Study on the Adsorption Behavior of a Cellulose Nanofibril/Tannic Acid/Polyvinyl Alcohol Aerogel for Cu(II), Cd(II), and Pb(II) Heavy Metal Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
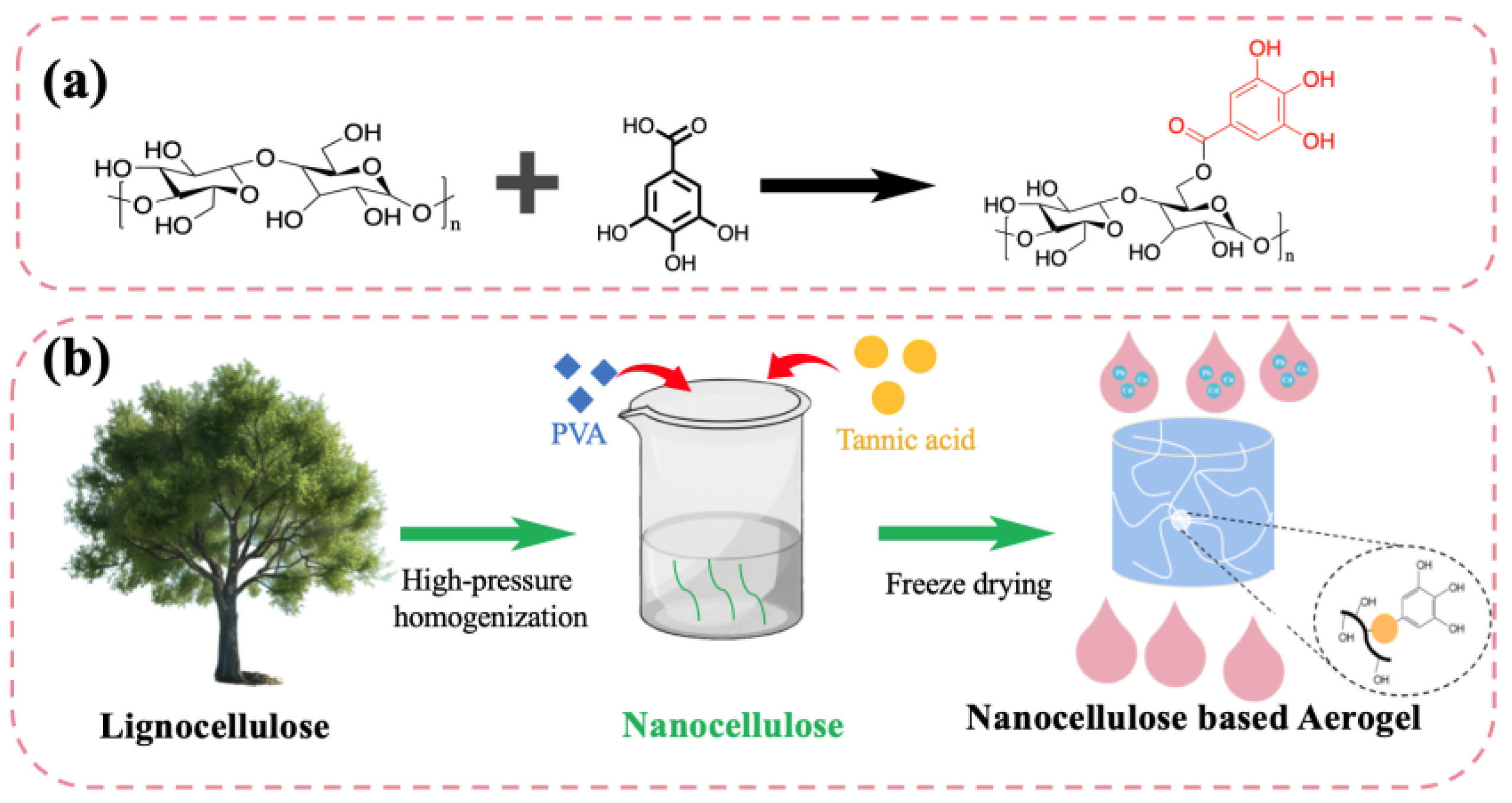
2.2. Preparation of the CNF/TA/PVA Hybrid Aerogel
2.3. Characterizations
2.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
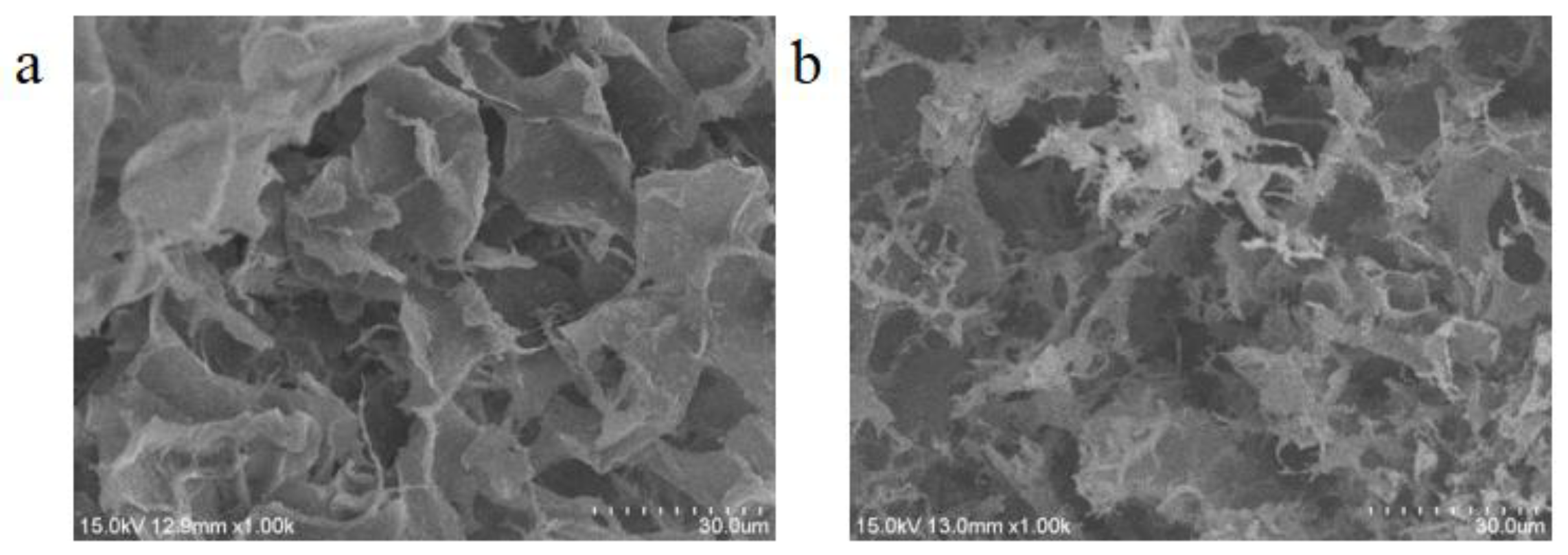
2.3.2. Scanning Electron Microscopy (SEM)
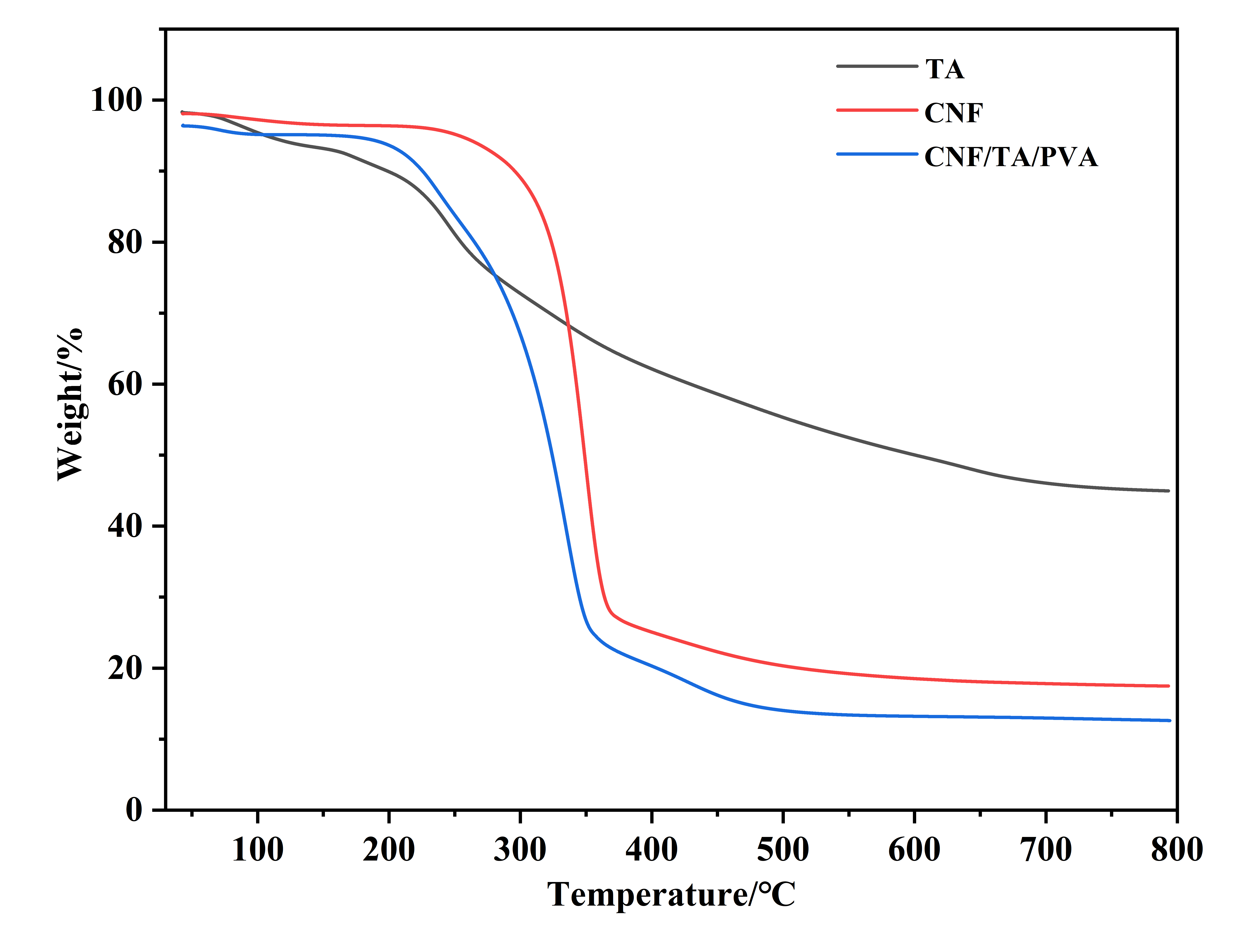
2.3.3. Thermogravimetric Analysis (TGA)
2.4. Adsorption Behaviors of the CNF/TA/PVA Hybrid Aerogel
2.4.1. Adsorption Kinetics
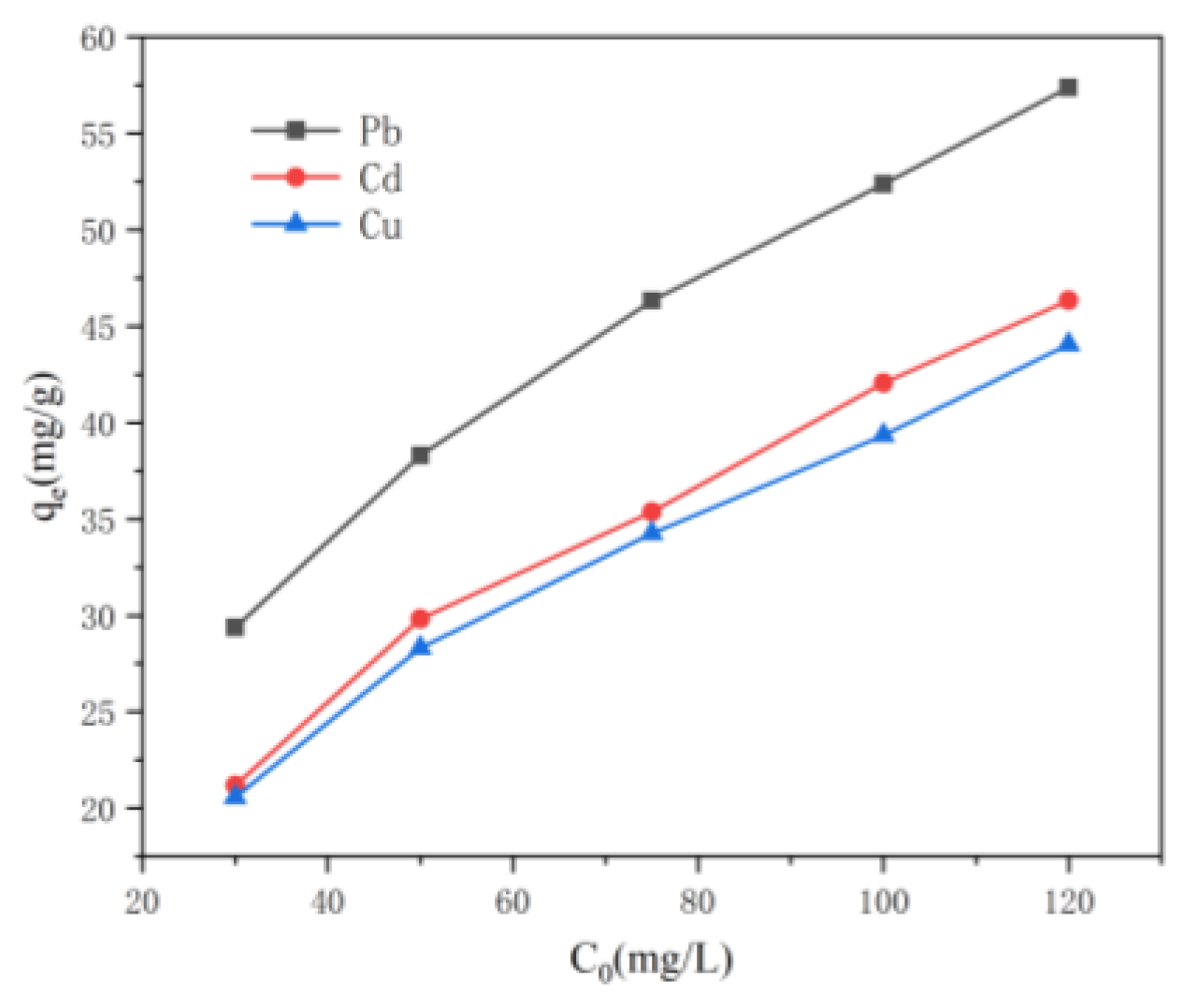
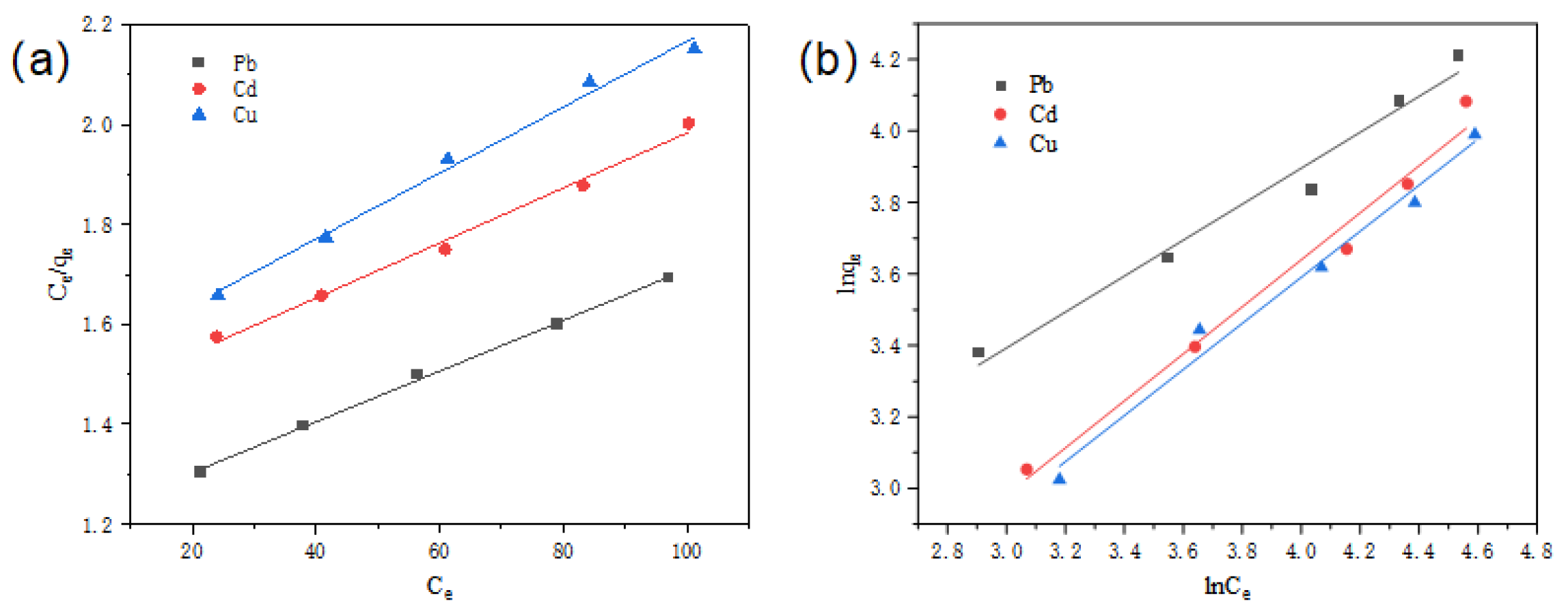
2.4.2. Adsorption Isotherm
3. Results and Discussion
3.1. Preparation and Characterizations of Aerogels
3.2. Adsorption Behavior of Aerogels
3.3. Adsorption Mechanism of the CNF/TA/PVA Hybrid Aerogel
3.4. Comparison of Adsorption Properties for Different Adsorbents
3.5. Studying the Regeneration of the Adsorbent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NCAs | Nanocellulose-based composite aerogels |
| CNFs | Cellulose nanofibrils |
| TA | Tannic acid |
| PVA | Polyvinyl alcohol |
| CNF/TA/PVA | Cellulose nanofibril/tannic acid/polyvinyl alcohol hybrid |
References
- Mohammad, O.; Saghir, K.M.; Almas, Z.; Ees, A. Soil Contamination, Nutritive Value, and Human Health Risk Assessment of Heavy Metals: An Overview. In Toxicity of Heavy Metals to Legumes and Bioremediation; Springer: Vienna, Austria, 2012; pp. 1–27. [Google Scholar]
- Huamain, C.; Chunrong, Z.; Cong, T.U.; Yongguan, Z. Heavy Metal Pollution in Soils in China: Status and Countermeasures. Ambio 1999, 28, 130–134. [Google Scholar]
