Nanofabrication Techniques for Enhancing Plant–Microbe Interactions in Sustainable Agriculture
Abstract
1. Introduction
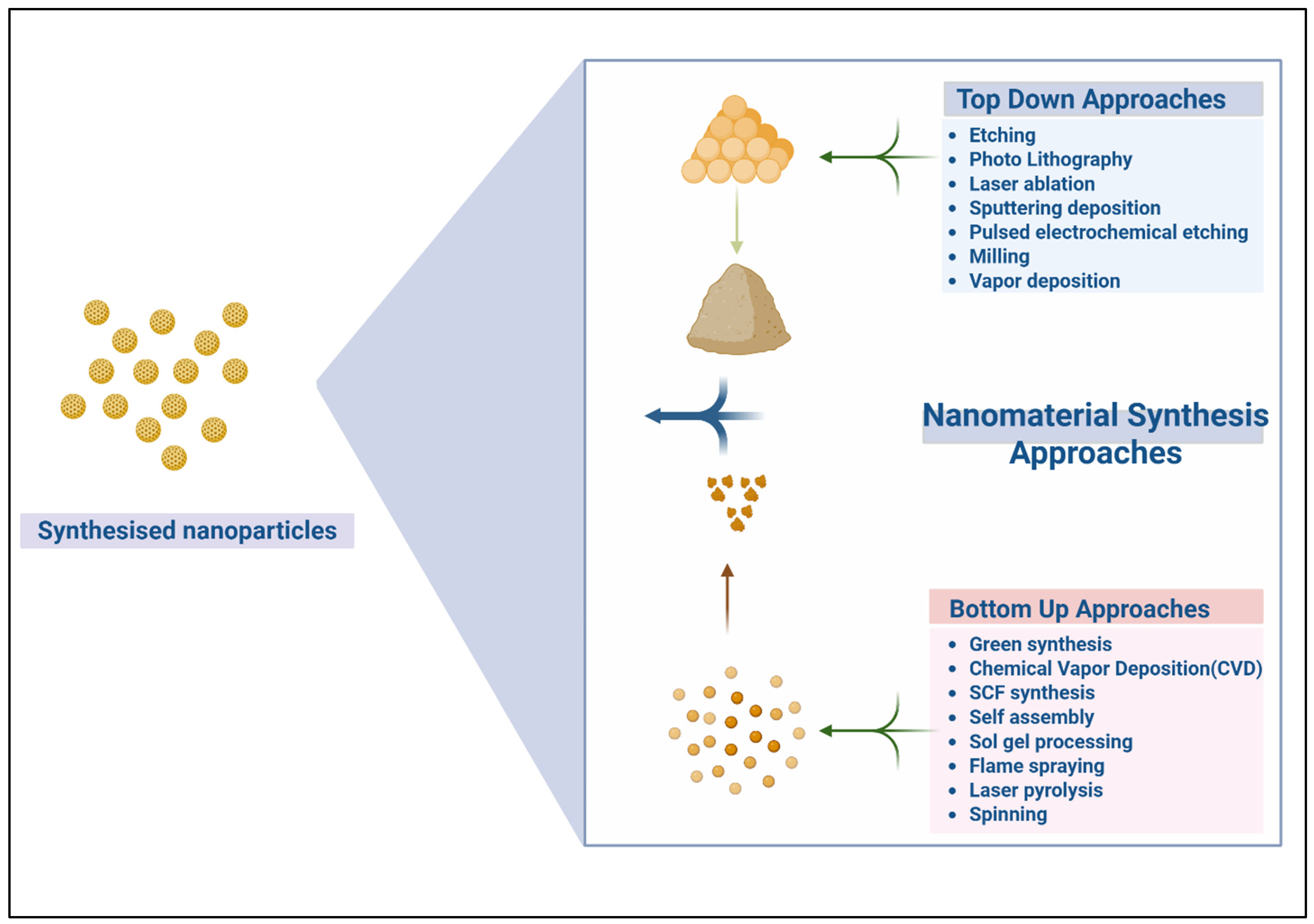
2. Overview of Nanofabrication Techniques
2.1. Top-Down vs. Bottom-Up Nanomanufacturing
2.2. Soft Lithography, Nanoimprinting, and Microfluidics
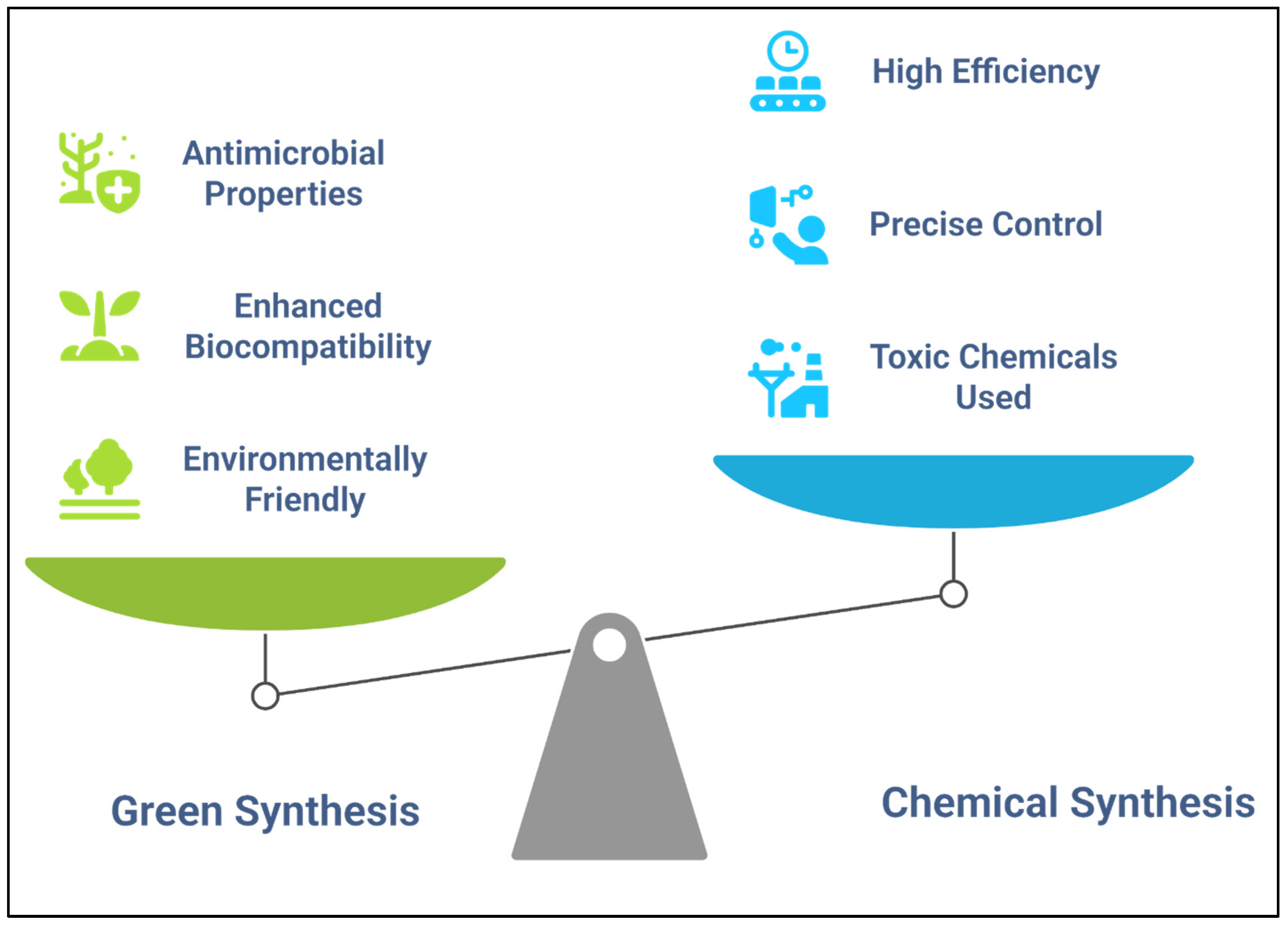
2.3. Nanoparticle Synthesis: Green vs. Chemical Methods
2.4. Electrospinning and Nanocoating
| Category | Application | Description | References |
|---|---|---|---|
| Seed Protection | Electrospun seed coatings | Electrospun nanofibers are used as seed coatings to protect seeds and enable the controlled release of agrichemicals, improving seedling development and crop protection | [58] |
| Nanocoatings for seed protection | Nanocoatings applied to seeds enhance microbial adhesion, promote plant growth, and protect against pathogens | [59] | |
| Biodegradable nanofiber coatings for seeds | Coatings prepared using biodegradable nanofibers control the release of agrochemicals while enhancing seedling growth and minimizing environmental harm | [50] | |
| Agrochemical Delivery | Agrochemical carriers | Nanofibers fabricated via electrospinning are used to encapsulate agrochemicals, improving controlled release and reducing environmental impact | [51] |
| Other Agricultural Uses | Biocompatible nanofiber membranes | Nanofibers provide a biocompatible porous membrane for storing seeds for protection while gradually releasing substances | [60] |
| Antimicrobial nanocoatings for agricultural tools | Nanocoatings with antimicrobial properties are applied to agricultural tools and equipment to reduce contamination and improve tool longevity | [61] | |
| Microbial complex nanocoatings on seeds | Nanocoatings formed using electrospinning incorporate microbial complexes to enhance the interactions between plants and beneficial microbes | [62] | |
| Multilayer nanocoatings for crop protection | Multilayer nanocoatings, such as chitosan/lignin with silver nanoparticles, protect seeds and plants from pathogens | [63] | |
| Edible nanoencapsulation for food applications | Nanoencapsulation technology for food coatings and preservation, improving shelf life and food safety | [64] |
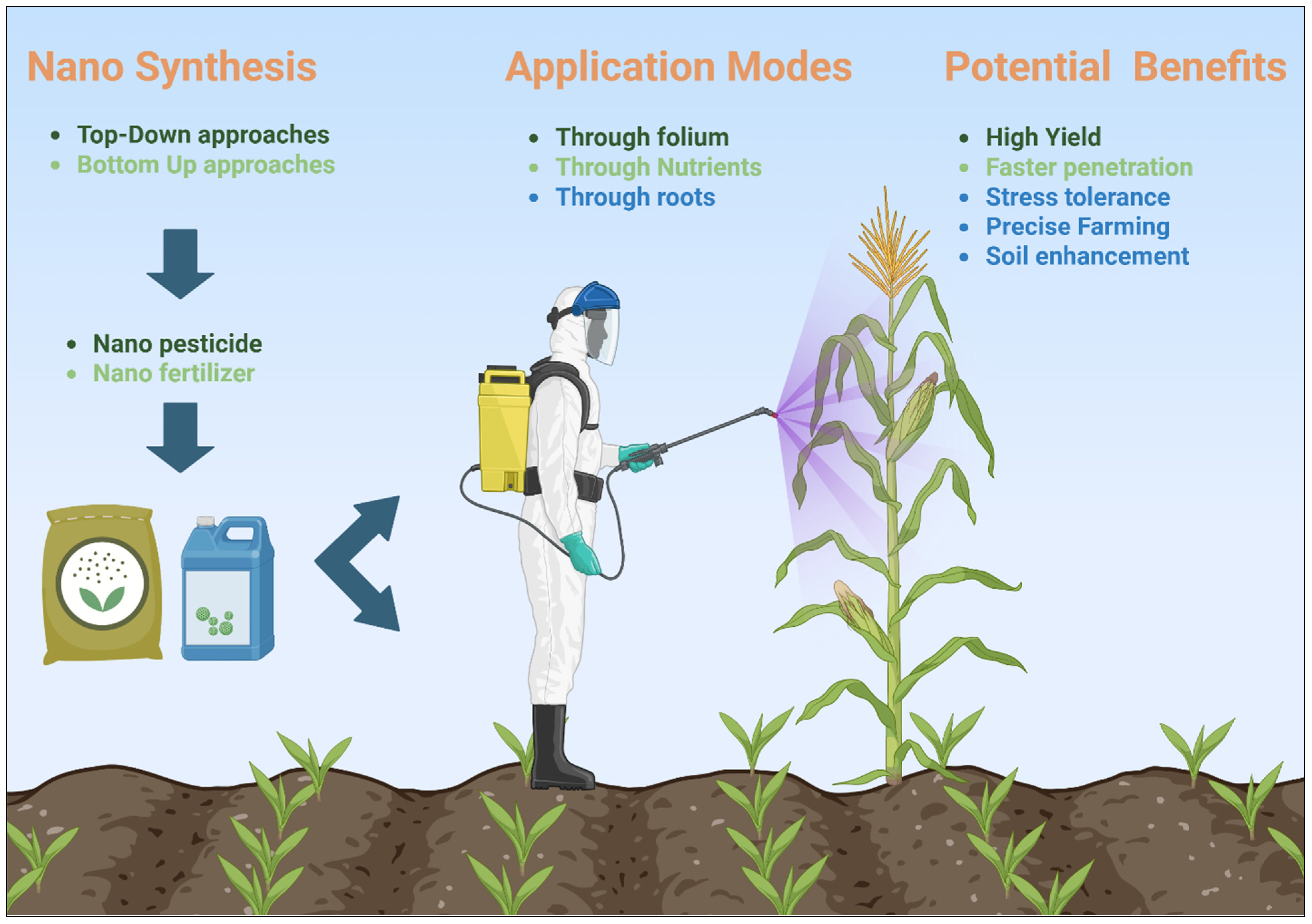
3. Nanoengineered Delivery Systems
3.1. Nanoformulations for Microbial Inoculants
3.2. Controlled Release of Biofertilizers and Biopesticides
3.3. Nanocarriers for Rhizobia, Mycorrhizae, and PGPR
| Nanocarrier Type | Microbe Type | Application | Functionality | References |
|---|---|---|---|---|
| Nanoparticles | Rhizobia | Encapsulation of rhizobia for sustained release to improve nitrogen fixation in legumes and reduce synthetic fertilizer use | Enhancing nitrogen fixation by maintaining a steady delivery of rhizobia to plant roots, thereby improving the symbiotic relationship between the plant and the microbe | [96] |
| Nanoemulsions | PGPR | Delivery system for PGPR that promotes plant growth by enabling the controlled release of beneficial microbes in the root zone | PGPR enhance plant growth via hormone production and pathogen suppression; nanoemulsions enable sustained release for consistent microbial activity | [83] |
| Bio-nanofertilizers | PGPR, microalgae | Functionalized nanoparticles with PGPR and microalgae for enhancing nutrient uptake and promoting plant growth | Improves nutrient availability and plant growth via synergistic effects of PGPR and microalgae; nanoparticles protect microbes from environmental stress | [93] |
| Nanofibers | Mycorrhizae | Delivery system for mycorrhizal fungi to promote phosphorus uptake and successfully colonize plant roots | Mycorrhizal fungi enhance nutrient uptake, particularly phosphorus; nanofibers provide physical protection and the controlled release of fungi to the root system | [97] |
| Polymeric nanoparticles | PGPR | Use of nanoparticles for the controlled delivery of PGPR, enhancing plant growth and resilience to drought conditions | Nanoparticles provide a stable environment for PGPR, promoting plant health under adverse conditions | [98] |
| Nanoparticles (zinc and iron) | PGPR, mycorrhizae | Zinc and iron nanoparticles functionalized with PGPR for enhancing plant growth and mycorrhizal colonization | These nanoparticles supply essential micronutrients to plants, whereas PGPR and mycorrhizae improve nutrient uptake and plant health | [99] |
| Silica nanoparticles | Rhizobia, PGPR | Delivery of rhizobia and PGPR using silica nanoparticles to improve microbial colonization and stress resistance in plants | Silica nanoparticles improve microbial survival under stressful environmental conditions by providing stability and protection | [100] |
| Chitosan-based nanogels | Mycorrhizae, PGPR | Chitosan nanogels for delivering mycorrhizal fungi and PGPR to increase soil fertility and promote plant growth | Chitosan-based nanogels are biodegradable and provide a slow-release mechanism for both mycorrhizal fungi and PGPR, ensuring sustained microbial activity in the root zone | [13] |
| Carbon nanotubes | Rhizobia, mycorrhizae | Delivery of rhizobia and mycorrhizae using carbon nanotubes to increase microbial efficacy and plant growth | Carbon nanotubes increase the efficiency of microbial delivery to plant roots, enhancing nutrient uptake and plant growth | [101] |
| Lipid-based nanocarriers | PGPR | Lipid-based nanoparticles for the controlled delivery of PGPR in the rhizosphere to enhance plant growth and resilience | Lipid carriers offer targeted delivery and increase microbial colonization by forming a protective barrier that prevents degradation | [102] |
4. Nanostructured Surfaces for Root–Microbe Interactions
4.1. Engineering Root-Mimetic Interfaces
4.2. Nanostructured Seed Coatings for Enhanced Microbe Colonization
4.3. Influence of Surface Charge, Porosity, and Hydrophobicity
| Surface Property | High Value (Effect) | Low Value (Effect) | References |
|---|---|---|---|
| Surface charge | Positive charge attracts negatively charged microbes, enhancing microbial adhesion (e.g., PGPR and mycorrhizae), facilitating nutrient cycling and plant growth | Negative charge repels some microbes, useful for preventing pathogen adhesion and can generate dynamic microbial environments for controlling harmful microbes | [126] |
| Porosity | High porosity increases surface area, promoting microbial colonization, enhances microbial activity, supports the retention of nutrients and moisture, and improves soil health and plant growth | Low porosity reduces surface area, limiting microbial colonization, but is useful for controlled release applications such as slow-release fertilizers and pesticide delivery systems | [129,146] |
