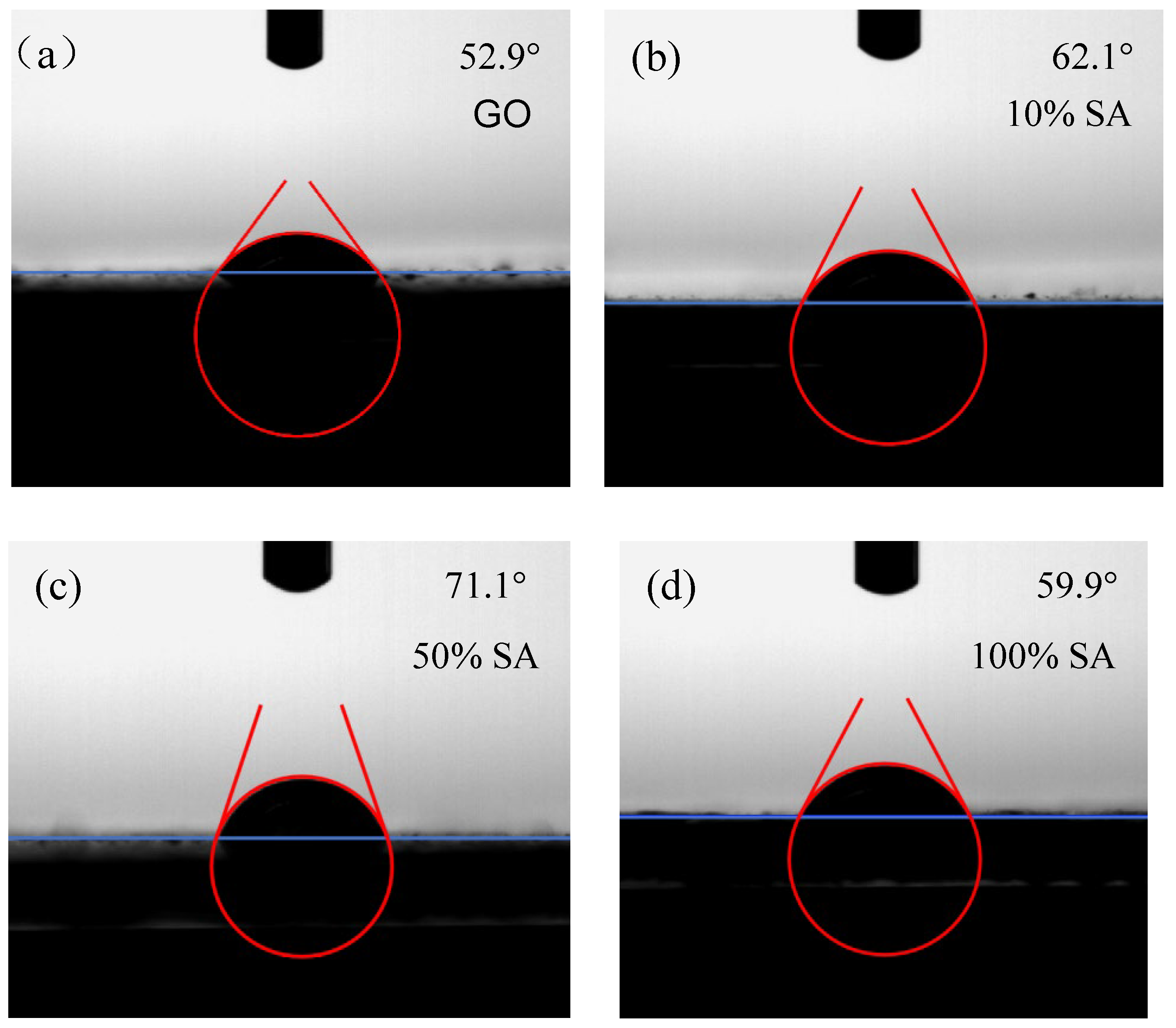
2.3. Microscopic Testing
3. Experimental Results and Discussion
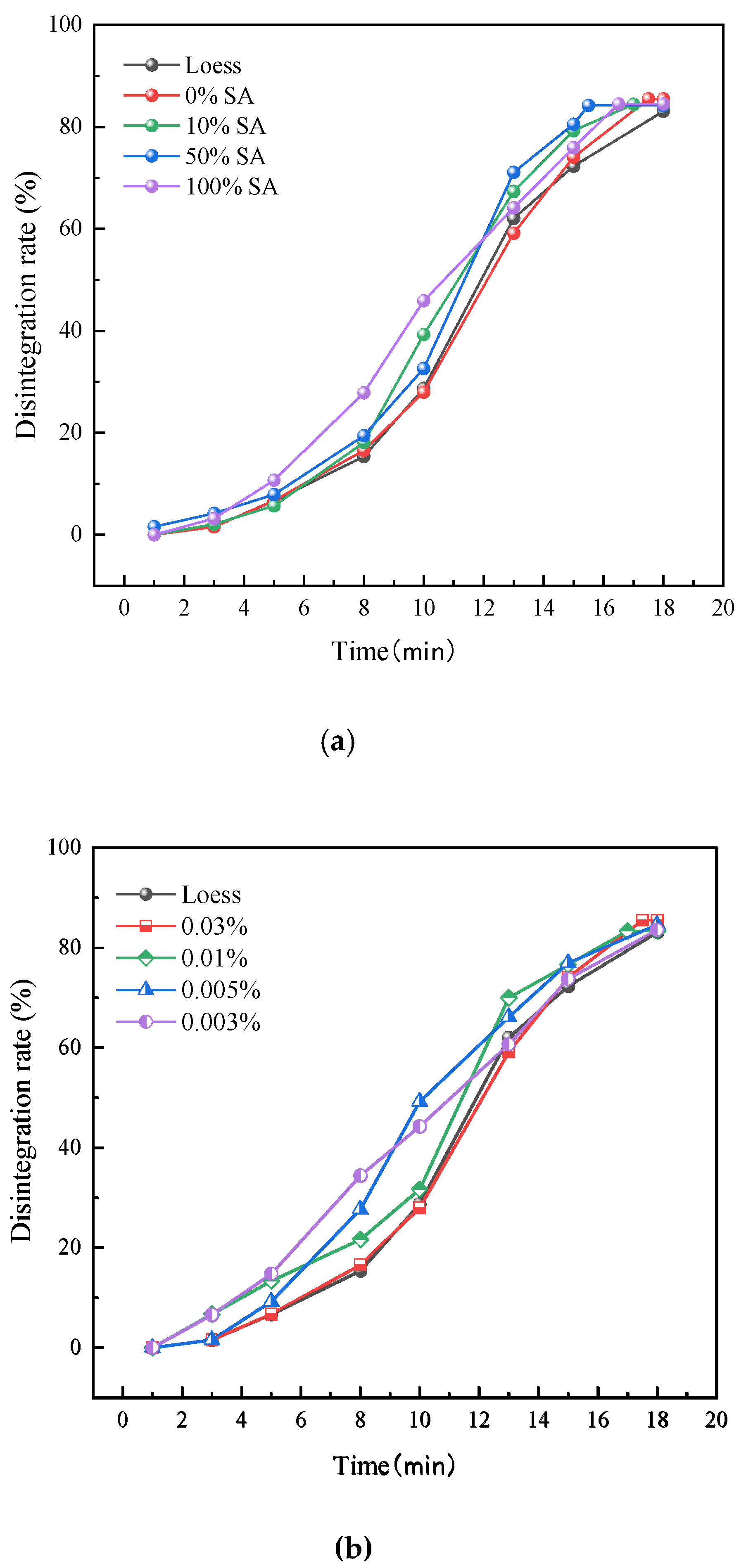
3.1. Influence on the Disintegration of Lanzhou Loess
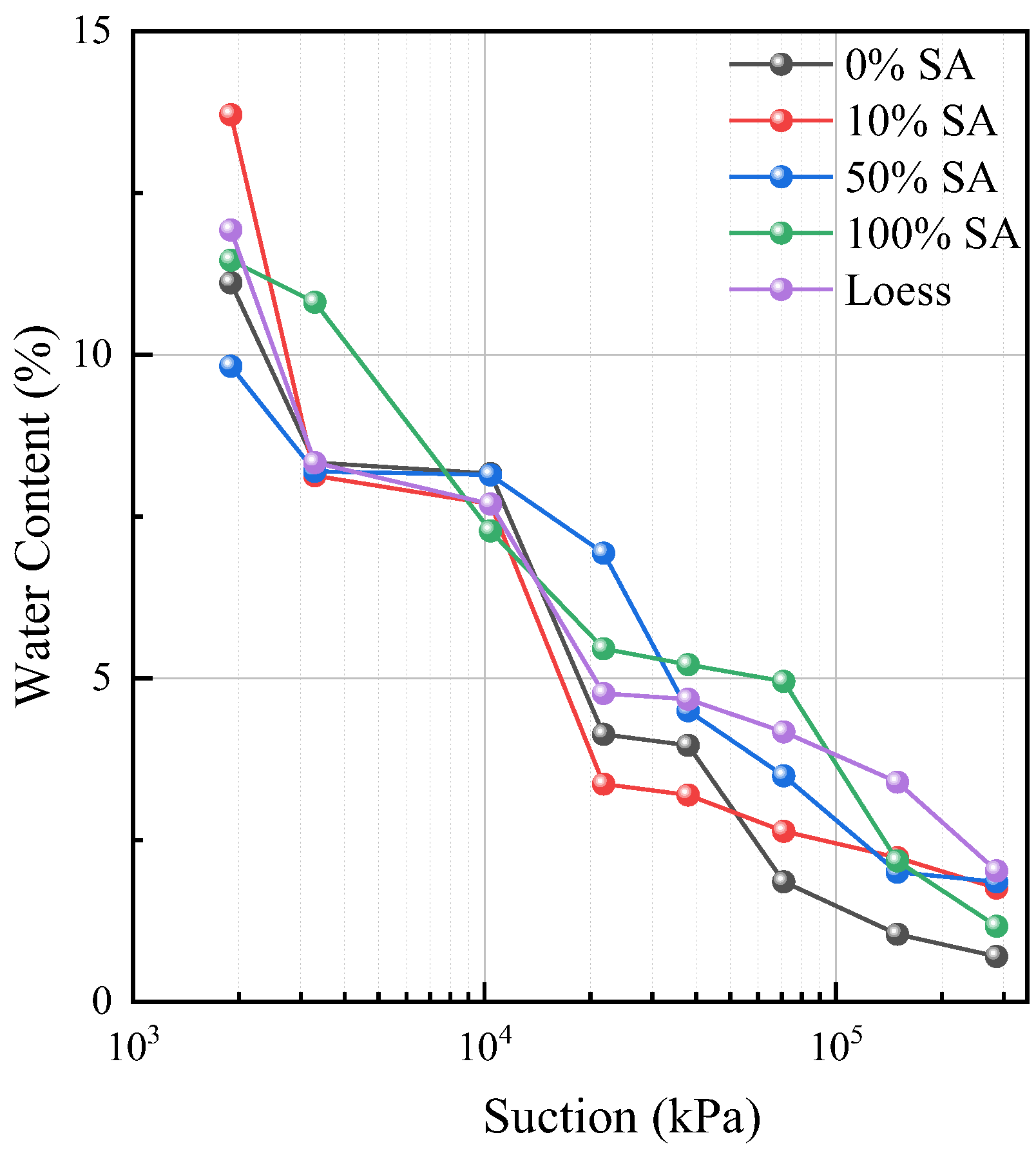
3.2. Impact on SWCC of Lanzhou Loess
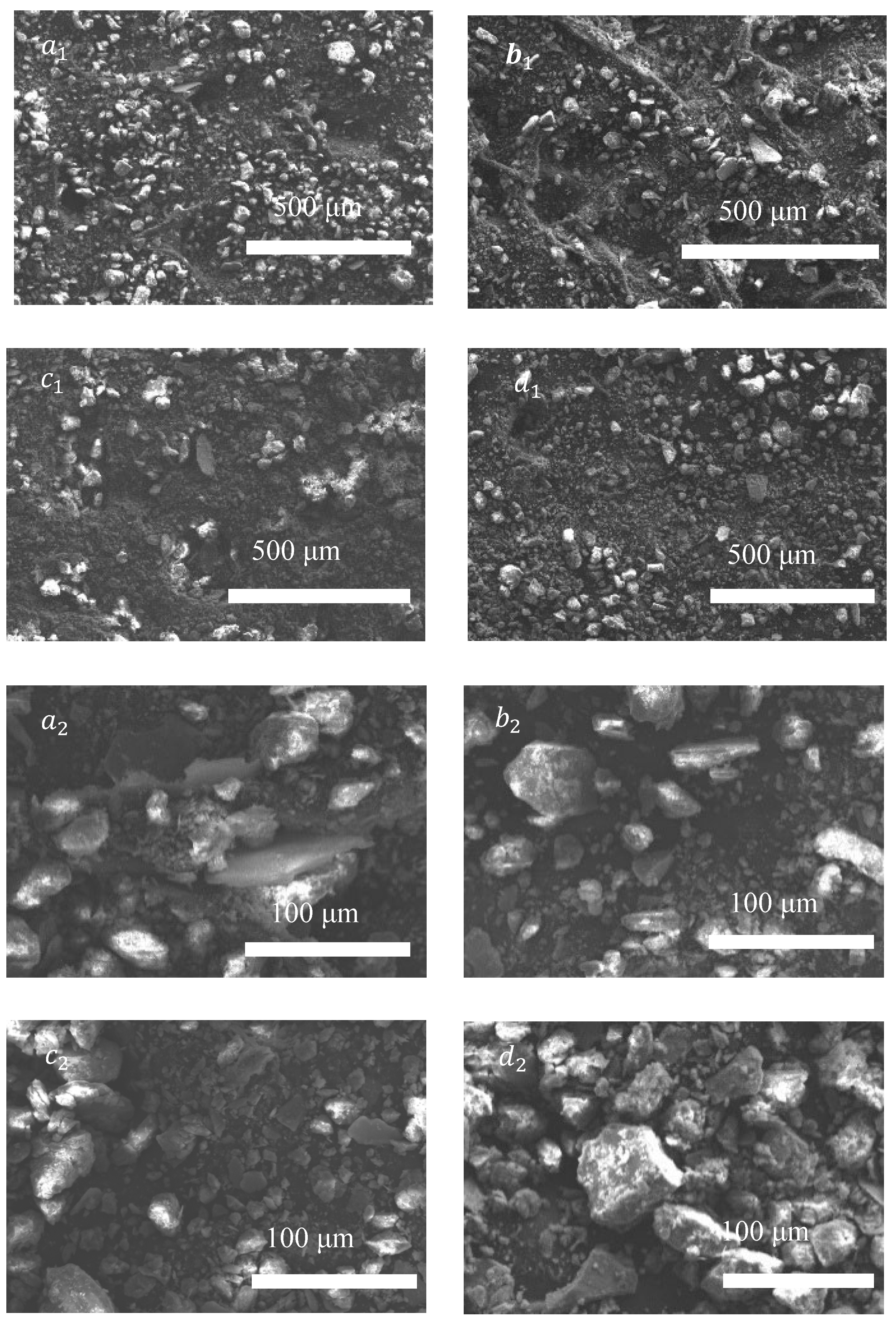
3.3. Influence on the Microstructure of Lanzhou Loess
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Zhao, J.; Li, B. Loess and Loess Geohazards in China; CRC Press: Boca Raton, FL, USA, 2017; p. 178. [Google Scholar]
- Tian, W.; Hu, W.; Li, J.; Gao, Z. Analysis of the Current Situation and Prevention Countermeasures of Soil Erosion in China. Res. Soil Water Conserv. 2008, 15, 204–209. [Google Scholar]
- Liu, D. The Accumulation of Loess in China; Science Press: Beijing, China, 1965. (In Chinese) [Google Scholar]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Shi, W.W. Studies on Ecological Effects of Secondary Salinization on Facility Culturing Soil and Bioremediation. Master’s Thesis, Xiameng University, Fujian, China, 2009. (In Chinese). [Google Scholar]
