Dual-Mode Integration of a Composite Nanoparticle in PES Membranes: Enhanced Performance and Photocatalytic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
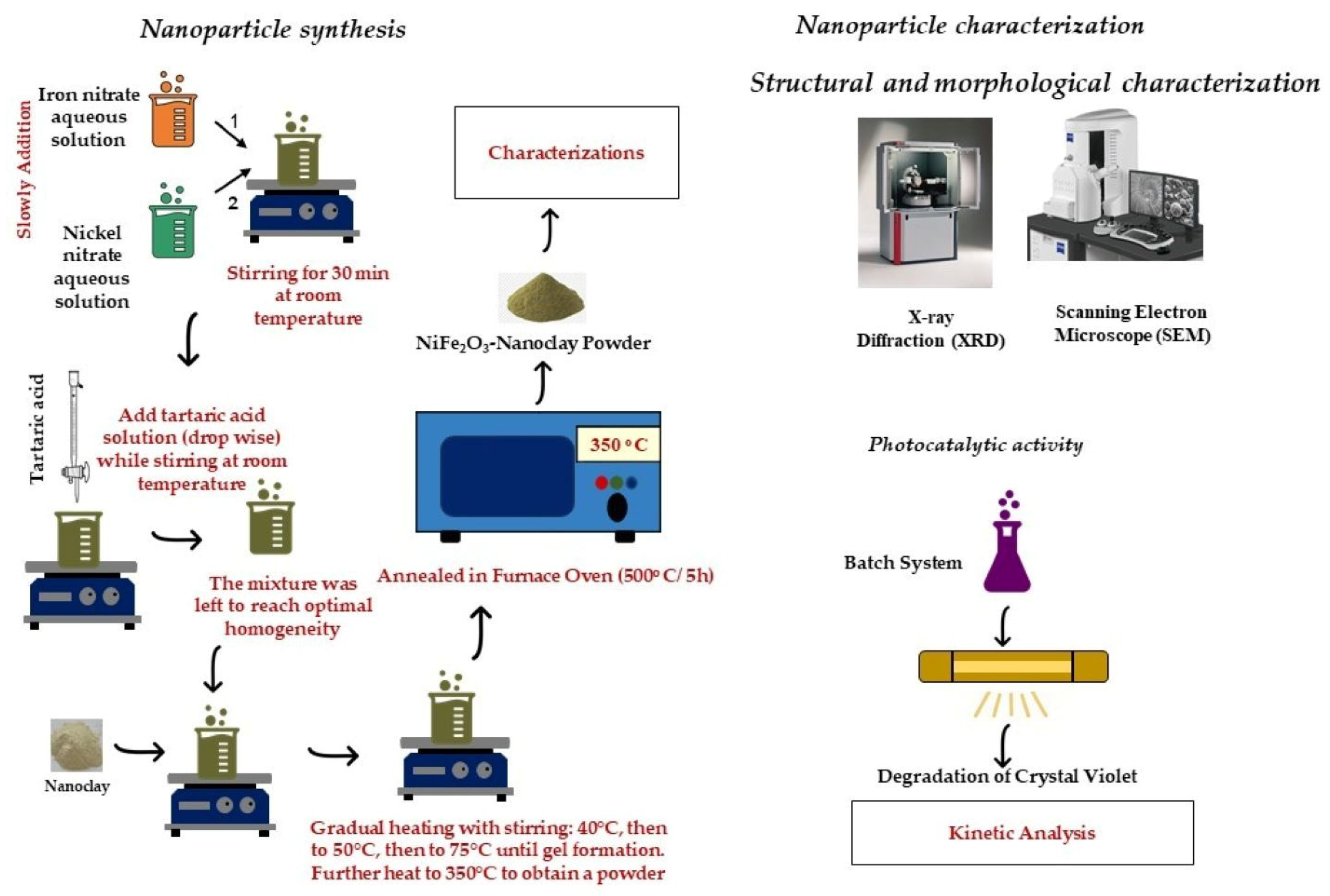
2.2. Nanoparticle Synthesis
2.3. Nanoparticle Characterization
2.3.1. Nanoparticle Structure
2.3.2. Nanoparticles’ Photocatalytic Activity
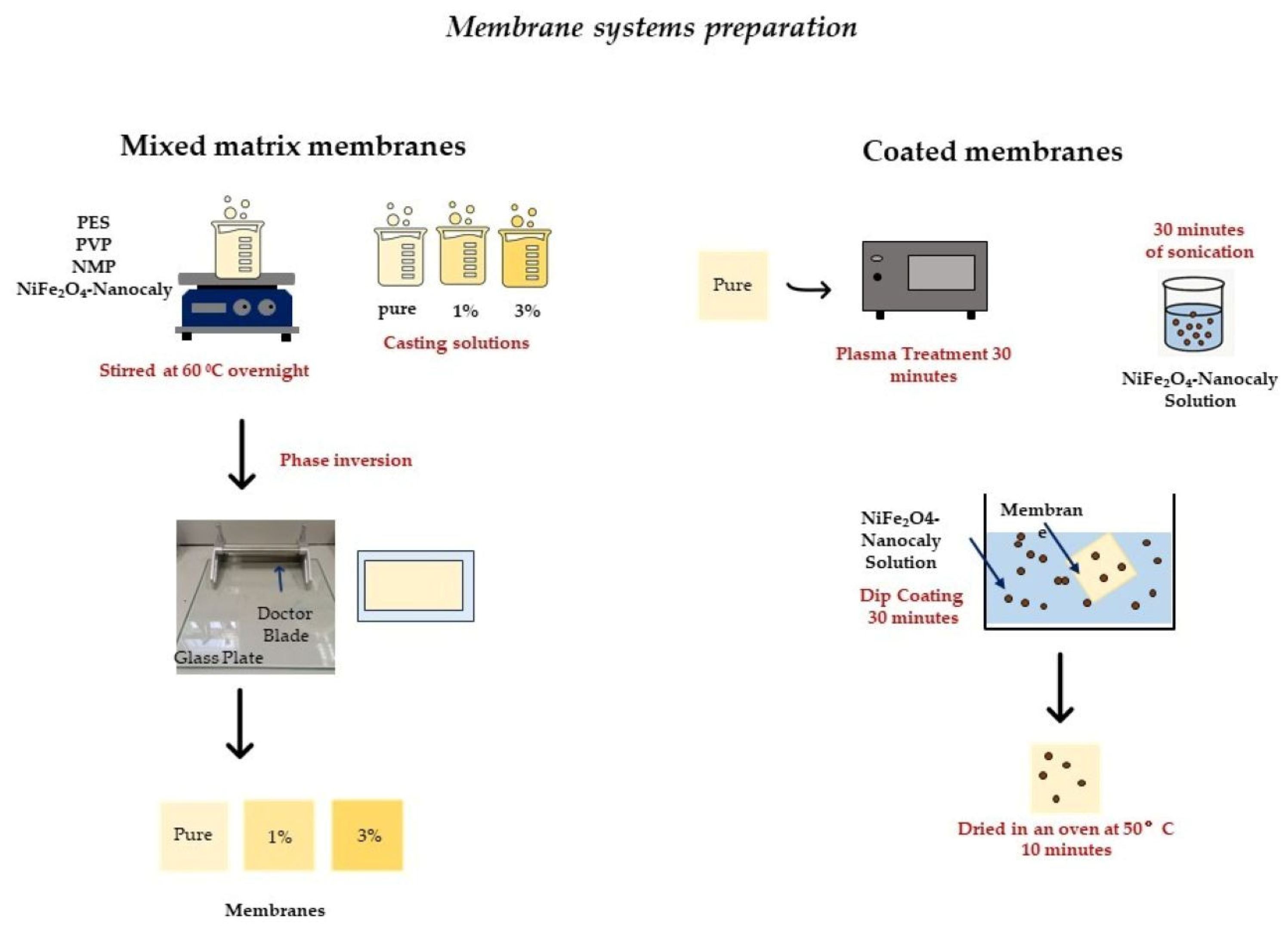
2.4. Membrane Systems Preparation
2.4.1. Mixed-Matrix Membranes
2.4.2. Coated Membranes
2.5. Membrane Systems Characterization
2.5.1. Surface Morphology and Structure Properties
2.5.2. Barrier Properties
2.5.3. Mechanical Properties
2.5.4. Photocatalytic Properties
3. Results and Discussion
3.1. Nanoparticle Structure Characterization
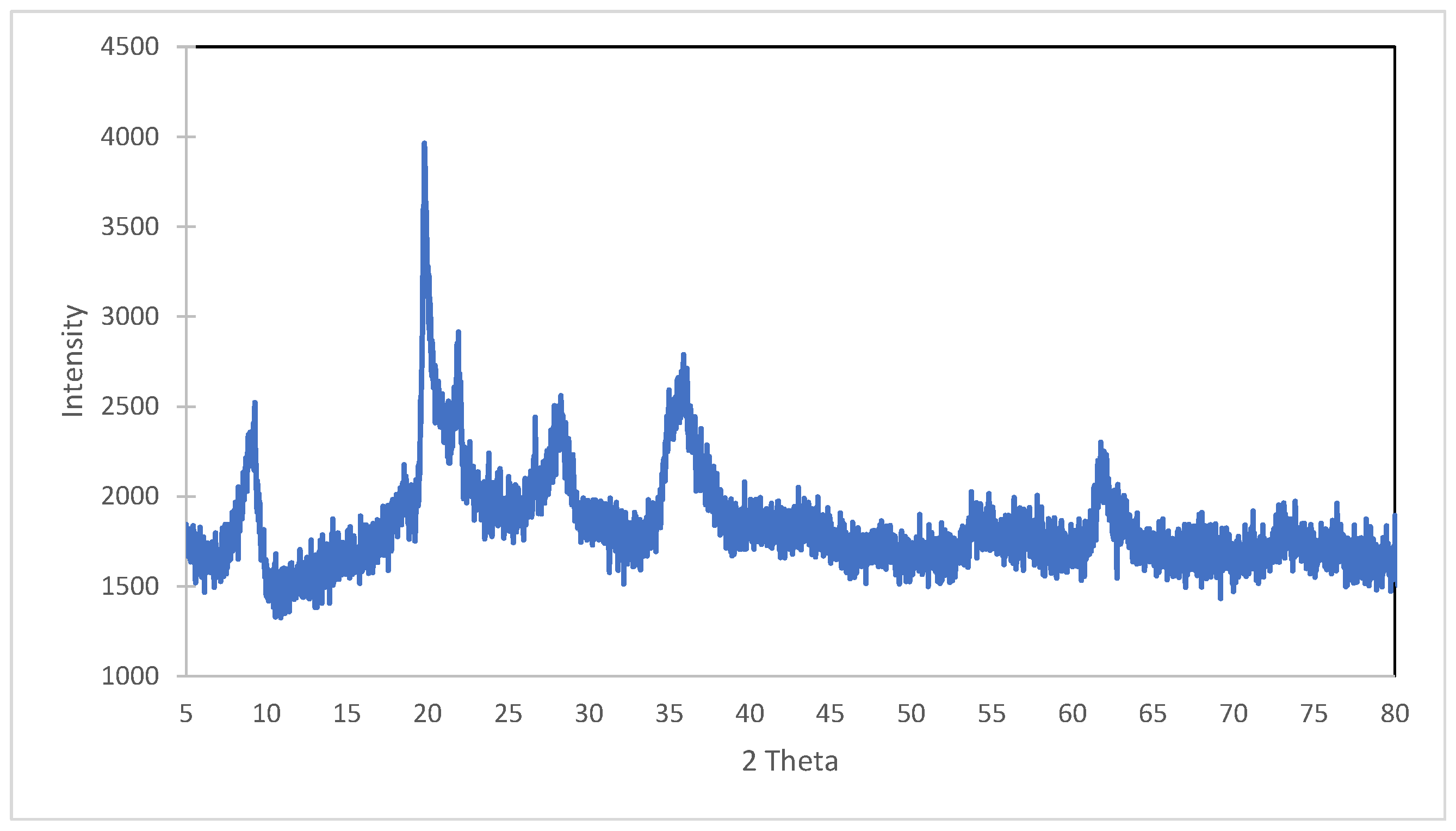
3.1.1. XRD
3.1.2. SEM
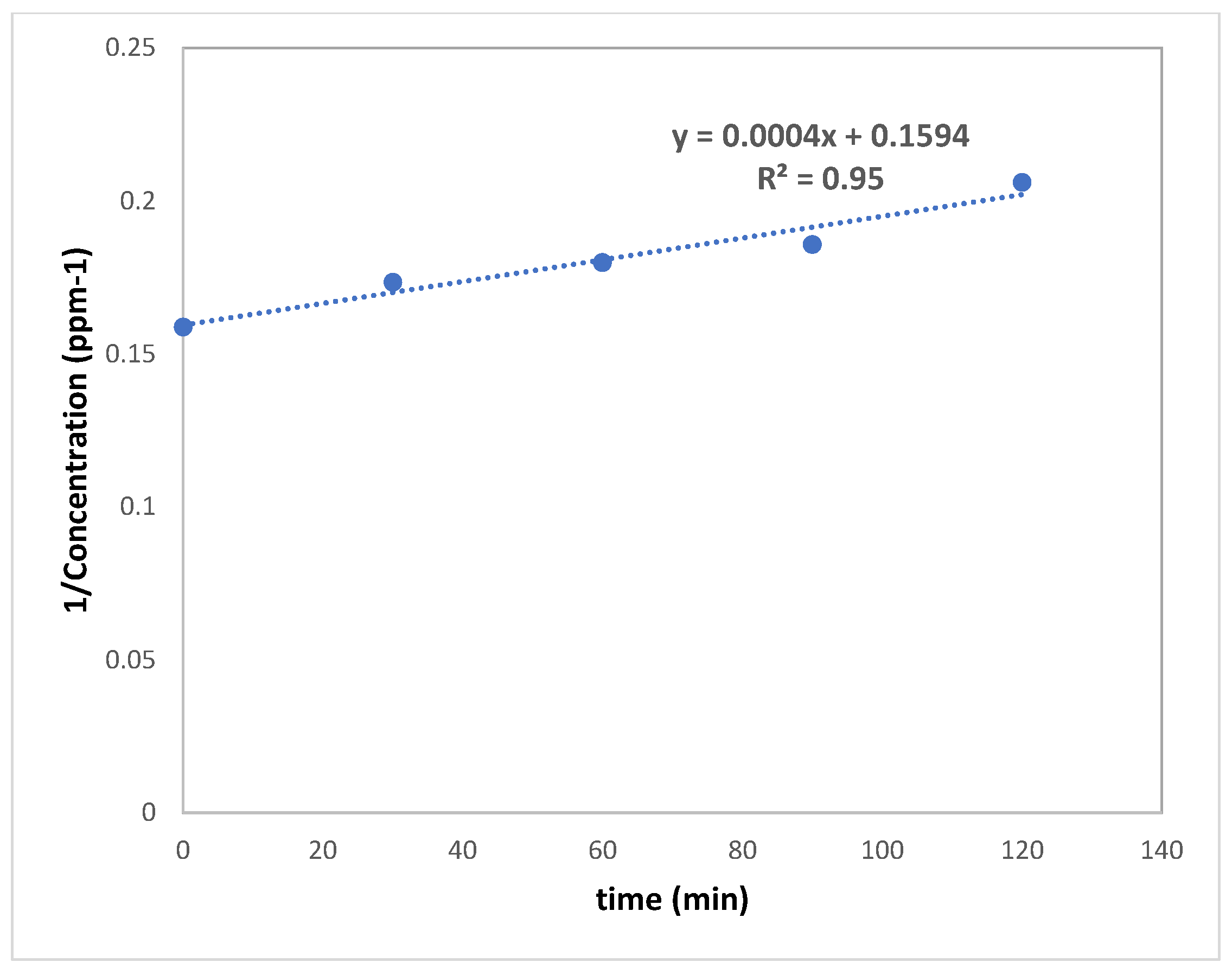
3.2. Photocatalytic Activity of Nanoparticles
3.3. Characterization of Membranes
3.3.1. Membrane Solution Viscosity
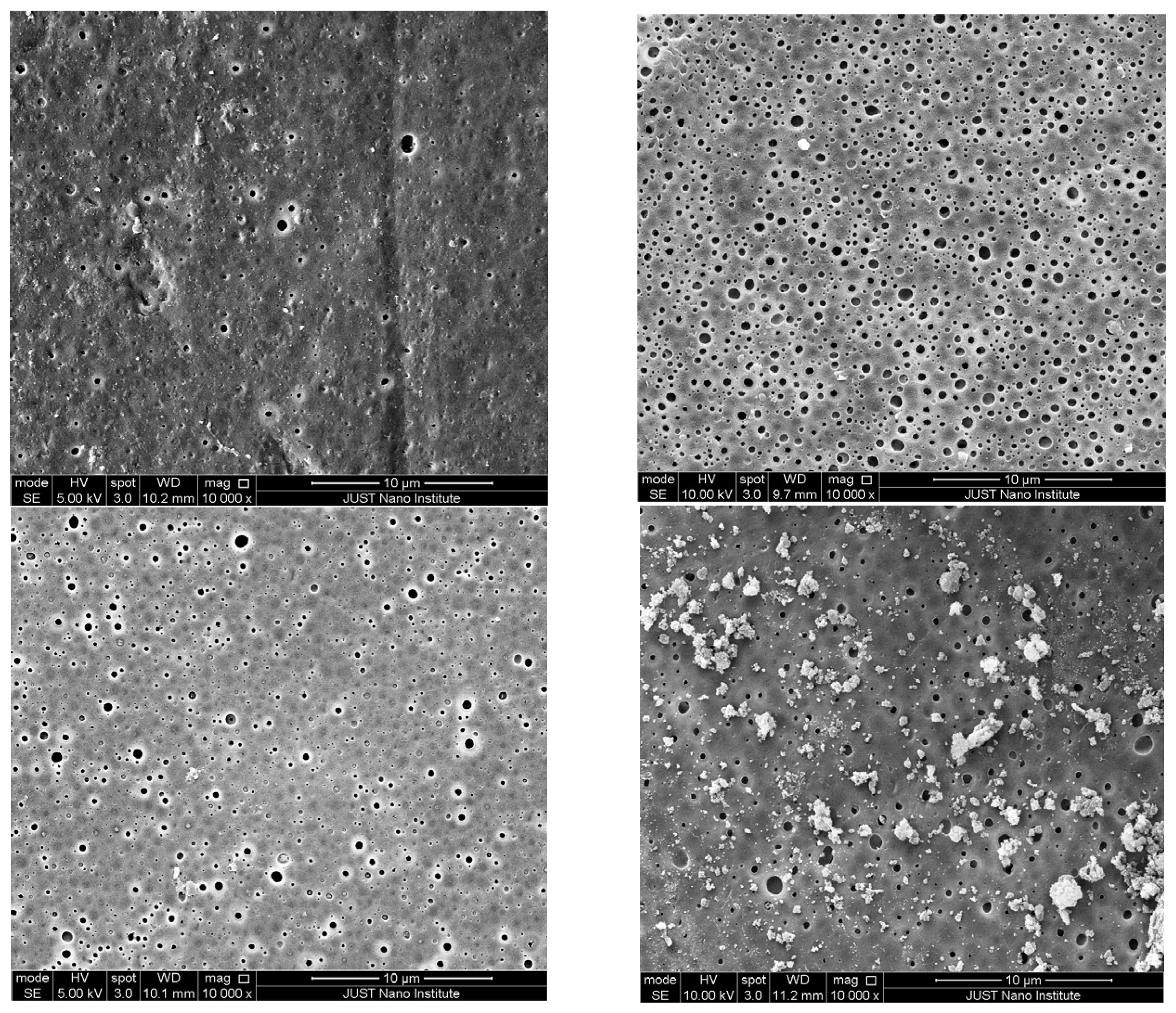
3.3.2. SEM
3.3.3. XRD
3.3.4. Surface Roughness and Hydrophilicity
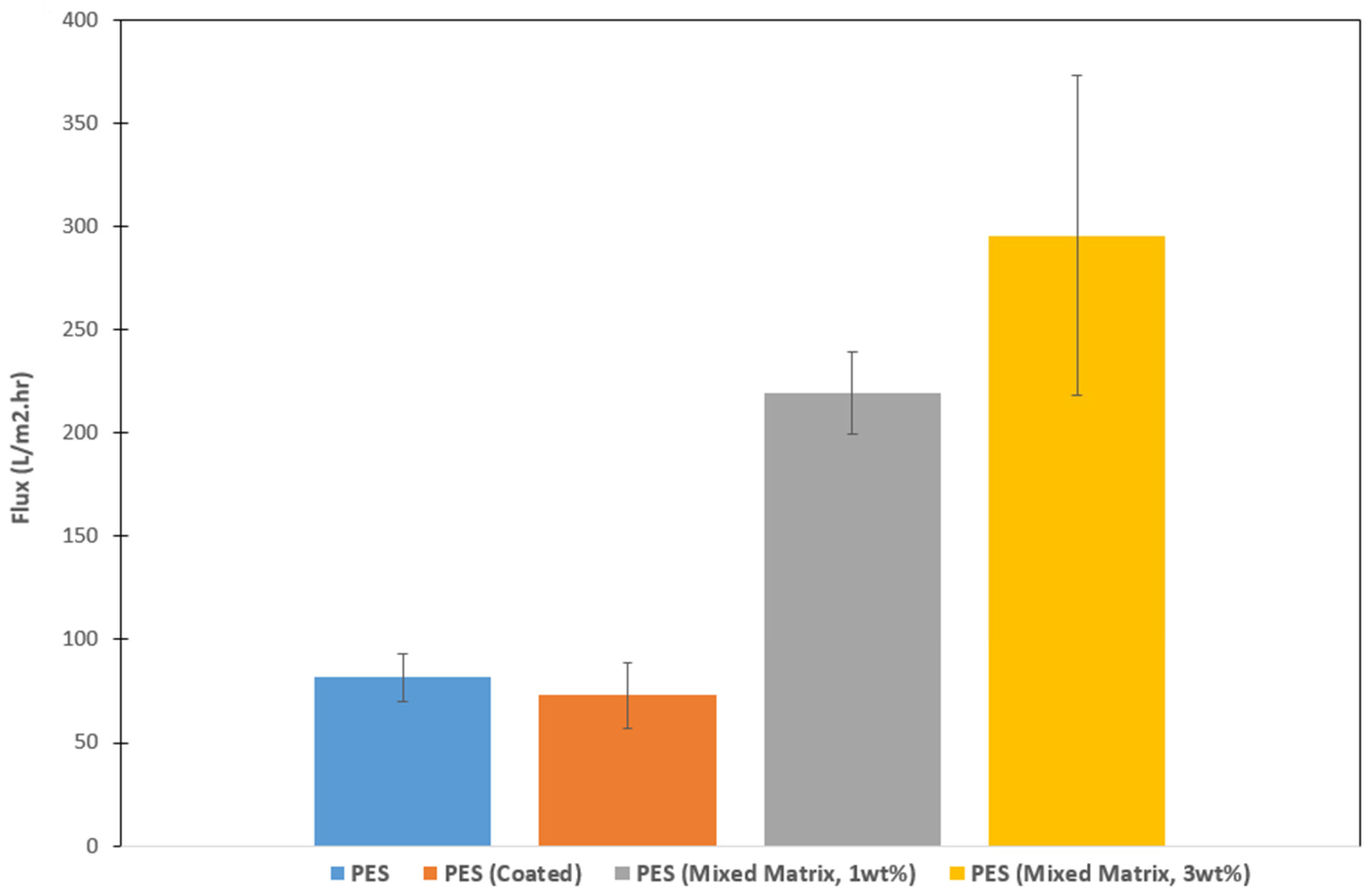
3.3.5. Permeability and Barrier Properties
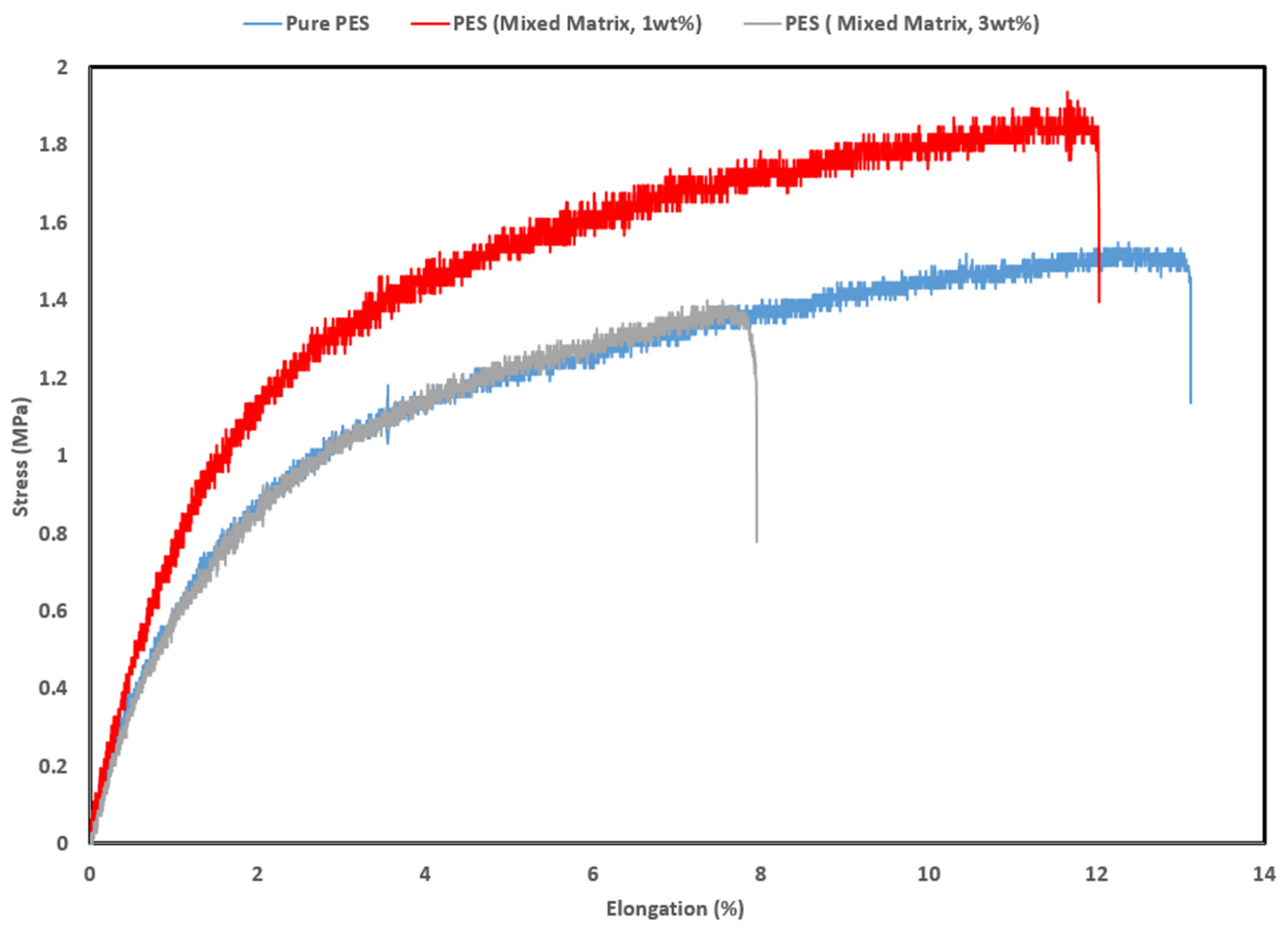
3.3.6. Mechanical Properties
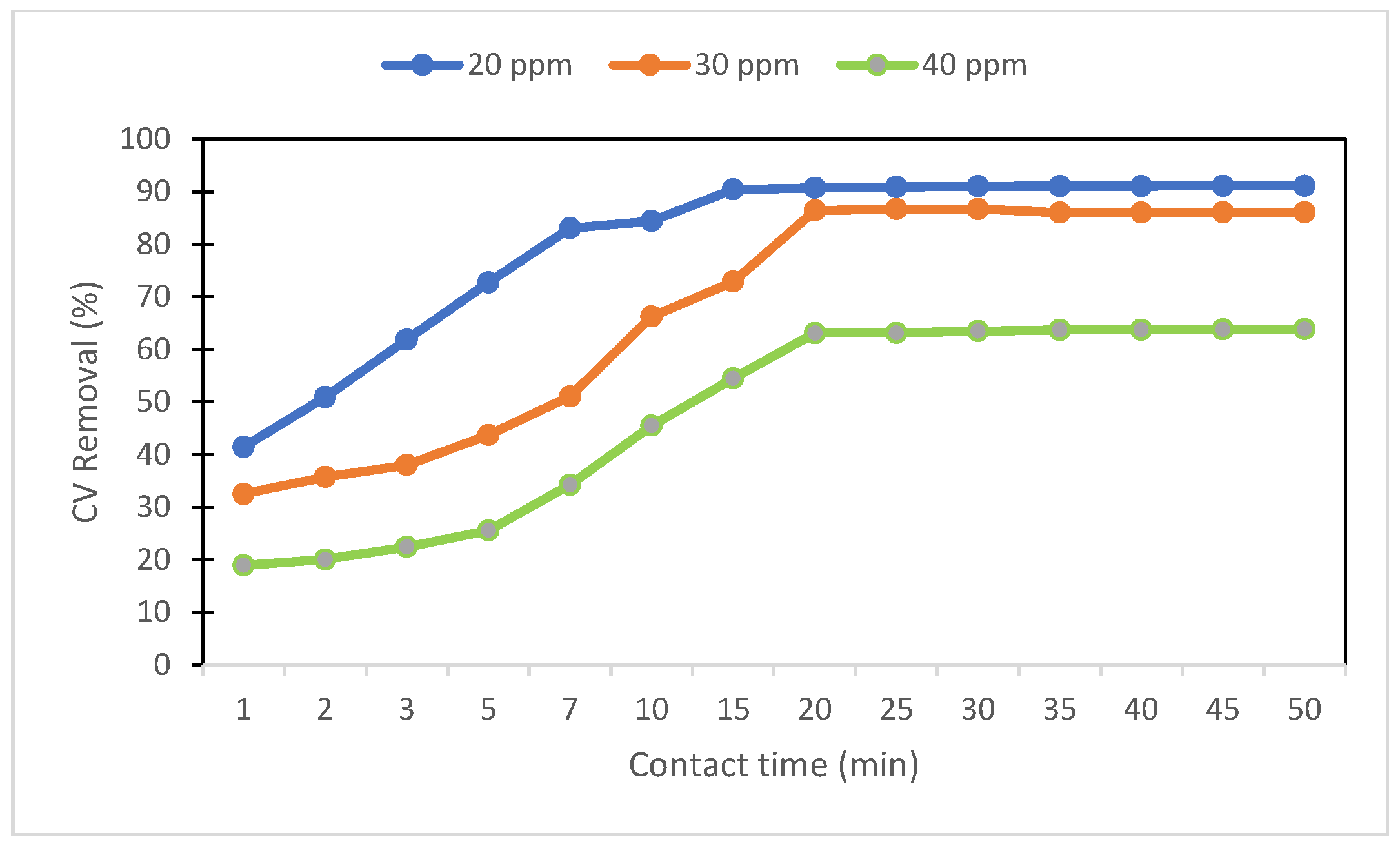
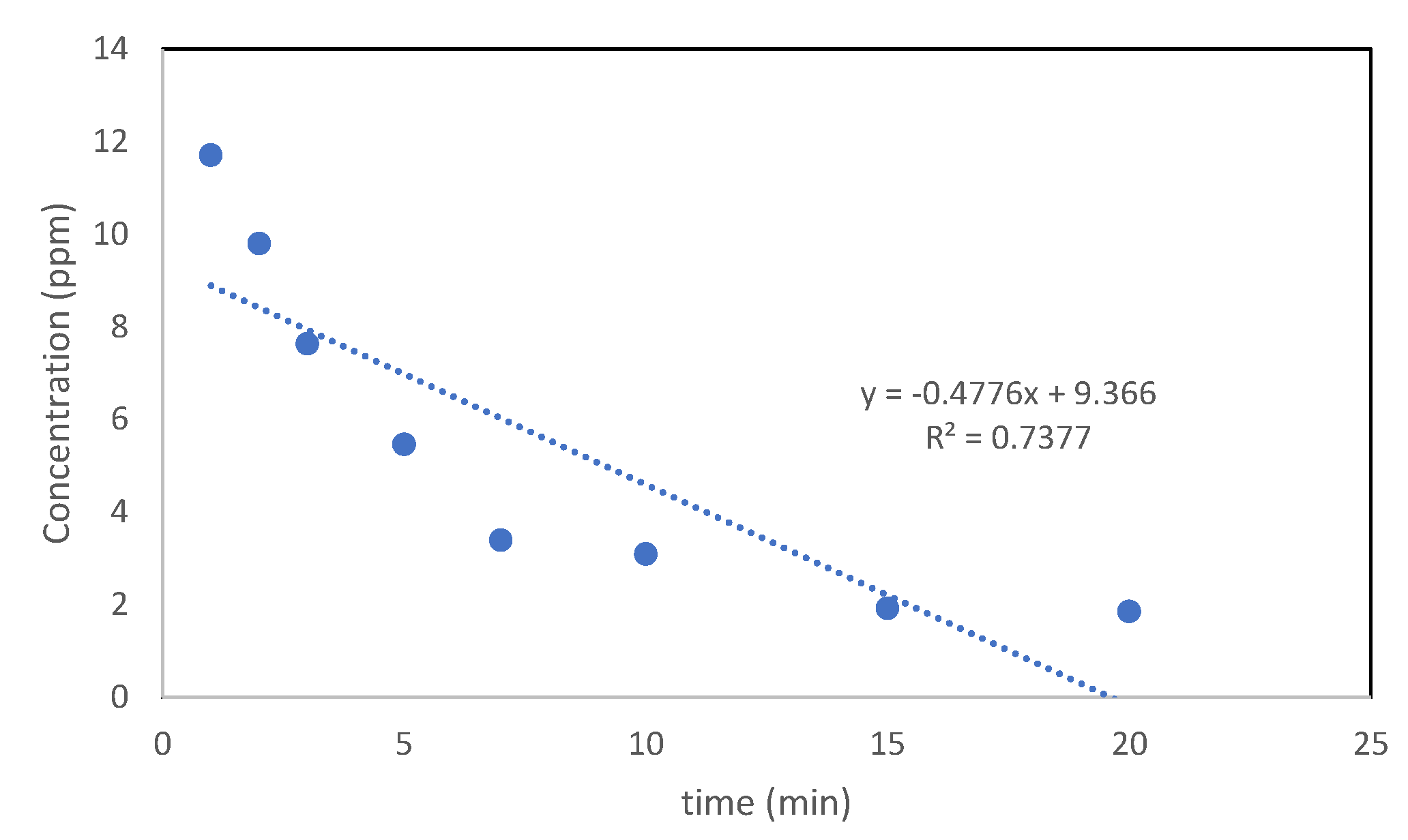
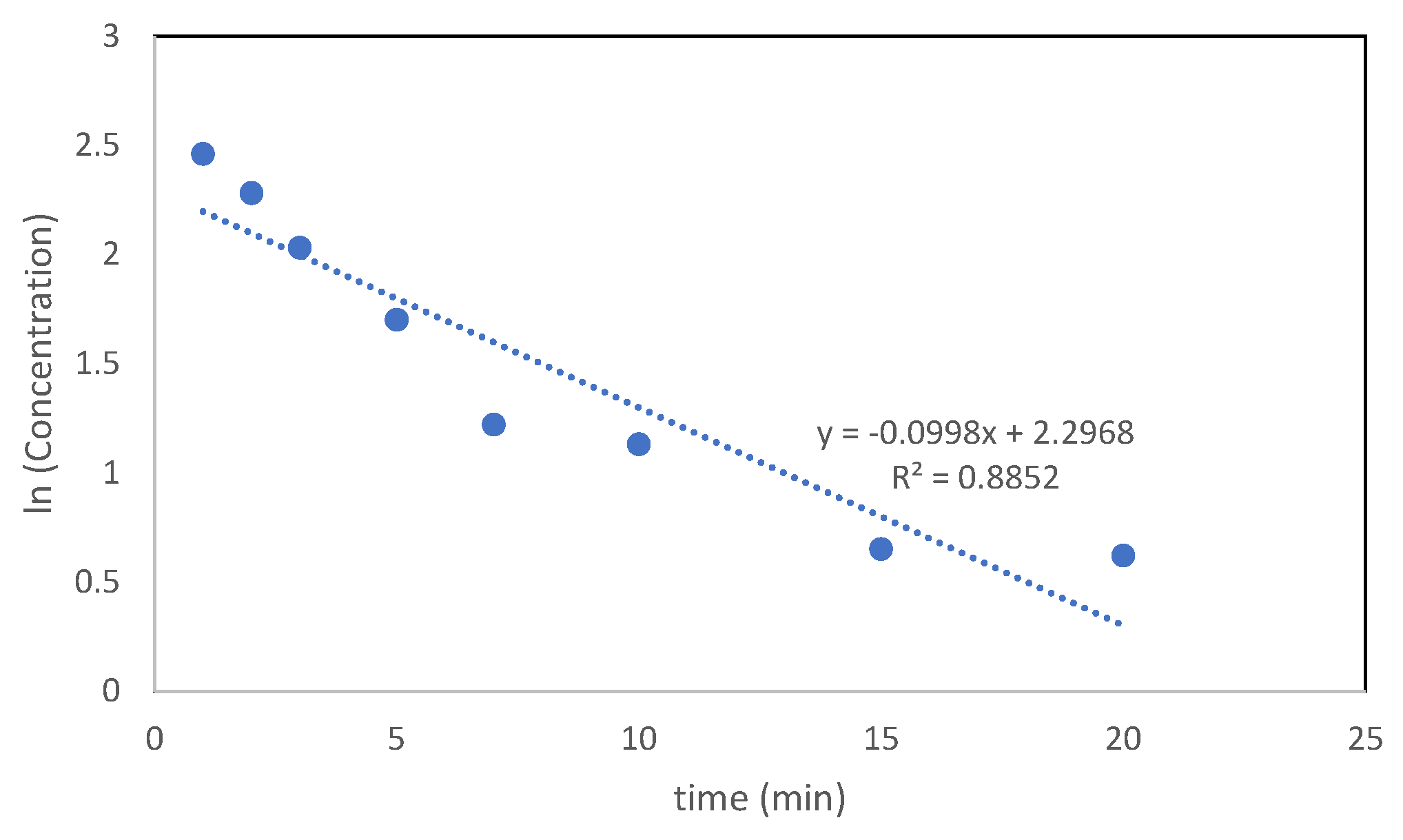
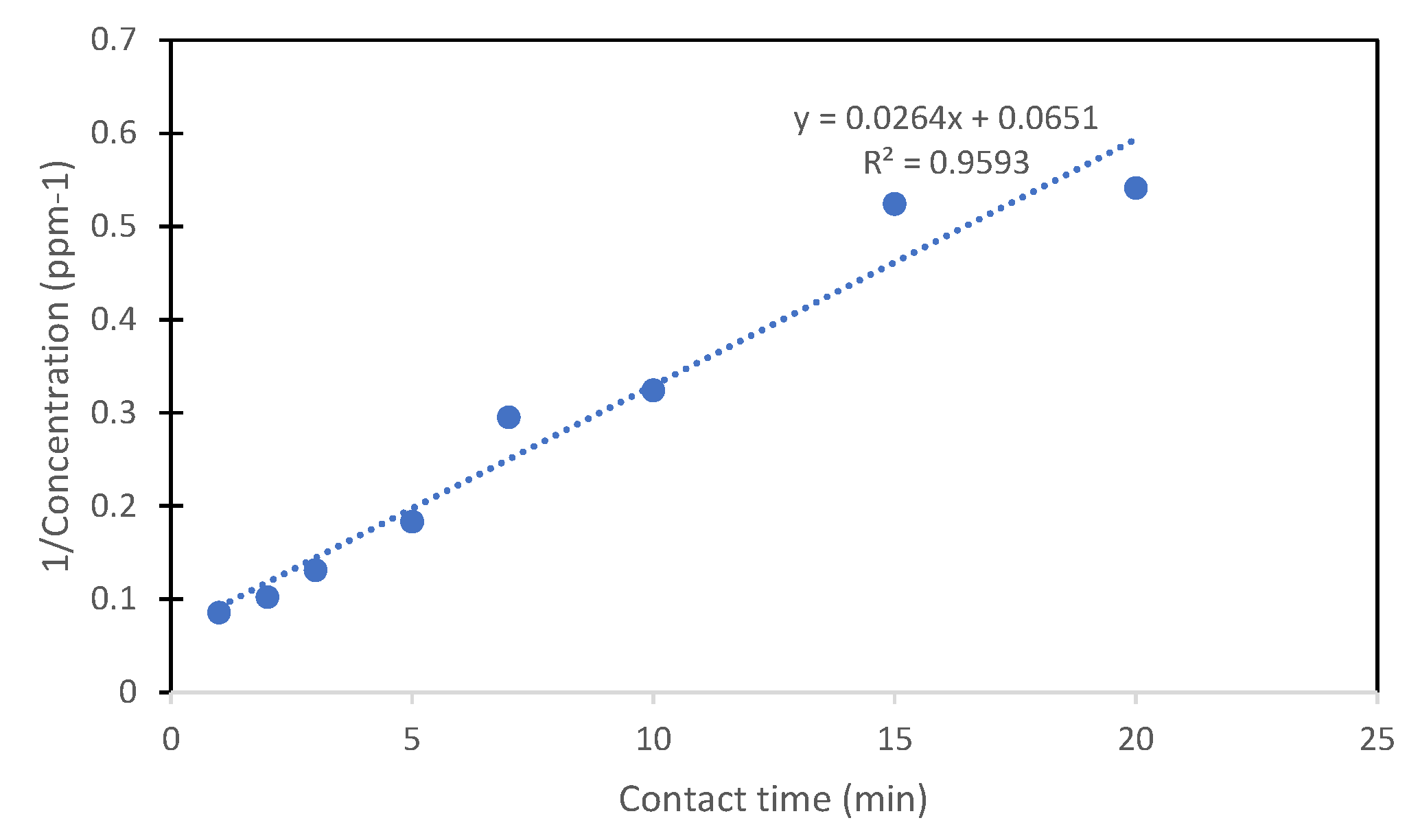
3.3.7. Photocatalysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousefi, H.; Hashemi, B.; Ghasemi, M. Investigating the photocatalytic properties of polyethersulfone/silver-doped zinc oxide nanoparticles/membranes. Chem. Phys. Lett. 2023, 830, 140787. [Google Scholar] [CrossRef]