| Hydrophobicity | Hydrophobic surfaces inhibit water-loving microbes, limiting microbial colonization. Useful for dry conditions or controlling microbial biofilms and pathogen growth on plant surfaces | Hydrophilic surfaces enhance microbial adhesion and water retention, promoting colonization of beneficial microbes, especially in moist environments such as plant roots | [144] |
| Surface roughness | High surface roughness increases available surface area for microbial attachment, supporting increased microbial colonization, especially for symbiotic microbes | Low surface roughness limits the available area for microbial attachment and may reduce microbial colonization potential | [106] |
| Surface functionalization | Functionalized surfaces can enhance or inhibit microbial attachment. For example, adding hydrophilic or hydrophobic functional groups allows for targeted microbial interactions (e.g., promoting beneficial microbe colonization or controlling pathogen biofilm formation) | Lack of surface functionalization can cause passive microbial attachment, causing inefficient or unintended microbial colonization | [147] |
| Environmental factors (pH and ionic strength) | Environmental conditions such as pH and ionic strength can influence the interactions between surface charge and microbial adhesion, promoting or reducing microbial attachment depending on the conditions | Inconsistent environmental factors can alter the effectiveness of surface charge and other properties, potentially reducing microbial colonization and unpredictable results | [148] |
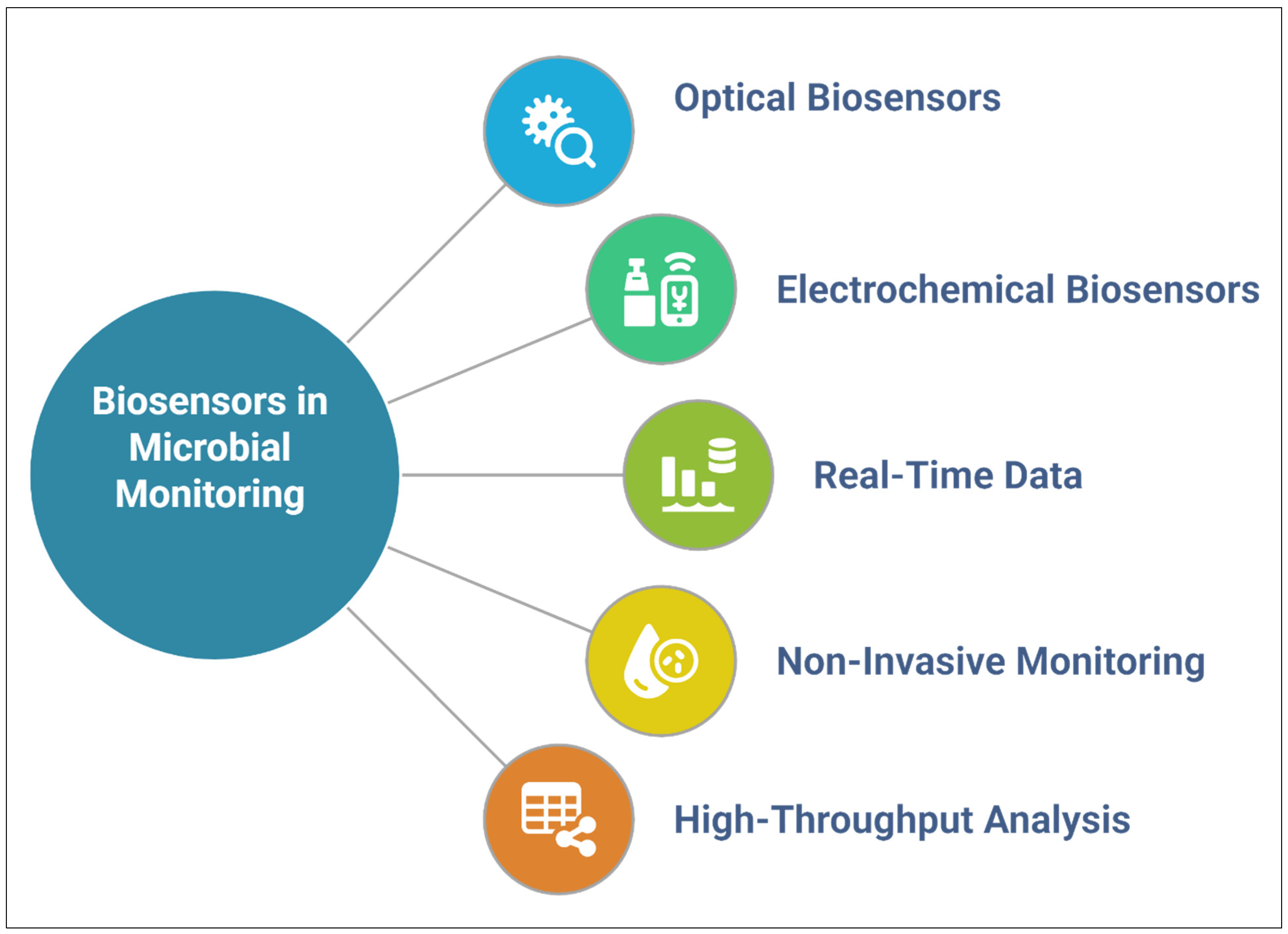
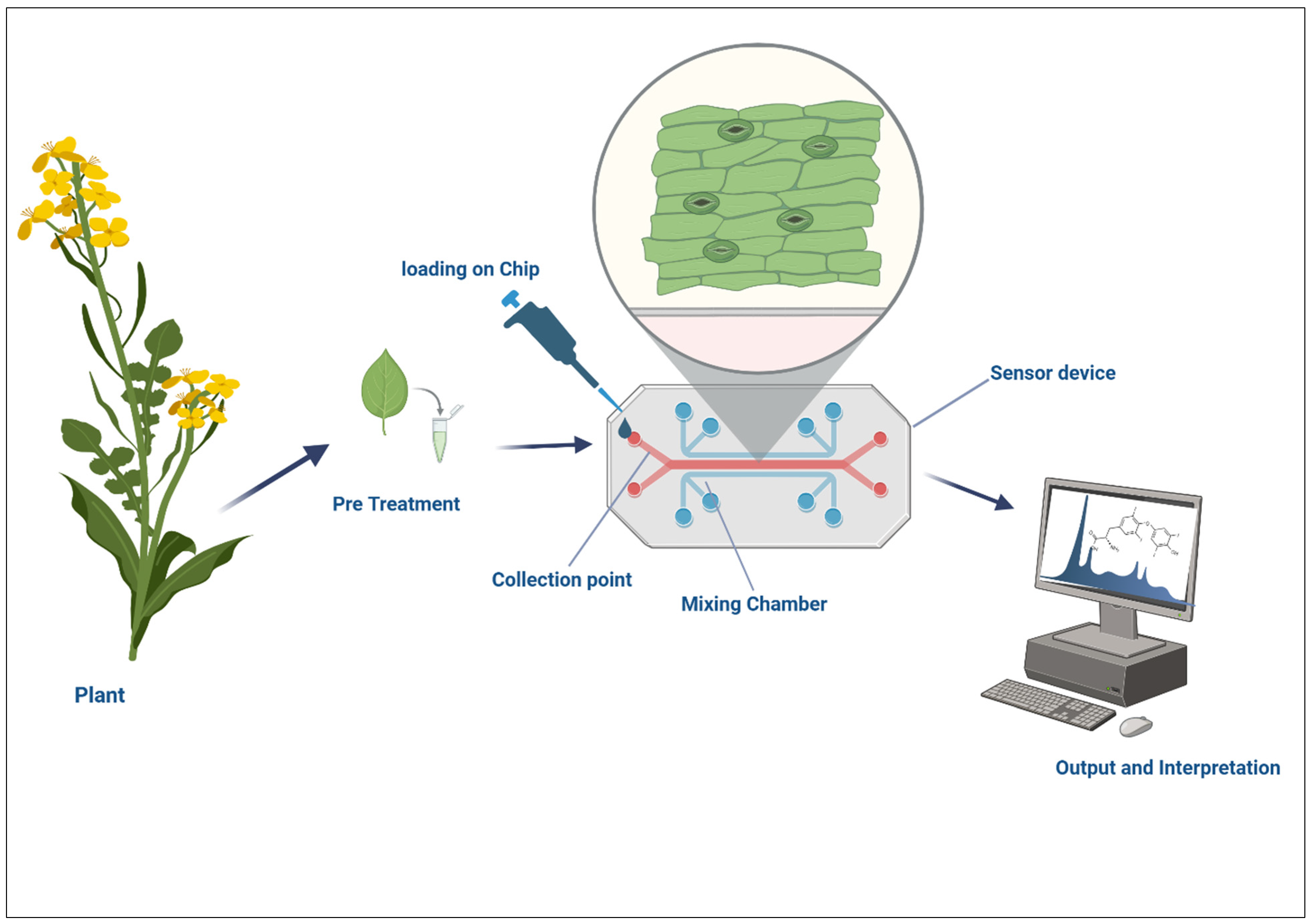
5. Biosensors and Microfluidics for Interaction Monitoring
5.1. Real-Time Monitoring of Microbial Colonization
5.2. Lab-On-A-Chip for Profiling Root Exudates
5.3. Nanosensors for pH, Reactive Oxygen Species, and Metabolite Detection
6. Environmental and Safety Considerations
6.1. Biodegradability of Nanomaterials in Agricultural Systems
6.2. Nanotoxicity to Soil Microbiota and Plants
7. Challenges and Future Directions
7.1. Integration of Nanotechnology into Field Applications
7.2. Scalability and Cost-Effectiveness of Nanofabrication Techniques
7.3. Synergies with AI, Synthetic Biology, and IoT in Agricultural Advancements
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhary, D.K.; Varma, A.; Tuteja, N. Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Mohanram, S.; Kumar, P. Rhizosphere microbiome: Revisiting the synergy of plant-microbe interactions. Ann. Microbiol. 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Mabrouk, Y.; Hemissi, I.; Salem, I.B.; Mejri, S.; Saidi, M.; Belhadj, O. Potential of rhizobia in improving nitrogen fixation and yields of legumes. Symbiosis 2018, 107. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil health and sustainable agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Xu, P.; Bhan, N.; Koffas, M.A. Engineering plant metabolism into microbes: From systems biology to synthetic biology. Curr. Opin. Biotechnol. 2013, 24, 291–299. [Google Scholar] [CrossRef]
- Su, C.; Lei, L.; Duan, Y.; Zhang, K.-Q.; Yang, J. Culture-independent methods for studying environmental microorganisms: Methods, application, and perspective. Appl. Microbiol. Biotechnol. 2012, 93, 993–1003. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Chavez, A.; Collins, J.J. CRISPR-based genomic tools for the manipulation of genetically intractable microorganisms. Nat. Rev. Microbiol. 2018, 16, 333–339. [Google Scholar] [CrossRef]
- Cremin, K.; Duxbury, S.J.; Rosko, J.; Soyer, O.S. Formation and emergent dynamics of spatially organized microbial systems. Interface Focus 2023, 13, 20220062. [Google Scholar] [CrossRef]
- Sodhi, G.K.; Wijesekara, T.; Kumawat, K.C.; Adhikari, P.; Joshi, K.; Singh, S.; Farda, B.; Djebaili, R.; Sabbi, E.; Ramila, F. Nanomaterials–plants–microbes interaction: Plant growth promotion and stress mitigation. Front. Microbiol. 2025, 15, 1516794. [Google Scholar] [CrossRef]
- Ariga, K. Manipulation of Nanoscale Materials: An Introduction to Nanoarchitectonics; Royal Society of Chemistry: London, UK, 2012. [Google Scholar]
- Ullah, M.W.; Shi, Z.; Shi, X.; Zeng, D.; Li, S.; Yang, G. Microbes as structural templates in biofabrication: Study of surface chemistry and applications. ACS Sustain. Chem. Eng. 2017, 5, 11163–11175. [Google Scholar] [CrossRef]
- Behl, K.; Jaiswal, P.; Pabbi, S. Recent advances in microbial and nano-formulations for effective delivery and agriculture sustainability. Biocatal. Agric. Biotechnol. 2024, 58, 103180. [Google Scholar] [CrossRef]
- Garg, D.; Sridhar, K.; Inbaraj, B.S.; Chawla, P.; Tripathi, M.; Sharma, M. Nano-biofertilizer formulations for agriculture: A systematic review on recent advances and prospective applications. Bioengineering 2023, 10, 1010. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; García-Simarro, M.P.; Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. A review on the encapsulation of “eco-friendly” compounds in natural polymer-based nanoparticles as next generation nano-agrochemicals for sustainable agriculture and crop management. Int. J. Biol. Macromol. 2024, 280, 136030. [Google Scholar] [CrossRef]
- Fu, X.; Sun, J.; Liang, R.; Guo, H.; Wang, L.; Sun, X. Application progress of microfluidics-integrated biosensing platforms in the detection of foodborne pathogens. Trend. Food. Sci & Technol. 2021, 116, 115–129. [Google Scholar]
- Gates, B.D.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.G.; Whitesides, G.M. New approaches to nanofabrication: Molding, printing, and other techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar]
- Hussain, M.; Shakoor, N.; Adeel, M.; Ahmad, M.A.; Zhou, H.; Zhang, Z.; Xu, M.; Rui, Y.; White, J.C. Nano-enabled plant microbiome engineering for disease resistance. Nano Today 2023, 48, 101752. [Google Scholar] [CrossRef]
- Del Campo, A.; Arzt, E. Fabrication approaches for generating complex micro-and nanopatterns on polymeric surfaces. Chem. Rev. 2008, 108, 911–945. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Fan, Y.; Jiao, F.; Geng, D.; Hu, W. From top to down—Recent advances in etching of 2D materials. Adv. Mater. Interfaces 2022, 9, 2201334. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro-and nanoscale patterning. Nat. Protoc. 2010, 5, 491. [Google Scholar] [CrossRef]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Ranjbaran, M.; Verma, M.S. Microfluidics at the interface of bacteria and fresh produce. Trends Food Sci. Technol. 2022, 128, 102–117. [Google Scholar] [CrossRef]
