Photosynthetic Microorganisms and Biogenic Synthesis of Nanomaterials for Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Cyanobacteria and Microalgae for Agriculture
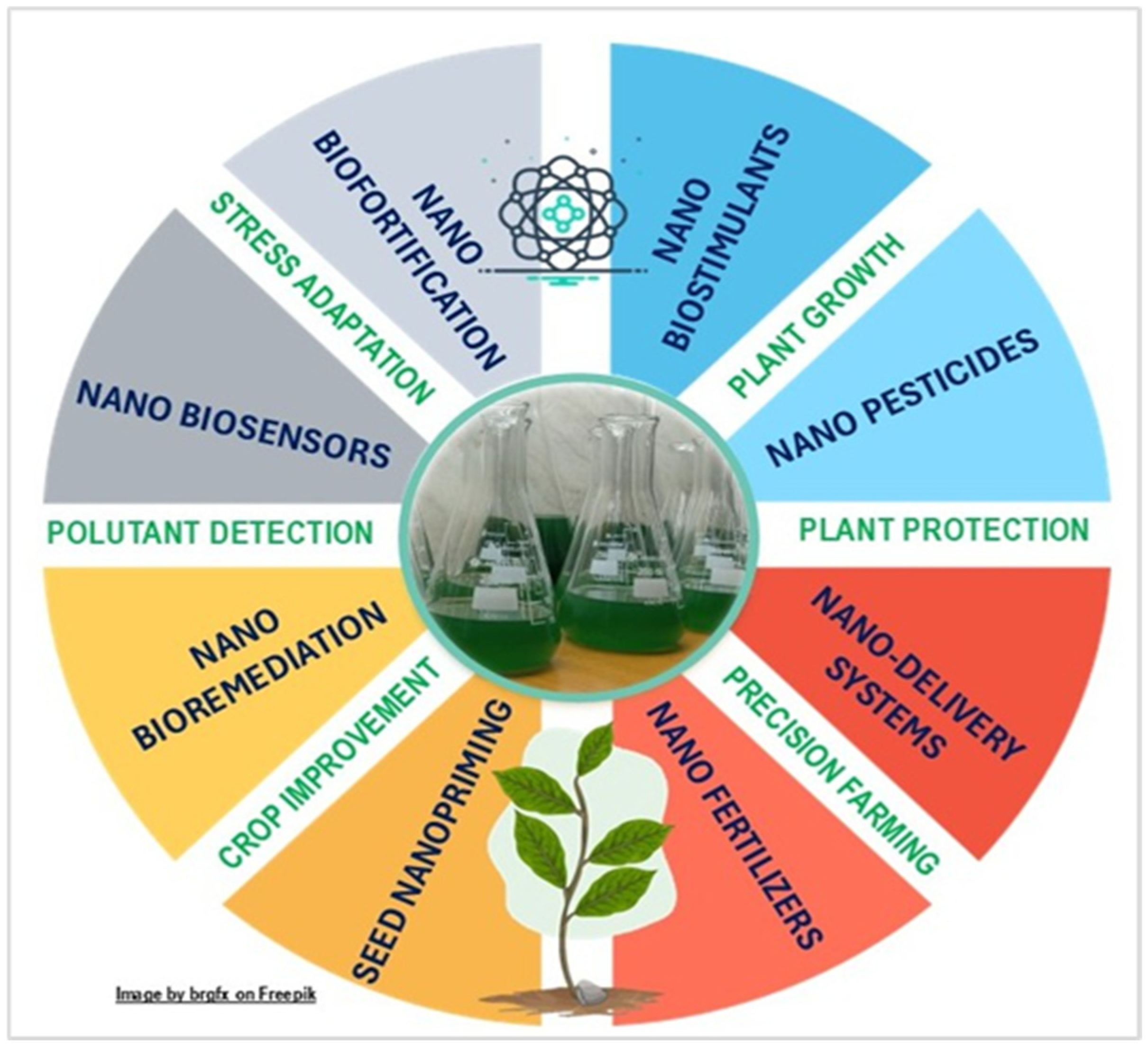
3.2. Cyanobacteria and Microalgae Applications in Nanotechnology
3.3. Cyanobacteria- and Microalgae-Based Nanosynthesis for Agriculture
3.3.1. Application in the Agri-Food Sector of Nanoparticles Synthesized by Photosynthetic Microorganisms
3.3.2. Nanoparticle-Enriched Extracts of Cyanobacteria and Microalgae in Plant Biotechnology
3.3.3. Cyanobacteria- and Microalgae-Based Nanosensors
3.3.4. Other Nanotechnological Applications of Cyanobacteria and Microalgae Impacting the Agri-Food Sector
4. Conclusions, Future Directions, and Final Remarks
- Despite ongoing discussions since the late 1990s about the potential of nanotechnology in agriculture, actual adoption, and application remain limited in this sector. For example, a bibliometric review of nanotechnology-related research from 2009 to 2019 found that only 10% of approximately 92,000 publications focused on agriculture [179]. Our updated analysis of publications from 2020 to 2024 confirms the continuation of this trend, indicating a persistent gap between laboratory innovation and agricultural implementation;
- The majority of literature examples related to the use of living organisms in nanosynthesis for agriculture focus primarily on plant-based materials;
- Although numerous nanoparticles have been successfully synthesized using cyanobacterial and microalgal strains, the vast majority of research has concentrated on medical and environmental applications rather than agriculture.
- -
- Future efforts should concentrate on identifying and characterizing novel strains with enhanced metabolic profiles and high nanoparticle synthesis capacity, along with optimizing their genetic or metabolic factors to improve yield and reproducibility;
- -
- While numerous studies have demonstrated the feasibility of both in vivo and in vitro nanoparticle synthesis, comparative investigations conducted under controlled experimental conditions are still necessary to determine the most efficient, reproducible, and economically viable approaches for specific applications;
- -
- Equally important is the development of standardized protocols for nanoparticle characterization, stability assessment, and biological safety validation, aligned with international regulatory frameworks, to support future commercial applications;
- -
- Applied research should extend beyond laboratory or controlled environments by implementing semi-industrial studies to validate the effectiveness of biogenic nanoparticles in real agricultural systems. Investigations are needed into their interactions with soil microbiota and potential ecological effects to ensure safe and sustainable use;
- -
- Integrating these nanoparticles into smart delivery systems, such as nano-encapsulated biofertilizers or targeted biopesticide formulations, could significantly enhance application precision and reduce environmental impact;
- -
- The integration of such biotechnological processes into circular economy models may also represent a strategic future direction, provided that their efficiency and safety are validated at the application scale.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Miu, B.A.; Dinischiotu, A. New Green Approaches in Nanoparticles Synthesis: An Overview. Molecules 2022, 27, 6472. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.M.O.; Bin-Meferij, M.M. Cyanobacteria—A Promising Platform in Green Nanotechnology: A Review on Nanoparticles Fabrication and Their Prospective Applications. Int. J. Nanomed. 2020, 15, 6033–6066. [Google Scholar] [CrossRef] [PubMed]
- Chugh, M.; Kumar, L.; Shah, M.P.; Bharadvaja, N. Algal Bioremediation of heavy metals: An insight into removal mechanisms, recovery of by-products, challenges, and future opportunities. Energy Nexus 2022, 7, 100129. [Google Scholar] [CrossRef]
- Pandey, S.; Rai, L.C.; Dubey, S.K. Cyanobacteria: Miniature Factories for Green Synthesis of Metallic Nanomaterials: A Review. Biometals 2022, 35, 653–674. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next Generation Plant Growth Additives: Functions, Applications, Challenges and Circular Bioeconomy Based Solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, D.; Sasmal, S. A Review of Green Synthesis of Metal Nanoparticles Using Algae. Front. Microbiol. 2021, 12, 693899. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, Z.; Sun, D.-W. Bioinspired Nanomodification Strategies: Moving from Chemical-Based Agrosystems to Sustainable Agriculture. ACS Nano 2021, 15, 12655–12686. [Google Scholar] [CrossRef]
- Paul, A.; Roychoudhury, A. Go Green to Protect Plants: Repurposing the Antimicrobial Activity of Biosynthesized Silver Nanoparticles to Combat Phytopathogens. Nanotechnol. Environ. Eng. 2021, 6, 10. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bano, A.; Waqar, A.; Khan, A.; Tariq, H. Phytostimulants in Sustainable Agriculture. Front. Sustain. Food Syst. 2022, 6, 801788. [Google Scholar] [CrossRef]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Naik, B.; Mishra, R.; Kumar, V.; Mishra, S.; Gupta, U.; Rustagi, S.; Gupta, A.K.; Preet, M.S.; Bhatt, S.C.; Rizwanuddin, S. Micro-Algae: Revolutionizing Food Production for a Healthy and Sustainable Future. J. Agric. Food Res. 2024, 15, 100939. [Google Scholar] [CrossRef]
- Santini, G.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Plant Biostimulants from Cyanobacteria: An Emerging Strategy to Improve Yields and Sustainability in Agriculture. Plants 2021, 10, 643. [Google Scholar] [CrossRef]
- Renaud, C.; Leys, N.; Wattiez, R. Photosynthetic Microorganisms, an Overview of Their Biostimulant Effects on Plants and Perspectives for Space Agriculture. J. Plant Interact. 2023, 18, 2242697. [Google Scholar] [CrossRef]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a Source of Biofertilizers for Sustainable Agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, P.; Wang, X.; Zou, M.; Liu, C.; Hao, J. Microalgae as Biofertilizer in Modern Agriculture. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 397–411. ISBN 978-981-15-0168-5. [Google Scholar]
- Cepoi, L. Secondary Metabolites in Cyanobacteria. In Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2024; pp. 283–311. ISBN 978-0-443-13231-5. [Google Scholar]
- Abreu, A.P.; Martins, R.; Nunes, J. Emerging Applications of Chlorella sp. and Spirulina (Arthrospira) sp. Bioengineering 2023, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Dmytryk, A.; Samoraj, M.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Bioactive Fatty Acids and Compounds from Spirulina (Arthrospira) platensis: Potential as Biostimulants for Plant Growth. Sustain. Chem. Pharm. 2022, 30, 100899. [Google Scholar] [CrossRef]
- Matthews, S.; Ali, A.; Siddiqui, Y.; Supramaniam, C.V. Plant Bio-Stimulant: Prospective, Safe and Natural Resources. J. Soil Sci. Plant Nutr. 2022, 22, 2570–2586. [Google Scholar] [CrossRef]
- Gitau, M.M.; Farkas, A.; Balla, B.; Ördög, V.; Futó, Z.; Maróti, G. Strain-Specific Biostimulant Effects of Chlorella and Chlamydomonas Green Microalgae on Medicago truncatula. Plants 2021, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- La Bella, E.; Baglieri, A.; Rovetto, E.I.; Stevanato, P.; Puglisi, I. Foliar Spray Application of Chlorella vulgaris Extract: Effect on the Growth of Lettuce Seedlings. Agronomy 2021, 11, 308. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Duraimurugan, M.; Sharmiladevi, N.; Lakshmi, L.P.; Rasheeq, A.A.; Arumugam, A.; Sampathkumar, P. Microalgal Liquid Biofertilizer and Biostimulant Effect on Green Gram (Vigna radiata L.) an Experimental Cultivation. Biomass Conv. Bioref. 2022, 12, 3007–3027. [Google Scholar] [CrossRef]