- Yang, T.; Gao, H.; Chen, H.; Xiao, X.; Zhao, C.; Gong, H. Insights and perspectives of chitosan-based hydrogels for the removal of heavy metals and dyes from wastewater. Int. J. Biol. Macromol. 2025, 292, 139280. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nandi, M.; Pakshirajan, K. Recent Advances in Heavy Metal Recovery from Wastewater by Biogenic Sulfide Precipitation. J. Environ. Manag. 2021, 278, 111555. [Google Scholar] [CrossRef] [PubMed]
- Peyman, S.; Forugh, V.; Hossein, M.A.; Kazem, N.; Reza, R.A. Nitrate Removal from Drinking Water by Point of Use Ion Exchange. J. Res. Health Sci. 2010, 10, 91–97. [Google Scholar]
- Zhang, C.; Wang, J.; Bai, J.; Zhao, Y. Recovering of Zinc from Solid Waste Bearing Sphalerite or Zinc Ferrite by Mechano-Chemical Extraction in Alkaline Solution. Procedia Environ. Sci. 2012, 16, 786–790. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of Three Dimensional Porous Aerogels as Adsorbent for Removal of Heavy Metal Ions from Water/Wastewater: A Review Study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Chen, M.; Li, Z.; Li, J.; Li, J.; Li, Q.; Zhang, L. The Extraction of Uranium Using Graphene Aerogel Loading Organic Solution. Talanta 2017, 166, 284–291. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Silica Aerogels/Xerogels Modified with Nitrogen-Containing Groups for Heavy Metal Adsorption. Molecules 2020, 25, 2788. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef]
- Syeda, H.I.; Yap, P.-S. A Review on Three-Dimensional Cellulose-Based Aerogels for the Removal of Heavy Metals from Water. Sci. Total Environ. 2022, 807, 150606. [Google Scholar] [CrossRef]