- Kumari, P.; Sayas, T.; Kleiman, M. Biomimetic Replication of Root Surface Microstructure using Alteration of Soft Lithography. J. Vis. Exp. 2020, 162, e61437. [Google Scholar]
- Barcelo, S.; Li, Z. Nanoimprint lithography for nanodevice fabrication. Nano Converg. 2016, 3, 21. [Google Scholar] [CrossRef]
- Yu, C.-C.; Chen, H.-L. Nanoimprint technology for patterning functional materials and its applications. Microelectron. Eng. 2015, 132, 98–119. [Google Scholar] [CrossRef]
- Zaher, O.; Mhada, M.; El Graoui, M.; Zvinavashe, A.T.; Kouisni, L.; Marelli, B. Plant microbiome modulation through seed coating: A novel approach for a smart and efficient microbial delivery. In Microbial Cross-Talk in the Rhizosphere; Springer: Berlin/Heidelberg, Germany, 2022; pp. 213–234. [Google Scholar]
- Jia, M.; Zhang, Z.; Li, J.; Ma, X.; Chen, L.; Yang, X. Molecular imprinting technology for microorganism analysis. TrAC Trends Anal. Chem. 2018, 106, 190–201. [Google Scholar] [CrossRef]
- Bezelya, A.; Küçüktürkmen, B.; Bozkır, A. Microfluidic devices for precision nanoparticle production. Micro 2023, 3, 822–866. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Yan, H.; Liu, C.; Zhu, X.; Chen, B. Microfluidics as an emerging platform for exploring soil environmental processes: A critical review. Environ. Sci. Technol. 2022, 56, 711–731. [Google Scholar] [CrossRef]
- Kane, R.S.; Stroock, A.D.; Jeon, N.L.; Ingber, D.E.; Whitesides, G.M. Soft lithography and microfluidics. In Optical Biosensors; Elsevier: Amsterdam, The Netherlands, 2002; pp. 571–595. [Google Scholar]
- Mele, E.; Pisignano, D. Nanobiotechnology: Soft lithography. In Biosilica in Evolution, Morphogenesis, and Nanobiotechnology: Case Study Lake Baikal; Springer: Berlin/Heidelberg, Germany, 2009; Volume 47, pp. 341–358. [Google Scholar]
- Guo, L.J. Nanoimprint lithography: Methods and material requirements. Adv. Mater. 2007, 19, 495–513. [Google Scholar] [CrossRef]
- Yang, B.; Yu, M.; Yu, H. Azopolymer-based nanoimprint lithography: Recent developments in methodology and applications. ChemPlusChem 2020, 85, 2166–2176. [Google Scholar] [CrossRef]
- Shankles, P.G.; Timm, A.C.; Doktycz, M.J.; Retterer, S.T. Fabrication of nanoporous membranes for tuning microbial interactions and biochemical reactions. J. Vac. Sci. Technol. B 2015, 33, 06FM03. [Google Scholar] [CrossRef] [PubMed]
- Mogi, K.; Sugii, Y.; Yamamoto, T.; Fujii, T. Rapid fabrication technique of nano/microfluidic device with high mechanical stability utilizing two-step soft lithography. Sens. Actuators B Chem. 2014, 201, 407–412. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2018, 25, 10164–10183. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, P.; Zhang, P.; Adeel, M.; Shakoor, N.; Li, Y.; Li, M.; Guo, M.; Zhao, W.; Lou, B. Green synthesis of metal-based nanoparticles for sustainable agriculture. Environ. Pollut. 2022, 309, 119755. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-Álvarez, J.; Vega-Fernández, L.; Montes de Oca-Vásquez, G.; Vega-Baudrit, J.R. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Hachem, K.; Ansari, M.J.; Saleh, R.O.; Kzar, H.H.; Al-Gazally, M.E.; Altimari, U.S.; Hussein, S.A.; Mohammed, H.T.; Hammid, A.T.; Kianfar, E. Methods of chemical synthesis in the synthesis of nanomaterial and nanoparticles by the chemical deposition method: A review. BioNanoScience 2022, 12, 1032–1057. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Beig, B.; Niazi, M.B.K.; Sher, F.; Jahan, Z.; Malik, U.S.; Khan, M.D.; Américo-Pinheiro, J.H.P.; Vo, D.-V.N. Nanotechnology-based controlled release of sustainable fertilizers. A review. Environ. Chem. Lett. 2022, 20, 2709–2726. [Google Scholar] [CrossRef]
- Kirubakaran, D.; Wahid, J.B.A.; Karmegam, N.; Jeevika, R.; Sellapillai, L.; Rajkumar, M.; SenthilKumar, K. A comprehensive review on the green synthesis of nanoparticles: Advancements in biomedical and environmental applications. Biomed. Mater. Devices 2025, 1–26. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Z.; Dhankher, O.P.; Xing, B. Nanotechnology as a new sustainable approach for controlling crop diseases and increasing agricultural production. J. Exp. Bot. 2020, 71, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Al-Abduljabbar, A.; Farooq, I. Electrospun polymer nanofibers: Processing, properties, and applications. Polymers 2022, 15, 65. [Google Scholar] [CrossRef]
- Reneker, D.; Yarin, A.; Zussman, E.; Xu, H. Electrospinning of nanofibers from polymer solutions and melts. Adv. Appl. Mech. 2007, 41, 43–346. [Google Scholar]
- Meraz-Dávila, S.; Pérez-García, C.; Feregrino-Perez, A.A. Challenges and advantages of electrospun nanofibers in agriculture: A review. Mater. Res. Express 2021, 8, 042001. [Google Scholar] [CrossRef]
- Colín-Orozco, J.; Colín-Orozco, E.; Valdivia-Barrientos, R. Production of Nanofibers by Electrospinning as Carriers of Agrochemical. Fibers 2024, 12, 64. [Google Scholar] [CrossRef]
- Venkatesan, M.; Veeramuthu, L.; Liang, F.-C.; Chen, W.-C.; Cho, C.-J.; Chen, C.-W.; Chen, J.-Y.; Yan, Y.; Chang, S.-H.; Kuo, C.-C. Evolution of electrospun nanofibers fluorescent and colorimetric sensors for environmental toxicants, pH, temperature, and cancer cells—A review with insights on applications. Chem. Eng. J. 2020, 397, 125431. [Google Scholar] [CrossRef]
- Aarif KO, M.; Alam, A.; Hotak, Y. Smart Sensor Technologies Shaping the Future of Precision Agriculture: Recent Advances and Future Outlooks. J. Sens. 2025, 2025, 2460098. [Google Scholar] [CrossRef]
- Mohammad, Z.H.; Ahmad, F. Nanocoating and its application as antimicrobials in the food industry: A review. Int. J. Biol. Macromol. 2024, 254, 127906. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, H. An insight on developing nanoformulations suitable for delivering plant beneficial microorganisms to crops under abiotic stresses. In Mitigation of Plant Abiotic Stress by Microorganisms; Elsevier: Amsterdam, The Netherlands, 2022; pp. 273–297. [Google Scholar]
- Dutta, P.; Das, G.; Boruah, S.; Kumari, A.; Mahanta, M.; Yasin, A.; Sharma, A.; Deb, L. Nanoparticles as nano-priming agent for antifungal and antibacterial activity against plant pathogens. Biol. Forum Int. J. 2021, 13, 476–482. [Google Scholar]
