A Review on Pre-, In-Process, and Post-Synthetic Strategies to Break the Surface Area Barrier in g-C3N4 for Energy Conversion and Environmental Remediation
Abstract
1. Introduction
2. The Method of Synthesizing High-SSA Carbon Nitride
2.1. Structure of Carbon Nitride
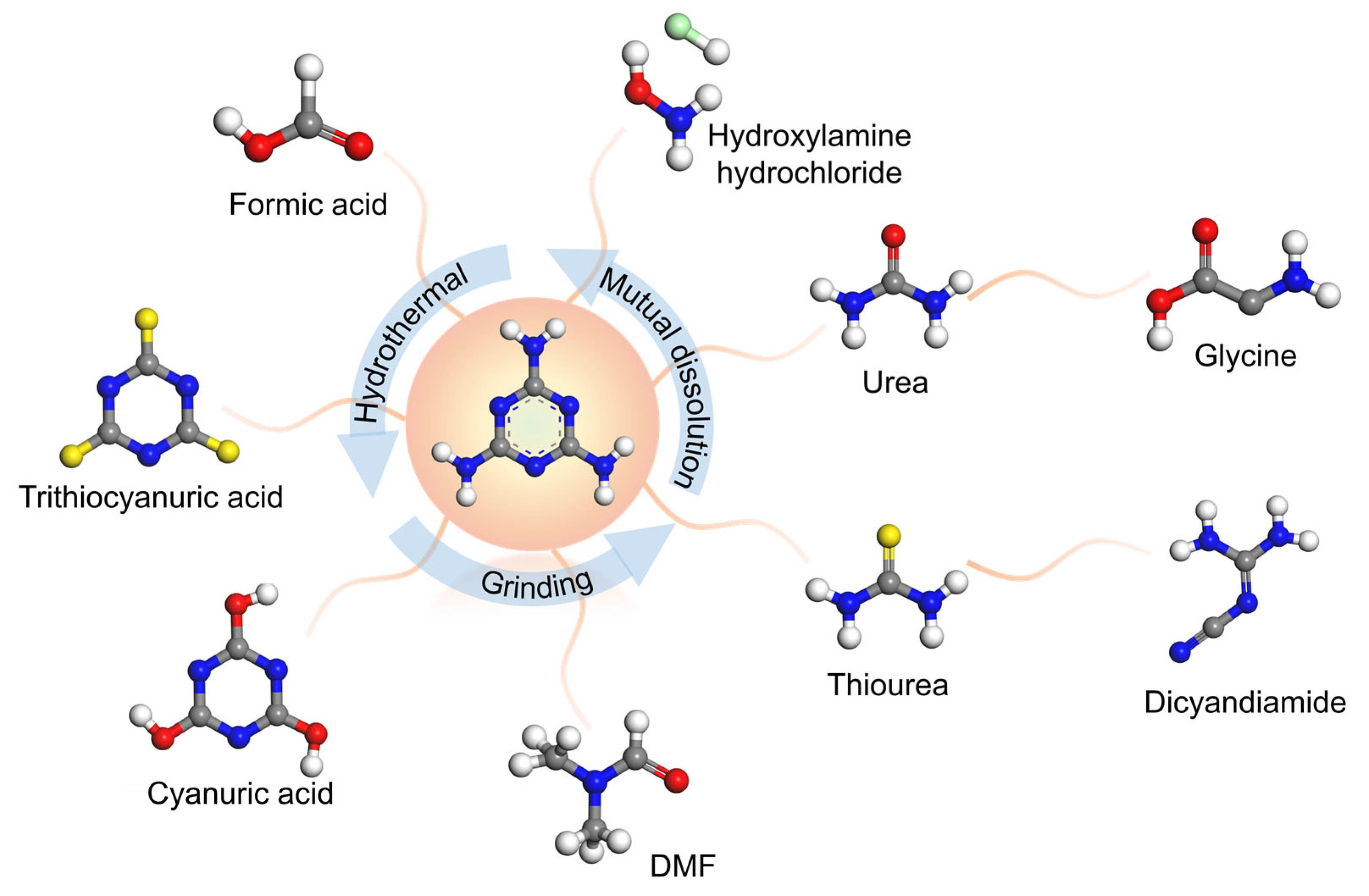
2.2. Preparation of Carbon Nitride
2.3. Preparation of High-SSA Carbon Nitride
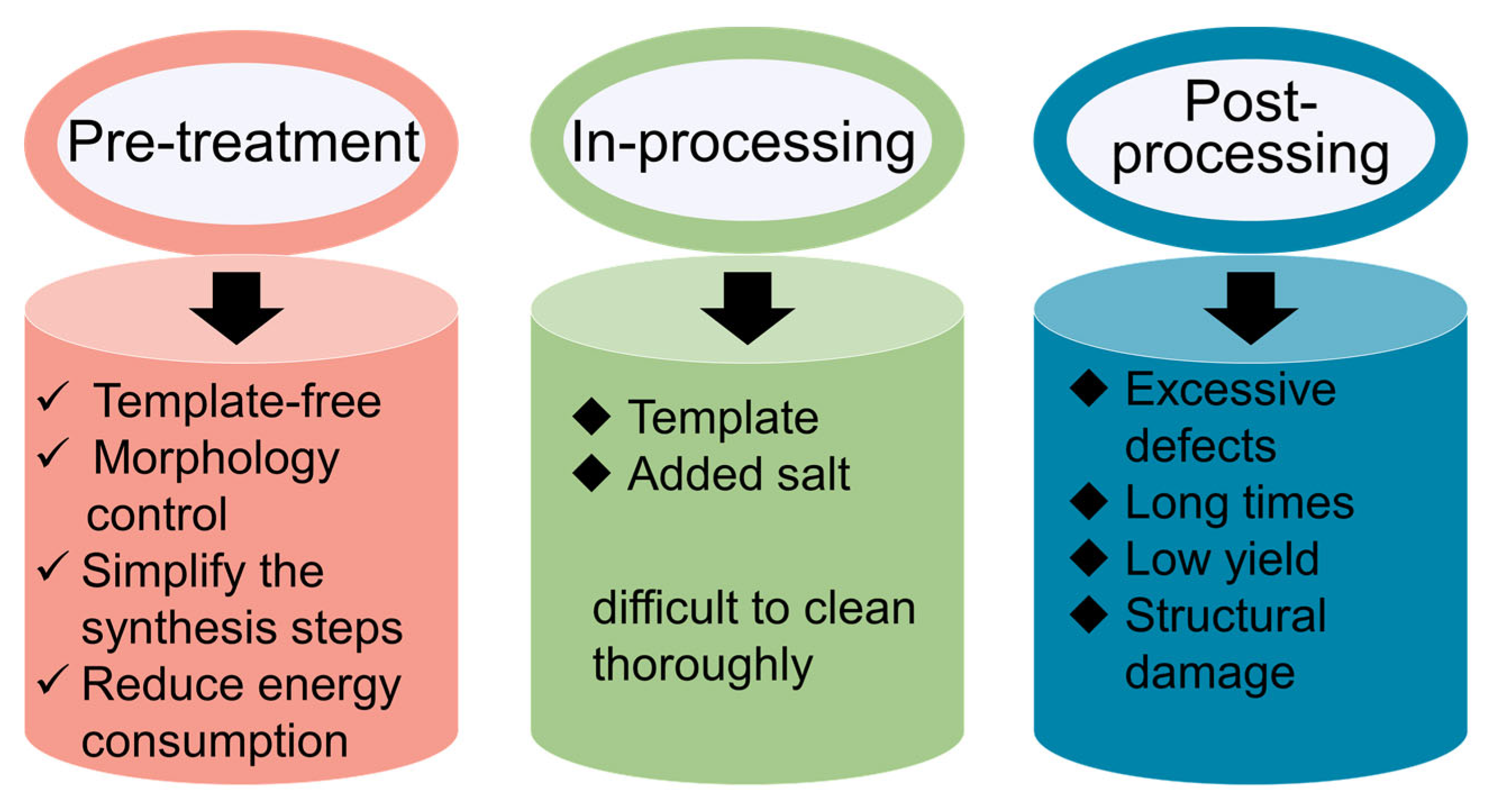
2.3.1. Pre-Treatment of Carbon Nitride
The Influence of Raw Materials on the Specific Surface Area and Morphology
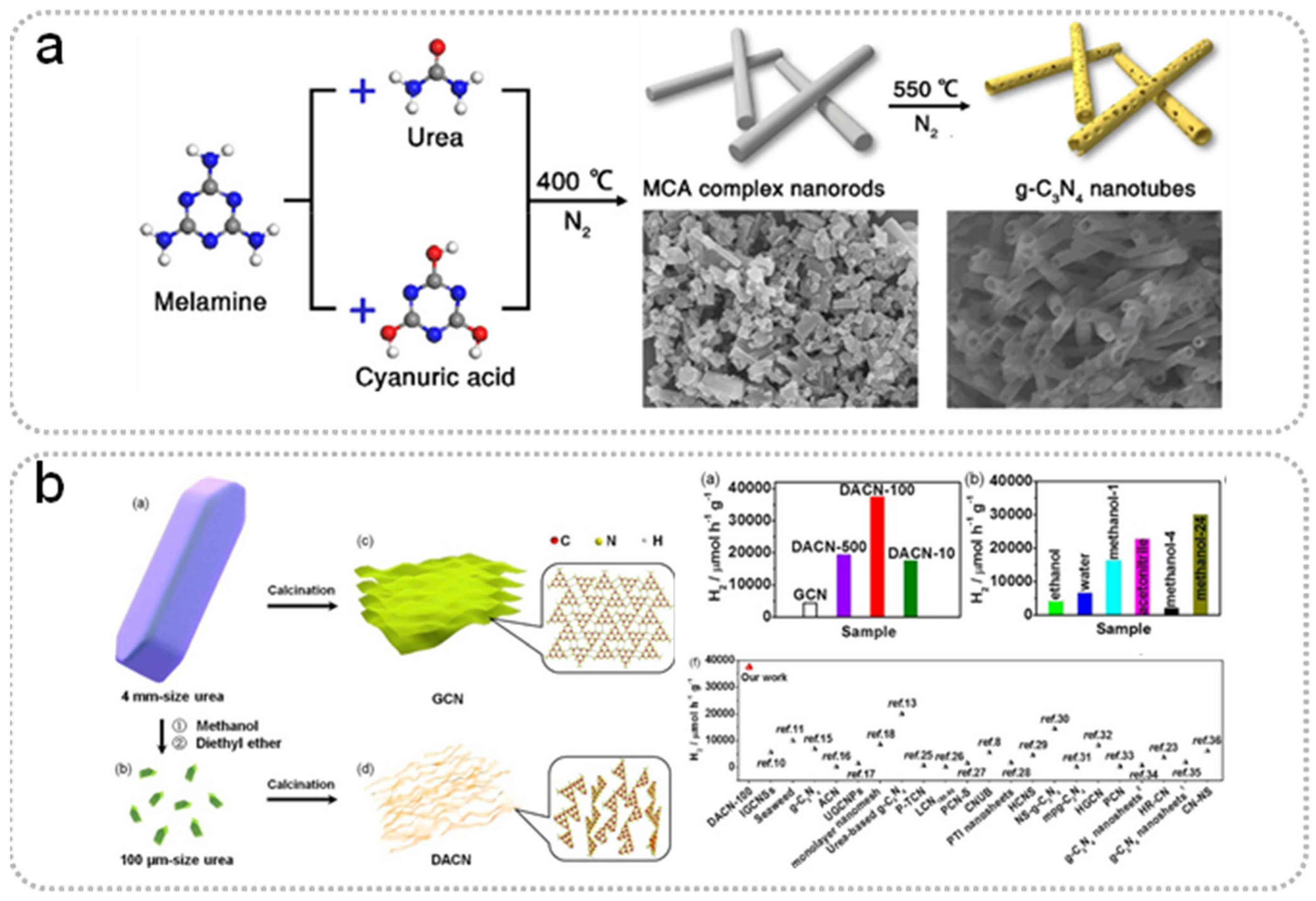
The Treatment for the Precursor of Carbon Nitride
2.3.2. In-Process Preparation of Carbon Nitride
Introducing the Template During the Synthesis
Introducing the Salt During the Synthesis
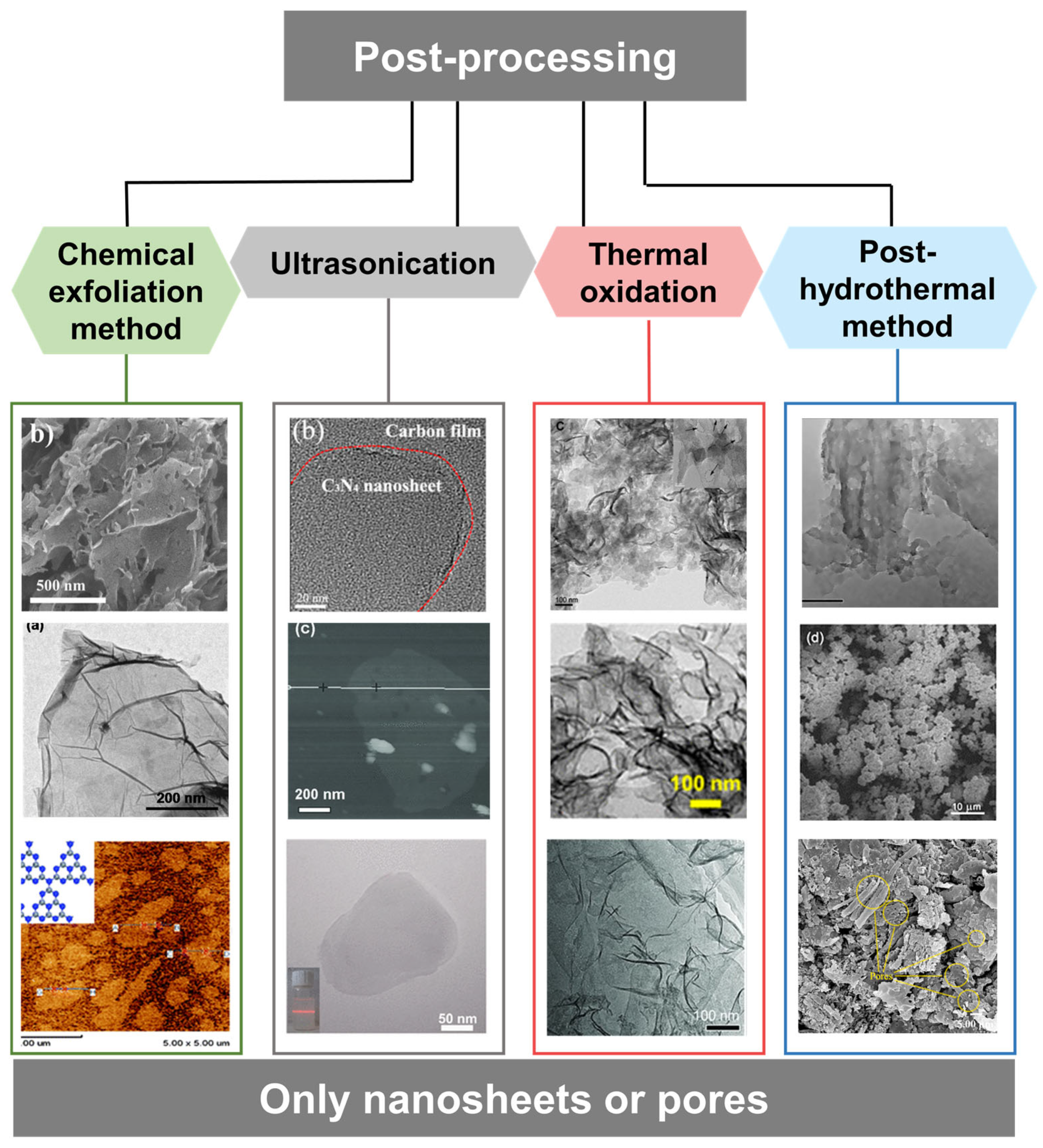
2.3.3. Post-Processing of Carbon Nitride
Acid/Chemical Treatment
| References | Synthesis of Bulk g-C3N4 | Acid | Assistant Method | Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mbulk g-C3N4 | Type | Concentration or Volume | Treated Time | Band Gap | BET | Morphology | Application | |||
| [60] | Dicyandiamide 550 °C for 4 h at 5 °C min−1 | 0.2 g | H3PO4 | 0.3 mL | 3 d | Dispersed in 20 mL dimethylformamide | 2.63 eV ↓ 2.74 eV | 7.6 m2/g ↓ 55.4 m2/g |  | Photocatalytic hydrogen evolution and CO2 conversion |
| [61] | Dicyandiamide 550 °C for 4 h at 2.3 °C min−1 | 4 g | H2SO4 (98%) | 52 g | 5 h | 20 g oleum NH4Cl (1.60 mol) | 2.83 eV ↓ 3.78 eV | 12 m2/g ↓ 42 m2/g |  | - |
| [62] | Melamine | 2 g | H2SO4 | 40 mL 98 wt% | 10 h | Sonicated with 8 h | 2.64 eV ↓ 2.68 eV | 8.1 m2/g ↓ 25.7 m2/g |  | Photo-reduction of p-nitrophenol |
| [63] | Dicyandiamide 550 °C for 4 h at 2.3 °C min−1 in static air | 1 g | HCl | 25 mL 10 M | 1 h | Sonication for 2 h | 2.57 eV ↓ 2.75 eV | 9 m2/g ↓ 305 m2/g |  | Excellent metal-/label-free biosensing platform |
| [64] | Melamine 550 °C for 4 h at 3 °C min−1 in static air | 1.0 g | H2SO4 | 10 mL 98 wt% | 8 h | Calcinated at 550 °C in N2 for 2 h | 2.65 eV ↓ 2.79 eV | 12 m2/g ↓ 54.3 m2/g |  | Photocatalytic degradation |