- Shaheed, N.; Javanshir, S.; Esmkhani, M.; Dekamin, M.G.; Naimi-Jamal, M.R. Synthesis of Nanocellulose Aerogels and Cu-BTC/Nanocellulose Aerogel Composites for Adsorption of Organic Dyes and Heavy Metal Ions. Sci. Rep. 2021, 11, 18553. [Google Scholar] [CrossRef] [PubMed]
- Henschen, J.; Illergård, J.; Larsson, P.A.; Ek, M.; Wågberg, L. Contact-Active Antibacterial Aerogels from Cellulose Nanofibrils. Colloids Surf. B 2016, 146, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; Hu, D.; Yang, R.; Zhang, J.; Guan, Y.; Lv, F.; Gao, H. A mesoporous nanocellulose/sodium alginate/carboxymethyl-chitosan gel beads for efficient adsorption of Cu2+ and Pb2+. Int. J. Biol. Macromol. 2021, 187, 922–930. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, C.; He, J.; Liu, L.; Yao, J.; Yang, Y.; Xu, K.; Feng, W.; Du, G.; Zhang, L. Cellulose/covalent organic framework aerogel for efficient removal of Cr(VI): Performance and mechanism study. Int. J. Biol. Macromol. 2025, 300, 140243. [Google Scholar] [CrossRef]
- Hu, Y.; Ju, S.; Luo, J.; Pan, H. Facile preparation of cellulose nanofiber/ZSM-5 zeolite/polyethyleneimine aerogel for selective adsorption of Cu2+ in wastewater. J. Solid State Chem. 2024, 337, 124751. [Google Scholar] [CrossRef]
- Fan., S.; Chen, J.; Fan, C.; Chen, G.; Liu, S.; Zhou, H.; Liu, R.; Zhang, Y.; Hu, H.; Huang, Z.; et al. Fabrication of a CO2 responsive chitosan aerogel as an effective adsorbent for the adsorption and desorption of heavy metal ions. Hazard. Mater. 2021, 416, 126225. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, D.; Song, Z.; Liu, W.; Shang, S.; Cao, H.; Du, J.; Ren, J.; Cui, S. Tannic acid-assisted construction of amino-functionalized cellulose nanofiber composite aerogel for high-performance adsorption of Cr(VI), Cu(II) and Congo red: Experimental and DFT studies. Sep. Purif. Technol. 2024, 350, 127979. [Google Scholar] [CrossRef]