- Thirugnanasambandan, T. Advances of engineered nanofertilizers for modern agriculture. In Plant-Microbes-Engineered Nano-Particles (PM-ENPs) Nexus in Agro-Ecosystems: Understanding the Interaction of Plant, Microbes and Engineered Nano-Particles (ENPS); Springer: Cham, Switzerland, 2021; pp. 131–152. [Google Scholar]
- Farias, B.V.; Pirzada, T.; Mathew, R.; Sit, T.L.; Opperman, C.; Khan, S.A. Electrospun polymer nanofibers as seed coatings for crop protection. ACS Sustain. Chem. Eng. 2019, 7, 19848–19856. [Google Scholar] [CrossRef]
- Xu, T.; Ma, C.; Aytac, Z.; Hu, X.; Ng, K.W.; White, J.C.; Demokritou, P. Enhancing agrichemical delivery and seedling development with biodegradable, tunable, biopolymer-based nanofiber seed coatings. ACS Sustain. Chem. Eng. 2020, 8, 9537–9548. [Google Scholar] [CrossRef]
- Chakkalakkal, N.D.; Thomas, M.; Chittillapilly, P.S.; Sujith, A.; Anjali, P. Electrospun polymer nanocomposite membrane as a promising seed coat for controlled release of agrichemicals and improved germination: Towards a better agricultural prospect. J. Clean. Prod. 2022, 377, 134479. [Google Scholar] [CrossRef]
- Meral, R.; Ceylan, Z.; Kutlu, N.; Kılıçer, A.; Çağlar, A.; Tomar, O. Antimicrobial nanocoating for food industry. In Handbook of Microbial Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 255–283. [Google Scholar]
- Thirumurugan, N.K.; Velu, G.; Murugaiyan, S.; Maduraimuthu, D.; Ponnuraj, S.; Subramanian, K. Nano-biofertilizers: Utilizing nanopolymers as coating matrix-a comprehensive review. Biofabrication 2024, 17, 012007. [Google Scholar] [CrossRef]
- Agrawal, S.; Kumar, V.; Kumar, S.; Shahi, S.K. Plant development and crop protection using phytonanotechnology: A new window for sustainable agriculture. Chemosphere 2022, 299, 134465. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Polo, J.; Monasterio, A.; Cantero-López, P.; Osorio, F.A. Combining edible coatings technology and nanoencapsulation for food application: A brief review with an emphasis on nanoliposomes. Food Res. Int. 2021, 145, 110402. [Google Scholar] [CrossRef]
- Jafari, S.M.; McClements, D.J. Nanotechnology approaches for increasing nutrient bioavailability. Adv. Food Nutr. Res. 2017, 81, 1–30. [Google Scholar] [PubMed]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial inoculants for soil quality and plant health. Sustain. Agric. Rev. 2017, 22, 281–307. [Google Scholar]
- Kour, H.; Khan, S.S.; Kour, D.; Singh, S.; Kumari, S.; Kaur, M.; Khan, R.T.; Yadav, A.N. Nanotechnologies for microbial inoculants as biofertilizers in the horticulture. In Sustainable Horticulture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–261. [Google Scholar]
- Elnahal, A.S.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Pereira, J.F.; Oliveira, A.L.M.; Sartori, D.; Yamashita, F.; Mali, S. Perspectives on the use of biopolymeric matrices as carriers for plant-growth promoting bacteria in agricultural systems. Microorganisms 2023, 11, 467. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Ahmad, R.; Abd-Elsalam, K.A. Emerging frontiers in nanotechnology for precision agriculture: Advancements, hurdles and prospects. Agrochemicals 2023, 2, 220–256. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Chitara, M.K.; Mishra, D.; Jha, M.N.; Jaiswal, A.; Kumari, G.; Ghosh, S.; Patel, V.K.; Naitam, M.G.; Singh, A.K.; et al. Synergistic impact of nanomaterials and plant probiotics in agriculture: A tale of two-way strategy for long-term sustainability. Front. Microbiol. 2023, 14, 1133968. [Google Scholar] [CrossRef]
- Yassin, Y.; Aseel, D.; Khalil, A.; Abdel-Megeed, A.; Al-Askar, A.; Elbeaino, T.; Moawad, H.; Behiry, S.; Abdelkhalek, A. Foliar application of Rhizobium leguminosarum bv. viciae strain 33504-Borg201 promotes faba bean growth and enhances systemic resistance against bean yellow mosaic virus infection. Curr. Microbiol. 2024, 81, 220. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Ye, B.-C.; Wang, J.; Guan, X.; Zhang, J. Viability evaluation of alginate-encapsulated Pseudomonas putida Rs-198 under simulated salt-stress conditions and its effect on cotton growth. Eur. J. Soil Biol. 2016, 75, 135–141. [Google Scholar] [CrossRef]
- Jin, W.; Li, L.; He, W.; Wei, Z. Application of Silica Nanoparticles Improved the Growth, Yield, and Grain Quality of Two Salt-Tolerant Rice Varieties under Saline Irrigation. Plants 2024, 13, 2452. [Google Scholar] [CrossRef] [PubMed]
- Kráľová, J.; Jampílek, J. Nanofertilizers: Recent approach in crop production. In Nanotechnology for Agriculture: Crop Production & Protection; Springer: Singapore, 2023; pp. 93–144. [Google Scholar]
- Deshmukh, S.K.; Kochar, M.; Kaur, P.; Singh, P.P. Nanotechnology in Agriculture and Environmental Science; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Zhang, W.; Xia, K.; Feng, Z.; Qin, Y.; Zhou, Y.; Feng, G.; Zhu, H.; Yao, Q. Tomato plant growth promotion and drought tolerance conferred by three arbuscular mycorrhizal fungi is mediated by lipid metabolism. Plant Physiol. Biochem. 2024, 208, 108478. [Google Scholar] [CrossRef]
- Hossain, M.E.; Shahrukh, S.; Hossain, S.A. Chemical fertilizers and pesticides: Impacts on soil degradation, groundwater, and human health in Bangladesh. In Environmental Degradation: Challenges and Strategies for Mitigation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 63–92. [Google Scholar]
- Gallucci, N.; De Cristofaro, I.; Krauss, I.R.; D’Errico, G.; Paduano, L. Eco-sustainable delivery strategies to drive agriculture forwards. Curr. Opin. Colloid Interface Sci. 2025, 77, 101917. [Google Scholar] [CrossRef]
- Betancourt, T.; Doiron, A.; Homan, K.A.; Brannon-Peppas, L. Controlled release and nanotechnology. In Nanotechnology in Drug Delivery; Springer: New York, NY, USA, 2009; pp. 283–312. [Google Scholar]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Nanofertilizers: Types, delivery and advantages in agricultural sustainability. Agrochemicals 2023, 2, 296–336. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Ravichandran, M.; Samiappan, S.C.; Rangaraj, S.; Murugan, K.; Al-Dhabi, N.A.; Karuppiah, P. Nanoemulsion formulations with plant growth promoting rhizobacteria (PGPR) for sustainable agriculture. In Bio-Based Nanoemulsions for Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 207–223. [Google Scholar]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O. Can nanotechnology deliver the promised benefits without negatively impacting soil microbial life? J. Basic Microbiol. 2014, 54, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A promising source of plant growth-promoting molecules and their non-legume interactions: Examining applications and mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Khaliq, A.; Perveen, S.; Alamer, K.H.; Haq, M.Z.U.; Rafique, Z.; Alsudays, I.M.; Althobaiti, A.T.; Saleh, M.A.; Hussain, S.; Attia, H. Arbuscular mycorrhizal fungi symbiosis to enhance plant–soil interaction. Sustainability 2022, 14, 7840. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, Z.; Wang, X.; Li, L.; Cai, K.; Han, H. Novel impacts of functionalized multi-walled carbon nanotubes in plants: Promotion of nodulation and nitrogenase activity in the rhizobium-legume system. Nanoscale 2017, 9, 9921–9937. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Pandey, S.; Pudake, R.N.; Tyagi, N.; Mishra, A.; Tyagi, J. Revolutions in Biotic Stress Management and Sustainable Agriculture Through Microbial-Mediated Nanoformulation. In Nano-Microbiology for Sustainable Development; Mohanta, Y.K., Mishra, B., Pudake, R.N., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 213–241. [Google Scholar]
