Advancements in Laser-Processed Functional Surfaces for Medical Devices: A Current Review
Abstract
1. Introduction
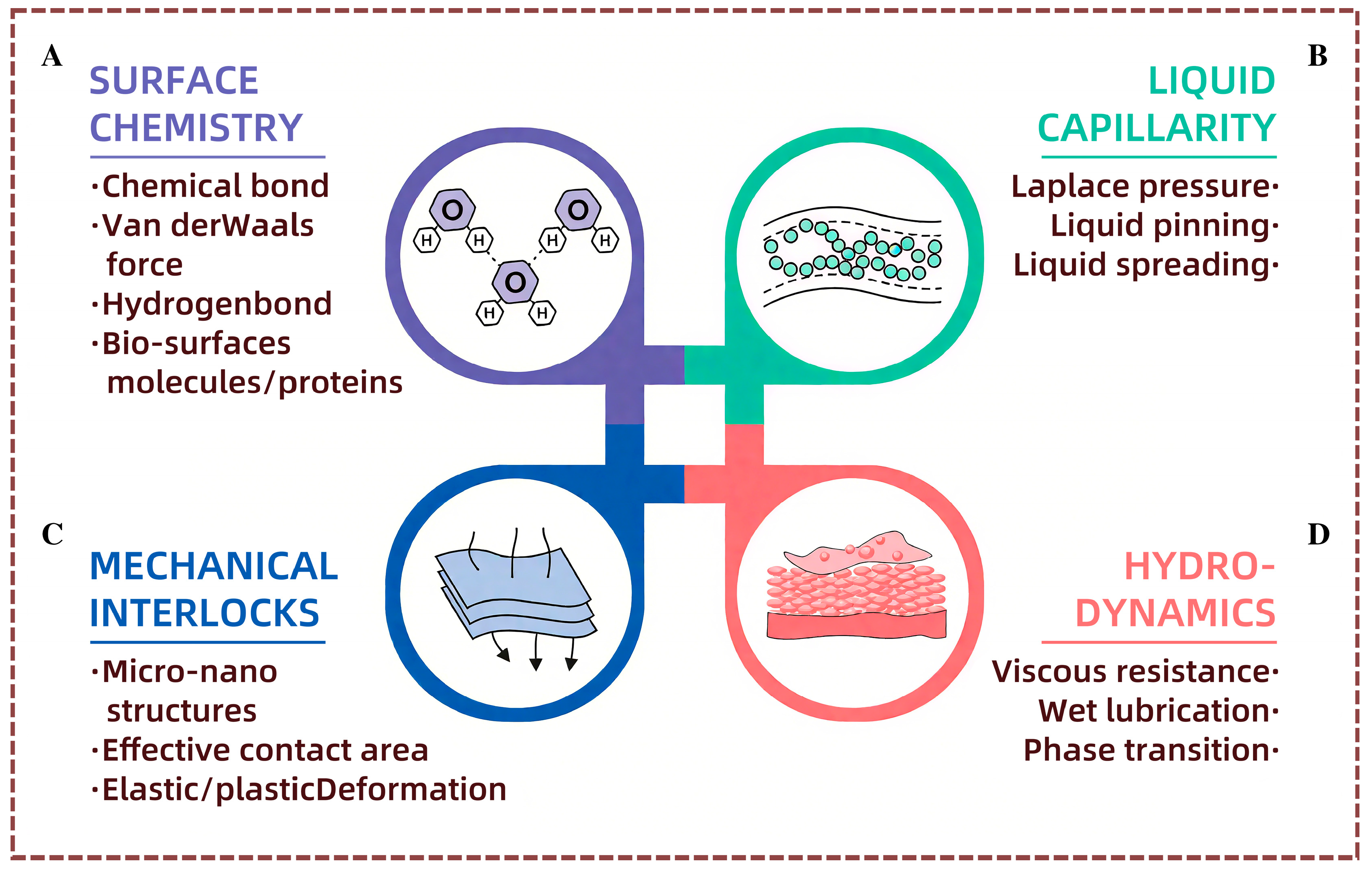
2. Potential Mechanisms of Functional Surfaces for Medical Devices
2.1. Fundamentals of Functional Surface Contacts
2.2. Anti-Adhesion and Super-Slip Properties of Natural Surfaces
3. Functional Surfaces of Medical Implants
3.1. Functional Surfaces for Cell Function Regulation

3.2. Antibacterial Functional Surfaces

3.3. Corrosion-Resistant Functional Surface
4. Functional Surfaces of Surgical Instruments
4.1. Functional Surfaces for Friction Regulation

4.2. Functional Surface Against Tissue Adhesion

5. Conclusions
- (1)
- The nanostructures on the surface of the devices have a significant impact on the modulation of cellular functions, antimicrobial properties, and corrosion resistance. These effects are multifaceted and can be influenced by multiple surface properties simultaneously. With the precise design and fabrication of these micro-nano structures, it is possible to achieve coordinated modulation of these characteristics and thus optimize the performance of the devices. However, there are relatively few studies focused on designing such surfaces with composite functions, and this area remains in an exploratory phase. Although some progress has been made, we are far from achieving the level of sophistication needed to design and fabricate multifunctional surfaces. More research is needed to deepen our understanding of the relationship between micro-nano structures and device performance and to further advance the field.
- (2)
- The field of medical devices continues to rely heavily on traditional metal materials like titanium alloys and stainless steel, which are favored for their excellent mechanical properties, corrosion resistance, and biocompatibility. However, with advancements in technology and the growing diversity of medical device needs, the exploration and application of new materials have become increasingly important. New medical device materials, such as composites, inorganic compounds, and amorphous alloys offer unique performance advantages that traditional metal materials do not have. For example, composite materials like zirconium-based amorphous alloys combine the advantages of multiple materials with good mechanical properties and corrosion resistance. Laser preparation technology also plays a key role in the functional surface treatment of these new materials, enabling the enhancement of their properties and expanding their application potential. The combination of innovative materials with laser preparation technology provides broader opportunities for the manufacture and development of medical devices and is expected to drive innovation and development in the medical device field.
- (3)