- Baniamerian, H.; Shokrollahzadeh, S.; Safavi, M.; Ashori, A.; Angelidaki, I. Visible-light-activated Fe2O3–TiO2 nanoparticles enhance biofouling resistance of polyethersulfone ultrafiltration membranes against marine algae Chlorella vulgaris. Sci. Rep. 2024, 14, 24831. [Google Scholar] [CrossRef] [PubMed]
- Bilal, A.; Yasin, M.; Akhtar, F.H.; Gilani, M.A.; Almohamadi, H.; Younas, M.; Mushtaq, A.; Aslam, M.; Hassan, M.; Nawaz, R.; et al. Enhancing Water Purification by Integrating Titanium Dioxide Nanotubes into Polyethersulfone Membranes for Improved Hydrophilicity and Anti-Fouling Performance. Membranes 2024, 14, 116. [Google Scholar] [CrossRef]
- Ren, S.; Guo, N.; Li, J.; Wang, Y. Integration of antibacterial and photocatalysis onto polyethersulfone membrane for fouling mitigation and contaminant degradation. J. Environ. Chem. Eng. 2023, 11, 110401. [Google Scholar] [CrossRef]
- Goyat, R.; Singh, J.; Umar, A.; Saharan, Y.; Kumar, V.; Ibrahim, A.A.; Akbar, S.; Baskoutas, S. Synergistic performance of polyethersulfone membranes embedded with graphene oxide-zinc oxide nanocomposites for efficient heavy metal and dye removal with strong antibacterial properties. Polym. Eng. Sci. 2024, 64, 4161–4180. [Google Scholar] [CrossRef]
- Güneş-Durak, S.; Acarer-Arat, S.; Tüfekci, M.; Pir, İ.; Üstkaya, Z.; Öz, N.; Tüfekci, N. Mechanical Enhancement and Water Treatment Efficiency of Nanocomposite PES Membranes: A Study on Akçay Dam Water Filtration Application. ACS Omega 2024, 9, 31556–31568. [Google Scholar] [CrossRef] [PubMed]