- Ahmed, T.; Luo, J.; Noman, M.; Ijaz, M.; Wang, X.; Masood, H.A.; Manzoor, N.; Wang, Y.; Li, B. Microbe-mediated nanoparticle intervention for the management of plant diseases. Crop Health 2023, 1, 3. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Requena, N.; Jimenez, I.; Toro, M.; Barea, J.M. Interactions between plant-growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytisoides, a model legume for revegetation in mediterranean semi-arid ecosystems. New Phytol. 1997, 136, 667–677. [Google Scholar] [CrossRef]
- Guardiola-Márquez, C.E.; López-Mena, E.R.; Segura-Jiménez, M.E.; Gutierrez-Marmolejo, I.; Flores-Matzumiya, M.A.; Mora-Godínez, S.; Hernández-Brenes, C.; Jacobo-Velázquez, D.A. Development and Evaluation of Zinc and Iron Nanoparticles Functionalized with Plant Growth-Promoting Rhizobacteria (PGPR) and Microalgae for Their Application as Bio-Nanofertilizers. Plants 2023, 12, 3657. [Google Scholar] [CrossRef]
- Panichikkal, J.; Prathap, G.; Nair, R.A.; Krishnankutty, R.E. Evaluation of plant probiotic performance of Pseudomonas sp. encapsulated in alginate supplemented with salicylic acid and zinc oxide nanoparticles. Int. J. Biol. Macromol. 2021, 166, 138–143. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Yin, M.; Shen, J.; Yan, S. Recent Advances in Nanoparticle-Mediated Co-Delivery System: A Promising Strategy in Medical and Agricultural Field. Int. J. Mol. Sci. 2023, 24, 5121. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Kah, M.; Kariman, K. Are nanoparticles a threat to mycorrhizal and rhizobial symbioses? A critical review. Front. Microbiol. 2019, 10, 1660. [Google Scholar] [CrossRef] [PubMed]
- Campaña, J.M.; Arias, M. Nanofibers as a delivery system for arbuscular mycorrhizal fungi. ACS Appl. Polym. Mater. 2020, 2, 5033–5038. [Google Scholar] [CrossRef]
- Meel, S.; Saharan, B.S. Enhancing crop resilience towards drought: By integrating nanotechnology, microbiomes, and growth-promoting rhizobacteria. Discov. Agric. 2024, 2, 112. [Google Scholar] [CrossRef]
- Khan, A. Promises and potential of in situ nano-phytoremediation strategy to mycorrhizo-remediate heavy metal contaminated soils using non-food bioenergy crops (Vetiver zizinoides & Cannabis sativa). Int. J. Phytoremediation 2020, 22, 900–915. [Google Scholar]
- Merinero, M.; Alcudia, A.; Begines, B.; Martínez, G.; Martín-Valero, M.J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Navarro-Torre, S.; Torres, Y. Assessing the biofortification of wheat plants by combining a plant growth-promoting rhizobacterium (PGPR) and polymeric Fe-nanoparticles: Allies or enemies? Agronomy 2022, 12, 228. [Google Scholar] [CrossRef]
- Khoshrou, B.; Fallah Nosratabad, A.; Khosravi, H.; Asgharzadeh, A.; Faridian, L. Enhancing agricultural productivity using PGPR and nanoparticles: Mechanisms, challenges, and future directions. J. Sol Biol. 2025, 12, 279–313. [Google Scholar]
- Khan, I.; Sultan, G.; Miskeen, S.; Madar, I.H.; Najeeb, S.; Sivanandan, P.K.; Chelliah, R.; Oh, D.H. Biobased nanomaterials and their interaction with plant growth-promoting rhizobacteria/blue-green algae/Rhizobium for sustainable plant growth and development. In Biostimulants in Plant Protection and Performance; Elsevier: Amsterdam, The Netherlands, 2024; pp. 33–60. [Google Scholar]
- Bhatia, R.; Gulati, D.; Sethi, G. Biofilms and nanoparticles: Applications in agriculture. Folia Microbiol. 2021, 66, 159–170. [Google Scholar] [CrossRef]
- McNear, D., Jr. The Rhizosphere—Roots, Soil and Everything In Between. Nat. Educ. Knowl. 2013, 4, 1. [Google Scholar]
- Bonebrake, M. Characterization of Biofilms in a Synthetic Rhizosphere Using Hollow Fiber Root-Mimetic Systems. Master’s Thesis, Utah State University, Logan, UT, USA, 2019. [Google Scholar]
- Mebert, A.M.; Villanueva, M.E.; Catalano, P.N.; Copello, G.J.; Bellino, M.G.; Alvarez, G.S.; Desimone, M.F. Surface chemistry of nanobiomaterials with antimicrobial activity. In Surface Chemistry of Nanobiomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 135–162. [Google Scholar]
- Harun-Ur-Rashid, M.; Jahan, I.; Foyez, T.; Imran, A.B. Bio-inspired nanomaterials for micro/nanodevices: A new era in biomedical applications. Micromachines 2023, 14, 1786. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface functionalization–The way for advanced applications of smart materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.d.C.; Glick, B.R. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef]
- Cartwright, A. Surface-Functionalized Silica Nanocarriers for Mitigating Water Stress in Wheat and Benefiting the Root Microbiome. Master’s Thesis, Utah State University, Logan, UT, USA, 2023. [Google Scholar]
- Mahmud, K.; Missaoui, A.; Lee, K.; Ghimire, B.; Presley, H.; Makaju, S. Rhizosphere microbiome manipulation for sustainable crop production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Shelar, A.; Singh, A.V.; Chaure, N.; Jagtap, P.; Chaudhari, P.; Shinde, M.; Chaskar, M.; Patil, R.; Nile, S.H. Nanoprimers in sustainable seed treatment: Molecular insights into abiotic-biotic stress tolerance mechanisms for enhancing germination and improved crop productivity. Sci. Total Environ. 2024, 951, 175118. [Google Scholar] [CrossRef]
- Sohail, M.; Pirzada, T.; Opperman, C.H.; Khan, S.A. Recent advances in seed coating technologies: Transitioning toward sustainable agriculture. Green Chem. 2022, 24, 6052–6085. [Google Scholar] [CrossRef]
- Govil, S.; Long, N.V.D.; Escribà-Gelonch, M.; Hessel, V. Controlled-release fertiliser: Recent developments and perspectives. Ind. Crops Prod. 2024, 219, 119160. [Google Scholar] [CrossRef]