- Qiao, L.; Li, Y.; Song, Y.; Zhai, J.; Wu, Y.; Chen, W.; Liu, G.; Xue, S. Effects of Vegetation Restoration on the Distribution of Nutrients, Glomalin-Related Soil Protein, and Enzyme Activity in Soil Aggregates on the Loess Plateau, China. Forests 2019, 10, 796. [Google Scholar] [CrossRef]
- Kananizadeh, N.; Ebadi, T.; Khoshniat, S.A.; Mousavirizi, S.E. The Positive Effects of Nanoclay on the Hydraulic Conductivity of Compacted Kahrizak Clay Permeated with Landfill Leachate. Clean-Soil Air Water 2011, 39, 605–611. [Google Scholar] [CrossRef]
- Ren, K.; Gao, L.; Yu, X.; Wang, C. Research on the Shear Strength Characteristics of Graphene-Modified Clay. J. Hebei Univ. Eng. Nat. Sci. Ed. 2018, 35, 62–66. (In Chinese) [Google Scholar]
- Zhang, X.R.; Wang, Y.S.; Guo, S.Y.; Li, H.Y.; Liu, J.F.; Zhang, Z.Y.; Yan, L.; Tan, C.H.; Yang, Z.C.; Guo, X.P. Concentration and speciation of trace metals and metalloids from road-deposited sediments in urban and rural areas of Beijing, China. J. Soils Sediments 2020, 20, 3487–3501. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Q.X.; Huang, G.X.; Fan, Q. Removal of heavy metals in aquatic environment by graphene oxide composites: A review. Environ. Sci. Pollut. Res. 2020, 27, 190–209. [Google Scholar] [CrossRef]