- Refaay, D.A.; El-Marzoki, E.M.; Abdel-Hamid, M.I.; Haroun, S.A. Effect of Foliar Application with Chlorella vulgaris, Tetradesmus dimorphus, and Arthrospira platensis as Biostimulants for Common Bean. J. Appl. Phycol. 2021, 33, 3807–3815. [Google Scholar] [CrossRef]
- Martini, F.; Beghini, G.; Zanin, L.; Varanini, Z.; Zamboni, A.; Ballottari, M. The Potential Use of Chlamydomonas reinhardtii and Chlorella sorokiniana as Biostimulants on Maize Plants. Algal Res. 2021, 60, 102515. [Google Scholar] [CrossRef] [PubMed]
- Righini, H.; Francioso, O.; Martel Quintana, A.; Roberti, R. Cyanobacteria: A Natural Source for Controlling Agricultural Plant Diseases Caused by Fungi and Oomycetes and Improving Plant Growth. Horticulturae 2022, 8, 58. [Google Scholar] [CrossRef]
- Sithole, N.; Gupta, S.; Dube, Z.; Ogbe, A.; Van Staden, J. Algae and Cyanobacteria-Based Biostimulants in Controlling Plant-Parasitic Nematodes: A Sustainable Approach for Crop Protection. Phytoparasitica 2023, 51, 803–813. [Google Scholar] [CrossRef]
- Poveda, J.; Díez-Méndez, A. Use of Elicitors from Macroalgae and Microalgae in the Management of Pests and Diseases in Agriculture. Phytoparasitica 2023, 51, 667–701. [Google Scholar] [CrossRef]
- Aldesuquy, H.; Abdel-Aziz, S.; Eltanahy, E. Applications Biotechnologiques des Microalgues Cultivées sur des Déchets Solides; Editions Notre Savoir: Edinburgh, UK, 2024; ISBN 978-620-8-08876-7. [Google Scholar]
- Dourou, M.; Dritsas, P.; Baeshen, M.N.; Elazzazy, A.; Al-Farga, A.; Aggelis, G. High-Added Value Products from Microalgae and Prospects of Aquaculture Wastewaters as Microalgae Growth Media. FEMS Microbiol. Lett. 2020, 367, fnaa081. [Google Scholar] [CrossRef]
- Saadat, E.; Ghorbanzadeh, N.; Farhangi, M.B.; Fazeli Sangani, M. Potential Application of Chlorella sp. Biomass Cultivated in Landfill Leachate as Agricultural Fertilizer. Arch. Agron. Soil. Sci. 2023, 69, 1193–1208. [Google Scholar] [CrossRef]
- Viegas, C.; Gouveia, L.; Gonçalves, M. Evaluation of Microalgae as Bioremediation Agent for Poultry Effluent and Biostimulant for Germination. Environ. Technol. Innov. 2021, 24, 102048. [Google Scholar] [CrossRef]
- Álvarez-González, A.; Greque De Morais, E.; Planas-Carbonell, A.; Uggetti, E. Enhancing Sustainability through Microalgae Cultivation in Urban Wastewater for Biostimulant Production and Nutrient Recovery. Sci. Total Environ. 2023, 904, 166878. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.M.; Satapathy, K.C.; Panda, B. Biofertilizers and Nanofertilizers for Sustainable Agriculture: Phycoprospects and Challenges. Sci. Total Environ. 2022, 803, 149990. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.; Parikh, K.D.; Lin, K.-Y.A.; Oza, R.; Larios, A.P.; Ghotekar, S. Algae-Based Synthesis to Generate Nanomaterials for Nanoremediation. In Green Nanoremediation; Policarpo Tonelli, F.M., Roy, A., Ananda Murthy, H.C., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 109–126. ISBN 978-3-031-30557-3. [Google Scholar]
- Arteaga-Castrejón, A.A.; Agarwal, V.; Khandual, S. Microalgae as a Potential Natural Source for the Green Synthesis of Nanoparticles. Chem. Commun. 2024, 60, 3874–3890. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, M.G.; Dias, D. Biogenic Synthesis of Metallic Nanoparticles from Algae. In Bioprospecting Algae for Nanosized Materials; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2021; pp. 71–91. ISBN 978-3-030-81556-1. [Google Scholar]
- Taghizadeh, S.-M.; Morowvat, M.H.; Negahdaripour, M.; Ebrahiminezhad, A.; Ghasemi, Y. Biosynthesis of Metals and Metal Oxide Nanoparticles Through Microalgal Nanobiotechnology: Quality Control Aspects. BioNanoScience 2021, 11, 209–226. [Google Scholar] [CrossRef]
- Khan, F.; Akhlaq, A.; Rasool, M.H.; Srinuanpan, S. Cyanobacterial Bioactive Compounds: Synthesis, Extraction, and Applications. In Pharmaceutical and Nutraceutical Potential of Cyanobacteria; Mehmood, M.A., Verma, P., Shah, M.P., Betenbaugh, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 215–243. ISBN 978-3-031-45522-3. [Google Scholar]
- Bhardwaj, A.K.; Naraian, R. Cyanobacteria as Biochemical Energy Source for the Synthesis of Inorganic Nanoparticles, Mechanism and Potential Applications: A Review. 3 Biotech 2021, 11, 445. [Google Scholar] [CrossRef]
- Ghosh, S.; Nag, M.; Lahiri, D.; Ghosh, S.; Dey, A.; Ray, R.R. Biogenic Nanoparticles from Cyanobacteria and Their Applications. In Agricultural Nanobiotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 79–103. ISBN 978-0-323-91908-1. [Google Scholar]
- Gupta, V.; Verma, N.K.; Choudhary, K.K. Cyanobacterial Nanoparticles: Application in Agriculture and Allied Sectors. In Cyanobacterial Lifestyle and Its Applications in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–40. ISBN 978-0-323-90634-0. [Google Scholar]
- Javed, M.R.; Shafiq, S.; Abd Allah, E.F.; Salman, M.; Perver, N.; Anwar, A.; Tul Zahra, F. Cyanobacteria-Based Green Synthesis of Nanoparticles for Industrial Applications. In Pharmaceutical and Nutraceutical Potential of Cyanobacteria; Mehmood, M.A., Verma, P., Shah, M.P., Betenbaugh, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 195–213. ISBN 978-3-031-45522-3. [Google Scholar]
- Chan, S.S.; Low, S.S.; Chew, K.W.; Ling, T.C.; Rinklebe, J.; Juan, J.C.; Ng, E.P.; Show, P.L. Prospects and Environmental Sustainability of Phyconanotechnology: A Review on Algae-Mediated Metal Nanoparticles Synthesis and Mechanism. Environ. Res. 2022, 212, 113140. [Google Scholar] [CrossRef]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green Synthesis of Silver Nanoparticles with Algae and the Importance of Capping Agents in the Process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Tauseef, A.; Hisam, F.; Hussain, T.; Caruso, A.; Hussain, K.; Châtel, A.; Chénais, B. Nanomicrobiology: Emerging Trends in Microbial Synthesis of Nanomaterials and Their Applications. J. Clust. Sci. 2023, 34, 639–664. [Google Scholar] [CrossRef]
- Das, B.S.; Das, A.; Mishra, A.; Arakha, M. Microbial Cells as Biological Factory for Nanoparticle Synthesis. Front. Mater. Sci. 2021, 15, 177–191. [Google Scholar] [CrossRef]
- Jacob, R.H.; Shanab, S.M.; Shalaby, E.A. Algal Biomass Nanoparticles: Chemical Characteristics, Biological Actions, and Applications. Biomass Conv. Bioref. 2023, 13, 11441–11455. [Google Scholar] [CrossRef]
- Adelere, I.A.; Lateef, A. Microalgal Nanobiotechnology and Its Applications—A Brief Overview. In Microbial Nanobiotechnology; Lateef, A., Gueguim-Kana, E.B., Dasgupta, N., Ranjan, S., Eds.; Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2021; pp. 233–255. ISBN 978-981-334-776-2. [Google Scholar]
- Shah, A.H.; Rather, M.A. Intracellular and Extracellular Microbial Enzymes and Their Role in Nanoparticle Synthesis. In Microbial Nanotechnology: Green Synthesis and Applications; Ansari, M.A., Rehman, S., Eds.; Springer: Singapore, 2021; pp. 41–59. ISBN 978-981-16-1922-9. [Google Scholar]
- Nahvi, I.; Belkahla, S.; Asiri, S.M.; Rehman, S. Overview and Prospectus of Algal Biogenesis of Nanoparticles. In Microbial Nanotechnology: Green Synthesis and Applications; Ansari, M.A., Rehman, S., Eds.; Springer: Singapore, 2021; pp. 121–134. ISBN 978-981-16-1922-9. [Google Scholar]
- El-Sheekh, M.M.; Morsi, H.H.; Hassan, L.H.S.; Ali, S.S. The Efficient Role of Algae as Green Factories for Nanotechnology and Their Vital Applications. Microbiol. Res. 2022, 263, 127111. [Google Scholar] [CrossRef]
- Li, X.; Mao, X.; Xie, W.; Liu, B.; Chen, F. Intracellular Biosynthesis of Gold Nanoparticles for Monitoring Microalgal Biomass via Surface-Enhanced Raman Spectroscopy. ACS Sustain. Chem. Eng. 2022, 10, 4872–4880. [Google Scholar] [CrossRef]