- Bionic structures, with their unique external forms and surface morphologies, present unique properties that allow them to play a vital role in many fields. For instance, bionic structures may be applied to surfaces to provide friction-reducing benefits, minimizing friction between contact surfaces, and extend the lifespan of equipment. At the same time, the surface could also be anti-adhesive, preventing the accumulation of unwanted materials and ensuring cleanliness and efficiency in operation. Furthermore, the bionic structures can offer antimicrobial properties, which is especially significant for medical implantable devices. As these devices are in constant contact with the human body, preventing the growth of bacteria and viruses on their surfaces is critical for maintaining safety and hygiene.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.; Zhang, Y.; Zhang, L.; Ding, X.; Zhang, D. Applications of bioinspired approaches and challenges in medical devices. Bio-Des. Manuf. 2021, 4, 146–148. [Google Scholar] [CrossRef]
- Saroia, J.; Yanen, W.; Wei, Q.; Zhang, K.; Lu, T.; Zhang, B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des. Manuf. 2018, 1, 265–279. [Google Scholar] [CrossRef]
- Massarweh, N.N.; Cosgriff, N.; Slakey, D.P. Electrosurgery: History, Principles, and Current and Future Uses. J. Am. Coll. Surg. 2006, 202, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.H.; Gibbons, G.D.; Innes, J.T.; Nichols, K.E.; Front, M.E.; Roby, S.R.; Ellison, E.C. Complications of laparoscopic cholecystectomy. Surgery 1991, 110, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, P.; Woisetschläger, R.; Rieger, R.; Wayand, W. Mechanism, management, and prevention of laparoscopic bowel injuries. Gastrointest. Endosc. 1996, 43, 572–574. [Google Scholar] [CrossRef]
- Bishoff, J.T.; Allaf, M.E.; Kirkels, W.; Moore, R.G.; Kavoussi, L.R.; Schroder, F. Laparoscopic bowel injury: Incidence and clinical presentation. J. Urol. 1999, 161, 887–890. [Google Scholar] [CrossRef]
- Choi, J.; Kang, D.; Han, S.; Kim, S.B.; Rogers, J.A. Thin, Soft, Skin-Mounted Microfluidic Networks with Capillary Bursting Valves for Chrono-Sampling of Sweat. Adv. Healthc. Mater. 2017, 6, 1601355. [Google Scholar] [CrossRef]
- Kim, T.; Park, J.; Sohn, J.; Cho, D.; Jeon, S. Bioinspired, Highly Stretchable, and Conductive Dry Adhesives Based on 1D–2D Hybrid Carbon Nanocomposites for All-in-One ECG Electrodes. ACS Nano 2016, 10, 4770–4778. [Google Scholar] [CrossRef]
- Xu, S.; Fan, H.; Li, Z.-Z.; Hua, J.-G.; Yu, Y.-H.; Wang, L.; Chen, Q.-D.; Sun, H.-B. Ultrafast laser-inscribed nanogratings in sapphire for geometric phase elements. Opt. Lett. 2021, 46, 536–539. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.-D.; Cao, X.-W.; Buividas, R.; Wang, X.; Juodkazis, S.; Sun, H.-B. Plasmonic nano-printing: Large-area nanoscale energy deposition for efficient surface texturing. Light Sci. Appl. 2017, 6, e17112. [Google Scholar] [CrossRef]
- Ahmmed, K.M.T.; Grambow, C.; Kietzig, A.-M. Fabrication of Micro/Nano Structures on Metals by Femtosecond Laser Micromachining. Micromachines 2014, 5, 1219–1253. [Google Scholar] [CrossRef]
- Kietzig, A.-M.; Hatzikiriakos, S.G.; Englezos, P. Ice friction: The effects of surface roughness, structure, and hydrophobicity. J. Appl. Phys. 2009, 106, 97. [Google Scholar] [CrossRef]
- Zorba, V.; Stratakis, E.; Barberoglou, M.; Spanakis, E.; Tzanetakis, P.; Anastasiadis, S.H.; Fotakis, C. Biomimetic Artificial Surfaces Quantitatively Reproduce the Water Repellency of a Lotus Leaf. Adv. Mater. 2008, 20, 4049–4054. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Metal pumps liquid uphill. Appl. Phys. Lett. 2009, 94, 224102. [Google Scholar] [CrossRef]