| [65] | Dicyandiamide 550 °C for 4 h at 2.3 °C min−1 in static air | 1 g | H2SO4 | 10 mL 98 wt% | 8 h | Sonication | 2.64 eV ↓ 2.92 eV | 4.3 m2/g ↓ 205.8 m2/g |  | Photocatalytic hydrogen evolution |
| [66] | - | 1 g | H2SO4 | 30 mL 98 wt% | - | Hummer’s method | 2.54 eV ↓ 3.07 eV | 3.5 m2/g ↓ 355.8 m2/g |  | - |
| [67] | Dicyandiamide 500 °C for 1 h 520 °C for 3 h | 10 g | H2SO4 | 230 mL 98 wt% | - | Hummer’s method | 2.72 eV ↓ 3.85 eV | - |  | Photocatalytic degradation |
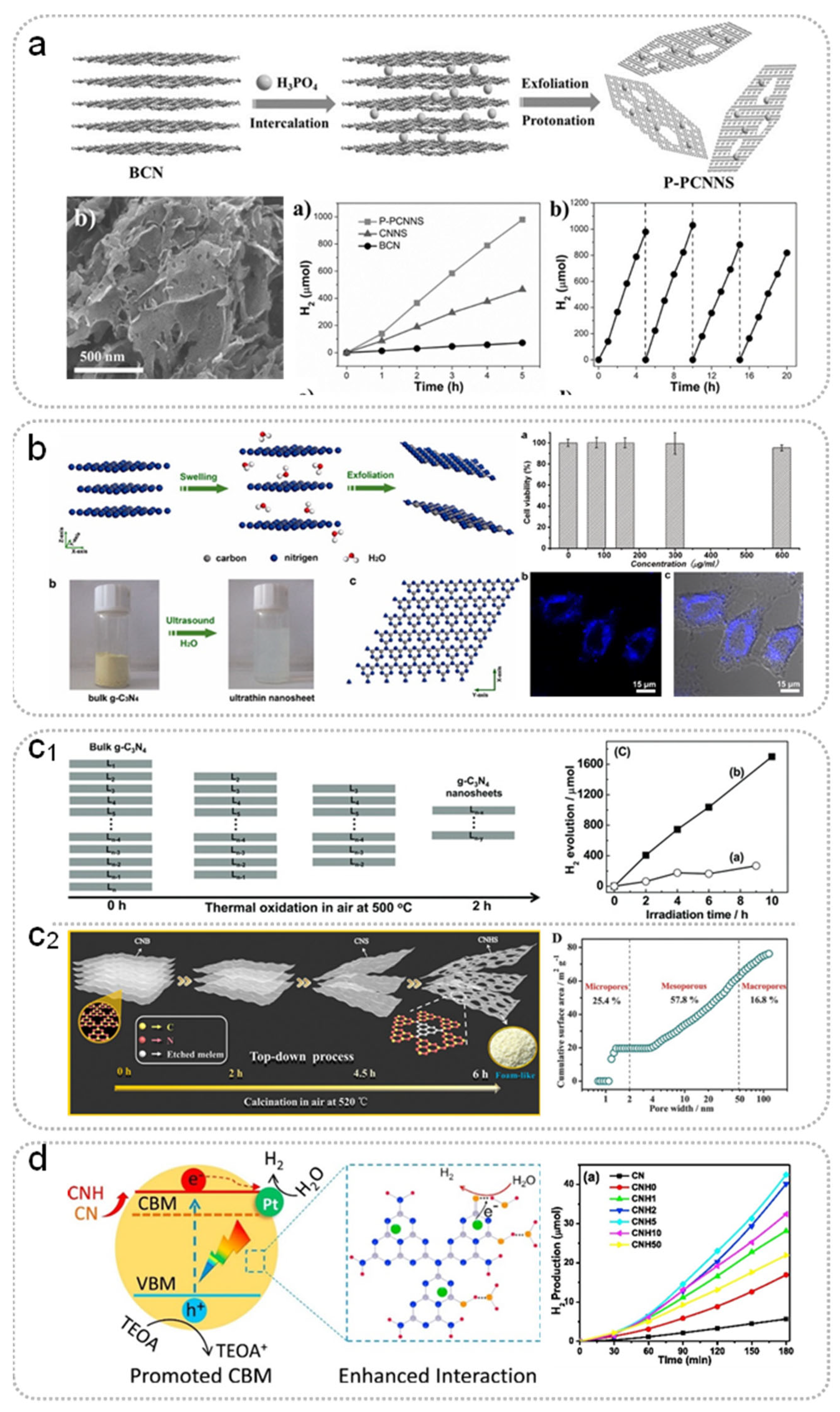
Ultrasonication
Thermal Oxidation Treatment
Post-Hydrothermal
2.4. Summary
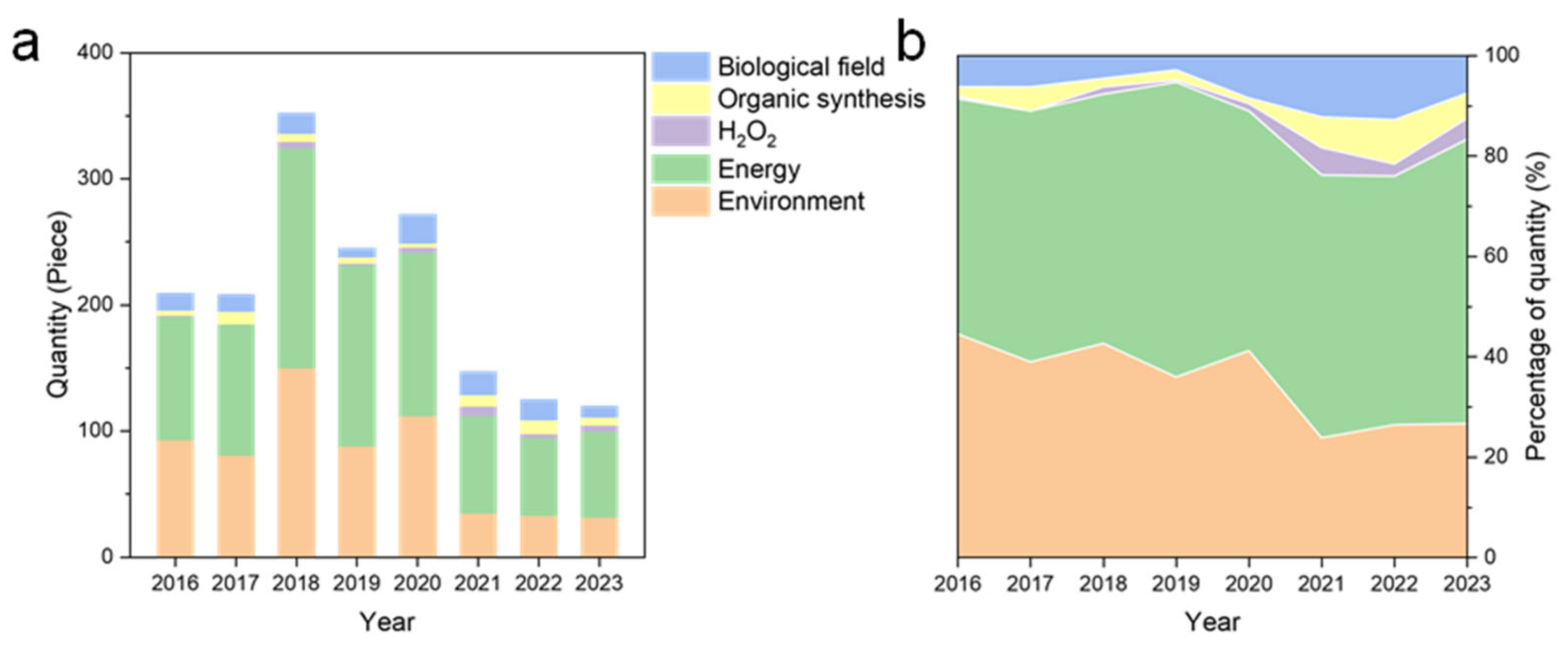
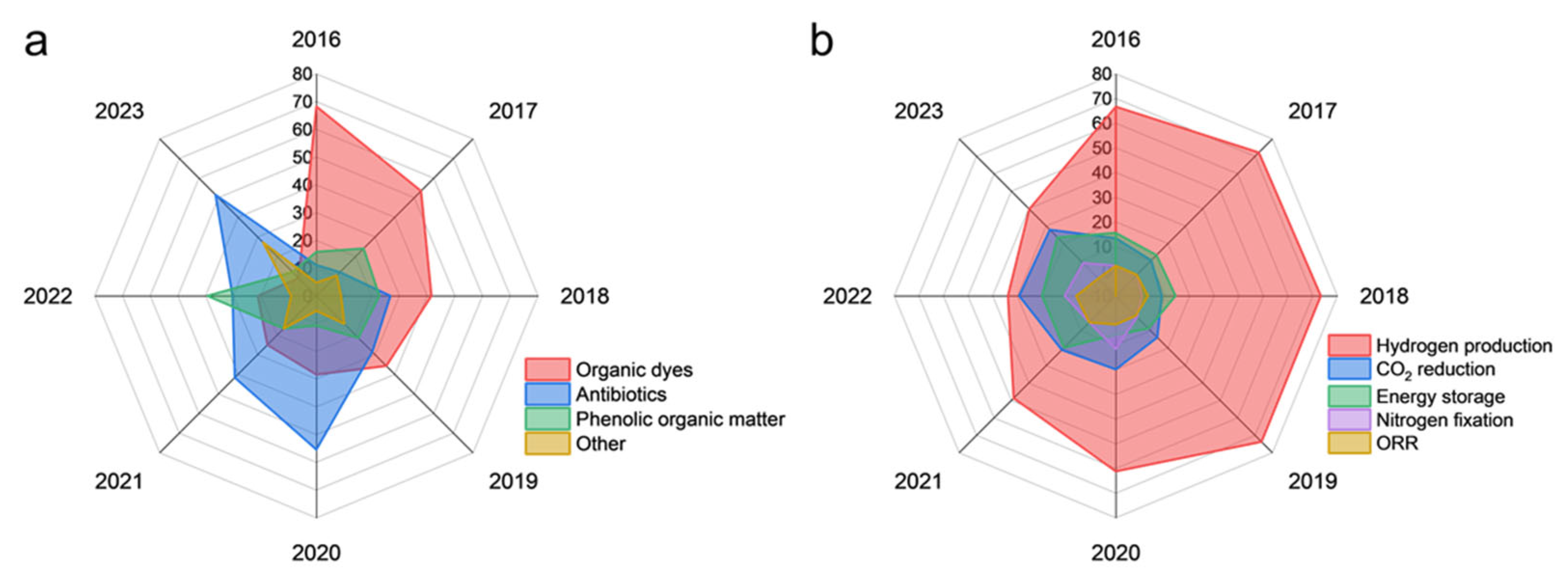
3. Research Distribution and Trend Analysis of Carbon Nitride Applications
3.1. Environmental Remediation: From Conventional Pollutant Removal to Emerging Contaminants
3.1.1. Aquatic Organic Contaminant Degradation
3.1.2. Heavy Metal Detoxification
3.1.3. Air Purification
3.1.4. Outlook
3.2. Energy Conversion and Storage: Bridging Photocatalysis to Practical Energy Systems
3.2.1. Solar Hydrogen Production
3.2.2. CO2 Photoreduction
3.2.3. Nitrogen Fixation
3.2.4. Energy Storage
3.2.5. Outlook
3.3. Biomedical Innovations: Antimicrobial and Sensing Platforms
3.3.1. Photodynamic Antimicrobial Therapy
3.3.2. Biosensing and Diagnostics
3.3.3. Outlook
4. Conclusions and Outlook
- (i)
- For the design and synthesis of g-C3N4 materials with excellent performance, overall consideration should be given to the specific surface area, band structure, and defects of g-C3N4 materials. For photocatalysis, the realization of excellent photocatalytic efficiency requires the combined efforts of the light absorption ability, charge transfer, and separation ability, and adsorption ability for the catalyst’s reactants. Mechanisms to promote the properties of g-C3N4-derived materials should be explored. The further functionalization mentioned in this work can improve the performances of g-C3N4 by promoting the transfer and separation of charge. However, the exact transfer pathway of charge in g-C3N4 derived material is still ambiguous or unsubstantiated. Further exploration of the mechanism is of great significance for the synthesis of g-C3N4 materials with target