- Chabalala, M.B.; Gumbi, N.N.; Mamba, B.B.; Al-Abri, M.Z.; Nxumalo, E.N. Photocatalytic Nanofiber Membranes for the Degradation of Micropollutants and Their Antimicrobial Activity: Recent Advances and Future Prospects. Membranes 2021, 11, 678. [Google Scholar] [CrossRef]
- Wan, P.; Zhang, Z.; Deng, B. Photocatalytic Polysulfone Hollow Fiber Membrane with Self-Cleaning and Antifouling Property for Water Treatment. Ind. Eng. Chem. Res. 2019, 58, 3339–3348. [Google Scholar] [CrossRef]
- Ramzan, M.; Abusalah, M.; Ahmed, N.; Yean, C.; Zeshan, B. Green Synthesis and Characterization of Silver Nanoparticles Using Zingiber officinale Extracts to Investigate Their Antibacterial Potential. Int. J. Nanomed. 2024, 19, 13319–13338. [Google Scholar] [CrossRef]
- Said, R.; Ghazzy, A.; Shakya, A.K.; Hunaiti, A.A. Iron oxide nanozymes as versatile analytical tools: An overview of their application as detection technique. Bioanalysis 2024, 16, 1261–1278. [Google Scholar] [CrossRef]
- Mousa, S.A.; Abdallah, H.; Khairy, S.A. Low-cost photocatalytic membrane modified with green heterojunction TiO2/ZnO nanoparticles prepared from waste. Sci. Rep. 2023, 13, 22150. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Bian, X.; Lu, X.; Shi, L.; Liu, Z.; Chen, L.; Hou, Z.; Fan, K. Preparation and characterization of ZnO/polyethersulfone (PES) hybrid membranes. Desalination 2012, 293, 21–29. [Google Scholar] [CrossRef]