- Cui, Z.; Lu, L.; Guan, Y.; Ramakrishna, S.; Hong, M. Enhancing SERS detection on a biocompatible metallic substrate for diabetes diagnosing. Opt. Lett. 2021, 46, 3801–3804. [Google Scholar] [CrossRef]
- Zupančič, M.; Može, M.; Gregorčič, P.; Sitar, A.; Golobič, I. Evaluation of enhanced nucleate boiling performance through wall-temperature distributions on PDMS-silica coated and non-coated laser textured stainless steel surfaces. Int. J. Heat Mass Transf. 2017, 111, 419–428. [Google Scholar] [CrossRef]
- Lei, S.; Devarajan, S.; Chang, Z. A study of micropool lubricated cutting tool in machining of mild steel. J. Mater. Process. Technol. 2009, 209, 1612–1620. [Google Scholar] [CrossRef]
- Kumar, B.A.; Babu, P.D.; Marimuthu, P.; Duraiselvam, M. Effect of laser surface texturing on tribological behaviour of grey cast iron. Int. J. Surf. Sci. Eng. 2019, 13, 220–235. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Y. The application of composite materials in the medical instrument. China Med. Device Inf. 2012, 18, 13–17. [Google Scholar] [CrossRef]
- Lu, L.; Wang, H.; Guan, Y.; Zhou, W. Laser microfabrication of biomedical devices. Chin. J. Lasers 2017, 44, 59–73. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, P.; Zhang, J. Effects of different implant surface properties on the biological behavior of Schwann cells. West China J. Stomatol. 2021, 39, 279–285. [Google Scholar] [CrossRef]
- Zaffora, A.; Di Franco, F.; Virtù, D.; Carfì Pavia, F.; Ghersi, G.; Virtanen, S.; Santamaria, M. Tuning of the Mg alloy AZ31 anodizing process for biodegradable implants. ACS Appl. Mater. Interfaces 2021, 13, 12866–12876. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, J.; Wang, X.; Wang, Z. Research progress on antibacterial properties of medical tantalum implants. J. Hebei Med. Univ. 2021, 42, 116–121. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Wang, Y.; Zhang, P.; Zhang, G.; Zhang, D. Bioinspired surgical grasper based on the strong wet attachment of tree frog’s toe pads. J. Mech. Eng. 2018, 54, 14–20. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, Y.; Zheng, Q.; Sun, J.; Liu, H. Study of drilling temperature on cortical bone based on micro-texture tool. J. Chin. Agric. Mech. 2016, 37, 207–211. [Google Scholar] [CrossRef]
- Han, Z.; Fu, J.; Fang, Y.; Zhang, J.; Niu, S.; Ren, L. Anti-adhesive property of maize leaf surface related with temperature and humidity. J. Bionic Eng. 2017, 14, 540–548. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, S.; Han, Z. Bionic structures and materials inspired by plant leaves: A comprehensive review for innovative problem-solving. Progress in Materials Science 2023, 139, 101181. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, H.; Zhang, D. Investigation of the anisotropic morphology-induced effects of the slippery zone in pitchers of Nepenthes alata. J. Bionic Eng. 2015, 12, 79–87. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, Q.; Deng, X.; Cui, J. Earthworm-inspired rough polymer coatings with self-replenishing lubrication for adaptive friction-reduction and antifouling surfaces. Adv. Mater. 