- Vijaykumar, S. Biopolymer-Based Multilayer Seed Coatings with Trichoderma, Rhizobium, or Bacillus and Compatible Fungicides Against Seed and Soil-Borne Diseases in Sesamum, Groundnut, and Soybean. Ph.D. Thesis, Professor Jayashankar Telangana State Agricultural University, Hyderabad, India, 2023. [Google Scholar]
- Bashan, Y.; de-Bashan, L.E. Chapter Two—How the Plant Growth-Promoting Bacterium Azospirillum Promotes Plant Growth—A Critical Assessment. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 108, pp. 77–136. [Google Scholar]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.K.; Singh, V.K.; Kumar, V. Nanotechnology-Based Sustainable Agriculture; John Wiley & Sons: Hoboken, NJ, USA, 2025. [Google Scholar]
- Teama, S.; Rabie, G.; Elhai, K.A.; El-Gazzar, N. Protection faba bean crop against root rot disease using chitosan nanoparticles and mycorrhizal fungi (A Review). Bull. Fac. Sci. Zagazig Univ. 2024, 2024, 91–101. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Mills, A.L. Keeping in touch: Microbial life on soil particle surfaces. Adv. Agron. 2003, 78, 2–45. [Google Scholar]
- Yan, X.; Chio, C.; Li, H.; Zhu, Y.; Chen, X.; Qin, W. Colonization characteristics and surface effects of microplastic biofilms: Implications for environmental behavior of typical pollutants. Sci. Total. Environ. 2024, 937, 173141. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, Z.; Fane, A.; Li, J.; Tang, C.; Zheng, C.; Lin, J.; Kong, L. Engineering antifouling reverse osmosis membranes: A review. Desalination 2021, 499, 114857. [Google Scholar] [CrossRef]
- Bushby, H. The role of bacterial surface charge in the ecology of root-nodule bacteria: An hypothesis. Soil Biol. Biochem. 1990, 22, 1–9. [Google Scholar] [CrossRef]
- Pereira, M.; Alves, M.; Azeredo, J.; Mota, M.; Oliveira, R. Influence of physico-chemical properties of porous microcarriers on the adhesion of an anaerobic consortium. J. Ind. Microbiol. Biotechnol. 2000, 24, 181–186. [Google Scholar] [CrossRef]
- Tiller, J.C. Antimicrobial surfaces. Bioact. Surf. 2011, 240, 193–217. [Google Scholar][Green Version]
- Montanaro, L.; Arciola, C.R. Studying bacterial adhesion to irregular or porous surfaces. In Handbook of Bacterial Adhesion: Principles, Methods, and Applications; Springer: Berlin/Heidelberg, Germany, 2000; pp. 331–343. [Google Scholar]
- Nath, A.; Molnár, M.A.; Albert, K.; Das, A.; Bánvölgyi, S.; Márki, E.; Vatai, G. Agrochemicals from nanomaterials—Synthesis, mechanisms of biochemical activities and applications. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 84, pp. 263–312. [Google Scholar][Green Version]
- Gunathilake, T.U.; Ching, Y.C.; Ching, K.Y.; Chuah, C.H.; Abdullah, L.C. Biomedical and microbiological applications of bio-based porous materials: A review. Polymers 2017, 9, 160. [Google Scholar] [CrossRef]
- Emrie, D.B. Sol–Gel Synthesis of Nanostructured Mesoporous Silica Powder and Thin Films. J. Nanomater. 2024, 2024, 6109770. [Google Scholar] [CrossRef]
- Galabova, B.B. Mesoporous silica nanoparticles: Synthesis, functionalization, drug loading and release—A review. Trop. J. Pharm. Res. 2022, 20, 1091–1100. [Google Scholar] [CrossRef]
- Perea, G.N.R.; Pavinatto, A.; Schneider, R.; Munk, M.; Brandão, H.M.; Correa, D.S. Electrospun nanofibers based on polyvinylpyrrolidone/chitosan and cloxacillin: Investigation of morphological features, antibiotic release and antimicrobial properties. J. Polym. Res. 2023, 30, 166. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Lu, W.; Guo, Y. Antibacterial Porous Coaxial Drug-Carrying Nanofibers for Sustained Drug-Releasing Applications. Nanomaterials 2021, 11, 1316. [Google Scholar] [CrossRef] [PubMed]
- Pinna, D. Microbial growth and its effects on inorganic heritage materials. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Springer: Cham, Switzerland, 2021; pp. 3–35. [Google Scholar]
- Easwaran, C.; Christopher, S.R.; Moorthy, G.; Mohan, P.; Marimuthu, R.; Koothan, V.; Nallusamy, S. Nano hybrid fertilizers: A review on the state of the art in sustainable agriculture. Sci. Total Environ. 2024, 929, 172533. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, S.; Qi, J.; Gong, Y.; Li, K. Pseudomonas fluorescens BsEB-1: An endophytic bacterium isolated from the root of Bletilla striata that can promote its growth. Plant Signal. Behav. 2022, 17, 2100626. [Google Scholar] [CrossRef]
- Lv, X.; Liu, S.; Cao, Y.; Wu, H.; Zhang, C.; Huang, B.; Wang, J. Multiwalled Carbon Nanotubes Promoted Biofilm Formation and Rhizosphere Colonization of Bacillus subtilis Tpb55. J. Agric. Food Chem. 2025, 73, 7087–7098. [Google Scholar] [CrossRef]
- Guastaldi, F.P.S.; Foggi, C.C.d.; Santana, L.C.L.; Vaz, L.G.; Vergani, C.E.; Guastaldi, A.C. Lower Susceptibility of Laser-irradiated Ti-15Mo Surface to Methicillin-resistant Staphylococcus aureus Cells Adhesion. Mater. Res. 2019, 22, e20190012. [Google Scholar] [CrossRef]
- Chebolu, A.; Laha, B.; Ghosh, M.; Nagahanumaiah. Investigation on bacterial adhesion and colonisation resistance over laser-machined micro patterned surfaces. Micro Nano Lett. 2013, 8, 280–283. [Google Scholar] [CrossRef]
- Aceti, D.M.; Daskalova, A.; Angelova, L.; Filipov, E.; Sotelo, L.; Andreeva, A.; Trifonov, A.; Buchvarov, I. Optimization of titanium and titanium alloy surface properties by ultra-short laser processing for improved antibacterial characteristics. J. Phys. Conf. Ser. 2022, 2240, 012040. [Google Scholar] [CrossRef]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 2014, 14, 234. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Ban, T.; Yamamoto, S. Adhesion of Microbial Cells to Porous Hydrophilic and Hydrophobic Solid Substrata. In Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 1993; Volume 39, pp. 159–169. [Google Scholar]
- Aung, K.; Jiang, Y.; He, S.Y. The role of water in plant–microbe interactions. Plant J. 2018, 93, 771–780. [Google Scholar] [CrossRef]
- Xia, T.; Lin, Y.; Li, S.; Yan, N.; Xie, Y.; He, M.; Guo, X.; Zhu, L. Co-transport of negatively charged nanoparticles in saturated porous media: Impacts of hydrophobicity and surface O-functional groups. J. Hazard. Mater. 2021, 409, 124477. [Google Scholar] [CrossRef]
- Feng, Z.-Y.; Liu, K.-K.; Jin, B.; Jiang, S.; Meng, L.-Y. N/S-Dual doped MnO modified spore-based ellipsoidal porous carbons for supercapacitors. Ceram. Int. 2021, 47, 29941–29948. [Google Scholar] [CrossRef]
- Krekeler, C.; Ziehr, H.; Klein, J. Influence of physicochemical bacterial surface properties on adsorption to inorganic porous supports. Appl. Microbiol. Biotechnol. 1991, 35, 484–490. [Google Scholar] [CrossRef]