- He, Y. Research on the Impact of Graphene Oxide on Plant Growth. Master’s Thesis, Tsinghua University, Beijing, China, 2019. (In Chinese). [Google Scholar]
- Ren, W.; Chang, H.; Teng, Y. Sulfonated graphene-induced hormesis is mediated through oxidative stress in the roots of maize seedlings. Sci. Total Environ. 2016, 572, 926–934. [Google Scholar] [CrossRef]
- Ren, W.; Ren, G.; Teng, Y.; Li, Z.; Li, L. Time-dependent effect of graphene on the structure, abundance, and function of the soil bacterial community. J. Hazard. Mater. 2015, 297, 286–294. [Google Scholar] [CrossRef]
- Zhao, G.X.; Li, J.X.; Ren, X.M.; Chen, C.L.; Wang, X.K. Few-Layered Graphene Oxide Nanosheets as Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef]
- Li, X.; Tang, X.P.; Fang, Y.F. Using graphene oxide as a superior adsorbent for the highly efficient immobilization of Cu(II) from aqueous solution. J. Mol. Liq. 2014, 199, 237–243. [Google Scholar] [CrossRef]
- Andelkovic, I.B.; Kabiri, S.; Tavakkoli, E.; Kirby, J.K.; McLaughlin, M.J.; Losic, D. Graphene oxide-Fe(III) composite containing phosphate—A novel slow release fertilizer for improved agriculture management. J. Clean. Prod. 2018, 185, 97–104. [Google Scholar] [CrossRef]
- Wang, T.J.; Bai, E.R.; Ren, B.; Xia, W.; Xu, J.Y. Strengthening effect of monolayer graphene oxide on the impact mechanical properties of concrete and the mechanism analysis based on fractal theory. Constr. Build. Mater. 2024, 428, 17. [Google Scholar] [CrossRef]
- Meng, F.X.; Qin, J.Z.; Wu, Q.H.; Dai, H.W.; Zhu, P.; Tang, T.; Zhang, L.X.; Zhang, K.C. Zuo Identifying the Critical Oxygenated Functional Groups on Graphene Oxide for Efficient Water Dissociation in Bipolar Membranes. Acs Energy Lett. 2024, 9, 5444–5451. [Google Scholar] [CrossRef]
- Zheng, H.Z.; Ma, R.L.; Gao, M.; Tian, X.; Li, Y.Q.; Zeng, L.W.; Li, R.B. Antibacterial applications of graphene oxides: Structure-activity relationships, molecular initiating events and biosafety. Sci. Bull. 2018, 63, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Xie, H.C.; Wang, Z.Y.; He, K.L.; Jing, D.P. Graphene oxide as a multifunctional synergist of insecticides against lepidopteran insect. Environ. Sci.-Nano 2019, 6, 75–84. [Google Scholar] [CrossRef]