2018, 30, 1802141. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Zhang, L.; Liu, H.; Jiang, Y.; Zhang, D.; Han, Z.; Jiang, L. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 2016, 532, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Yang, X.; Shen, Z.; Feng, Q. Effects of hierarchical micro/nano-topographies on the morphology, proliferation and differentiation of osteoblast-like cells. Colloids Surf. B Biointerfaces 2016, 145, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Dumas, V.; Guignandon, A.; Vico, L.; Mauclair, C.; Zapata, X.; Linossier, M.T.; Bouleftour, W.; Granier, J.; Peyroche, S.; Dumas, J.-C. Femtosecond laser nano/micro patterning of titanium influences mesenchymal stem cell adhesion and commitment. Biomed. Mater. 2015, 10, 055002. [Google Scholar] [CrossRef]
- Li, C.; Yang, L.; Liu, N.; Yang, Y.; Zhao, J.; Yang, P.; Cheng, G. Bioinspired surface hierarchical microstructures of Ti6Al4V alloy with a positive effect on osteoconduction. Surf. Coat. Technol. 2020, 388, 125594. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, W.; Zhang, G.; Li, Z.; Hu, H.; Wang, C.; Zeng, X.; Zhao, S.; Zhang, Y.; Ren, T. Friction stability and cellular behaviors on laser textured Ti–6Al–4V alloy implants with bioinspired micro-overlapping structures. J. Mech. Behav. Biomed. Mater. 2020, 109, 103823. [Google Scholar] [CrossRef]
- Carvalho, A.; Cangueiro, L.; Oliveira, V.; Vilar, R.; Fernandes, M.H.; Monteiro, F.J. Femtosecond laser microstructured Alumina toughened Zirconia: A new strategy to improve osteogenic differentiation of hMSCs. Appl. Surf. Sci. 2018, 435, 1237–1245. [Google Scholar] [CrossRef]
- Iaroslav, G.; Leonid, D.; Aile, T.; Ana, M.F.; Kateryna, D.; Sergei, K.; Yaroslav, T.; Santa, V.; Vincent, P.; Maksym, P. Enhanced osteointegration and osteogenesis of osteoblast cells by laser-induced surface modification of Ti implants. Nanomed. Nanotechnol. Biol. Med. 2024, 62, 102785. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, G.; Zhang, W.; Hu, J. Investigating the effect of picosecond laser texturing on microstructure and biofunctionalization of titanium alloy. J. Mater. Process. Technol. 2018, 255, 129–136. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnology 2017, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Elie, A.-M.; Plawinski, L.; Serro, A.P.; do Rego, A.M.B.; Almeida, A.; Urdaci, M.C.; Durrieu, M.-C.; Vilar, R. Femtosecond laser texturing of titanium as a method to reduce the adhesion of Staphylococcus aureus and biofilm formation. Appl. Surf. Sci. 2016, 360, 485–493. [Google Scholar] [CrossRef]
- Peter, A.; Lutey, A.H.; Faas, S.; Romoli, L.; Onuseit, V.; Graf, T. Direct laser interference patterning of stainless steel by ultrashort pulses for antibacterial surfaces. Optics & Laser Technology 2020, 123, 105954. [Google Scholar] [CrossRef]
- Sahar, S.; Hedieh, P.; Bijan, G.; Mahmood, M.; Mohsen, M. Improving the antibacterial performance of 304 stainless steel using Nd-YAG laser irradiation. Appl. Phys. A 2024, 130, 104. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, P.; Yu, Z.; Zhang, X.; Shen, L.; Shi, H.; Yan, H.; Wang, L.; Tian, Y. Effects of periodic surface structures induced by femtosecond laser irradiation on the antibacterial properties of Zr-based amorphous material. Optik 2022, 268, 169760. [Google Scholar] [CrossRef]