- Pudake, R.N.; Jain, U.; Kole, C. Biosensors in Agriculture: Recent Trends and Future Perspectives; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Neethirajan, S.; Kobayashi, I.; Nakajima, M.; Wu, D.; Nandagopal, S.; Lin, F. Microfluidics for food, agriculture and biosystems industries. Lab A Chip 2011, 11, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, I.; Fulk, E.M.; Kalvapalle, P.; Silberg, J.J.; Masiello, C.A.; Stadler, L.B. Translating new synthetic biology advances for biosensing into the earth and environmental sciences. Front. Microbiol. 2021, 11, 618373. [Google Scholar] [CrossRef] [PubMed]
- Lobete, M.M.; Fernandez, E.N.; Van Impe, J.F. Recent trends in non-invasive in situ techniques to monitor bacterial colonies in solid (model) food. Front. Microbiol. 2015, 6, 148. [Google Scholar] [CrossRef]
- Neelam, A.; Tabassum, S. Optical sensing technologies to elucidate the interplay between plant and microbes. Micromachines 2023, 14, 195. [Google Scholar] [CrossRef]
- Ehosioke, S.; Nguyen, F.; Rao, S.; Kremer, T.; Placencia-Gomez, E.; Huisman, J.A.; Kemna, A.; Javaux, M.; Garré, S. Sensing the electrical properties of roots: A review. Vadose Zone J. 2020, 19, e20082. [Google Scholar] [CrossRef]
- Walton, C.L.; Khalid, M.; Bible, A.N.; Kertesz, V.; Retterer, S.T.; Morrell-Falvey, J.; Cahill, J.F. In situ detection of amino acids from bacterial biofilms and plant root exudates by liquid microjunction surface-sampling probe mass spectrometry. J. Am. Soc. Mass Spectrom. 2022, 33, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Doornbos, R.F.; van Loon, L.C.; Bakker, P.A. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Huang, X.-F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Yang, L.; Qian, X.; Zhao, Z.; Wang, Y.; Ding, G.; Xing, X. Mechanisms of rhizosphere plant-microbe interactions: Molecular insights into microbial colonization. Front. Plant Sci. 2024, 15, 1491495. [Google Scholar] [CrossRef]
- Kaiser, C.-F.; Perilli, A.; Grossmann, G.; Meroz, Y. Studying root–environment interactions in structured microdevices. J. Exp. Bot. 2023, 74, 3851–3863. [Google Scholar] [CrossRef]
- Patko, D.; Gunatilake, U.B.; Gonzalez-Gaya, B.; Dupuy, L.X.; Basabe-Desmonts, L.; Benito-Lopez, F. Spatial and temporal detection of root exudates with a paper-based microfluidic device. Soil Biol. Biochem. 2024, 195, 109456. [Google Scholar] [CrossRef]
- Xie, A.; Zhou, Q.; Fu, L.; Zhan, L.; Wu, W. From Lab to Field: Advancements and Applications of On-the-Go Soil Sensors for Real-Time Monitoring. Eurasian Soil Sci. 2024, 57, 1730–1745. [Google Scholar]
- Bharti, A.; Jain, U.; Chauhan, N. From lab to field: Nano-biosensors for real-time plant nutrient tracking. Plant Nano Biol. 2024, 9, 100079. [Google Scholar] [CrossRef]
- Shaw, D.S.; Honeychurch, K.C. Nanosensor applications in plant science. Biosensors 2022, 12, 675. [Google Scholar] [CrossRef]
- Jones, D.L.; Darrah, P.R. Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 1994, 166, 247–257. [Google Scholar] [CrossRef]
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Nanostructured materials-based nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- Husson, O.; Sarthou, J.-P.; Bousset, L.; Ratnadass, A.; Schmidt, H.-P.; Kempf, J.; Husson, B.; Tingry, S.; Aubertot, J.-N.; Deguine, J.-P. Soil and plant health in relation to dynamic sustainment of Eh and pH homeostasis: A review. Plant Soil 2021, 466, 391–447. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.; Kumar, R. Sensing methodologies in agriculture for monitoring biotic stress in plants due to pathogens and pests. Inventions 2021, 6, 29. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Zhang, D.; Qi, S.; Liu, Y. Metabolite interactions between host and microbiota during health and disease: Which feeds the other? Biomed. Pharmacother. 2023, 160, 114295. [Google Scholar] [CrossRef]
- Dimri, A.; Pathak, N.; Sharma, S. Nanosensors for root zone parameters influencing plant growth. In Nanomaterials for Agriculture and Forestry Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 387–406. [Google Scholar]
- Pramanik, P.; Krishnan, P.; Maity, A.; Mridha, N.; Mukherjee, A.; Rai, V. Application of nanotechnology in agriculture. Environ. Nanotechnol. 2020, 4, 317–348. [Google Scholar]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Ullah, S.; Abdi, G.; Shah, G.M.; Zaman, W.; Ayaz, A. Agriculture and environmental management through nanotechnology: Eco-friendly nanomaterial synthesis for soil-plant systems, food safety, and sustainability. Sci. Total Environ. 2024, 926, 171862. [Google Scholar] [CrossRef]
- Bellanthudawa, B.; Nawalage, N.; Handapangoda, H.; Suvendran, S.; Wijayasenarathne, K.; Rathnasuriya, M.; Wickramasinghe, P.; Aberathna, A.; Tennakoon, A.; Perera, I. A perspective on biodegradable and non-biodegradable nanoparticles in industrial sectors: Applications, challenges, and future prospects. Nanotechnol. Environ. Eng. 2023, 8, 975–1013. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Verma, K.; Saha, S.; Sarkar, C. Exploration of Biodegradable Polymeric Particles in Agriculture: A Holistic Approach for Sustainable Farming. Environ. Sci. Adv. 2025, 4, 409–431. [Google Scholar] [CrossRef]
- Karnwal, A.; Dohroo, A.; Malik, T. Unveiling the potential of bioinoculants and nanoparticles in sustainable agriculture for enhanced plant growth and food security. BioMed Res. Int. 2023, 2023, 6911851. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.; Ali, S.; Jan, R.; Kim, K.-M. Lignin Nanoparticles: Transforming Environmental Remediation. Nanomaterials 2024, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z.; Khan, M.; Khan, M. Engineered nanomaterials in soil: Their impact on soil microbiome and plant health. Plants 2021, 11, 109. [Google Scholar] [CrossRef]
- Xu, Z.; Long, X.; Jia, Y.; Zhao, D.; Pan, X. Occurrence, transport, and toxicity of nanomaterials in soil ecosystems: A review. Environ. Chem. Lett. 2022, 20, 3943–3969. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Khan, M.; Khan, M.A.; Ali, S. Ameliorative symbiosis of Serratia fonticola (S1T1) under salt stress condition enhance growth-promoting attributes of Cucumis sativus L. Symbiosis 2023, 89, 283–297. Symbiosis 2023, 89, 283–297. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.; Moon, Y.-S. Synergistic Effect of Serratia fonticola and Pseudomonas koreensis on Mitigating Salt Stress in Cucumis sativus L. Curr. Issues Mol. Biol. 2025, 47, 194. [Google Scholar] [CrossRef]