- Gao, C.; Wang, X.; An, Q.; Xiao, Z.; Zhai, S. Synergistic preparation of modified alginate aerogel with melamine/chitosan for efficiently selective adsorption of lead ions. Carbohydr. Polym. 2021, 256, 117564. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.; Wang, N.; Zhao, G.; Song, G.; Chen, D.; Liang, Y.; Wen, T.; Wang, H.; Hayat, T.; et al. In-Situ Growth of Hierarchical Layered Double Hydroxide on Polydopamine-Encapsulated Hollow Fe3O4 Microspheres for Efficient Removal and Recovery of U(VI). J. Clean. Prod. 2018, 172, 2033–2044. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y.; Liang, J.; Ou, M.; Chen, J.; Li, G. Extraction, Purification and Anti-Radiation Activity of Persimmon Tannin from Diospyros Kaki Lf. J. Environ. Radioact. 2016, 162, 182–188. [Google Scholar] [CrossRef]
- Sun, X.; Huang, X.; Liao, X.; Shi, B. Adsorptive Removal of Cu(II) from Aqueous Solutions Using Collagen-Tannin Resin. J. Hazard. Mater. 2011, 186, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, C.; Zhu, W.; He, G.; Wei, Y.; Zhou, J. Hydrous Titanium Oxide and Bayberry Tannin Co-Immobilized Nano Collagen Fibrils for Uranium Extraction from Seawater and Recovery from Nuclear Wastewater. Chemosphere 2022, 286, 131626. [Google Scholar] [CrossRef] [PubMed]
- Koopmann, A.-K.; Malfait, W.J.; Sepperer, T.; Huesing, N. A Systematic Study on Bio-Based Hybrid Aerogels Made of Tannin and Silica. Materials 2021, 14, 5231. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Jin, L.; Wang, Y.; Qin, M. Adsorption of Cu (II), Pb (II) and Cr (VI) from Aqueous Solutions Using Black Wattle Tannin-Immobilized Nanocellulose. J. Hazard. Mater. 2017, 339, 91–99. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Meng, Q.; Wu, S.; Shen, C. Polyethylenimine-Grafted-Corncob as a Multifunctional Biomaterial for Removing Heavy Metal Ions and Killing Bacteria from Water. Ind. Eng. Chem. Res. 2020, 59, 17476–17482. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Peng, Y.; Feng, L.; Li, X.; Zhao, C.; Sarfaraz, K. Novel cationic polymer modified magnetic chitosan beads for efficient adsorption of heavy metals and dyes over a wide pH range. Int. J. Biol. Macromol. 2020, 156, 289–301. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.; Cheng, R. Thermal Degradation Behaviors of Spherical Cellulose Nanocrystals with Sulfate Groups. Polymer 2007, 48, 3486–3493. [Google Scholar] [CrossRef]
- Zhou, K.; Yin, L.; Gong, K. 3D Vascular-structured Flame-retardant Cellulose-based photothermal aerogel for Solar-driven interfacial evaporation and wastewater purification. Chem. Eng. J. 2023, 464, 142616. [Google Scholar] [CrossRef]
- Hong, H.; Ban, G.; Kim, H. Fabrication of cylindrical 3D cellulose nanofibril (CNF) aerogel for continuous removal of copper (Cu2+) from wastewater. Chemosphere 2021, 278, 130288. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, D.; Shang, S.; Song, Z.; You, Q.; Huang, L.; Cui, S. A novel magnetic Fe3O4/cellulose nano-fiber/polyethyleneimine/thiol-modified montmorillonite aerogel for efficient removal of heavy metal ions: Adsorption behavior and mechanism study. Int. J. Biol. Macromol. 2023, 253, 126634. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Q.; Sun, G.; Chen, C.; Zhu, M.; Huang, X. The synthesis of tannin-based graphene aerogel by hydrothermal treatment for removal of heavy metal ions. Ind. Crop. Prod. 2022, 176, 114304. [Google Scholar] [CrossRef]