- Rajabi, H.; Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Astinchap, B.; Zinadini, S.; Razavizadeh, S.H. Nano-ZnO embedded mixed matrix polyethersulfone (PES) membrane: Influence of nanofiller shape on characterization and fouling resistance. Appl. Surf. Sci. 2015, 349, 66–77. [Google Scholar] [CrossRef]
- Dinkar, D.K.; Das, B.; Gopalan, R.; Dehiya, B.S. Magnetic and optical properties of green synthesized nickel ferrite nanoparticles and its application into photocatalysis. Nanotechnology 2021, 32, 505725. [Google Scholar] [CrossRef] [PubMed]
- Maghiani, I.; Souza, L.V.; Bach-Toledo, L.; Faria, A.M.; Ortega, P.P.; Amoresi, R.A.C.; Simões, A.Z.; Mazon, T. Application of NiFe2O4 nanoparticles towards the detection of ovarian cancer marker. Mater. Res. Bull. 2024, 177, 112835. [Google Scholar] [CrossRef]
- Sivadasan, S.; Renuga, V.; Dineshbabu, N.; Dinesh, A.; Babu, K.; Sakthivel, S.; Radhakrishnan, K.; Mamani, R.; Shanmugam, A.; Ayyar, M. Biomedical, Sensor and Photocatalytic Applications of NiFe2O4 Nanoparticles: A Review. Semiconductors 2024, 58, 797–808. [Google Scholar] [CrossRef]
- Šafařík, I.; Šafaříková, M. Magnetic Nanoparticles and Biosciences. In Nanostructured Materials; Hofmann, H., Rahman, Z., Schubert, U., Eds.; Springer: Vienna, Austria, 2002; pp. 1–23. ISBN 978-3-7091-6740-3. [Google Scholar]