- McKee, M.S.; Filser, J. Impacts of metal-based engineered nanomaterials on soil communities. Environ. Sci. Nano 2016, 3, 506–533. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Handa, N.; Kaur, H.; Ohri, P.; Bhardwaj, R.; Yousaf, B.; Rinklebe, J.; Ahmad, P. Enthralling the impact of engineered nanoparticles on soil microbiome: A concentric approach towards environmental risks and cogitation. Ecotoxicol. Environ. Saf. 2021, 222, 112459. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, A.; Verma, S.K.; Pandey, H.; Pandey, M. Impact of nanoparticles on plant physiology, nutrition, and toxicity: A short review. Next Nanotechnol. 2024, 6, 100081. [Google Scholar] [CrossRef]
- Lewis, R.W.; Bertsch, P.M.; McNear, D.H. Nanotoxicity of engineered nanomaterials (ENMs) to environmentally relevant beneficial soil bacteria—A critical review. Nanotoxicology 2019, 13, 392–428. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Mauter, M.S.; Zucker, I.; Perreault, F.; Werber, J.R.; Kim, J.-H.; Elimelech, M. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 2018, 1, 166–175. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Z.; Ullah, S.; Melagraki, G.; Afantitis, A.; Lynch, I. Nanotechnology and artificial intelligence to enable sustainable and precision agriculture. Nat. Plants 2021, 7, 864–876. [Google Scholar] [CrossRef]
- de Souza, T.F.; Dias Ferreira, G.M. Biochars as Adsorbents of Pesticides: Laboratory-Scale Performances and Real-World Contexts, Challenges, and Prospects. ACS EST Water 2024, 4, 4264–4282. [Google Scholar] [CrossRef]
- Saleh, H.M.; Hassan, A.I. Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices. Sustainability 2023, 15, 10891. [Google Scholar] [CrossRef]
- Falsini, S.; Bardi, U.; Abou-Hassan, A.; Ristori, S. Sustainable strategies for large-scale nanotechnology manufacturing in the biomedical field. Green Chem. 2018, 20, 3897–3907. [Google Scholar] [CrossRef]
- Zabot, G.L.; Rodrigues, F.S.; Ody, L.P.; Tres, M.V.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Cooper, K.; Wachter, R. Challenges and Opportunities in Nanomanufacturing; SPIE: Bellingham, WA, USA, 2011; Volume 8105. [Google Scholar]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef]
- Subhan, M.A.; Choudhury, K.P.; Neogi, N. Advances with Molecular Nanomaterials in Industrial Manufacturing Applications. Nanomanufacturing 2021, 1, 75–97. [Google Scholar] [CrossRef]
- Uzhinskiy, A. Advanced Technologies and Artificial Intelligence in Agriculture. AppliedMath 2023, 3, 799–813. [Google Scholar] [CrossRef]
- Gupta, G.; Kumar Pal, S. Applications of AI in precision agriculture. Discov. Agric. 2025, 3, 61. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, F.; Wang, F.; Le, L.; Pu, L. Synthetic biology and artificial intelligence in crop improvement. Plant Commun. 2025, 6, 101220. [Google Scholar] [CrossRef]
- Cabanás, C.G.-L.; Mercado-Blanco, J. Groundbreaking Technologies and the Biocontrol of Fungal Vascular Plant Pathogens. J. Fungi 2025, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Dhanaraju, M.; Chenniappan, P.; Ramalingam, K.; Pazhanivelan, S.; Kaliaperumal, R. Smart Farming: Internet of Things (IoT)-Based Sustainable Agriculture. Agriculture 2022, 12, 1745. [Google Scholar] [CrossRef]
- Sumel, A.; Amisha, R.; Sabahat, J.; Lavanya, L.; Shilpa Amit, G.; Sneha, D.; Shubneesh, K. Artificial Intelligence Integration with Nanotechnology: A New Frontier for Sustainable and Precision Agriculture. Curr. Nanosci. 2025, 21, 242–273. [Google Scholar]
- Obaideen, K.; Yousef, B.A.A.; AlMallahi, M.N.; Tan, Y.C.; Mahmoud, M.; Jaber, H.; Ramadan, M. An overview of smart irrigation systems using IoT. Energy Nexus 2022, 7, 100124. [Google Scholar] [CrossRef]


| Technology | Description | Application in Agriculture | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Soft lithography | Involves the formation of a mold using an elastomeric material (e.g., PDMS) to transfer micro- and nanoscale patterns onto substrates such as silicon or plastic | Used to fabricate microfluidic devices and nanopatterned surfaces that influence microbial behavior. Natural root surfaces are mimicked to promote microbial colonization and modulate microbial communication, enhancing plant growth and stress resistance | Low cost, flexible, suitable for application to various substrate materials, and ideal for applications in biological systems | Limited resolution with respect to other methods; may not be suitable for certain high-precision applications | [23,33,34] |
| Nanoimprinting | A high-throughput method for transferring nanoscale patterns onto substrates by pressing a mold onto the material | Used to fabricate nanostructured surfaces for seed coatings, microbial inoculants, and drug delivery systems, improving plant–microbe interactions and agricultural productivity | High resolution, low cost, scalable, and applicable to various substrates | Requires high precision; molds can be expensive and slow in certain cases | [35,36] |
| Microfluidics | Involves the manipulation of small fluid volumes within micro-sized channels, generating controlled environments for biological studies | Essential for simulating the rhizosphere, microfluidic systems are used for high-throughput screening of plant–microbe interactions or for monitoring exudate release from plant roots | Enables the real-time observation of microbial behavior; efficient and scalable for plant–microbe studies | Requires a complex setup, can be expensive, and has high operational requirements to ensure precision | [37,38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, W.; Khalil, A.A.K.; Amin, A.; Ali, S. Nanofabrication Techniques for Enhancing Plant–Microbe Interactions in Sustainable Agriculture. Nanomaterials 2025, 15, 1086. https://doi.org/10.3390/nano15141086
Zaman W, Khalil AAK, Amin A, Ali S. Nanofabrication Techniques for Enhancing Plant–Microbe Interactions in Sustainable Agriculture. Nanomaterials. 2025; 15(14):1086. https://doi.org/10.3390/nano15141086
Chicago/Turabian StyleZaman, Wajid, Atif Ali Khan Khalil, Adnan Amin, and Sajid Ali. 2025. "Nanofabrication Techniques for Enhancing Plant–Microbe Interactions in Sustainable Agriculture" Nanomaterials 15, no. 14: 1086. https://doi.org/10.3390/nano15141086
APA StyleZaman, W., Khalil, A. A. K., Amin, A., & Ali, S. (2025). Nanofabrication Techniques for Enhancing Plant–Microbe Interactions in Sustainable Agriculture. Nanomaterials, 15(14), 1086. https://doi.org/10.3390/nano15141086