- Rani, K.; Gomathi, T.; Vijayalakshmi, K.; Saranya, M.; Sudha, P.N. Banana Fiber Cellulose Nano Crystals Grafted with Butyl Acrylate for Heavy Metal Lead (II) Removal. Int. J. Biol. Macromol. 2019, 131, 461–472. [Google Scholar]
- Wang, N.; Jin, R.-N.; Omer, A.M.; Ouyang, X. Adsorption of Pb(II) from Fish Sauce Using Carboxylated Cellulose Nanocrystal: Isotherm, Kinetics, and Thermodynamic Studies. Int. J. Biol. Macromol. 2017, 102, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhu, C.; Dang, Z.; Zhang, H.; Yi, X.; Liu, C. Preparation of Cellulose Derived from Corn Stalk and Its Application for Cadmium Ion Adsorption from Aqueous Solution. Carbohydr. Polym. 2012, 90, 1008–1015. [Google Scholar] [CrossRef]
- Daochalermwong, A.; Chanka, N.; Songsrirote, K.; Dittanet, P.; Niamnuy, C.; Seubsai, A. Removal of Heavy Metal Ions Using Modified Celluloses Prepared from Pineapple Leaf Fiber. ACS Omega 2020, 5, 5285–5296. [Google Scholar] [CrossRef]
- Kenawy, I.M.; Hafez, M.A.H.; Ismail, M.A.; Hashem, M.A. Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from Aqueous Single Metal Solutions by Guanyl-Modified Cellulose. Int. J. Biol. Macromol. 2018, 107, 1538–1549. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, Y.; Meng, P.; Peng, D. Absorption of Cadmium (II) via Sulfur-Chelating Based Cellulose: Characterization, Isotherm Models and Their Error Analysis. Carbohydr. Polym. 2019, 209, 38–50. [Google Scholar] [CrossRef]
- Pei, Y.; Xu, G.; Wu, X.; Tang, K.; Wang, G. Removing Pb(II) Ions from Aqueous Solution by a Promising Absorbent of Tannin-Immobilized Cellulose Microspheres. Polymers 2019, 11, 548. [Google Scholar] [CrossRef]