- Gebre, S.H. Bio-Inspired Synthesis of Metal and Metal Oxide Nanoparticles: The Key Role of Phytochemicals. J. Clust. Sci. 2023, 34, 665–704. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Fais, G.; Casula, M.; Borselli, M.; Giannaccare, G.; Locci, A.M.; Lai, N.; Orrù, R.; Cao, G.; Concas, A. Nanoparticles from Microalgae and Their Biomedical Applications. Mar. Drugs 2023, 21, 352. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; Hassan, L.H.S.; Morsi, H.H. Evaluation of Antimicrobial Activities of Blue-Green Algae-Mediated Silver and Gold Nanoparticles. Rend. Fis. Acc. Lincei 2021, 32, 747–759. [Google Scholar] [CrossRef]
- Mondal, A.; Mukherjee, A.; Pal, R. Phycosynthesis of Nanoiron Particles and Their Applications—A Review. Biocatal. Agric. Biotechnol. 2024, 55, 102986. [Google Scholar] [CrossRef]
- Choudhary, S.; Sangela, V.; Saxena, P.; Saharan, V.; Pugazhendhi, A. Harish Recent Progress in Algae-Mediated Silver Nanoparticle Synthesis. Int. Nano Lett. 2023, 13, 193–207. [Google Scholar] [CrossRef]
- Behera, M.; Behera, P.R.; Bhuyan, P.P.; Singh, L.; Pradhan, B. Algal Nanoparticles and Their Antibacterial Activity: Current Research Status and Future Prospectives. Drugs Drug Candidates 2023, 2, 554–570. [Google Scholar] [CrossRef]
- Gul, A.; Baig, M.N.; Ahmed, D.; Najam, Z.; Aslam, T.; Ali, S. Green Synthesis of Silver Nanoparticles from Spirulina platensis Extract: Antibacterial and Antioxidant Potential. BioNanoScience 2024, 14, 2327–2336. [Google Scholar] [CrossRef]
- Zayadi, R.A.; Abu Bakar, F. Comparative Study on Stability, Antioxidant and Catalytic Activities of Bio-Stabilized Colloidal Gold Nanoparticles Using Microalgae and Cyanobacteria. J. Environ. Chem. Eng. 2020, 8, 103843. [Google Scholar] [CrossRef]
- Jakhu, S.; Sharma, Y.; Sharma, K.; Vaid, K.; Dhar, H.; Kumar, V.; Singh, R.P.; Shekh, A.; Kumar, G. Production and Characterization of Microalgal Exopolysaccharide as a Reducing and Stabilizing Agent for Green Synthesis of Gold-Nanoparticle: A Case Study with a Chlorella sp. from Himalayan High-Altitude Psychrophilic Habitat. J. Appl. Phycol. 2021, 33, 3899–3914. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Elhadary, A.M.A.; Abuelmagd, M.A. Extruded Polysaccharide/Protein Matrix from Arthrospira platensis Cultures Mediated Silver Nanoparticles Biosynthesis and Capping. Appl. Nanosci. 2020, 10, 3839–3855. [Google Scholar] [CrossRef]
- Gusain, D.; Renuka, N.; Guldhe, A.; Bux, F. Use of Microalgal Lipids and Carbohydrates for the Synthesis of Carbon Dots via Hydrothermal Microwave Treatment. Inorg. Chem. Commun. 2021, 134, 109021. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Alkhateeb, M.A.; Alfassam, H.E.; Momenah, M.A.; Bin-Meferij, M.M. Harnessing Desmochloris Edaphica Strain CCAP 6006/5 for the Eco-Friendly Synthesis of Silver Nanoparticles: Insights into the Anticancer and Antibacterial Efficacy. Molecules 2024, 29, 3750. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green Synthesis and Characterization of Fe3O4 Nanoparticles Using Chlorella-K01 Extract for Potential Enhancement of Plant Growth Stimulating and Antifungal Activity. Sci. Rep. 2021, 11, 21996. [Google Scholar] [CrossRef]
- Koli, S.H.; Patil, S.V.; Mohite, B.V.; Otari, S.V. Microbial Pigment-Mediated Synthesis of Metal Nanoparticles. J. Clust. Sci. 2024, 35, 1625–1642. [Google Scholar] [CrossRef]
- Ismail, G.A.; El-Sheekh, M.M.; Samy, R.M.; Gheda, S.F. Antimicrobial, Antioxidant, and Antiviral Activities of Biosynthesized Silver Nanoparticles by Phycobiliprotein Crude Extract of the Cyanobacteria Spirulina platensis and Nostoc linckia. BioNanoScience 2021, 11, 355–370. [Google Scholar] [CrossRef]
- Annamalai, J.; Ummalyma, S.B.; Pandey, A.; Bhaskar, T. Recent Trends in Microbial Nanoparticle Synthesis and Potential Application in Environmental Technology: A Comprehensive Review. Environ. Sci. Pollut. Res. 2021, 28, 49362–49382. [Google Scholar] [CrossRef]
- Deb, D.; Sutradhar, A. Microalgal Nanobiotechnology for Biosynthesis of Metallic Nanoparticles: In-Depth into the Strategies, Mechanism and Nanofluidic Hydrodynamics. Biocatal. Agric. Biotechnol. 2024, 56, 103046. [Google Scholar] [CrossRef]
- Malode, U.; Patil, Y.S.; Selokar, Y.N.; Yadav, P.R.; Bhagat, R.P.; Nikose, V.M.; Thakare, R.U.; Nimbarte, S. Sustainable Approaches for the Synthesis of Biogenic Platinum Nanoparticles. Bull. Natl. Res. Cent. 2023, 47, 130. [Google Scholar] [CrossRef]
- Shera, S.S.; Banik, R.M. Algal Nanoparticles: Synthesis and Characterization. In Bioprospecting Algae for Nanosized Materials; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2021; pp. 25–69. ISBN 978-3-030-81556-1. [Google Scholar]
- Singh, G.; Chandra, S. Insights on Microbes-Mediated Greener Synthesis of Nanoparticles: Advantages and Challenges. In Applications of Nanotechnology in Microbiology; Chaughule, R.S., Lokur, A.S., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 41–57. ISBN 978-3-031-49932-6. [Google Scholar]
- Lamilla-Tamayo, L.; Escobar-Calderón, F.; Skalický, M. Reviewing the Potential of Algae Species as a Green Alternative to Produce Nanoparticles: Findings from a Database Analysis. Water 2023, 15, 2208. [Google Scholar] [CrossRef]
- Hamida, R.S.; AlMotwaa, S.M.; Al-Otaibi, W.A.; Alqhtani, H.A.; Ali, M.A.; Bin-Meferij, M.M. Apoptotic Induction by Biosynthesized Gold Nanoparticles Using Phormidesmis Communis Strain AB_11_10 against Osteosarcoma Cancer. Biomedicines 2024, 12, 1570. [Google Scholar] [CrossRef] [PubMed]
- Cepoi, L.; Rudi, L.; Zinicovscaia, I.; Chiriac, T.; Miscu, V.; Rudic, V. Biochemical Changes in Microalga Porphyridium Cruentum Associated with Silver Nanoparticles Biosynthesis. Arch. Microbiol. 2021, 203, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Bacteria in Heavy Metal Remediation and Nanoparticle Biosynthesis. ACS Sustain. Chem. Eng. 2020, 8, 5395–5409. [Google Scholar] [CrossRef]
- Singh, S.K.; Sharma, A.; Sharma, L.; Sundaram, S. Green Synthesis of Algal Nanoparticles: Harnessing Nature’s Biofactories for Sustainable Nanomaterials. In Biogenic Wastes-Enabled Nanomaterial Synthesis; Bhardwaj, A.K., Srivastav, A.L., Rai, S., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 257–284. ISBN 978-3-031-59082-5. [Google Scholar]
- Choudhary, S.; Kumawat, G.; Khandelwal, M.; Khangarot, R.K.; Saharan, V.; Nigam, S. Harish Phyco-Synthesis of Silver Nanoparticles by Environmentally Safe Approach and Their Applications. Sci. Rep. 2024, 14, 9568. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, G.; Mutaf, T.; Agba, H.C.; Elibol, M. Green Synthesis and Characterization of Titanium Nanoparticles Using Microalga, Phaeodactylum tricornutum. Geomicrobiol. J. 2022, 39, 83–96. [Google Scholar] [CrossRef]
- Choudhary, S.; Kumawat, G.; Khandelwal, M.; Khangarot, R.K.; Sangela, V.; Kumar, M.; Deora, S.; Rai, N.; Saharan, V. Harish Sustainable Phyco-Fabrication of Silver Nanoparticles Using Coelastrella Terrestris and Their Multiple Downstream Applications. Biocatal. Agric. Biotechnol. 2023, 53, 102854. [Google Scholar] [CrossRef]
- Bi, L.; Chen, Y.-P.; Wang, C.; Su, J.; Pan, G. Microalgae-Derived Cellulose/Inorganic Nanocomposite Rattle-Type Microspheres as an Advanced Sensor for Pollutant Detection. Chem. Eng. J. 2020, 395, 125073. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Mugren, N.; Al-Zaban, M.I.; Bin-Meferij, M.M.; Redhwan, A. Planophila laetevirens-Mediated Synthesis of Silver Nanoparticles: Optimization, Characterization, and Anticancer and Antibacterial Potentials. ACS Omega 2023, 8, 29169–29188. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.; Ali, M.A.; Sharif, F.T.; Sonbol, H.; Bin-Meferij, M.M. Biofabrication of Silver Nanoparticles Using Nostoc Muscorum Lukesova 2/91: Optimization, Characterization, and Biological Applications. Int. J. Nanomed. 2023, 18, 5625–5649. [Google Scholar] [CrossRef]