- Coey, J.M.D.; Venkatesan, M.; Fitzgerald, C.B. Donor impurity band exchange in dilute ferromagnetic oxides. Nat. Mater. 2005, 4, 173–179. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Uddin, M.N.; Hossain, M.T.; Mahmud, N.; Alam, S.; Jobaer, M.; Mahedi, S.I.; Ali, A. Research and applications of nanoclays: A review. SPE Polym. 2024, 5, 507–535. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Cojocaru, S.; Borhan, A.; Iordan, A.; Palamaru, M.N.; Cucu-Man, S.; Hulea, V.; Melniciuc Puica, N.; Dumitru, I.; Roman, T.; Breabăn, I. Synergistic effect of fuel agents and mass ratio for morpho-structural optimization of magnetic claybased nanocomposites with high adsorption capacity. Environ. Eng. Manag. J. 2020, 19, 849–860. [Google Scholar] [CrossRef]
- Popa, A.; Toloman, D.; Stan, M.; Stefan, M.; Radu, T.; Vlad, G.; Ulinici, S.; Baisan, G.; Macavei, S.; Barbu-Tudoran, L.; et al. Tailoring the RhB removal rate by modifying the PVDF membrane surface through ZnO particles deposition. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1642–1652. [Google Scholar] [CrossRef]
- Naidu, T.; Narayana, P.V. Synthesis and Characterization of Fe-TiO2 and NiFe2O4 Nanoparticles and Its Thermal Properties. J. Nanosci. Technol. 2019, 5, 769–772. [Google Scholar] [CrossRef]
- Baul, S.; Paul, T.C.; Hoque, K.; Khan, M.N.I.; Islam, S.; Sen, S.K.; Kamal, M.M.; Bala, P. Investigation of the structural, magnetic and dielectric properties of NiFe2O4/nanoclay composites synthesized via sol-gel autocombustion. J. Mater. Res. Technol. 2023, 27, 6606–6618. [Google Scholar] [CrossRef]