- Ji, Y.; Wen, Y.; Wang, Z.; Zhang, S.; Guo, M. Eco-Friendly Fabrication of a Cost-Effective Cellulose Nanofiber-Based Aerogel for Multifunctional Applications in Cu(II) and Organic Pollutants Removal. J. Clean. Prod. 2020, 255, 120276. [Google Scholar] [CrossRef]


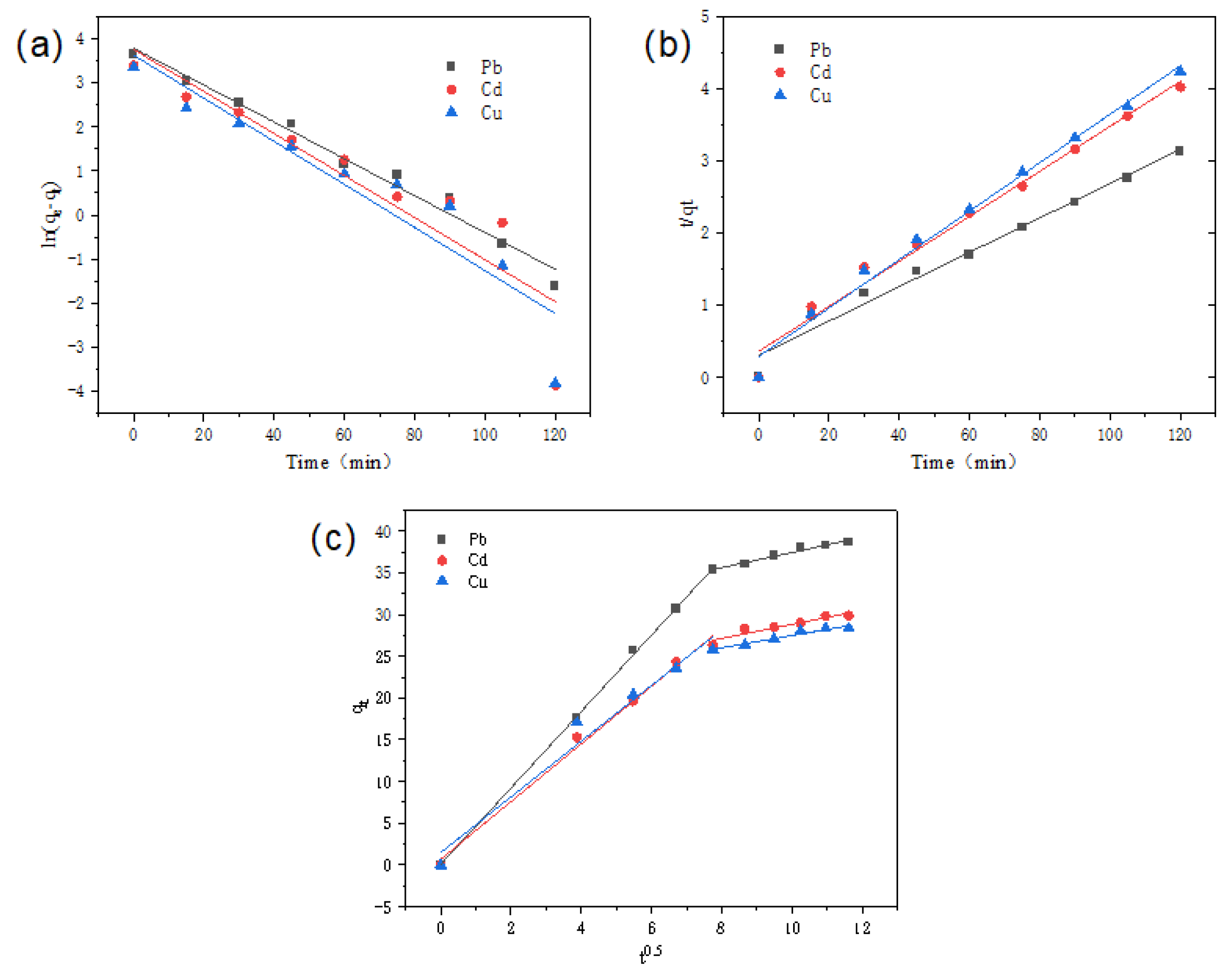
| Metal Ions | Pb2+ | Cd2+ | Cu2+ | |
|---|---|---|---|---|
| Pseudo-first-order kinetic model | R2 | 0.9722 | 0.8146 | 0.8238 |
| K1 | 0.0374 | 0.0477 | 0.0517 | |
| qe, cal (mg/g) | 36.432 | 22.781 | 16.415 | |
| Pseudo-second-order kinetic model | R2 | 0.996 | 0.981 | 0.989 |
| K2 | 0.304 | 0.360 | 0.290 | |
| qe, cal (mg/g) | 38.0 | 29.2 | 27.8 | |
| Ion diffusion model | R12 | 0.999 | 0.992 | 0.970 |
| Kid1 | 4.576 | 3.451 | 3.336 | |
| C1 | 0.075 | 0.697 | 1.489 | |
| R22 | 0.979 | 0.913 | 0.953 | |
| Kid2 | 0.911 | 0.843 | 0.725 | |
| C2 | 28.301 | 20.394 | 20.2 | |
| qe, exp (mg/g) | 52.4 | 42.1 | 39.3 |
| Metal Ions | Pb2+ | Cd2+ | Cu2+ | |
|---|---|---|---|---|
| Langmuir | R2 | 0.998 | 0.992 | 0.991 |
| KL | 0.005 | 0.006 | 0.007 | |
| RL | 0.800 | 0.769 | 0.741 | |
| qmax (mg/g) | 197 | 181 | 152 | |
| Freundlich | R2 | 0.976 | 0.981 | 0.983 |
| n | 1.99 | 1.520 | 1.55 | |
| KF | 6.573 | 2.737 | 2.748 |
| Materials | Metal Ions | Adsorbent Concentration | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|
| Corn stalk-grafted cellulose (AGCS-Cell) | Cd2+ | 100 mg/L | 21.4 | [36] |
| Carboxylated cellulose (Cell-EDTA) | Cd2+ | 100 ppm | 33.2 | [37] |
| Pb2+ | 41.2 | |||
| Schiff base cellulose (Gu-Mc) | Cu2+ | 100 mg/L | 83.0 | [38] |
| Cd2+ | 68.0 | |||
| Pb2+ | 52.0 | |||
| Multifunctionalized cellulose (TMCS) Carbon nanotube | Cd2+ | 200 mg/L | 54.7 | [39] |
| Tannin/cellulose microspheres | Pb2+ | 100 mg/L | 23.8 | [40] |
| Tannin/nanocellulose composite | Cu2+ | 100 mg/L | 46.1 | [25] |
| Pb2+ | 50.0 | |||