- Romoli, L.; Lazzini, G.; Lutey, A.H.; Fuso, F. Influence of ns laser texturing of AISI 316L surfaces for reducing bacterial adhesion. CIRP Ann. 2020, 69, 529–532. [Google Scholar] [CrossRef]
- Xu, J.; Ji, M.; Li, L.; Wu, Y.; Yu, Q.; Chen, M. Improving wettability, antibacterial and tribological behaviors of zirconia ceramics through surface texturing. Ceram. Int. 2022, 48, 3702–3710. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Yan, Y.; Yang, K.; Lu, L. Influences of protein adsorption on the in vitro corrosion of biomedical metals. Acta Metall. Sin. 2021, 57, 1–15. [Google Scholar] [CrossRef]
- Talha, M.; Ma, Y.; Kumar, P.; Lin, Y.; Singh, A. Role of protein adsorption in the bio corrosion of metallic implants–A review. Colloids Surf. B Biointerfaces 2019, 176, 494–506. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; Li, Z.; Zeng, X.; Xu, Y.; Zhao, S.; Hu, H.; Zhang, Y.; Ren, T. Tribological behavior of Ti-6Al-4V against cortical bone in different biolubricants. J. Mech. Behav. Biomed. Mater. 2019, 90, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Anandkumar, B.; Choubey, A.; George, R.; Ganesh, P.; Upadhyaya, B.; Philip, J.; Bindra, K.; Kaul, R. Antibacterial and corrosion studies on nanosecond pulse laser textured 304 L stainless steel surfaces. Lasers Manuf. Mater. Process. 2019, 6, 332–343. [Google Scholar] [CrossRef]
- Lu, Y.; Guan, Y.; Li, Y.; Yang, L.; Wang, M.; Wang, Y. Nanosecond laser fabrication of superhydrophobic surface on 316L stainless steel and corrosion protection application. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125259. [Google Scholar] [CrossRef]
- Saqib, M.; Beshchasna, N.; Pelaccia, R.; Roshchupkin, A.; Yanko, I.; Husak, Y.; Kyrylenko, S.; Reggiani, B.; Cuniberti, G.; Pogorielov, M. Tailoring surface properties, biocompatibility and corrosion behavior of stainless steel by laser induced periodic surface treatment towards developing biomimetic stents. Surf. Interfaces 2022, 34, 102365. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Zhang, G.; Wang, G.; Zeng, Z.; Wang, C.; Wang, C.; Zhao, S.; Zhang, Y.; Ren, T. Electrochemical corrosion and anisotropic tribological properties of bioinspired hierarchical morphologies on Ti-6Al-4V fabricated by laser texturing. Tribol. Int. 2019, 134, 352–364. [Google Scholar] [CrossRef]
- Kuczyńska-Zemła, D.; Sotniczuk, A.; Pisarek, M.; Chlanda, A.; Garbacz, H. Corrosion behavior of titanium modified by direct laser interference lithography. Surf. Coat. Technol. 2021, 418, 127219. [Google Scholar] [CrossRef]
- Nishizaki, C.; Nishikawa, M.; Yata, T.; Yamada, T.; Takahashi, Y.; Oku, M.; Yurimoto, H.; Sakai, Y.; Nakanishi, K.; Takakura, Y. Inhibition of surgical trauma-enhanced peritoneal dissemination of tumor cells by human catalase derivatives in mice. Free Radic. Biol. Med. 2011, 51, 773–779. [Google Scholar] [CrossRef]