structures. Combination of improved methods is required. The published methods for modification of g-C3N4 have usually aimed to improve a particular aspect of performance. Therefore, the synthesis of high-performance g-C3N4 can be realized by combining various modification methods according to the requirements of specific application fields. The design and assembly of material reactors should be considered. Generally, most prepared g-C3N4 materials are powder, which is not suitable for all applications. The design of suitable reactors can meet the particular requirements of some reactions to broaden the application range of g-C3N4.
- (ii)
- Further exploration of reaction mechanisms should include interface reactions between reactants and g-C3N4 materials. Many applied reactions of g-C3N4 materials have occurred in water or other media where the reaction isdirectly affected by the interface effect between reactants and materials. Regarding the pathways of g-C3N4 material reactions with reactants, our experimental process was a macroscopic process, so that the specific reaction path between g-C3N4 materials and the reactants could not be fully assessed. Such exploration is very important for the targeted synthesis and application of high-performance g-C3N4 materials. Theoretical calculations related to g-C3N4 materials can be combined with experimental results to clarify the specific active mechanisms of g-C3N4 materials, which is conducive to understanding the properties of materials and the occurrence of reactions. However, the direction of calculation needs to be further explored.
- (iii)
- Application fields of g-C3N4 materials include practical industrial application of g-C3N4 materials in “old” fields. The degradation activity of the g-C3N4 material under actual water conditions should be studied, including the coexistence of various pollutants, fluid medium, sunlight irradiation, and so on. With regard to improving the performances of g-C3N4 material in “new” fields, g-C3N4 materials have been subject to a relatively few studies in the fields of nitrogen fixation, antibacterial activity, and sensing, leaving large room for improvement.