- Caprì, A.; Gatto, I.; Lo Vecchio, C.; Trocino, S.; Carbone, A.; Baglio, V. Anion Exchange Membrane Water Electrolysis Based on Nickel Ferrite Catalysts. ChemElectroChem 2023, 10, e202201056. [Google Scholar] [CrossRef]
- Ihsan, A.; Irshad, A.; Warsi, M.F.; Din, M.I.; Zulfiqar, S. NiFe2O4/ZnO nanoparticles and its composite with flat 2D rGO sheets for efficient degradation of colored and colorless effluents photocatalytically. Opt. Mater. 2022, 134, 113213. [Google Scholar] [CrossRef]
- Roshani, R.; Ardeshiri, F.; Peyravi, M.; Jahanshahi, M. Highly permeable PVDF membrane with PS/ZnO nanocomposite incorporated for distillation process. RSC Adv. 2018, 8, 23499–23515. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A novel thin film composite forward osmosis membrane prepared from PSf–TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Abu-Zurayk, R.; Alnairat, N.; Waleed, H.; Al-Khaial, M.Q.; Khalaf, A.; Bozeya, A.; Abu-Dalo, D.; Al-Yousef, S.; Afaneh, R. Polyvinylidene Fluoride (PVDF) and Nanoclay Composites’ Mixed-Matrix Membranes: Exploring Structure, Properties, and Performance Relationships. Polymers 2025, 17, 1120. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, K.; Zhang, W.; Bai, Y.; Sun, Y.; Gu, J. Graphene Oxide Quantum Dots Incorporated into a Thin Film Nanocomposite Membrane with High Flux and Antifouling Properties for Low-Pressure Nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 11082–11094. [Google Scholar] [CrossRef]
- Choi, W.; Choi, J.; Bang, J.; Lee, J.-H. Layer-by-Layer Assembly of Graphene Oxide Nanosheets on Polyamide Membranes for Durable Reverse-Osmosis Applications. ACS Appl. Mater. Interfaces 2013, 5, 12510–12519. [Google Scholar] [CrossRef]