| Cr6+ | 103 | |||
| TA@CNF–cardanol-derived siloxane | Cu2+ | 100 mg/L | 47.6 | [41] |
| CNF/TA/PVA hybrid aerogel | Pb2+ | 100 mg/L | 62.4 | This work |
| Cd2+ | 52.1 | |||
| Cu2+ | 49.3 |
| Heavy Metal Ions | Adsorption Capacity (mg/g) | Adsorption Efficiency of Reused Aerogels (%) | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Pb2+ | 62.4 | 95.2 | 93.2 | 90.5 |
| Cd2+ | 52.1 | 96.6 | 94.7 | 91.8 |
| Cu2+ | 49.3 | 98.4 | 97.4 | 97.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tian, Y.; Chen, H.; Liu, Y.; Han, S.; Chang, M.; Zhuang, J.; Ma, Q. Study on the Adsorption Behavior of a Cellulose Nanofibril/Tannic Acid/Polyvinyl Alcohol Aerogel for Cu(II), Cd(II), and Pb(II) Heavy Metal Ions. Nanomaterials 2025, 15, 1063. https://doi.org/10.3390/nano15141063
Zhang X, Tian Y, Chen H, Liu Y, Han S, Chang M, Zhuang J, Ma Q. Study on the Adsorption Behavior of a Cellulose Nanofibril/Tannic Acid/Polyvinyl Alcohol Aerogel for Cu(II), Cd(II), and Pb(II) Heavy Metal Ions. Nanomaterials. 2025; 15(14):1063. https://doi.org/10.3390/nano15141063
Chicago/Turabian StyleZhang, Xuejin, Yulong Tian, Huanhuan Chen, Ying Liu, Shuaichuang Han, Minmin Chang, Jingshun Zhuang, and Qingzhi Ma. 2025. "Study on the Adsorption Behavior of a Cellulose Nanofibril/Tannic Acid/Polyvinyl Alcohol Aerogel for Cu(II), Cd(II), and Pb(II) Heavy Metal Ions" Nanomaterials 15, no. 14: 1063. https://doi.org/10.3390/nano15141063
APA StyleZhang, X., Tian, Y., Chen, H., Liu, Y., Han, S., Chang, M., Zhuang, J., & Ma, Q. (2025). Study on the Adsorption Behavior of a Cellulose Nanofibril/Tannic Acid/Polyvinyl Alcohol Aerogel for Cu(II), Cd(II), and Pb(II) Heavy Metal Ions. Nanomaterials, 15(14), 1063. https://doi.org/10.3390/nano15141063