- Mondal, A.; Dey, I.; Mukherjee, A.; Ismail, A.; Satpati, G.G.; Banerjee, S.; Paul, S.; Paul, S.; Pal, R. Spirulina Biomass Loaded with Iron Nanoparticles: A Novel Biofertilizer for the Growth and Enrichment of Iron Content in Rice Plants. Biocatal. Agric. Biotechnol. 2024, 61, 103387. [Google Scholar] [CrossRef]
- Savvidou, M.G.; Kontari, E.; Kalantzi, S.; Mamma, D. Green Synthesis of Silver Nanoparticles Using the Cell-Free Supernatant of Haematococcus pluvialis Culture. Materials 2023, 17, 187. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.E.; El Semary, N.A.; Younis, N.S. Silver Nanoparticle Production by the Cyanobacterium Cyanothece sp.: De Novo Manipulation of Nano-Biosynthesis by Phytohormones. Life 2022, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Abdelmeguid, N.E.; Ali, M.A.; Bin-Meferij, M.M.; Khalil, M.I. Synthesis of Silver Nanoparticles Using a Novel Cyanobacteria desertifilum sp. Extract: Their Antibacterial and Cytotoxicity Effects. Int. J. Nanomed. 2020, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Zolfaghari, M.R.; Aghaei, S.S.; Zargar, M.; Noghabi, K.A. Desertifilum sp. EAZ03 Cell Extract as a Novel Natural Source for the Biosynthesis of Zinc Oxide Nanoparticles and Antibacterial, Anticancer and Antibiofilm Characteristics of Synthesized Zinc Oxide Nanoparticles. J. Appl. Microbiol. 2022, 132, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.L.; Hamouda, H.M.; Goda, H.A.; Sadik, M.W.; Moghanm, F.S.; Ghoneim, A.M.; Alenezi, M.A.; Alnomasy, S.F.; Alam, P.; Elsayed, T.R. Biosynthesis and Characterization of Silver Nanoparticles Produced by Phormidium ambiguum and Desertifilum tharense Cyanobacteria. Bioinorg. Chem. Appl. 2022, 2022, 9072508. [Google Scholar] [CrossRef] [PubMed]
- Nowruzi, B.; Jalil, B.S.; Metcalf, J.S. Antifungal Screening of Selenium Nanoparticles Biosynthesized by Microcystin-Producing Desmonostoc Alborizicum. BMC Biotechnol. 2023, 23, 41. [Google Scholar] [CrossRef]
- Aslam, F.; Minhas, L.A.; Kaleem, M.; Jabeen, A.; Akram, A.; Malik, H.A.; Farooqi, H.M.U.; Amin, M.W.; Mumtaz, A.S. Sustainable Synthesis of Cobalt Oxide Nanoparticles (Co3O4-NPs) Using Extract of Nodosilinea nodulosa: Characterization and Potential Biological Applications. BioNanoScience 2024, 14, 4764–4778. [Google Scholar] [CrossRef]
- Arya, A.; Gupta, K.; Chundawat, T.S. In Vitro Antimicrobial and Antioxidant Activity of Biogenically Synthesized Palladium and Platinum Nanoparticles Using Botryococcus braunii. Turk. J. Pharm. Sci. 2020, 17, 299–306. [Google Scholar] [CrossRef]
- Saritha, G.N.G.; Anju, T.; Kumar, A. Nanotechnology—Big Impact: How Nanotechnology Is. Changing the Future of Agriculture? J. Agric. Food Res. 2022, 10, 100457. [Google Scholar] [CrossRef]
- Sarma, H.; Joshi, S.J.; Prasad, R.; Jampilek, J. (Eds.) Biobased Nanotechnology for Green Applications; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-61984-8. [Google Scholar]
- El-Ramady, H.; Abdalla, N.; Sári, D.; Ferroudj, A.; Muthu, A.; Prokisch, J.; Fawzy, Z.F.; Brevik, E.C.; Solberg, S.Ø. Nanofarming: Promising Solutions for the Future of the Global Agricultural Industry. Agronomy 2023, 13, 1600. [Google Scholar] [CrossRef]
- Ernst, D.; Kolenčík, M.; Straka, V.; Šebesta, M.; Ďurišová, Ľ.; Tomovičová, L.; Kratošová, G.; Ravza, I.; Žitniak Čurná, V.; Černý, I.; et al. Appropriate Agro-Environmental Strategy for ZnO-Nanoparticle Foliar Application on Soybean. Soil Sci. Ann. 2024, 75, 193449. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, A.; Seth, A.; Guleria, I.; Arya, V.; Shahi, S.K. Nanotechnology Intervention for Sustainable Agriculture: Challenges and Possibilities. In Nanotechnology: Agriculture, Environment and Health; Balkrishna, A., Thakur, N., Arya, V., Kumar, A., Eds.; Springer Nature: Singapore, 2024; ISBN 978-981-97-6813-4. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Chen, J.; Zhu, Y.; Zhang, Z. The Effects of Several Metal Nanoparticles on Seed Germination and Seedling Growth: A Meta-Analysis. Coatings 2022, 12, 183. [Google Scholar] [CrossRef]
- Mariyam, S.; Upadhyay, S.K.; Chakraborty, K.; Verma, K.K.; Duhan, J.S.; Muneer, S.; Meena, M.; Sharma, R.K.; Ghodake, G.; Seth, C.S. Nanotechnology, a Frontier in Agricultural Science, a Novel Approach in Abiotic Stress Management and Convergence with New Age Medicine—A Review. Sci. Total Environ. 2024, 912, 169097. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Rojas, C.; Pérez-de-Luque, A. Nanobiosensors and Nanoformulations in Agriculture: New Advances and Challenges for Sustainable Agriculture. Emerg. Top. Life Sci. 2023, 7, 229–238. [Google Scholar] [CrossRef]
- Neme, K.; Nafady, A.; Uddin, S.; Tola, Y.B. Application of Nanotechnology in Agriculture, Postharvest Loss Reduction and Food Processing: Food Security Implication and Challenges. Heliyon 2021, 7, e08539. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.N.; Verma, I.; Kumar, M. Cyanobacteria: Potential Source of Biofertilizer and Synthesizer of Metallic Nanoparticles. In Advances in Cyanobacterial Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 351–367. ISBN 978-0-12-819311-2. [Google Scholar]
- Singh, K.; Chauhan, A.; Sonu; Kumar, P.; Verma, R.; Kumar, V.; Thakur, P. Nano-Biofertilizers and Nano-Biopesticides: Impact of Future Agrochemicals. In Nanotechnology: Agriculture, Environment and Health; Balkrishna, A., Thakur, N., Arya, V., Kumar, A., Eds.; Springer Nature: Singapore, 2024; ISBN 978-981-97-6813-4. [Google Scholar] [CrossRef]
- Singh, V.; Bhat, R.A.; Dar, G.H. (Eds.) Nanobiostimulants: Emerging Strategies for Agricultural Sustainability; Springer Nature: Cham, Switzerland, 2024; ISBN 978-3-031-68137-0. [Google Scholar] [CrossRef]
- Shahcheraghi, N.; Golchin, H.; Sadri, Z.; Tabari, Y.; Borhanifar, F.; Makani, S. Nano-Biotechnology, an Applicable Approach for Sustainable Future. 3 Biotech 2022, 12, 65. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in Agriculture: Use of Nanomaterials to Promote Plant Growth and Stress Tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef]
- Ghahari, S.; Ghahari, S.; Ramzani, M.; Nematzadeh, G.A. Microbial Nanotechnology in Life Sciences: An Opportunity for Green Applications. In Biobased Nanotechnology for Green Applications; Sarma, H., Joshi, S.J., Prasad, R., Jampilek, J., Eds.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2021; pp. 239–269. ISBN 978-3-030-61984-8. [Google Scholar]
- Ouzakar, S.; Skali Senhaji, N.; Saidi, M.Z.; El Hadri, M.; El Baaboua, A.; El Harsal, A.; Abrini, J. Antibacterial and Antifungal Activity of Zinc Oxide Nanoparticles Produced by Phaeodactylum Tricornutum Culture Supernatants and Their Potential Application to Extend the Shelf Life of Sweet Cherry (Prunus avium L.). Biocatal. Agric. Biotechnol. 2023, 49, 102666. [Google Scholar] [CrossRef]
- Osborne, C.J.; Norton, A.E.; Whitworth, R.J.; Silver, K.S.; Cohnstaedt, L.W. Tiny Silver Bullets: Silver Nanoparticles Are Insecticidal to Culicoides Sonorensis (Diptera: Ceratopogonidae) Biting Midge Larvae. J. Med. Entomol. 2024, 61, 1427–1434. [Google Scholar] [CrossRef]
- Anand, U.; Chandel, A.K.S.; Oleksak, P.; Mishra, A.; Krejcar, O.; Raval, I.H.; Dey, A.; Kuca, K. Recent Advances in the Potential Applications of Luminescence-Based, SPR-Based, and Carbon-Based Biosensors. Appl. Microbiol. Biotechnol. 2022, 106, 2827–2853. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Wang, B.; Verma, M.L. Development and Applications of Nanobiosensors for Sustainable Agricultural and Food Industries: Recent Developments, Challenges and Perspectives. Environ. Technol. Innov. 2022, 26, 102371. [Google Scholar] [CrossRef]