- Gai, W.; Xu, S.; Pan, J.; Pan, X.; Liu, Y.; Li, Q.; Huang, L.; Ma, Y. High dye separation performance of novel Cu-BTC/PES MMMs with water-stabilized Cu-BTC nanoparticles. J. Appl. Polym. Sci. 2023, 140, e54506. [Google Scholar] [CrossRef]
- Guo, J.; Kim, J. Modifications of polyethersulfone membrane by doping sulfated-TiO2 nanoparticles for improving anti-fouling property in wastewater treatment. RSC Adv. 2017, 7, 33822–33828. [Google Scholar] [CrossRef]
- Abu-Zurayk, R.; Alnairat, N.; Bozeya, A.; Khalaf, A.; Abu-Dalo, D. Enhanced properties of PVDF membranes using green Ag-nanoclay composite nanoarchitectonics. Mater. Res. Express 2024, 11, 045007. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Liu, W.; Fu, R.; Tu, S.; Zhao, Y.; Dong, L.; Yan, B.; Gu, Y. High Performance Piezoelectric Nanogenerators Based on Electrospun ZnO Nanorods/Poly(vinylidene fluoride) Composite Membranes. J. Phys. Chem. C 2019, 123, 11378–11387. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bui, H.M.; Wang, Y.-F.; You, S.-J. Antifouling CuO@TiO2 coating on plasma-grafted PAA/PES membrane based on photocatalysis and hydrogen peroxide activation. Environ. Sci. Pollut. Res. 2023, 30, 12929–12943. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, M.; Piao, H.; Zuo, S.; Shi, X.; Quan, Q.; Zhu, R.; Huang, Q.; Xiao, C. ZnS/Ag2S decorated PES membrane with efficient near-infrared response and enhanced photocatalysis for pollutants photodegradation on high-turbidity water. Appl. Surf. Sci. 2023, 635, 157728. [Google Scholar] [CrossRef]