Funding
Acknowledgments
Conflicts of Interest
References
- Tong, T.; Zhang, M.; Chen, W.; Huo, X.; Xu, F.; Yan, H.; Lai, C.; Wang, W.; Hu, S.; Qin, L.; et al. Recent advances in carbon-based material semiconductor composite photoelectrocatalysts: Synthesis, improvement strategy, and organic pollutant removal. Coord. Chem. Rev. 2024, 500, 215498. [Google Scholar] [CrossRef]
- Tang, L.; Zou, J. p-type two-dimensional semiconductors: From materials preparation to electronic applications. Nano-Micro Lett. 2023, 15, 230. [Google Scholar] [CrossRef]
- Liu, L.; Bai, B.; Yang, X.; Du, Z.; Jia, G. Anisotropic heavy-metal-free semiconductor nanocrystals: Synthesis, properties, and applications. Chem. Rev. 2023, 123, 3625–3692. [Google Scholar] [CrossRef]
- Liras, M.; Barawi, M.; de la Peña O’Shea, V.A. Hybrid materials based on conjugated polymers and inorganic semiconductors as photocatalysts: From environmental to energy applications. Chem. Soc. Rev. 2019, 48, 5454–5487. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Li, Y.; Yang, Y.; Xiao, X.; Chen, G. Boosting Li-metal anode performance with lithiophilic Li-Zn seeds in a 2D reduced graphene oxide scaffold. Adv. Energy Mater. 2024, 15, 2403640. [Google Scholar] [CrossRef]
- Ning, M.; Wang, Y.; Wu, L.; Yang, L.; Chen, Z.; Song, S.; Yao, Y.; Bao, J.; Chen, S.; Ren, Z. Hierarchical interconnected NiMoN with large specific surface area and high mechanical strength for efficient and stable alkaline water/seawater hydrogen evolution. Nano-Micro Lett. 2023, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Kuang, P.; Sayed, M.; Fan, J.; Cheng, B.; Yu, J. 3D Graphene-based H2-production photocatalyst and electrocatalyst. Adv. Energy Mater. 2020, 10, 1903802. [Google Scholar] [CrossRef]
- Zang, Y.; Pei, F.; Huang, J.; Fu, Z.; Xu, G.; Fang, X. Large-area preparation of crack-free crystalline microporous conductive membrane to upgrade high energy lithium-sulfur batteries. Adv. Energy Mater. 2018, 8, 1802052. [Google Scholar] [CrossRef]
- Lu, S.Y.; Jin, M.; Zhang, Y.; Niu, Y.B.; Gao, J.C.; Li, C.M. Chemically exfoliating biomass into a graphene-like porous active carbon with rational pore structure, good conductivity, and large surface area for high-performance supercapacitors. Adv. Energy Mater. 2017, 8, 1702545. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, K.; Xie, C.; Yang, P. Vertically implanting MoSe2 nanosheets on superior thin C-doped g-C3N4 nanosheets towards interface-enhanced electrochemical activities. Carbon 2024, 220, 118884. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, A.; Ding, J.; Hu, R.; Gong, Y.; Li, X.; Chen, L.; Chen, P.; Tian, X. Amino modulation on the surface of graphitic carbon nitride for enhanced photocatalytic hydrogen production. Carbon 2024, 219, 118841. [Google Scholar] [CrossRef]
- Xu, T.; Hur, J.; Niu, P.; Wang, S.; Lee, S.; Chun, S.-E.; Li, L. Synthesis of crystalline g-C3N4 with rock/molten salts for efficient photocatalysis and piezocatalysis. Green Energy Environ. 2024, 9, 890–898. [Google Scholar] [CrossRef]
- Ding, X.; Gao, R.; Chen, Y.; Wang, H.; Liu, Y.; Zhou, B.; Wang, C.; Bai, G.; Qiu, W. Carbon vacancies in graphitic carbon nitride-driven high catalytic performance of Pd/CN for phenol-selective hydrogenation to cyclohexanone. ACS Catal. 2024, 14, 3308–3319. [Google Scholar] [CrossRef]
- Wang, W.; He, Q.; Yi, Y.; Xiao, Y.; Xiao, X.; Yang, H.; Dong, X. Boosting piezocatalytic activity of graphitic carbon nitride for degrading antibiotics through morphologic regulation and chlorine doping. J. Clean. Prod. 2023, 415, 137818. [Google Scholar] [CrossRef]
- Tabarkhoon, F.; Abolghasemi, H.; Rashidi, A.; Bazmi, M.; Alivand, M.S.; Tabarkhoon, F.; Farahani, M.V.; Esrafili, M.D. Synthesis of novel and tunable micro-mesoporous carbon nitrides for ultra-high CO2 and H2S capture. Chem. Eng. J. 2023, 456, 140973. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Hu, X.; Xue, H.; Pang, H. Metal/graphitic carbon nitride composites: Synthesis, structures, and applications. Chem.-Asian J. 2016, 11, 3305–3328. [Google Scholar] [CrossRef]
- Tang, J.; Xu, R.; Sui, G.; Guo, D.; Zhao, Z.; Fu, S.; Yang, X.; Li, Y.; Li, J. Double-shelled porous g-C3N4 nanotubes modified with amorphous Cu-doped FeOOH nanoclusters as 0D/3D non-homogeneous photo-Fenton catalysts for effective removal of organic dyes. Small 2023, 19, 2208232. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, C.; Zheng, Z.; Sun, P.; She, Y.; Huang, F.; Mo, Z.; Yuan, J.; Li, H.; Xu, H. Porous carbon nitride nanotubes efficiently promote two-electron O2 reduction for photocatalytic H2O2 production. J. Alloys Compd. 2023, 934, 167901. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Zhao, C.; Zhao, Q.; Niu, T.; Pan, L.; Xu, P.; Zhang, F.; Wu, W.; Ni, T. Facile synthesis of three-dimensional hollow porous carbon doped polymeric carbon nitride with highly efficient photocatalytic performance. Chem. Eng. J. 2022, 438, 135623. [Google Scholar] [CrossRef]
- Li, Q.; Tong, Y.; Zeng, Y.; Gu, X.-K.; Ding, M. Carbohydrate-regulated synthesis of ultrathin porous nitrogen-vacancy polymeric carbon nitride for highly efficient visible-light hydrogen evolution. Chem. Eng. J. 2022, 450, 138010. [Google Scholar] [CrossRef]
- Attri, P.; Garg, P.; Sharma, P.; Singh, R.; Chauhan, M.; Lim, D.-K.; Kumar, S.; Chaudhary, G.R. Precursor-dependent fabrication of exfoliated graphitic carbon nitride (gCN) for enhanced photocatalytic and antimicrobial activity under visible light irradiation. J. Clean. Prod. 2023, 422, 138538. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Wang, X. Two-dimensional covalent carbon nitride nanosheets: Synthesis, functionalization, and applications. Energy Environ. Sci. 2015, 8, 3092–3108. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, S.; Liu, Y.; Zhai, Y. Formic acid assisted fabrication of Oxygen-doped Rod-like carbon nitride with improved photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 624, 338–347. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, J.; Yu, L.; Peng, J.; Song, Z.; Xiong, Z.; Li, N.; Xiang, K.; Zou, J.; Hsu, J.P.; et al. Tailoring advanced N-defective and S-doped g-C3N4 for photocatalytic H2 evolution. Small 2023, 19, 2301116. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiao, S.; Zhang, H.; Qiao, Y.; Liu, J.; Li, Y. Highly sensitive and selective demethylase FTO detection using a DNAzyme-mediated CRISPR/Cas12a signal cascade amplification electrochemiluminescence biosensor with C-CN/PCNV heterojunction as emitter. Biosens. Bioelectron. 2024, 256, 116276. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, C.; Shi, R.; Liu, Q.; Waterhouse, G.I.N.; Wu, L.; Tung, C.-H.; Zhang, T. Supramolecular precursor strategy for the synthesis of holey graphitic carbon nitride nanotubes with enhanced photocatalytic hydrogen evolution performance. Nano Res. 2019, 12, 2385–2389. [Google Scholar] [CrossRef]
- Chen, X.; Shi, R.; Chen, Q.; Zhang, Z.; Jiang, W.; Zhu, Y.; Zhang, T. Three-dimensional porous g-C3N4 for highly efficient photocatalytic overall water splitting. Nano Energy 2019, 59, 644–650. [Google Scholar] [CrossRef]
- Barrio, J.; Shalom, M. Rational design of carbon nitride materials by supramolecular preorganization of monomers. ChemCatChem 2018, 10, 5573–5586. [Google Scholar] [CrossRef]
- Bao, J.; Bai, W.; Wu, M.; Gong, W.; Yu, Y.; Zheng, K.; Liu, L. Template-mediated copper doped porous g-C3N4 for efficient photodegradation of antibiotic contaminants. Chemosphere 2022, 293, 133607. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Ho, W.; Li, Y.; Zhang, L. Facile synthesis of porous graphene-like carbon nitride (C6N9H3) with excellent photocatalytic activity for NO removal. Appl. Catal. B Environ. 2015, 174–175, 477–485. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Hu, J.-Y.; Jiang, H. Facile modification of a graphitic carbon nitride catalyst to improve its photoreactivity under visible light irradiation. Chem. Eng. J. 2014, 256, 230–237. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Feng, J.; Yan, S.; Zhang, H.; Li, Z.; Zou, Z. Improvement in photocatalytic H2 evolution over g-C3N4 prepared from protonated melamine. Appl. Surf. Sci. 2014, 295, 253–259. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Y.; Xu, S. Synthesis of graphitic carbon nitride by directly heating sulfuric acid treated melamine for enhanced photocatalytic H2 production from water under visible light. Int. J. Hydrogen Energy 2012, 37, 125–133. [Google Scholar] [CrossRef]
- Han, Q.; Cheng, Z.; Wang, B.; Zhang, H.; Qu, L. Significant enhancement of visible-light-driven hydrogen evolution by structure regulation of carbon nitrides. ACS Nano 2018, 12, 5221–5227. [Google Scholar] [CrossRef]