- Hu, J.; Lao, Z.; Wu, K.; Fan, H. Research Progress on the Environmental Behavior and Toxic Effects of Graphene Oxide. J. Ecol. Environ. 2017, 26, 2169–2176. (In Chinese) [Google Scholar]
- Navarro, D.A.; Kah, M.; Losic, D.; Kookana, R.S.; McLaughlin, M.J. Mineralisation and release of 14C-graphene oxide (GO) in soils. Chemosphere 2020, 238, 7. [Google Scholar] [CrossRef]
- Zhang, J.L.; Yang, H.J.; Shen, G.X.; Cheng, P.; Zhang, J.Y.; Guo, S.W. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Li, D.D.; Wang, T.; Hu, X.G. Leaching of graphene oxide nanosheets in simulated soil and their influences on microbial communities. J. Hazard. Mater. 2021, 404, 11. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.M.; Jang, M.; Son, A.; Her, N.; Yu, M.; Snyder, S.; Kim, D.H.; Yoon, Y. Aqueous removal of inorganic and organic contaminants by graphene-based nanoadsorbents: A review. Chemosphere 2018, 212, 1104–1124. [Google Scholar] [CrossRef]
- Iakunkov, A.; Talyzin, A.V. Swelling properties of graphite oxides and graphene oxide multilayered materials. Nanoscale 2020, 12, 21060–21093. [Google Scholar] [CrossRef]