- Zain, M.; Ma, H.; Nuruzzaman, M.; Chaudhary, S.; Nadeem, M.; Shakoor, N.; Azeem, I.; Duan, A.; Sun, C.; Ahamad, T. Nanotechnology Based Precision Agriculture for Alleviating Biotic and Abiotic Stress in Plants. Plant Stress 2023, 10, 100239. [Google Scholar] [CrossRef]
- Aswathi, V.P.; Meera, S.; Maria, C.G.A.; Nidhin, M. Green Synthesis of Nanoparticles from Biodegradable Waste Extracts and Their Applications: A Critical Review. Nanotechnol. Environ. Eng. 2023, 8, 377–397. [Google Scholar] [CrossRef]
- Bora, K.A.; Hashmi, S.; Zulfiqar, F.; Abideen, Z.; Ali, H.; Siddiqui, Z.S.; Siddique, K.H.M. Recent Progress in Bio-Mediated Synthesis and Applications of Engineered Nanomaterials for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 999505. [Google Scholar] [CrossRef] [PubMed]
- Gauba, A.; Hari, S.K.; Ramamoorthy, V.; Vellasamy, S.; Govindan, G.; Valan Arasu, M. The Versatility of Green Synthesized Zinc Oxide Nanoparticles in Sustainable Agriculture: A Review on Metal-Microbe Interaction That Rewards Agriculture. Physiol. Mol. Plant Pathol. 2023, 125, 102023. [Google Scholar] [CrossRef]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.-O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef]
- Kour, D.; Khan, S.S.; Kumari, S.; Singh, S.; Khan, R.T.; Kumari, C.; Kumari, S.; Dasila, H.; Kour, H.; Kaur, M.; et al. Microbial Nanotechnology for Agriculture, Food, and Environmental Sustainability: Current Status and Future Perspective. Folia Microbiol. 2024, 69, 491–520. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of Green Nanoparticles for Energy, Biomedical, Environmental, Agricultural, and Food Applications: A Review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Flores-Contreras, E.A.; González-González, R.B.; Pablo Pizaña-Aranda, J.J.; Parra-Arroyo, L.; Rodríguez-Aguayo, A.A.; Iñiguez-Moreno, M.; González-Meza, G.M.; Araújo, R.G.; Ramírez-Gamboa, D.; Parra-Saldívar, R.; et al. Agricultural Waste as a Sustainable Source for Nanoparticle Synthesis and Their Antimicrobial Properties for Food Preservation. Front. Nanotechnol. 2024, 6, 1346069. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Boddu, V.M.; Chadha, N.K.; Chakraborty, P.; Kumar, J.; Krishna, G.; Pathak, H. Metallic and Non-Metallic Nanoparticles from Plant, Animal, and Fisheries Wastes: Potential and Valorization for Application in Agriculture. Environ. Sci. Pollut. Res. 2022, 29, 81130–81165. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Alghuthaymi, M.A. (Eds.) Nanofertilizers for Sustainable Agroecosystems: Recent Advances and Future Trends; Nanotechnology in the Life Sciences; Springer Nature: Cham, Switzerland, 2024; ISBN 978-3-031-41328-5. [Google Scholar]
- Akhtar, N.; Ilyas, N.; Meraj, T.A.; Pour-Aboughadareh, A.; Sayyed, R.Z.; Mashwani, Z.-R.; Poczai, P. Improvement of Plant Responses by Nanobiofertilizer: A Step towards Sustainable Agriculture. Nanomaterials 2022, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Sridhar, K.; Stephen Inbaraj, B.; Chawla, P.; Tripathi, M.; Sharma, M. Nano-Biofertilizer Formulations for Agriculture: A Systematic Review on Recent Advances and Prospective Applications. Bioengineering 2023, 10, 1010. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Nayarisseri, A.; Choudhary, R.; Bhat, R.A. Nanobiostimulants: Precision Tools for Harnessing Soil Microbes and Elevating Agricultural Productivity. In Nanobiostimulants; Singh, V., Bhat, R.A., Dar, G.H., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 135–163. ISBN 978-3-031-68137-0. [Google Scholar]
- Ullah, Z.; Haq, S.I.U.; Ullah, A.; Asghar, M.A.; Seleiman, M.F.; Saleem, K.; Zeng, F.; Sama, N.U.; Kamran, K.; Ahmad, S. Effect of Green Synthesized Silver Nanoparticles on Growth and Physiological Responses of Pearl Millet under Salinity Stress. Environ. Dev. Sustain. 2024, 27, 625–644. [Google Scholar] [CrossRef]
- Shaikhaldein, H.O.; Al-Qurainy, F.; Babiker, K.A.; Nadeem, M.; Khan, S.; Tarroum, M.; Salih, A.M. Evaluating Impacts of Biosynthetic Silver Nanoparticles on Morphophysiological Responses in Barley (Hordeum vulgare L.). J. Nanomater. 2024, 2024, 1–13. [Google Scholar] [CrossRef]
- Shaikhaldein, H.O.; Al-Qurainy, F.; Nadeem, M.; Khan, S.; Tarroum, M.; Salih, A.M.; Al-Hashimi, A. Biosynthesis of Copper Nanoparticles Using Solenostemma Argel and Their Effect on Enhancing Salt Tolerance in Barley Plants. Sci. Rep. 2024, 14, 12701. [Google Scholar] [CrossRef] [PubMed]
- Jampílek, J.; Kráľová, K.; Fedor, P. Bioactivity of Nanoformulated Synthetic and Natural Insecticides and Their Impact on Environment. In Nanopesticides; Fraceto, L.F., S.S. De Castro, V.L., Grillo, R., Ávila, D., Caixeta Oliveira, H., Lima, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 165–225. ISBN 978-3-030-44872-1. [Google Scholar]
- Babaniyi, B.R.; Ogundele, O.D.; Thompson, S.O.; Aransiola, S.A. Microbial Nanomaterial Synthesis: Types and Applications. In Microbial Processes for Synthesizing Nanomaterials; Maddela, N.R., Rodríguez Díaz, J.M., Branco Da Silva Montenegro, M.C., Prasad, R., Eds.; Environmental and Microbial Biotechnology; Springer Nature: Singapore, 2023; pp. 3–28. ISBN 978-981-99-2807-1. [Google Scholar]
- Jampílek, J.; Kráľová, K. Nanoparticles for Improving and Augmenting Plant Functions. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 171–227. ISBN 978-0-12-820092-6. [Google Scholar]
- El-Sayyad, G.S.; Elfadil, D.; Mosleh, M.A.; Hasanien, Y.A.; Mostafa, A.; Abdelkader, R.S.; Refaey, N.; Elkafoury, E.M.; Eshaq, G.; Abdelrahman, E.A.; et al. Eco-Friendly Strategies for Biological Synthesis of Green Nanoparticles with Promising Applications. BioNanoScience 2024, 14, 3617–3659. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, Extraction and Characterization of Chlorella vulgaris Soluble Polysaccharides and Their Applications in AgNPs Biosynthesis and Biostimulation of Plant Growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef] [PubMed]
- Shamshad, A.; Ijaz, S.; Arooj, I.; Tahir, S. Characterization and Sustainable Applications of Multifunctional, Non-Genotoxic ZnO-NPs Biofabricated by Organic Biomass Extract of Nostoc sp. SI-SN. BioNanoScience 2024, 14, 5527–5549. [Google Scholar] [CrossRef]
- Mahawar, H.; Prasanna, R.; Gogoi, R.; Singh, A.K. Differential Modes of Disease Suppression Elicited by Silver Nanoparticles Alone and Augmented with Calothrix elenkinii against Leaf Blight in Tomato. Eur. J. Plant Pathol. 2020, 157, 663–678. [Google Scholar] [CrossRef]
- Mahawar, H.; Prasanna, R.; Gogoi, R.; Singh, S.B.; Chawla, G.; Kumar, A. Synergistic Effects of Silver Nanoparticles Augmented Calothrix Elenkinii for Enhanced Biocontrol Efficacy against Alternaria Blight Challenged Tomato Plants. 3 Biotech 2020, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, H.M.; Ahmed, O.K.; Fayed, S.A.-K.; Hammam, K.A.-M.; Yousef, R.S. Enhancing French Basil Growth through Synergistic Foliar Treatment with Copper Nanoparticles and Spirulina sp. BMC Plant Biol. 2024, 24, 512. [Google Scholar] [CrossRef] [PubMed]
- Cepoi, L.; Chiriac, T.; Rudi, L.; Zosim, L.; Iatco, I.; Rudic, V. Extracts Derived from Spirulina Cultivated on Media with Cu and CuO Nanoparticles as Active Agents for Triticale Seed Priming. In Proceedings of the International Scientific Conference Ed. 8, Chişinău, Moldova, 7–8 October 2024; pp. 519–524. [Google Scholar]
- El Semary, N.A.H.; Alouane, M.H.H.; Nasr, O.; Aldayel, M.F.; Alhaweti, F.H.; Ahmed, F. Salinity Stress Mitigation Using Encapsulated Biofertilizers for Sustainable Agriculture. Sustainability 2020, 12, 9218. [Google Scholar] [CrossRef]
- Abuo El-Kasem, S.A.A.; Tony, E.; Darwesh, O.D.; Matter, I.A. Effect of Scenedesmus Obliquus Extract and Its Biosynthesized Zinc Oxide Nanoparticles as Foliar Application on Growth and Yield of Tomato Grown in Late Summer Seasons. SVU-Int. J. Agric. Sci. 2024, 6, 45–65. [Google Scholar] [CrossRef]