- Wu, S.; Wen, S.; Xu, X.; Huang, G.; Cui, Y.; Li, J.; Qu, A. Facile synthesis of porous graphene-like carbon nitride nanosheets with high surface area and enhanced photocatalytic activity via one-step catalyst-free solution self-polymerization. Appl. Surf. Sci. 2018, 436, 424–432. [Google Scholar] [CrossRef]
- Mo, Z.; Xu, H.; Chen, Z.; She, X.; Song, Y.; Wu, J.; Yan, P.; Xu, L.; Lei, Y.; Yuan, S.; et al. Self-assembled synthesis of defect-engineered graphitic carbon nitride nanotubes for efficient conversion of solar energy. Appl. Catal. B Environ. 2018, 225, 154–161. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Chen, M.; Shi, X.; Zhang, Y.; Cao, J.; Ho, W.; Lee, S.C. Roles of N-vacancies over porous g-C3N4 microtubes during photocatalytic NOx removal. ACS Appl. Mater. Interfaces 2019, 11, 10651–10662. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, Z.; Li, Q.; Li, X.; Fang, S.; Wu, X.; Li, M.; Ding, Y.; Liu, B.; Yang, C.; et al. Fabrication of high photoreactive carbon nitride nanosheets by polymerization of amidinourea for hydrogen production. Appl. Catal. B Environ. 2019, 245, 197–206. [Google Scholar] [CrossRef]
- Mondal, S.; Mark, G.; Tashakory, A.; Volokh, M.; Shalom, M. Porous carbon nitride rods as an efficient photoanode for water splitting and benzylamine oxidation. J. Mater. Chem. A 2024, 12, 11502–11510. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, Y.; Zhao, Z. Porous defect-modified graphitic carbon nitride via a facile one-step approach with significantly enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Catal. B Environ. 2018, 226, 1–9. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Liang, F.; Wang, J.; Li, S.; Zhang, H.; Zhang, S. Molten salt synthesis of tetragonal carbon nitride hollow tubes and their application for removal of pollutants from wastewater. Appl. Catal. B Environ. 2018, 225, 307–313. [Google Scholar] [CrossRef]
- Vinu, A.; Ariga, K.; Mori, T.; Nakanishi, T.; Hishita, S.; Golberg, D.; Bando, Y. Preparation and characterization of well-ordered hexagonal mesoporous carbon nitride. Adv. Mater. 2005, 17, 1648–1652. [Google Scholar] [CrossRef]
- Vinu, A. Two-dimensional hexagonally-ordered mesoporous carbon nitrides with tunable pore diameter, surface area and nitrogen content. Adv. Funct. Mater. 2008, 18, 816–827. [Google Scholar] [CrossRef]
- Chen, X.; Jun, Y.-S.; Takanabe, K.; Maeda, K.; Domen, K.; Fu, X.; Antonietti, M.; Wang, X. Ordered mesoporous SBA-15 type graphitic carbon nitride: A semiconductor host structure for photocatalytic hydrogen evolution with visible light. Chem. Mater. 2009, 21, 4093–4095. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; Zhang, G.; Huang, J.; Liu, P.; Antonietti, M.; Wang, X. Synthesis of bulk and nanoporous carbon nitride polymers from ammonium thiocyanate for photocatalytic hydrogen evolution. J. Mater. Chem. 2011, 21, 13032. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, Y.; Lin, J.; Wang, G. Highly-ordered mesoporous carbon nitride with ultrahigh surface area and pore volume as a superior dehydrogenation catalyst. Chem. Mater. 2014, 26, 3151–3161. [Google Scholar] [CrossRef]
- Wang, F.; Feng, Y.; Chen, P.; Wang, Y.; Su, Y.; Zhang, Q.; Zeng, Y.; Xie, Z.; Liu, H.; Liu, Y.; et al. Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: Kinetics, mechanism, and antibacterial activity elimination. Appl. Catal. B Environ. 2018, 227, 114–122. [Google Scholar] [CrossRef]
- Srinivasu, P.; Vinu, A.; Hishita, S.; Sasaki, T.; Ariga, K.; Mori, T. Preparation and characterization of novel microporous carbon nitride with very high surface area via nanocasting technique. Microporous Mesoporous Mater. 2008, 108, 340–344. [Google Scholar] [CrossRef]
- Kailasam, K.; Epping, J.D.; Thomas, A.; Losse, S.; Junge, H. Mesoporous carbon nitride-silica composites by a combined sol-gel/thermal condensation approach and their application as photocatalysts. Energy Environ. Sci. 2011, 4, 4668. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kofuji, Y.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Effects of surface defects on photocatalytic H2O2 production by mesoporous graphitic carbon nitride under visible light irradiation. ACS Catal. 2015, 5, 3058–3066. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Li, Z.; Nan, Y.; Ding, F.; Shen, Y.; Jiang, Z. Thylakoid-inspired multishell g-C3N4 nanocapsules with enhanced visible-light harvesting and electron transfer properties for high-efficiency photocatalysis. ACS Nano 2017, 11, 1103–1112. [Google Scholar] [CrossRef]
- Lin, W.; Hong, W.; Sun, L.; Yu, D.; Yu, D.; Chen, X. Bioinspired mesoporous chiral nematic graphitic carbon nitride photocatalysts modulated by polarized light. ChemSusChem 2018, 11, 114–119. [Google Scholar] [CrossRef]
- Liu, X.; Pang, F.; He, M.; Ge, J. Confined reaction inside nanotubes: New approach to mesoporous g-C3N4 photocatalysts. Nano Res. 2017, 10, 3638–3647. [Google Scholar] [CrossRef]
- Cui, L.; Song, J.; McGuire, A.F.; Kang, S.; Fang, X.; Wang, J.; Yin, C.; Li, X.; Wang, Y.; Cui, B. Constructing highly uniform onion-ring-like graphitic carbon nitride for efficient visible-light-driven photocatalytic hydrogen evolution. ACS Nano 2018, 12, 5551–5558. [Google Scholar] [CrossRef]
- Si, Y.; Huang, T.; Li, Q.; Huang, Y.; Gao, S.P.; Chen, M.; Wu, L. Hierarchical macro-mesoporous polymeric carbon nitride microspheres with narrow bandgap for enhanced photocatalytic hydrogen production. Adv. Mater. Interfaces 2018, 5, 1801241. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Yang, C.; Wang, X. Nanospherical carbon nitride frameworks with sharp edges accelerating charge collection and separation at a soft photocatalytic interface. Adv. Mater. 2014, 26, 4121–4126. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Xing, L.; He, Q.; Zhou, F.; Yang, X.; Wu, T.; Tong, G.; Wang, D.; Wu, W. Selective synthesis and defects steering superior microwave absorption capabilities of hollow graphitic carbon nitride micro-polyhedrons. Chem. Eng. J. 2022, 435, 135086. [Google Scholar] [CrossRef]
- Shi, L.; Chang, K.; Zhang, H.; Hai, X.; Yang, L.; Wang, T.; Ye, J. Drastic enhancement of photocatalytic activities over phosphoric acid protonated porous g-C3N4 nanosheets under visible light. Small 2016, 12, 4431–4439. [Google Scholar] [CrossRef]
- Du, X.; Zou, G.; Wang, Z.; Wang, X. A scalable chemical route to soluble acidified graphitic carbon nitride: An ideal precursor for isolated ultrathin g-C3N4 nanosheets. Nanoscale 2015, 7, 8701–8706. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yuan, A.; Yao, C.; Liu, J.; Li, B.; Xi, F.; Dong, X. Highly efficient photo-reduction of p-Nitrophenol by protonated graphitic carbon nitride nanosheets. ChemCatChem 2018, 10, 4747–4754. [Google Scholar] [CrossRef]
- Ma, T.Y.; Tang, Y.; Dai, S.; Qiao, S.Z. Proton-functionalized two-dimensional graphitic carbon nitride nanosheet: An excellent metal-/label-free biosensing platform. Small 2014, 10, 2382–2389. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, C.; Jin, Y.; Jing, Q.; Yang, Y.; Shen, X.; Tang, Q.; Mu, Y.; Du, A.K. Facile synthesis of 3D porous thermally exfoliated g-C3N4 nanosheet with enhanced photocatalytic degradation of organic dye. J. Colloid Interface Sci. 2016, 468, 211–219. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766. [Google Scholar] [CrossRef]
- Teng, Z.; Lv, H.; Wang, C.; Xue, H.; Pang, H.; Wang, G. Bandgap engineering of ultrathin graphene-like carbon nitride nanosheets with controllable oxygenous functionalization. Carbon 2017, 113, 63–75. [Google Scholar] [CrossRef]
- Feng, J.; Chen, T.; Liu, S.; Zhou, Q.; Ren, Y.; Lv, Y.; Fan, Z. Improvement of g-C3N4 photocatalytic properties using the Hummers method. J. Colloid Interface Sci. 2016, 479, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Luo, B.; Song, R.; Jing, D. Significantly enhanced photocatalytic hydrogen generation over graphitic carbon nitride with carefully modified intralayer structures. Chem. Eng. J. 2018, 332, 499–507. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Lin, Q.; Li, L.; Liang, S.; Liu, M.; Bi, J.; Wu, L. Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2015, 163, 135–142. [Google Scholar] [CrossRef]
- Gao, M.; Feng, J.; Zhang, Z.; Gu, M.; Wang, J.; Zeng, W.; Lv, Y.; Ren, Y.; Wei, T.; Fan, Z. Wrinkled ultrathin graphitic C3N4 nanosheets for photocatalytic degradation of organic wastewater. ACS Appl. Nano Mater. 2018, 1, 6733–6741. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Lu, L.; Si, Y.; Zhang, S.; Chen, Y.; Dai, K.; Duan, P.; Duan, L.; Liu, J. Graphitic carbon nitride nanosheet for photocatalytic hydrogen production: The impact of morphology and element composition. Appl. Surf. Sci. 2017, 391, 369–375. [Google Scholar] [CrossRef]