| Membrane | Surface Roughness (RMS) (nm) | Contact Angle (°) |
|---|---|---|
| PES | 15.74 | 80 |
| PES-1% Mixed matrix | 15.53 | 75 |
| PES-3% Mixed matrix | 21.91 | 76 |
| PES-Coated | 17.03 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Zurayk, R.; Alnairat, N.; Waleed, H.; Khalaf, A.; Abu-Dalo, D.; Bozeya, A.; Afaneh, R. Dual-Mode Integration of a Composite Nanoparticle in PES Membranes: Enhanced Performance and Photocatalytic Potential. Nanomaterials 2025, 15, 1055. https://doi.org/10.3390/nano15141055
Abu-Zurayk R, Alnairat N, Waleed H, Khalaf A, Abu-Dalo D, Bozeya A, Afaneh R. Dual-Mode Integration of a Composite Nanoparticle in PES Membranes: Enhanced Performance and Photocatalytic Potential. Nanomaterials. 2025; 15(14):1055. https://doi.org/10.3390/nano15141055
Chicago/Turabian StyleAbu-Zurayk, Rund, Nour Alnairat, Haneen Waleed, Aya Khalaf, Duaa Abu-Dalo, Ayat Bozeya, and Razan Afaneh. 2025. "Dual-Mode Integration of a Composite Nanoparticle in PES Membranes: Enhanced Performance and Photocatalytic Potential" Nanomaterials 15, no. 14: 1055. https://doi.org/10.3390/nano15141055
APA StyleAbu-Zurayk, R., Alnairat, N., Waleed, H., Khalaf, A., Abu-Dalo, D., Bozeya, A., & Afaneh, R. (2025). Dual-Mode Integration of a Composite Nanoparticle in PES Membranes: Enhanced Performance and Photocatalytic Potential. Nanomaterials, 15(14), 1055. https://doi.org/10.3390/nano15141055