- Ahmed, H.; Gaafar, R.; Elsherif, D.; Haider, A.; Shanshory, A. Effect of Silver Nanoparticles and Chlorella sp. Suspension on Vegetative Growth of Eruca Sativa. Egypt J. Exp. Biol. 2020, 16, 27. [Google Scholar] [CrossRef]
- Munaro, D.; Mazo, C.H.; Bauer, C.M.; Da Silva Gomes, L.; Teodoro, E.B.; Mazzarino, L.; Da Rocha Veleirinho, M.B.; Moura E Silva, S.; Maraschin, M. A Novel Biostimulant from Chitosan Nanoparticles and Microalgae-Based Protein Hydrolysate: Improving Crop Performance in Tomato. Sci. Hortic. 2024, 323, 112491. [Google Scholar] [CrossRef]
- Antonacci, A.; Frisulli, V.; Carvalho, L.B.; Fraceto, L.F.; Miranda, B.; De Stefano, L.; Johanningmeier, U.; Giardi, M.T.; Scognamiglio, V. An All-Green Photo-Electrochemical Biosensor Using Microalgae Immobilized on Eco-Designed Lignin-Based Screen-Printed Electrodes to Detect Sustainable Nanoherbicides. Int. J. Mol. Sci. 2023, 24, 10088. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.P.; Biswas, A.; Vanlalveni, C.; Lalfakzuala, R.; Gupta, I.; Ingle, P.; Rokhum, L.; Rai, M. Biogenic Synthesis of Nanoparticles and Their Role in the Management of Plant Pathogenic Fungi. In Microbial Nanotechnology; Rai, M., Golińska, P., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 135–161. ISBN 978-0-429-27633-0. [Google Scholar]
- Abdel-Aziz, S.M.; Prasad, R.; El Enshasy, H.; Sukmawati, D. Prospects of Microbial Nanotechnology for Promoting Climate Resilient Agriculture. In Nanoparticles and Plant-Microbe Interactions; Elsevier: Amsterdam, The Netherlands, 2023; pp. 163–186. ISBN 978-0-323-90619-7. [Google Scholar]
- Ali, M.A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef]
- El-Refaey, A.A.; Salem, S.S. Algae Materials for Bionanopesticides. In Algae Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 219–230. ISBN 978-0-443-18816-9. [Google Scholar]
- Singh, Y.; Kaushal, S.; Sodhi, R.S. Biogenic Synthesis of Silver Nanoparticles Using Cyanobacterium Leptolyngbya sp. WUC 59 Cell-Free Extract and Their Effects on Bacterial Growth and Seed Germination. Nanoscale Adv. 2020, 2, 3972–3982. [Google Scholar] [CrossRef]
- Abideen, Z.; Waqif, H.; Munir, N.; El-Keblawy, A.; Hasnain, M.; Radicetti, E.; Mancinelli, R.; Nielsen, B.L.; Haider, G. Algal-Mediated Nanoparticles, Phycochar, and Biofertilizers for Mitigating Abiotic Stresses in Plants: A Review. Agronomy 2022, 12, 1788. [Google Scholar] [CrossRef]
- Sowmiya, K.; Praveen, K.; Krishna Kumar, S.; Priya, M. Algal Nanobiofertilizers: Prospects and Challenges. In Metabolomics, Proteomics and Gene Editing Approaches in Biofertilizer Industry; Kaur, S., Dwibedi, V., Sahu, P.K., Eds.; Springer Nature: Singapore, 2024; pp. 177–200. ISBN 978-981-97-2909-8. [Google Scholar]
- Somna, G.; Challabathula, D.; Bakka, K. Nanobiofertilizers: Applications, Crop Productivity, and Sustainable Agriculture. In Nanofertilizers for Sustainable Agroecosystems; Abd-Elsalam, K.A., Alghuthaymi, M.A., Eds.; Nanotechnology in the Life Sciences; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 233–258. ISBN 978-3-031-41328-5. [Google Scholar]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Bleakley, B.; Zhou, R. Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology. Molecules 2024, 29, 2534. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, J.; Chen, C.; Zhao, L.; Wu, Z.; Liu, L.; Cai, D. Promoting Desert Biocrust Formation Using Aquatic Cyanobacteria with the Aid of MOF-Based Nanocomposite. Sci. Total Environ. 2020, 708, 134824. [Google Scholar] [CrossRef] [PubMed]
- Allouzi, M.M.A.; Allouzi, S.; Al-Salaheen, B.; Khoo, K.S.; Rajendran, S.; Sankaran, R.; Sy-Toan, N.; Show, P.L. Current Advances and Future Trend of Nanotechnology as Microalgae-Based Biosensor. Biochem. Eng. J. 2022, 187, 108653. [Google Scholar] [CrossRef]
- Dwivedi, S.; Ahmad, I.Z. Microalgal Nanotechnology for the Remediation of Environmental Pollutants. In Bioprospecting Algae for Nanosized Materials; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2021; pp. 403–428. ISBN 978-3-030-81556-1. [Google Scholar]
- Mahmoud, R.H.; Hassan, R.Y.A. Algae-Derived Biosensor Materials and Their Applications. In Algae Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 203–218. ISBN 978-0-443-18816-9. [Google Scholar]
- Gupta, A.; Rayeen, F.; Mishra, R.; Tripathi, M.; Pathak, N. Nanotechnology Applications in Sustainable Agriculture: An Emerging Eco-Friendly Approach. Plant Nano Biol. 2023, 4, 100033. [Google Scholar] [CrossRef]
- Moreira, J.B.; De Morais, E.G.; De Almeida, A.C.A.; Pontes, J.F.; Grenha, A.; Barreira, L.; Varela, J.; Costa, J.A.V.; De Morais, M.G. Microalgal Applications in Nanotechnology: An Outstanding Tool for Nanocompounds Synthesis and Bioproducts Obtention. In Bioprospecting Algae for Nanosized Materials; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2021; pp. 95–116. ISBN 978-3-030-81556-1. [Google Scholar]
- Yucetepe, A. Strategies for Nanoencapsulation of Algal Proteins, Protein Hydrolysates and Bioactive Peptides: The Effect of Encapsulation Techniques on Bioactive Properties. In Bioprospecting Algae for Nanosized Materials; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2021; pp. 211–227. ISBN 978-3-030-81556-1. [Google Scholar]
- Blanco-Llamero, C.; Galindo-Camacho, R.M.; Fonseca, J.; Santini, A.; Señoráns, F.J.; Souto, E.B. Development of Lipid Nanoparticles Containing Omega-3-Rich Extract of Microalga Nannochlorpsis Gaditana. Foods 2022, 11, 3749. [Google Scholar] [CrossRef]
- Govindasamy, R.; Gayathiri, E.; Sankar, S.; Venkidasamy, B.; Prakash, P.; Rekha, K.; Savaner, V.; Pari, A.; Thirumalaivasan, N.; Thiruvengadam, M. Emerging Trends of Nanotechnology and Genetic Engineering in Cyanobacteria to Optimize Production for Future Applications. Life 2022, 12, 2013. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, C.; Lin, Y.; Gu, A.Z. Nano-Titanium Dioxide Exposure Impacts Nitrogen Metabolism Pathways in Cyanobacteria. Environ. Eng. Sci. 2021, 38, 469–480. [Google Scholar] [CrossRef]
- Kumar, M.; Sabu, S.; Sangela, V.; Meena, M.; Rajput, V.D.; Minkina, T.; Vinayak, V. Harish The Mechanism of Nanoparticle Toxicity to Cyanobacteria. Arch. Microbiol. 2023, 205, 30. [Google Scholar] [CrossRef] [PubMed]
- Padash, A.; Heydarnajad Giglou, R.; Torabi Giglou, M.; Azarmi, R.; Mokhtari, A.M.; Gohari, G.; Amini, M.; Cruz, C.; Ghorbanpour, M. Comparing the Toxicity of Tungsten and Vanadium Oxide Nanoparticles on Spirulina platensis. Environ. Sci. Pollut. Res. 2023, 30, 45067–45076. [Google Scholar] [CrossRef] [PubMed]
- Fazelian, N.; Movafeghi, A.; Yousefzadi, M.; Rahimzadeh, M.; Zarei, M. Impact of Silver Nanoparticles on the Growth, Fatty Acid Profile, and Antioxidative Response of Nannochloropsis oculata. Acta Physiol. Plant 2020, 42, 126. [Google Scholar] [CrossRef]
- Wang, F.; Liu, T.; Guan, W.; Xu, L.; Huo, S.; Ma, A.; Zhuang, G.; Terry, N. Development of a Strategy for Enhancing the Biomass Growth and Lipid Accumulation of Chlorella sp. UJ-3 Using Magnetic Fe3O4 Nanoparticles. Nanomaterials 2021, 11, 2802. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Gao, X.; Liu, C.; Liang, W.; Xue, H.; Li, Z.; Jin, H. Application of Nanomaterials in the Production of Biomolecules in Microalgae: A Review. Mar. Drugs 2023, 21, 594. [Google Scholar] [CrossRef] [PubMed]
- Mansor, M.; Xu, J. Benefits at the Nanoscale: A Review of NANOPARTICLE-ENABLED Processes Favouring Microbial Growth and Functionality. Environ. Microbiol. 2020, 22, 3633–3649. [Google Scholar] [CrossRef] [PubMed]
- Alishah Aratboni, H.; Rafiei, N.; Mehdizadeh Allaf, M.; Abedini, S.; Naseema Rasheed, R.; Seif, A.; Barati, B.; Wang, S.; Morones-Ramírez, J.R. Nanotechnology: An Outstanding Tool for Increasing and Better Exploitation of Microalgae Valuable Compounds. Algal Res. 2023, 71, 103019. [Google Scholar] [CrossRef]