- Nie, H.; Ou, M.; Zhong, Q.; Zhang, S.; Yu, L. Efficient visible-light photocatalytic oxidation of gaseous NO with graphitic carbon nitride (g-C3N4) activated by the alkaline hydrothermal treatment and mechanism analysis. J. Hazard. Mater. 2015, 300, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Tsutsui, S.; Koike, K.; Hirakawa, T.; Teramoto, Y.; Negishi, N.; Takeuchi, K. Activation of graphitic carbon nitride (g-C3N4) by alkaline hydrothermal treatment for photocatalytic NO oxidation in gas phase. J. Mater. Chem. A 2013, 1, 6489. [Google Scholar] [CrossRef]
- Li, Y.; Jin, R.; Xing, Y.; Li, J.; Song, S.; Liu, X.; Li, M.; Jin, R. Macroscopic foam-like holey ultrathin g-C3N4 nanosheets for drastic improvement of visible-light photocatalytic activity. Adv. Energy Mater. 2016, 6, 1601273. [Google Scholar] [CrossRef]
- She, X.; Xu, H.; Xu, Y.; Yan, J.; Xia, J.; Xu, L.; Song, Y.; Jiang, Y.; Zhang, Q.; Li, H. Exfoliated graphene-like carbon nitride in organic solvents: Enhanced photocatalytic activity and highly selective and sensitive sensor for the detection of trace amounts of Cu2+. J. Mater. Chem. A 2014, 2, 2563. [Google Scholar] [CrossRef]
- Dong, F.; Li, Y.; Wang, Z.; Ho, W.-K. Enhanced visible light photocatalytic activity and oxidation ability of porous graphene-like g-C3N4 nanosheets via thermal exfoliation. Appl. Surf. Sci. 2015, 358, 393–403. [Google Scholar] [CrossRef]
- Zhang, Y.; Zong, S.; Cheng, C.; Shi, J.; Guo, P.; Guan, X.; Luo, B.; Shen, S.; Guo, L. Rapid high-temperature treatment on graphitic carbon nitride for excellent photocatalytic H2-evolution performance. Appl. Catal. B Environ. 2018, 233, 80–87. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, J.; Dong, C.-L.; Zhou, W.; Huang, Y.-C.; Mao, S.S.; Guo, L.; Shen, S. Interlayer interaction in ultrathin nanosheets of graphitic carbon nitride for efficient photocatalytic hydrogen evolution. J. Catal. 2017, 352, 491–497. [Google Scholar] [CrossRef]
- Huangfu, Y.; Zheng, T.; Zhang, K.; She, X.; Xu, H.; Fang, Z.; Xie, K. Facile fabrication of permselective g-C3N4 separator for improved lithium-sulfur batteries. Electrochim. Acta 2018, 272, 60–67. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, J.; Lei, C.; Yang, B.; Li, Z.; Xie, Y.; Zhang, X.; Lei, L.; Chen, J. Nitrogen vacancy structure driven photoeletrocatalytic degradation of 4-Chlorophenol using porous graphitic carbon nitride nanosheets. ACS Sustain. Chem. Eng. 2018, 6, 6497–6506. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. Holey graphitic carbon nitride nanosheets with carbon vacancies for highly improved photocatalytic hydrogen production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Song, X.; Yang, Q.; Jiang, X.; Yin, M.; Zhou, L. Porous graphitic carbon nitride nanosheets prepared under self-producing atmosphere for highly improved photocatalytic activity. Appl. Catal. B Environ. 2017, 217, 322–330. [Google Scholar] [CrossRef]
- Song, T.; Zhang, P.; Wang, T.; Ali, A.; Zeng, H. Alkali-assisted fabrication of holey carbon nitride nanosheet with tunable conjugated system for efficient visible-light-driven water splitting. Appl. Catal. B Environ. 2018, 224, 877–885. [Google Scholar] [CrossRef]
- Hu, P.; Chen, C.; Zeng, R.; Xiang, J.; Huang, Y.; Hou, D.; Li, Q.; Huang, Y. Facile synthesis of bimodal porous graphitic carbon nitride nanosheets as efficient photocatalysts for hydrogen evolution. Nano Energy 2018, 50, 376–382. [Google Scholar] [CrossRef]
- Nie, Y.; Zhu, Y.A.; Lu, X.; Qiu, J.; Wang, B.; Xie, Z.; Le, Z. Cu doped crystalline carbon nitride with increased carrier migration efficiency for uranyl photoreduction. Chem. Eng. J. 2023, 477, 146908. [Google Scholar] [CrossRef]
- Li, Y.; Xue, Y.; Gao, X.; Wang, L.; Liu, X.; Wang, Z.; Shen, S. Cayanamide group functionalized crystalline carbon nitride aerogel for efficient CO2 photoreduction. Adv. Funct. Mater. 2023, 34, 2312634. [Google Scholar] [CrossRef]
- Wang, X.-J.; Tian, X.; Li, F.-T.; Li, Y.-P.; Zhao, J.; Hao, Y.-J.; Liu, Y. Synchronous surface hydroxylation and porous modification of g-C3N4 for enhanced photocatalytic H2 evolution efficiency. Int. J. Hydrogen Energy 2016, 41, 3888–3895. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, H.; Luo, J.; Yuan, J.; Wang, L.; Liu, C.; Xia, Y.; Liu, M.; Luo, S.; Cai, T.; et al. Sea-urchin-structure g-C3N4 with narrow bandgap (˜2.0 eV) for efficient overall water splitting under visible light irradiation. Appl. Catal. B Environ. 2019, 249, 275–281. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, J.; Luo, W.; Zhang, Z.; Cheng, X.; Wu, J.; Yang, Y.; Chen, G.; Sun, S.; et al. Post-redox engineering electron configurations of atomic thick C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 270, 118855. [Google Scholar] [CrossRef]
- Ai, L.; Zhao, Z.; Song, X.; Tang, Y.; Feng, K.; Wang, X.; Zhang, B.; Bi, H.; Qiu, J. Curved-Slit Effect Induced Nucleation for g-C3N4 Nanorings with Double Concaves and Enhanced Photocatalysis. Adv. Funct. Mater. 2025, 35, 2506395. [Google Scholar] [CrossRef]
- An, N.; Zhang, X.; Chen, Y.; Wang, Z.; Qiu, J.; Gao, B.; Li, Q. A Self-floating Photothermal/Photocatalytic Evaporator for Simultaneous High-Efficiency Evaporation and Purification of Volatile Organic Wastewater. Adv. Funct. Mater. 2025, 35, 2500777. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Díez, A.M.; Silva, C.G.; Faria, J.L.; Sanromán, M.Á.; Silva, A.M.T.; Pazos, M. Photoelectrocatalytic degradation of pharmaceuticals promoted by a metal-free g-C3N4 catalyst. Chem. Eng. J. 2023, 476, 146761. [Google Scholar] [CrossRef]
- He, S.; Wang, X.; Tang, L.; Wang, J.; Chen, J.; Zhang, Y. A Novel G-C3N4 Modified Biogenic Mackinawite Mediated by SRB for Boosting Highly Efficient Adsorption and Catalytic Degradation of Antibiotics in Photo-Fenton Process. Small 2024, 20, 2408723. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, G. Achieving Near Infrared Photodegradation by the Synergistic Effect of Z-Scheme Heterojunction and Antenna of Rare Earth Single Atoms. Small 2025, 21, 2412148. [Google Scholar] [CrossRef]
- Jiang, W.; Luo, W.; Zong, R.; Yao, W.; Li, Z.; Zhu, Y. Polyaniline/carbon nitride nanosheets composite hydrogel: A separation-free and high-efficient photocatalyst with 3D hierarchical structure. Small 2016, 12, 4370–4378. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, Z.; Guo, Y.; Wong, P.K.; Zhou, X.; Bai, R. In-situ growth of all-solid Z-scheme heterojunction photocatalyst of Bi7O9I3/g-C3N4 and high efficient degradation of antibiotic under visible light. Appl. Catal. B Environ. 2020, 261, 118212. [Google Scholar] [CrossRef]
- An, S.; Zhang, G.; Wang, T.; Zhang, W.; Li, K.; Song, C.; Miller, J.T.; Miao, S.; Wang, J.; Guo, X. High-density ultra-small clusters and single-atom Fe sites embedded in graphitic carbon nitride (g-C3N4) for hghly efficient catalytic advanced oxidation processes. ACS Nano 2018, 12, 9441–9450. [Google Scholar] [CrossRef]
- Gao, M.; Feng, J.; He, F.; Zeng, W.; Wang, X.; Ren, Y.; Wei, T. Carbon microspheres work as an electron bridge for degrading high concentration MB in CoFe2O4@carbon microsphere/g-C3N4 with a hierarchical sandwich-structure. Appl. Surf. Sci. 2020, 507, 145167. [Google Scholar] [CrossRef]
- Le, Z.; Xiong, C.; Gong, J.; Wu, X.; Pan, T.; Chen, Z.; Xie, Z. Self-cleaning isotype g-C3N4 heterojunction for efficient photocatalytic reduction of hexavalent uranium under visible light. Environ. Pollut. 2020, 260, 114070. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Chen, X.; Zhou, X.; Hu, W.; Li, P.; Li, Y.; Zhang, Y.; Wang, Y. A novel g-C3N4 modified biosynthetic Fe(III)-hydroxysulfate for efficient photoreduction of Cr(VI) in wastewater treatment under visible light irradiation. Chem. Eng. J. 2020, 398, 125632. [Google Scholar] [CrossRef]
- Liao, Q.; Pan, W.; Zou, D.; Shen, R.; Sheng, G.; Li, X.; Zhu, Y.; Dong, L.; Asiri, A.M.; Alamry, K.A.; et al. Using of g-C3N4 nanosheets for the highly efficient scavenging of heavy metals at environmental relevant concentrations. J. Mol. Liq. 2018, 261, 32–40. [Google Scholar] [CrossRef]
- Lei, Z.-D.; Xue, Y.-C.; Chen, W.-Q.; Li, L.; Qiu, W.-H.; Zhang, Y.; Tang, L. The influence of carbon nitride nanosheets doping on the crystalline formation of MIL-88B(Fe) and the photocatalytic activities. Small 2018, 14, 1802045. [Google Scholar] [CrossRef]
- Chen, S.; Lu, W.; Han, J.; Zhong, H.; Xu, T.; Wang, G.; Chen, W. Robust three-dimensional g-C3N4@cellulose aerogel enhanced by cross-linked polyester fibers for simultaneous removal of hexavalent chromium and antibiotics. Chem. Eng. J. 2019, 359, 119–129. [Google Scholar] [CrossRef]
- Wu, J.-H.; Shao, F.-Q.; Luo, X.-Q.; Xu, H.-J.; Wang, A.-J. Pd nanocones supported on g-C3N4: An efficient photocatalyst for boosting catalytic reduction of hexavalent chromium under visible-light irradiation. Appl. Surf. Sci. 2019, 471, 935–942. [Google Scholar] [CrossRef]
- Luo, J.; Dong, G.; Zhu, Y.; Yang, Z.; Wang, C. Switching of semiconducting behavior from n-type to p-type induced high photocatalytic NO removal activity in g-C3N4. Appl. Catal. B Environ. 2017, 214, 46–56. [Google Scholar] [CrossRef]