- Sadjadi, H.; Hashtrudi-Zaad, K.; Fichtinger, G. Needle deflection estimation: Prostate brachytherapy phantom experiments. Int. J. Comput. Assist. Radiol. Surg. 2014, 9, 921–929. [Google Scholar] [CrossRef]
- Wang, X.; Han, P.; Kang, M.; Ehmann, K. Surface-blended texturing of medical needles for friction reduction using a picosecond laser. Appl. Phys. A 2016, 122, 286. [Google Scholar] [CrossRef]
- Wang, X.; Giovannini, M.; Xing, Y.; Kang, M.; Ehmann, K. Fabrication and tribological behaviors of corner-cube-like dimple arrays produced by laser surface texturing on medical needles. Tribol. Int. 2015, 92, 553–558. [Google Scholar] [CrossRef]
- Pan, C.; Xu, C.; Huang, Z.; Zhou, J. Antifriction effect of 316L stainless steel textured surface with superhydrophilic properties in brain tissue insertion. Mater. Res. Express 2021, 8, 105401. [Google Scholar] [CrossRef]
- Butler-Smith, P.; See, T.; Humphrey, E.; Vilar, J.G.; Steege, T.; Kunze, T.; Schell, F.; Serey, N.; Tomic, D. A comparison of the tactile friction and cutting performance of textured scalpel blades modified by Direct Laser Writing and Direct Laser Interference Patterning processes. Procedia CIRP 2022, 111, 657–661. [Google Scholar] [CrossRef]
- Nitta, I.; Tsukiyama, Y.; Nomura, S.; Takatsu, N. Frictional characteristics of clamp surfaces of aneurysm clips finished by laser processing. J. Adv. Mech. Des. Syst. Manuf. 2016, 10, JAMDSM0026. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Yang, L.; Shi, Z. Biomimetic anti-adhesive surface microstructures on electrosurgical blade fabricated by long-pulse laser inspired by pangolin scales. Micromachines 2019, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, X.; Huang, Y.; Zhou, C.; Xu, B.; Fu, Q. Fabrication and cutting performance of bionic micro-serrated scalpels based on the miscanthus leaves. Tribol. Int. 2020, 145, 106162. [Google Scholar] [CrossRef]
- Meakin, L.B.; Murrell, J.C.; Doran, I.C.; Knowles, T.G.; Tivers, M.S.; Chanoit, G.P. Electrosurgery reduces blood loss and immediate postoperative inflammation compared to cold instruments for midline celiotomy in dogs: A randomized controlled trial. Vet. Surg. 2017, 46, 515–519. [Google Scholar] [CrossRef]
- Zheng, L.; Wan, J.; Long, Y.; Fu, H.; Zheng, J.; Zhou, Z. Effect of high-frequency electric field on the tissue sticking of minimally invasive electrosurgical devices. R. Soc. Open Sci. 2018, 5, 180125. [Google Scholar] [CrossRef]
- Sutton, P.A.; Awad, S.; Perkins, A.C.; Lobo, D.N. Comparison of lateral thermal spread using monopolar and bipolar diathermy, the Harmonic Scalpel™ and the Ligasure™. J. Br. Surg. 2010, 97, 428–433. [Google Scholar] [CrossRef]
- Tesler, A.B.; Kim, P.; Kolle, S.; Howell, C.; Ahanotu, O.; Aizenberg, J. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat. Commun. 2015, 6, 8649. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, J.; Wang, X. Adhesion behavior of textured electrosurgical electrode in an electric cutting process. Coatings 2020, 10, 596. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, H.J.; Lin, Y.H.; Sugiatno, E.; Ruslin, M.; Su, C.Y.; Ou, K.L.; Cheng, H.Y. Micro/nanostructured surface modification using femtosecond laser pulses on minimally invasive electrosurgical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 865–873. [Google Scholar] [CrossRef]