- Bhaskar, R.; Pandey, S.P.; Kumar, U.; Kim, H.; Jayakodi, S.K.; Gupta, M.K.; Han, S.S. Nanobionics for Sustainable Crop Production: Recent Development to Regulate Plant Growth and Protection Strategies from Pests. OpenNano 2024, 15, 100198. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Tripathi, D.K.; Prasad, S.M.; Chauhan, D.K. (Eds.) Plant Responses to Nanomaterials: Recent Interventions, and Physiological and Biochemical Responses; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-36739-8. [Google Scholar]
- Pant, D.; Tiwari, B.; Taruna; Sharma, H. Nanobionics in Bioenergy and Crop Production. In Stress Biology in Photosynthetic Organisms; Mishra, A.K., Ed.; Springer Nature: Singapore, 2024; pp. 311–345. ISBN 978-981-97-1882-5. [Google Scholar]
- Salem, S.S. A Mini Review on Green Nanotechnology and Its Development in Biological Effects. Arch. Microbiol. 2023, 205, 128. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Khan, F.; Shahid, A.; Zhu, H.; Wang, N.; Javed, M.R.; Ahmad, N.; Xu, J.; Alam, M.A.; Mehmood, M.A. Prospects of Algae-Based Green Synthesis of Nanoparticles for Environmental Applications. Chemosphere 2022, 293, 133571. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green Synthesis of Metal Nanoparticles Using Microorganisms and Their Application in the Agrifood Sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Arahou, F.; Lijassi, I.; Wahby, A.; Rhazi, L.; Arahou, M.; Wahby, I. Spirulina-Based Biostimulants for Sustainable Agriculture: Yield Improvement and Market Trends. Bioenerg. Res. 2023, 16, 1401–1416. [Google Scholar] [CrossRef]
- Ndaba, B.; Roopnarain, A.; Rama, H.; Maaza, M. Biosynthesized Metallic Nanoparticles as Fertilizers: An Emerging Precision Agriculture Strategy. J. Integr. Agric. 2022, 21, 1225–1242. [Google Scholar] [CrossRef]
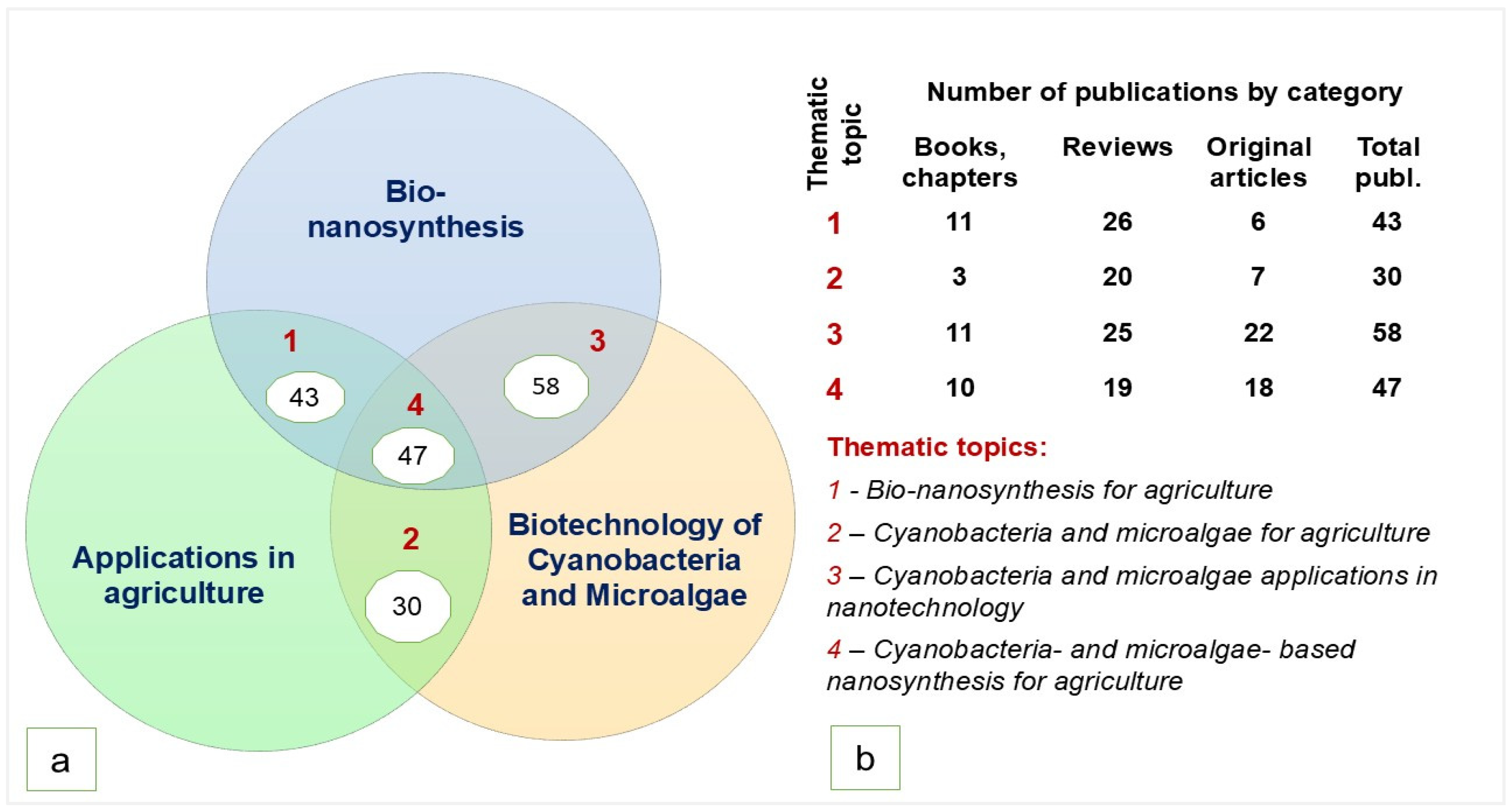
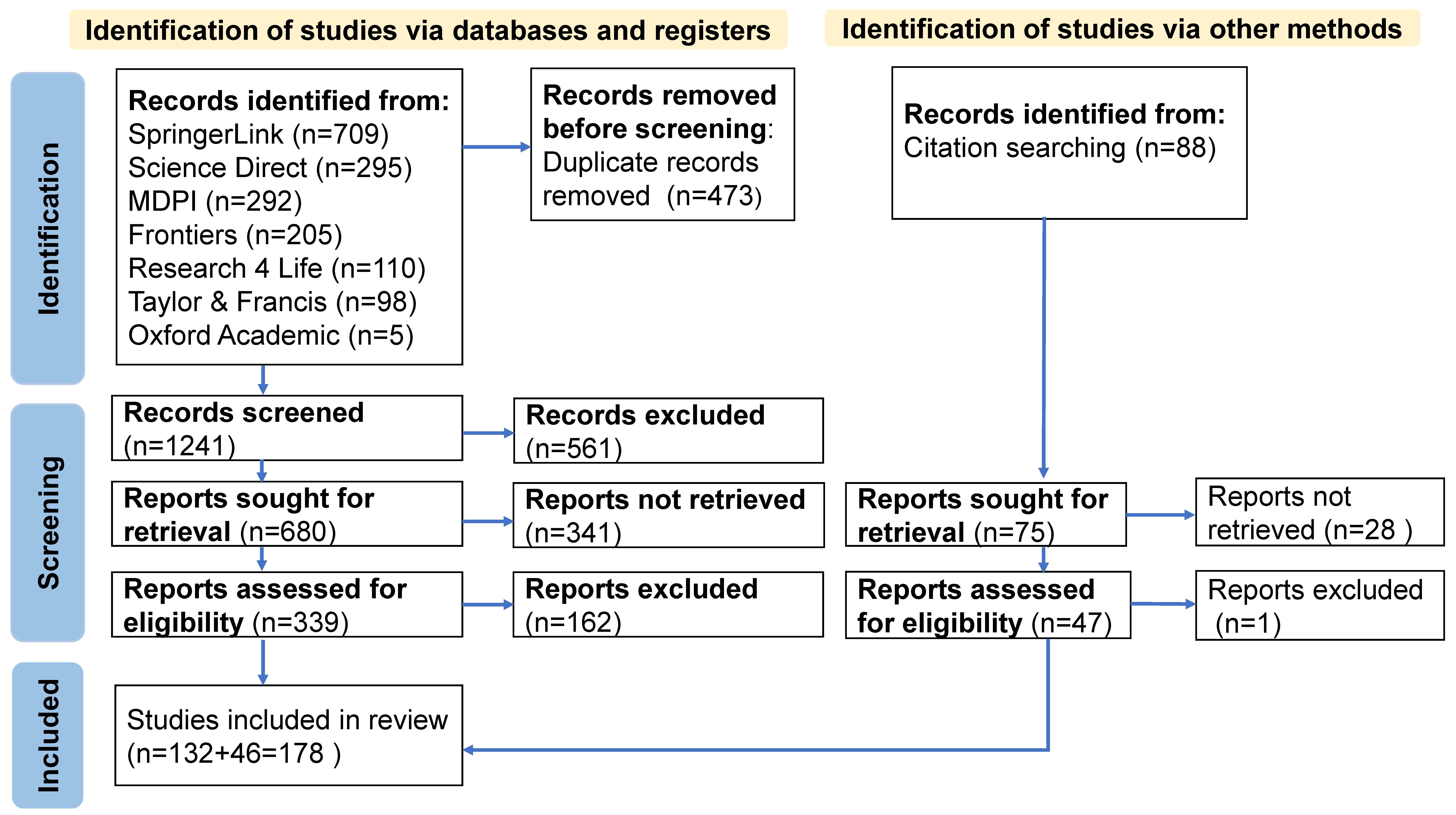

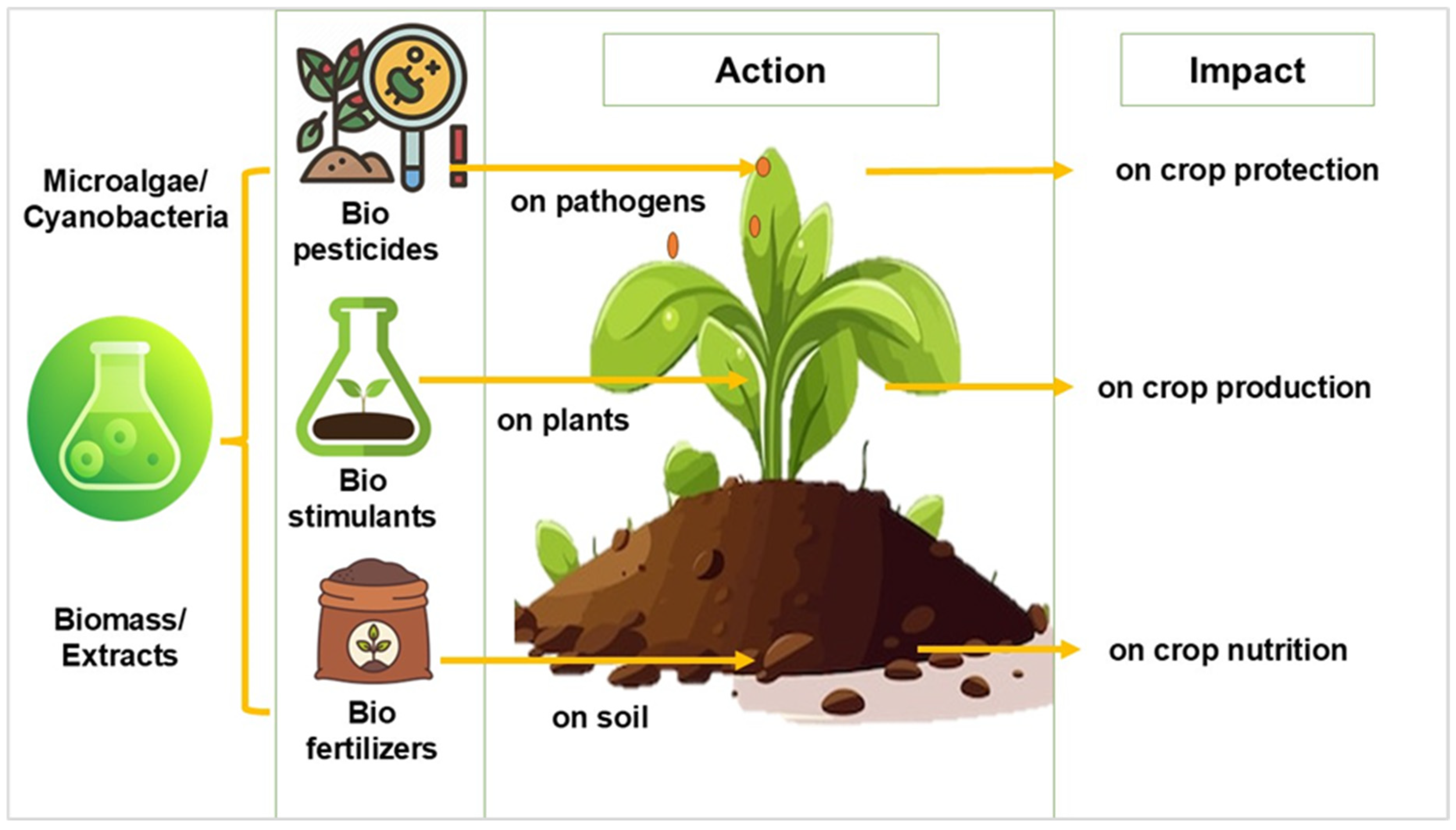

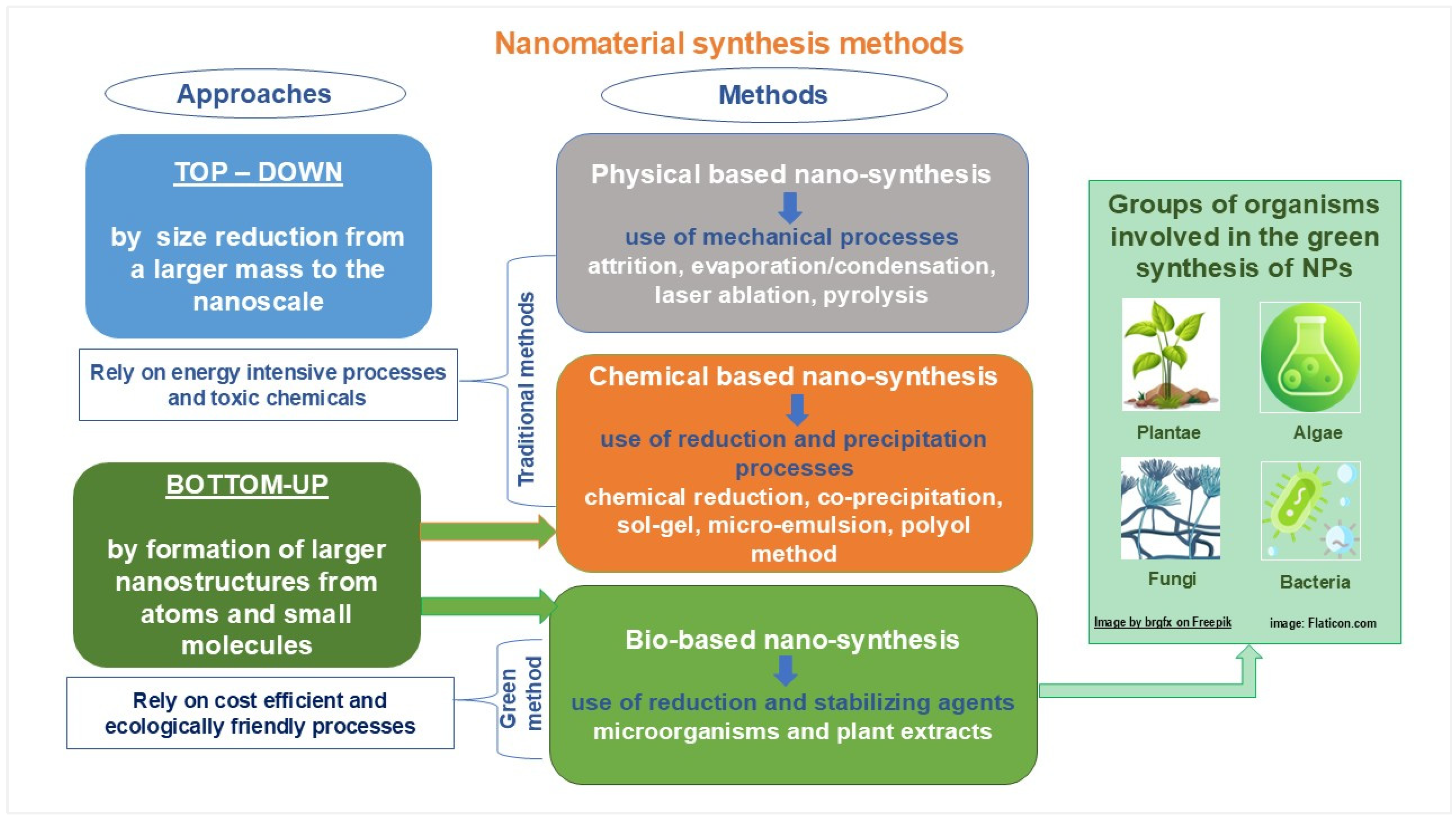
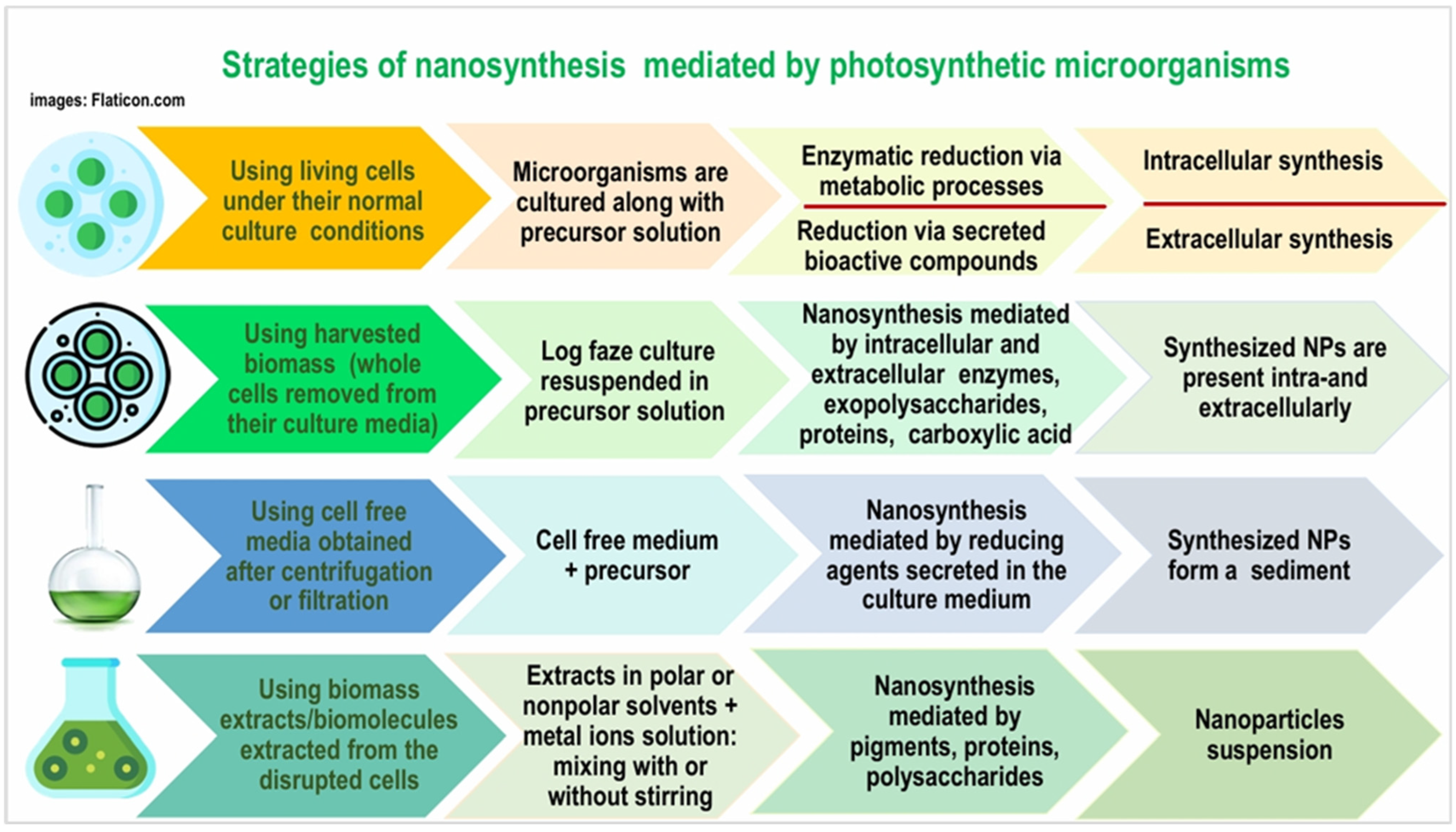
| Topic | Main Ideas |
|---|---|
| Bio-based mechanism and techniques of NPs synthesis | - Intracellular and extracellular biochemical reduction of metallic ions, supported by metabolic processes (nitrogen fixation, photosynthesis, cellular respiration); - Nanosynthesis can be performed using dried biomass or living cells - Techniques: (1) using algal or cyanobacteria suspension, (2) using cell filtrate, (3) using metabolites in the culture medium, and (4) using extracted biomolecules; - Stages of NPs synthesis: nucleation, development, and stabilization |
| Capping, reducing, and stabilizing agents | Carboxylic acid; polyphosphates; carbohydrates, including polysaccharides; proteins, including Histone H4; enzymes (NADH reductases, nitrate reductase, nitrite reductase); polyunsaturated fatty acids, antioxidants (polyphenols and tocopherols); pigments like carotenoids, chlorophylls, and phycobilins (phycocyanin, allophycocyanin and phycoerythrin) |
| Genera used for nanosynthesis | Cyanobacteria: Anabena, Arthrospira/Spirulina, Aphanizomenon, Aphanocapsa, Aphanothece, Aulosira, Calothrix, Cyanothece, Cylindrospermopsis, Cylindrospermum, Desertifilum, Desmonostoc, Gloeocapsa, Leptolingbya, Limnotrix, Lyngbya, Microchaete, Microcoleus, Microcystis, Nannochloropsis, Nostoc, Oscilatoria/Phormidium, Plectonema, Porphyridium, Scytonema, Synechocystis, Synechococcus, Trichodesmium, Westiellopsis; Microalgae: Acutodesmu/Amphora, Asterarcys, Botryococcus, Chlamydomonas, Chlorella, Chlorococcum, Chromochloris, Coelastrum, Cosmarium, Desmochloris, Dunaliella, Euglena, Galdieria, Kirchneriella, Klebsormidium, Nannochloropsis, Neochloris, Pediastrum, Picochorum, Pithophora, Planophila, Rhizoclonium, Tetradesmus, Tetraselmis, Scenedesmus, Diatoms: Amphora, Chaetoceros, Diadesmus, Navicula, Nitzschia, Phaeodactylum, Skeletonema, Thalassosira |
| Factors influencing the NPs synthesis | Cyanobacteria and microalgae species, precursor concentration, incubation period, illumination, pH, temperature, biomass concentration |
| Precursors (bulk material) | AgNO3, AgC2H3O2, AgClO4, Ag2SO4, Au (S2O3)2, AuCl4, HAuCl4, Pd (NO3)2, H2PtCl6, Pd (NO3)2, CdCl2 and Na2S, Zn (NO3)2, ZnSO4 |
| Precursor concentration | 0.1–5 mM AgNO3, 0.5 mM HAuCl4, 0.1M FeCl3/FeCl2, 0.05M FeCl3 |
| Bio reductant/precursor ratio, v:v | 1:4, 1:2, 1:9, 9:1—for AgNO3 |
| Incubation period | Minimal: 5 min; maximal: 1 month |
| pH | The diapason of 4–14 was applied, optimum 7, 4; 9; exceptionally 14 |
| Temperature | Usually: 20–25 °C; exceptionally: 37 °C, 100 °C, 180 °C, 200 °C |
| Illumination | Synthesis was performed in both light and dark conditions, but the most successful synthesis was registered under illumination |
| Type of synthesized NPs | Metallic (Ag, Au, Pt, Cd, Pd), metalloid (Se); metallic oxide (CuO, ZnO, TiO2, Fe3O4); metallic chalcogenides (CdS, HgS, ZnS) and other NPs (carbon nanodots, QDs, and Ag–Au nanoalloy, FeOOH, AgCl, Fe3O4/Ag nanocomposite) |
| Characteristics of nanoparticles | Size: from 2 nm to 200 nm Shape: spherical, elongated, triangular, cubic, pentagonal, hexagonal, octahedral, stars, nanorods |
| Possible application of synthesized NPs | Biomedical (drug delivery, anti-cancer, anti-bacterial, anti-fungal, anti-inflammatory, anti-hemolytic, anti-aging), environmental bioremediation, agricultural (nano-fertilizers), dye decolorization agent |
| Feature | Intracellular Synthesis | Extracellular Synthesis |
|---|---|---|
| Location | Inside the cell (cytoplasm, cell membrane, thylakoid membrane) | Outside the cell (in the culture medium) |
| Process | Metal ions enter cells and are reduced internally | Biomolecules secreted by cells reduce metal ions in the surrounding medium |
| Mechanism | Cells absorb metal ions, which are then bio-reduced into NPs via metabolic processes | The secreted biomolecules act as reducing, capping, and stabilizing agents, promoting nanoparticle formation |
| Recovery | Requires cell lysis and extraction | Nanoparticles are already in the medium |
| Advantages | Controlled NP size and shape | Easier recovery |
| Application | Nanomaterials | References |
|---|---|---|
| Seed priming, growth, and reproduction stimulation | AgNPs, AlNPs, CeNPs, CuNPs, CuONPs, FeNPs, Fe2O3NPS, FeNPs with SiO2, MgONPs, multi-walled carbon nanotubes (MWCNTs), NiONPs, quantum dots (QD), SeO2NPs, TiO2NPs, ZnONPs | [98,99,100] |
| Fertilizers | AlNPs, B2O3NPs, nano-CaCO3, carbon nanotubes (CNT), CuNPs, CuONPs, Cu-Chitosan NPs, copper nanowires, FeNPs, FeONPs, FeO(OH)NPs, Fe2O3NPs, Fe3O4 NPs, MgNPs, MgONPs, MnNPs, MoNPs, MWCNTs, KFeO2NPs, SeNPs, SiNPs, SiO2NPs, TiO2NPs, ZnNPs, ZnONPs, Zn-chitosan NPs, ZnNPs, hydroxyapatite NPs, 2-D graphite carbon NPs, vermiculite, nanoclay, zeolite | [1,99,101,102,103,104,105,106,107,108] |
| Fungicides | AgNPs, alumino silicates (3Al2O3·2SiO2) NPs, CNTs, CuNPs, FeNPs, MgNPs, MgONPs, NiNPs, SeNPs, SiNPs, Silver-coated carbon nanotubes hybrid NPs, TiO2NPs, ZnONPs | [99,106,109,110] |
| Bactericides | AgNPs, Ag, Cu and Ti nanocomposites, Al2O3NPs, CuNPs CuONPs, graphene oxide, Fe3O4–Ag core–shell magnetic NPs, MgONPs, TiO2NPs, ZnONPs | [99,106,110] |
| Herbicide nano-carriers | Carboxymethyl chitosan-modified carbon NPs, chitosan NMs, poly ε-caprolactone, nanocapsules | [99] |
| Insecticides | AgNPs, CuNPs, CuONPs, NiONPs, PbNPs, SiO2NPs, ZnONPs, TiO2NPs, electrospun nanofibers | [99,111] |
| Hit stress tolerance | AgNPs, CaNPs, CeONPs, SeNPs, SiNPs, TiO2 NPs, ZnONPs | [99,108] |
| Drought stress tolerance | AgNPs, CuNPs, CNT, Fe3O4 NPs, fullerenes, ZnNPs, MWNTs, SiNPs, single-walled carbon nanotubes (SWNTs), TiO2NPs, ZnONPs, | [96,99,106,108] |
| Salt stress tolerance | AgNPs, CeONPs, FeNPs, Fe3O4NPs, MWCNT, SiNPs, TiO2NPs | [99,108] |