- Ou, M.; Wan, S.; Zhong, Q.; Zhang, S.; Song, Y.; Guo, L.; Cai, W.; Xu, Y. Hierarchical Z-scheme photocatalyst of g-C3N4@Ag/BiVO4 (040) with enhanced visible-light-induced photocatalytic oxidation performance. Appl. Catal. B Environ. 2018, 221, 97–107. [Google Scholar] [CrossRef]
- Zhou, M.; Dong, G.; Yu, F.; Huang, Y. The deep oxidation of NO was realized by Sr multi-site doped g-C3N4 via photocatalytic method. Appl. Catal. B Environ. 2019, 256, 117825. [Google Scholar] [CrossRef]
- Liao, J.; Cui, W.; Li, J.; Sheng, J.; Wang, H.; Dong, X.A.; Chen, P.; Jiang, G.; Wang, Z.; Dong, F. Nitrogen defect structure and NO+ intermediate promoted photocatalytic NO removal on H2 treated g-C3N4. Chem. Eng. J. 2020, 379, 122282. [Google Scholar] [CrossRef]
- Hu, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. 3D aerogel of graphitic carbon nitride modified with perylene imide and graphene oxide for highly efficient nitric oxide removal under visible light. Small 2018, 14, e1800416. [Google Scholar] [CrossRef]
- Li, X.; Dong, G.; Guo, F.; Zhu, P.; Huang, Y.; Wang, C. Enhancement of photocatalytic NO removal activity of g-C3N4 by modification with illite particles. Environ. Sci. Nano 2020, 7, 1990–1998. [Google Scholar] [CrossRef]
- dos Santos, G.; Tian, L.; Gonçalves, R.; García, H.; Rossi, L. Boosting CO2 Photoreduction Efficiency of Carbon Nitride via S-scheme g-C3N4/Fe2TiO5 Heterojunction. Adv. Funct. Mater. 2025, 35, 2422055. [Google Scholar] [CrossRef]
- Liu, M.; Huang, L.; Su, K.; Liang, J.; Wu, Y.; Luo, P.; Song, H.; Du, L.; Cui, Z. Multiple Ion Rectification Strategy Regulated Polyethylene Glycol-Based Polymer Electrolyte for Stable High-Voltage Lithium Metal Batteries. Adv. Funct. Mater. 2025, 35, 2425417. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, E.; Tai, X.; Zong, H.; Yi, J.; Yuan, Z.; Zhao, X.; Huang, P.; Xu, H.; Jiang, Z. g-C3N4 S-Scheme Homojunction through Van der Waals Interface Regulation by Intrinsic Polymerization Tailoring for Enhanced Photocatalytic H2 Evolution and CO2 Reduction. Angew. Chem. Int. Ed. 2025, 64, e202425439. [Google Scholar] [CrossRef]
- Yadav, D.K.; Latiyan, S.; Devan, R.S.; Urkude, R.R.; Rajput, P.; Singh, A.; Deka, S. Breaking Barriers: Synergistic Interactions Between Pt Single Atoms and Nitrogen-Rich g-C3N4 for Maximized Photocatalytic Hydrogen Production. Small 2025, 21, 2503843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Zhai, B.; Li, X.; Niu, P.; Odent, J.; Wang, S.; Li, L. Highly Crystalline Poly(heptazine imide)-Based Carbonaceous Anodes for Ultralong Lifespan and Low-Temperature Sodium-Ion Batteries. ACS Nano 2024, 18, 3456–3467. [Google Scholar] [CrossRef]
- Zhuang, C.; Chang, Y.; Li, W.; Li, S.; Xu, P.; Zhang, H.; Zhang, Y.; Zhang, C.; Gao, J.; Chen, G.; et al. Light-Induced Variation of Lithium Coordination Environment in g-C3N4 Nanosheet for Highly Efficient Oxygen Reduction Reactions. ACS Nano 2024, 18, 5206–5217. [Google Scholar] [CrossRef]
- Sun, Y.-J.; He, J.-Y.; Zhang, D.; Wang, X.-J.; Zhao, J.; Liu, R.-H.; Li, F.-T. Simultaneous construction of dual-site phosphorus modified g-C3N4 and its synergistic mechanism for enhanced visible-light photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 517, 146192. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Nie, T.; Wang, R.; He, B.; Han, B.; Wang, H.; Tian, Y.; Gong, Y. Enhanced visible-light photocatalytic H2 production of hierarchical g-C3N4 hexagon by one-step self-assembly strategy. Appl. Surf. Sci. 2020, 499, 143942. [Google Scholar] [CrossRef]
- Che, W.; Cheng, W.; Yao, T.; Tang, F.; Liu, W.; Su, H.; Huang, Y.; Liu, Q.; Liu, J.; Hu, F.; et al. Fast photoelectron transfer in (Cring)-C3N4 plane heterostructural nanosheets for overall water splitting. J. Am. Chem. Soc. 2017, 139, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Gao, Y.; Zhang, L.; Zhang, J.; Zhang, Q.; Li, Q.; Bao, H.; Zhou, J.; Miao, S.; Chen, N.; et al. A promoted charge separation/transfer system from Cu single atoms and C3N4 layers for efficient photocatalysis. Adv. Mater. 2020, 32, 2003082. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Yadav, R.; Kumar, A.; Kumar Sinha, A.; Srivastava, R. Surface modified C, O co-doped polymeric g-C3N4 as an efficient photocatalyst for visible light assisted CO2 reduction and H2O2 production. Appl. Catal. B Environ. 2019, 259, 118054. [Google Scholar] [CrossRef]
- Mo, Z.; Zhu, X.; Jiang, Z.; Song, Y.; Liu, D.; Li, H.; Yang, X.; She, Y.; Lei, Y.; Yuan, S.; et al. Porous nitrogen-rich g-C3N4 nanotubes for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2019, 256, 117854. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; An, W.; Wang, H.; Guo, H.; Liang, Y.; Cui, W. Ultrathin porous g-C3N4 nanosheets modified with AuCu alloy nanoparticles and C-C coupling photothermal catalytic reduction of CO2 to ethanol. Appl. Catal. B Environ. 2020, 266, 118618. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Guo, Y.; Yang, L.; Lv, M.; Tang, X.; Li, S. Enhanced photoreduction CO2 activity on g-C3N4: By synergistic effect of nitrogen defective-enriched and porous structure, and mechanism insights. Chem. Eng. J. 2020, 388, 124288. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Q.; Bai, X.; Ge, Z.; Yang, Q.; Yin, C.; Kang, S.; Dong, M.; Li, X. Carbothermal activation synthesis of 3D porous g-C3N4/carbon nanosheets composite with superior performance for CO2 photoreduction. Appl. Catal. B Environ. 2018, 239, 196–203. [Google Scholar] [CrossRef]
- Cheng, L.; Yin, H.; Cai, C.; Fan, J.; Xiang, Q. Single Ni atoms anchored on porous few-layer g-C3N4 for photocatalytic CO2 reduction: The role of edge confinement. Small 2020, 16, 2002411. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, W.; Huang, J.; Du, Z.; Hong, X.; Chen, X.; Hu, X.; Wang, X. Enhanced photocatalytic nitrogen fixation of Ag/B-doped g-C3N4 nanosheets by one-step in-situ decomposition-thermal polymerization method. Appl. Catal. A Gen. 2020, 601, 117647. [Google Scholar] [CrossRef]
- Kwon, N.H.; Shin, S.-J.; Jin, X.; Jung, Y.; Hwang, G.-S.; Kim, H.; Hwang, S.-J. Monolayered g-C3N4 nanosheet as an emerging cationic building block for bifunctional 2D superlattice hybrid catalysts with controlled defect structures. Appl. Catal. B Environ. 2020, 277, 119191. [Google Scholar] [CrossRef]
- Qiu, P.; Xu, C.; Zhou, N.; Chen, H.; Jiang, F. Metal-free black phosphorus nanosheets-decorated graphitic carbon nitride nanosheets with C P bonds for excellent photocatalytic nitrogen fixation. Appl. Catal. B Environ. 2018, 221, 27–35. [Google Scholar] [CrossRef]
- Cao, S.; Fan, B.; Feng, Y.; Chen, H.; Jiang, F.; Wang, X. Sulfur-doped g-C3N4 nanosheets with carbon vacancies: General synthesis and improved activity for simulated solar-light photocatalytic nitrogen fixation. Chem. Eng. J. 2018, 353, 147–156. [Google Scholar] [CrossRef]
- Yin, H.; Li, S.-L.; Gan, L.-Y.; Wang, P. Pt-embedded in monolayer g-C3N4 as a promising single-atom electrocatalyst for ammonia synthesis. J. Mater. Chem. A 2019, 7, 11908–11914. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.-Y.; Wang, W.-P.; Zhang, X.-H.; Tan, X.-H.; Chu, W.-G.; Guo, Y.-G. Microemulsion assisted assembly of 3D porous S/Graphene@g-C3N4 hybrid sponge as free-standing cathodes for high energy density Li-S batteries. Adv. Energy Mater. 2018, 8, 1702839. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Chen, J.; Wang, X.; Wang, D.; Mao, Z. Templated transformation of g-C3N4 nanosheets into nitrogen-doped hollow carbon sphere with tunable nitrogen-doping properties for application in Li-ions batteries. Carbon 2020, 168, 458–467. [Google Scholar] [CrossRef]
- Wang, M.; Liang, Q.; Han, J.; Tao, Y.; Liu, D.; Zhang, C.; Lv, W.; Yang, Q.-H. Catalyzing polysulfide conversion by g-C3N4 in a graphene network for long-ife lithium-sulfur batteries. Nano Res. 2018, 11, 3480–3489. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.; Fan, C.; Liu, J.; Li, Y.; Wu, X.-L.; Xie, H.; Zhang, J.; Sun, H. Layered g-C3N4@reduced graphene oxide composites as anodes with improved rate performance for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 30330–30336. [Google Scholar] [CrossRef]
- Kang, S.; Huang, W.; Zhang, L.; He, M.; Xu, S.; Sun, D.; Jiang, X. Moderate bacterial etching allows scalable and clean delamination of g-C3N4 with enriched unpaired electrons for highly improved photocatalytic water disinfection. ACS Appl. Mater. Interfaces 2018, 10, 13796–13804. [Google Scholar] [CrossRef]