- Han, Z.; Fu, J.; Feng, X.; Niu, S.; Zhang, J.; Ren, L. Bionic anti-adhesive electrode coupled with maize leaf microstructures and TiO2 coating. RSC Adv. 2017, 7, 45287–45293. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, F.; Gu, H.; Feng, J.; Huang, D.; Zheng, L.; Yao, G.; Chen, Z.; Wang, C. Adhesion failure and anti-adhesion bionic structure optimization of surgical electrodes in soft tissue cutting. J. Manuf. Process. 2023, 89, 444–457. [Google Scholar] [CrossRef]
- Li, K.; Yao, W.; Xie, Y.; Zhang, J.; Li, B.; Wan, Z.; Zhang, Z.; Lu, L.; Tang, Y. A strongly hydrophobic and serum-repelling surface composed of CrN films deposited on laser-patterned microstructures that was optimized with an orthogonal experiment. Surf. Coat. Technol. 2020, 391, 125708. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Li, D.; Zhang, X.; Li, Q.; Liu, Z.; Fang, Y.; Zhang, S.; Man, J. In vivo blood-repellent performance of a controllable facile-generated superhydrophobic surface. ACS Appl. Mater. Interfaces 2021, 13, 29021–29033. [Google Scholar] [CrossRef]


| Classification | Instruments | Application | Required Functions |
|---|---|---|---|
| Implantable Devices | Artificial Bone | Replace human bone to assist in repairing bone tissue defects | Excellent bio-compatibility; promote the growth and adhesion of bone tissue and cells |
| Bone Plates + Screws | Connect and fixate, maintain the position of bones | High abrasion resistance; promote bone cell growth | |
| Vascular Stents | Support narrowed or occluded blood vessels, maintain patency of blood flow in the lumen | Good surface drag reduction and anti-adhesion properties, prevent restenosis | |
| Surgical Instruments | Electrosurgical Knife | Achieve tissue separation and coagulation, serve the purpose of cutting and hemostasis | Excellent anti-adhesion properties, reduce adhesion of biological tissues due to high surface temperatures |
| Surgical Forceps, Vascular Clamps | Grasp dense tissue, hold the ends of severed tissues | Provide stable grasping, strong wet friction ability to prevent slipping | |
| Scalpel | Used to cut skin and muscle | Low friction during cutting, reduce resistance to ensure a smooth incision |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Wang, Y.A.; Ng, V.; Yin, H.; Xu, S. Advancements in Laser-Processed Functional Surfaces for Medical Devices: A Current Review. Nanomaterials 2025, 15, 999. https://doi.org/10.3390/nano15130999
Xu Z, Wang YA, Ng V, Yin H, Xu S. Advancements in Laser-Processed Functional Surfaces for Medical Devices: A Current Review. Nanomaterials. 2025; 15(13):999. https://doi.org/10.3390/nano15130999
Chicago/Turabian StyleXu, Ziyi, Yanxiao Austin Wang, Vivian Ng, Hongyan Yin, and Shuai Xu. 2025. "Advancements in Laser-Processed Functional Surfaces for Medical Devices: A Current Review" Nanomaterials 15, no. 13: 999. https://doi.org/10.3390/nano15130999
APA StyleXu, Z., Wang, Y. A., Ng, V., Yin, H., & Xu, S. (2025). Advancements in Laser-Processed Functional Surfaces for Medical Devices: A Current Review. Nanomaterials, 15(13), 999. https://doi.org/10.3390/nano15130999