- Adhikari, S.; Timms, W.; Mahmud, M.A.P. Optimising water holding capacity and hydrophobicity of biochar for soil amendment-A review. Sci. Total. Environ. 2022, 851, 15. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Lu, H.; Li, Z.; Wu, Y.; Ren, J. Elucidating the Role of Graphene Oxide Surface Architecture and Properties in Loess Soil Remediation Efficacy. Nanomaterials 2025, 15, 1098. https://doi.org/10.3390/nano15141098
Wang Z, Lu H, Li Z, Wu Y, Ren J. Elucidating the Role of Graphene Oxide Surface Architecture and Properties in Loess Soil Remediation Efficacy. Nanomaterials. 2025; 15(14):1098. https://doi.org/10.3390/nano15141098
Chicago/Turabian StyleWang, Zirui, Haotian Lu, Zhigang Li, Yuwei Wu, and Junping Ren. 2025. "Elucidating the Role of Graphene Oxide Surface Architecture and Properties in Loess Soil Remediation Efficacy" Nanomaterials 15, no. 14: 1098. https://doi.org/10.3390/nano15141098
APA StyleWang, Z., Lu, H., Li, Z., Wu, Y., & Ren, J. (2025). Elucidating the Role of Graphene Oxide Surface Architecture and Properties in Loess Soil Remediation Efficacy. Nanomaterials, 15(14), 1098. https://doi.org/10.3390/nano15141098