| Metal stress tolerance | FeONPs, SiO2NPs, ZnONPs | [99,108] |
| Gene delivery | Cationic polymers, CuNPs, dimethylamino ethyl methacrylate (DMAEM) polymer NPs, lipid NPs (LNPs), nanotubes, quantum dots (QDs) | [99] |
| Nanosensors | AgNPs, AuNPs, CdTeQD4-Rd, CNT, CuNPs, MWCNTs, SWNTs, fullerenes, graphene oxide NPs, nanoTiO2/nafion composite, nanoscale wires, Pt nanoparticle-anchored zirconium-based metal–organic framework nanocomposites, SiO2NPs, silicate/glucose oxidase, TiNPs, ZnONPs, ZnONP–chitosan nanocomposite QDs | [99,102,106,107,112,113,114] |
| Vegetable and fruit preservation | AgNPs, cellulose nanocrystal, chitosan-assisted nano-silica, chitosan film-based nano-SiO2, nano-ZnO | [103,109] |
| Cyanobacterial/ Microalgal Strain | NM Name and Characteristics | Synthesis Methods | Crops | Outcomes | Ref. |
|---|---|---|---|---|---|
| Application of nanoparticles synthesized by photosynthetic microorganisms in the agri-food sector | |||||
| Chlorella K01 | Fe3O4 NPs, 76.5 nm | Using microalgal aqueous extract, t = 65 °C, pH = 6–12 | - Rice, maize, mustard, green gram, watermelon seed priming | - Stimulatory effect on germination and seedling vigor index ranging from 35% to 100% above control, maximal effect observed in green gram - Antifungal activity against Fusarium, Rhizoctonia, and Pythium | [67] |
| Leptolyngbya sp. WUC 59 | Spherical Ag NPs, 20–35 nm | Using cell-free aqueous extract, t = 70 °C, pH = 6–12 | Triticum aestivum L. seed priming | - Increase in the root and shoot length of 3.0 cm and 8.0 cm at 25 mg L−1 concentration of Ag NPs and decrease at higher concentrations | [72] |
| Chlorella vulgaris | Spherical Ag NPs, 5.76 nm | Using solution of soluble polysaccharides, 24 h, in the dark | Triticum vulgare Phaseolus vulgaris seed priming | - Increase in shoot height by apr. 23%, root length by apr. 30%, and first vegetative leaves by apr. 60% above control level | [134] |
| Desmonostoc alborizicum | Spherical Se NPs, 58.8 nm | Using cell-free extract, t = 60 °C, mechanical stirring | - | - Antifungal activity against plant pathogens. Alternaria alternata showed the highest sensitivity | [92] |
| Nodosilinea nodulosa | Spherical Co3O4 NPs, 41 nm | Using cell-free extract, t = 60 °C, mechanical stirring | - | - Antifungal activity against Fusarium oxysporum with a zone of inhibition of 3 ± 0.04 µg/mL, Aspergillus flavus with a zone of inhibition of 2 ± 0.04 µg/mL | [93] |
| Nostoc sp. | Spherical ZnO NPs, 18.47 nm | Using acetone extract, T = 60 °C, mechanical stirring 1 h | corn, wheat seed priming | - Enhanced corn seedlings parameters by apr. 60–75% and chlorophyll content by 30% at NP concentr. of 10 µg/mL; wheat seedling parameters by 140–170% and chlorophyll content by 60% at NP concentr. of 15 µg/mL | [135] |
| Nanoparticle-enriched extracts of cyanobacteria and microalgae in plant biotechnology | |||||
| Calothrix elenkinii | AgNPs | NPs from Sigma-Aldrich Chemical Pvt. Ltd. applied in synergy with cyanobacterium | Lycopersicon esculentum (tomato) detached leaf assay | Biocontrol efficacy against Alternaria alternata: higher leaf chlorophyll accumulation and lower endoglucanase activity | [136] |
| Calothrix elenkinii | AgNPs | NPs from Sigma-Aldrich Chemical Pvt. Ltd. applied in synergy with cyanobacterium | Lycopersicon esculentum Mill(tomato) foliar application on plants infected by Alternaria alternata | - Disease severity reduced by 47–58% - Leaf chlorophyll, carotenoid content, polyphenol oxidase activity significantly increased by 44–45%, 40–46%, and 23–33%, respectively - Ergosterol content decreased by 63–79% | [137] |
| Spirulina sp. | CuNPs | Chemically synthesized NPs, combined with spirulina extract | French basil foliar treatment 2 seasons, pot experiment in natural field conditions | - Maximum effects were achieved with 500 mg/L CuNPs and 1.5 g/L spirulina: a 4–5-fold increase in oil yield, the highest chlorophyll and carotenoid levels, and a 45% increase in plant height compared to the control | [138] |
| Arthrospira platensis | CuNPs CuONPs 100 nm | Cyanobacterium cultivated with NPs (Merck KGaA) | Triticale seed priming | - Extracts from cyanobacterium cultured in media supplemented with CuONPs increased chlorophyll content in leaves by 42.8% and carotenoid content by 37.4% - Extracts from biomass cultured with CuNPs increased antioxidant activity in triticale leaves by 48–65.3% | [139] |
| Cyanothece sp. | Graphene, graphene oxide, carbon nanotubes | Chemically synthesized NMs, in combination with methyl salicylate and Cyanothece sp. and Enterobacter cloacae | Hordeum vulgare, Vicia faba adding to soil, presoaking in biofertilizer | - The germination of both plants in the presence of graphene and carbon nanotubes, combined with biofertilizer treatment under the highest salinity stress level, almost completely alleviated the effects of salinity stress, | [140] |
| Scenedesmus obliquus | ZnONPs | Biosynthetized ZnONPs, using cell-free algal extract incubated overnight at 28 °C, applied along with microalga extract | Tomato, foliar spray | Algal extract + bio-ZnONPs increased total fruit yield by 37% and marketable yield by 43.1%; raised soil microbial and dehydrogenase activity by 369.4% and 298.8%, respectively; boosted zinc accumulation by 74.8% in roots, 182.7% in leaves, and 104.2% in fruit | [141] |
| Spirulina sp. | Spindle-shaped FeNPs, 24.77 ± 5.264 nm | Biosynthetized FeNPs (using spirulina biomass), dried spirulina biomass, nano-iron-loaded spirulina biomass | Rice, fertilizer type | Nano-iron-loaded Spirulina sp. biomass increased shoot length by 37.6%, crop productivity by 47%, and grain weight by 15% | [58] |
| Chlorella sp. MF1 | AgNPs | Using algal biomass aqueous extract 28 °C, 1 h, pH-6 | Eruca sativa seed soaking, foliar spray | - Increase in root length, fresh weight, and dry weight in response to increased concentrations of AgNPs and Chlorella | [142] |
| Arthrospiraplatensis | Lecithin/chitosan NPs | Nanoliposome system employing lecithin/chitosan as carriers of Arthrospira protein hydrolysate | Tomato, weekly sprays | NPs and protein hydrolysate treatments significantly stimulated plant growth, up to a 49.5% increase in plant height during the vegetative phase | [143] |
| Cyanobacteria- and microalgae-based nanosensors | |||||
| Chlamydomonas reinhardtii UV180 | Nano-formulated atrazine, encapsulated into zein and chitosan poly-ε-caprolactone nanoparticles | Whole algal cells immobilized on carbonized lignin electrodes and integrated into a photo-electrochemical transducer | - | The biosensor demonstrated a linear response for atrazine detection in the 0.1–5 μM range, with detection limits of 0.9 nM and 1.1 nM for atrazine–zein and atrazine-PCL-Ch nanoparticles, respectively | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codreanu, S.; Cepoi, L.; Rudi, L.; Chiriac, T. Photosynthetic Microorganisms and Biogenic Synthesis of Nanomaterials for Sustainable Agriculture. Nanomaterials 2025, 15, 990. https://doi.org/10.3390/nano15130990
Codreanu S, Cepoi L, Rudi L, Chiriac T. Photosynthetic Microorganisms and Biogenic Synthesis of Nanomaterials for Sustainable Agriculture. Nanomaterials. 2025; 15(13):990. https://doi.org/10.3390/nano15130990
Chicago/Turabian StyleCodreanu, Svetlana, Liliana Cepoi, Ludmila Rudi, and Tatiana Chiriac. 2025. "Photosynthetic Microorganisms and Biogenic Synthesis of Nanomaterials for Sustainable Agriculture" Nanomaterials 15, no. 13: 990. https://doi.org/10.3390/nano15130990
APA StyleCodreanu, S., Cepoi, L., Rudi, L., & Chiriac, T. (2025). Photosynthetic Microorganisms and Biogenic Synthesis of Nanomaterials for Sustainable Agriculture. Nanomaterials, 15(13), 990. https://doi.org/10.3390/nano15130990