- Li, R.; Ren, Y.; Zhao, P.; Wang, J.; Liu, J.; Zhang, Y. Graphitic carbon nitride (g-C3N4) nanosheets functionalized composite membrane with self-cleaning and antibacterial performance. J. Hazard. Mater. 2019, 365, 606–614. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yu, X.; Gao, F.; Shen, Z.; Zhang, X.; Ge, S.; Liu, J.; Gu, Z.; Chen, C. An all-organic semiconductor C3N4/PDINH heterostructure with advanced antibacterial photocatalytic therapy activity. Adv. Mater. 2019, 31, 1901965. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Visconti, M.C.; Desipio, M.M.; Thorpe, R. Inactivation of antibiotic resistance gene by ternary nanocomposites of carbon nitride, reduced graphene oxide and iron oxide under visible light. Chem. Eng. J. 2020, 382, 122857. [Google Scholar] [CrossRef]
- Chen, S.; Lv, Y.; Shen, Y.; Ji, J.; Zhou, Q.; Liu, S.; Zhang, Y. Highly sensitive and quality self-testable electrochemiluminescence assay of DNA methyltransferase activity using multifunctional sandwich-assembled carbon nitride nanosheets. ACS Appl. Mater. Interfaces 2018, 10, 6887–6894. [Google Scholar] [CrossRef]
- Niazi, S.; Khan, I.M.; Yu, Y.; Pasha, I.; Lv, Y.; Mohsin, A.; Mushtaq, B.S.; Wang, Z. A novel fluorescent aptasensor for aflatoxin M1 detection using rolling circle amplification and g-C3N4 as fluorescence quencher. Sens. Actuators B Chem. 2020, 315, 128049. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, L.; Tang, Y.; Zhang, X. A facile Pt-doped g-C3N4 photocatalytic biosensor for visual detection of superoxide dismutase in serum samples. Sens. Actuators B Chem. 2020, 318, 128238. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, L.; Wen, Z.; Zhang, M.; Jiang, D.; Guo, Y.; Ding, C.; Wang, K. Core-shell LaFeO3@g-C3N4 p-n heterostructure with improved photoelectrochemical performance for fabricating streptomycin aptasensor. Appl. Surf. Sci. 2020, 511, 145571. [Google Scholar] [CrossRef]


| References | Precursor 1 | Precursor 2 | Mixing Mode | Calcining Process | Results | |||
|---|---|---|---|---|---|---|---|---|
| Bulk g-C3N4 BET | Increased BET | Morphology | Application | |||||
| [28] | Melamine 0.5 g | Urea 5 g | - | 550 °C for 4 h 5 °C min−1 in a nitrogen | 5.6 m2/g | 42.2 m2/g |  | Photocatalytic hydrogen evolution |
| [21] | Cyanuric acid 2.58 g | Melamine 2.52 g | Stirred for 2 h | 550 °C for 4 h | 17.74 m2/g | 81.58 m2/g |  | H2 production activity and degradation rate |
| [25] | 5 g of formic acid | 3 g of melamine | Hydrothermal treatment | 550 °C for 4 h | - | 81.4 m2/g |  | Photocatalytic hydrogen evolution |
| [20] | 1.0 g melamine | 2.0 g hydroxylamine hydrochloride | Hydrothermal process | 520 °C for 4 h in air | 3.9 m2/g | 129.4 m2/g |  | Photocatalytic H2O2 production |
| [26] | 4 g of melamine | 50 mL of N,N- dimethylformamide | Fully mixed at 25 °C for 0.5 h | 550 °C for 4 h under air | 11.23 m2/g | 181.74 m2/g |  | Photocatalytic hydrogen evolution |
| [27] | Melamine | Cyanuric acid Phosphorous acid | Hydrothermal process | 520 °C for 4 h | - | - |  | Electrochemiluminescence |
| [29] | 0.01 mol melamine | 0.01 mol cyanuric acid | Stirred for 12 h at room temperature | 550 °C for 4 h 5 °C min−1 | 10.83 m2/g | 130 m2/g |  | Photocatalytic overall water splitting |
| References | Precursor | Added Reagent | Treatment Mode | Calcining Process | Results | |||
|---|---|---|---|---|---|---|---|---|
| Bulk g-C3N4 BET | Increased BET | Morphology | Application | |||||
| [32] | Melamine | 1 mL HCl (37%) | In 80 mL of hot distilled water Stirring for 30 min | 500 °C for 2 h 20 °C/min 520 °C for 2 h | 8.5 m2/g | 345 m2/g |  | Photocatalytic activity for NO removal |
| [33] | Melamine | 3 mL concentrated HCl (1:1, v/v) | In 30 mL of absolute alcohol Stirring for 30 min | 550 °C for 4 h | 12.7 m2/g | 26.2 m2/g |  | Photocatalytic degradation |
| [34] | Melamine | 0.6 M HNO3 solution (50 mL) | 50 mL of ethylene glycol Stirring at room temperature | 550 °C for 2 h | 16.6 m2/g | 86.4 m2/g | 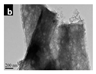 | Photocatalytic hydrogen evolution |
| [35] | Melamine | H2SO4:H2O 1:1 in volume | In distilled water (100 mL) Stirring for 2 h | 380 °C in 5 min 600 °C for 4 h 1 °C min−1 in Ar | 8.6 m2/g | 15.6 m2/g | - | Photocatalytic hydrogen evolution |
| [36] | Urea | 50 mL of methanol | Diethyl until white jellylike crystallization occurred | 600 °C for 2 h 2.3 °C min−1 in Ar | 43.1 m2/g | 228.4 m2/g |  | Photocatalytic hydrogen evolution |
| [37] | Melamine | Dried dimethyl sulfoxide 100 mL | 180 °C under magnetic stirring | - | 7.94 m2/g | 669.15 m2/g |  | Photocatalytic degradation |
| [42] | Dicyandiamide | NH4Cl | Frozen in liquid nitrogen | 550 °C for 4 h 3 °C min−1 in N2 | - | 65 m2/g |  | Photocatalytic hydrogen evolution |
| [43] | Melamine | 352 °C for LiCl-KCl | Milled together | 450 °C for 5 h 4 °C min−1 in air | 7 m2/g | 128 m2/g |  | Photocatalytic degradation |
| [38] | Melamine | Deionized water (40 mL) | 200 °C for 12 h | 550 °C for 4 h 2 °C min−1 | 8.6 m2/g | 127.8 m2/g |  | Photocatalytic hydrogen evolution |
| [39] | Melamine | Deionized water (70 mL) | 180 °C for 12 h | 550 °C for 3 h 2.5 °C min−1 | 19.9 m2/g | 67.5 m2/g |  | Photocatalytic activity for NO removal |
| [40] | Dicyandiamide | Deionized water (65 mL) | 200 °C for 2 h | 550 °C for 4 h 5 °C min−1 | 12.2 m2/g | 59.8 m2/g |  | Photocatalytic hydrogen evolution |
| References | Template | Precursor | Mixing Mode | Calcining Process | Removing Template | Results | |||
|---|---|---|---|---|---|---|---|---|---|
| Bulk g-C3N4 BET | Increased BET | Morphology | Application | ||||||
| [44] | SBA-15 | Ethane diamine CCl4 | Refluxed and stirred at 90 °C for 6 h | 600 °C for 5 h 3.0 °C min−1 in a nitrogen | 5 wt. % hydrofluoric acid | - | 505 m2/g |  | - |
| [45] | SBA-15 10.7 nm | Ethane diamine CCl4 | Refluxed and stirred at 90 °C for 6 h | 600 °C for 5 h 3.0 °C min−1 in nitrogen | 5 wt. % hydrofluoric acid | - | 830 m2/g |  | The Friedel-Crafts acylation of benzene |
| [46] | SBA-15 | Cyanamide | Stirred for 1 h | 550 °C for 4 h 2.3 °C min−1 | NH4HF2 4 M | - | 239 m2/g |  | Photocatalytic Hydrogen Evolution |
| [47] | SBA-15 | Ammonium thiocyanate | Stirred at 100 °C to remove water | 550 °C for 2 h | NH4HF2 4 M | 9 m2/g | 239 m2/g |  | Photocatalytic Hydrogen Evolution |
| SiO2 | 188 m2/g |  | |||||||
| [48] | SBA-15 | Hexamethylene-tetramine | Stirred at room temperature | 750 °C in nitrogen | 40% of HF | - | 1116 m2/g |  | Dehydrogenation of ethylbenzene to styrene |
| [49] | SBA-15 | Dicyandiamide | Vaporized at 70 °C | 550 °C for 3 h | NH4HF2 4 M | 16.7 m2/g | 50.1 m2/g |  | Photocatalytic degradation of fluoroquinolone antibiotics |
| [50] | MCM-22 | Ethane diamine CCl4 | Refluxed at 90 °C for 6 h | 600 °C for 5 h 3.0 °C min−1 in nitrogen | 5 wt. % hydrofluoric acid | less than 25 m2/g | 739 m2/g | 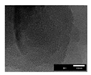 | - |
| [51] | SiO2 | Cyanamide | Stirred for 30 min (CA at 0.01 N HCl and ethanol pH 2, adding TEOS) | 550 °C for 4 h 2.3 °C min−1 in argon | NH4HF2 4 M | - | 131 m2/g |  | Photocatalytic Hydrogen Evolution |
| [52] | SiO2 12 nm | Cyanamide | Stirred at 333 K for 12 h | 823 K for 4 h 2.3 °C min−1 under N2 | NH4HF2 4 M | 10 m2/g | 160 m2/g 228 m2/g |  | Photocatalytic H2O2 Production |
| [53] | Multishell SiO2 nanospheres | Cyanamide | Stirred at 40 °C for 8 h | 550 °C for 3h under N2 | Na2CO3 0.3 M | - | 310.7 m2/g |  | Photocatalytic Hydrogen Evolution |
| [54] | Chiral mesoporous SiO2 films | Cyanamide | Sonicated at 55 °C for 4 h | 550 °C for 4 h 4 °C min−1 in N2 | NH4HF2 4 M | 6.03 m2/g | 132.26 m2/g |  | Photocatalytic Hydrogen Evolution |
| [55] | SiO2 nanotubes with porous shells | Cyanamide | Stirring for 10 min, separating, and drying (three times) | 550 °C for 4 h in N2 | 10% of HF | 4.6 m2/g | 135.1 m2/g |  | Photocatalytic Hydrogen Evolution |
| [56] | SiO2 microspheres | Melamine | In-air CVD method 320 °C for 2 h | 550 °C for 3 h | NH4HF2 4 M | 10.1 m2/g | 29.9 m2/g |  | Photocatalytic Hydrogen Evolution |
| [57] | SiO2 | Cyanamide | Stirred at 25 °C for 24 h NH4OH Stirred for about 5 min | 550 °C for 4 h 1 °C min−1 in N2 | NH5F2 4 M | - | 69.1 m2/g | 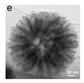 | Photocatalytic Hydrogen Evolution |
| [58] | KCC-1 | Cyanamide | Sonication at 55 °C for 4 h (HCl-treated KCC-1) | 550 °C for 4 h | NH4HF2 4 M | 9 m2/g | 160 m2/g |  | Photocatalytic Hydrogen Evolution |
| References | Synthesis of Bulk g-C3N4 | Ultrasonic Process | Assistant Method | Results | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mbulk g-C3N4 | Solvent/VSolvent | Treated Time | Band Gap | BET | Morphology | Application | |||
| [68] | Melamine 600 °C for 2 h 3 °C/min in air | 0.1 g | Water 100 mL | 16 h | - | 2.64 eV ↓ 2.70 eV | - |  | Bioimaging |
| [71] | Commercial g-C3N4 | 0.03 g | IPA 10 mL | 10 h | - | 2.35 eV ↓ 2.65 eV | 384 m2/g | 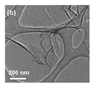 | Hydrogen Evolution |
| [78] | Dicyandiamide 350 °C for 2 h 600 °C for 2 h | 0.06 g | 1,3-BUT 25 mL | 24 h | - | 2.65 eV ↓ 2.79 eV | 3.3 m2/g ↓ 32.54 m2/g |  | The sensor for trace amounts of Cu2+ determination Photocatalytic degradation |
| [72] | Melamine 550 °C for 4 h in static air 2.3 °C/min. | 0.5 g | Ethanol–water 150 mL | 10 h | - | 2.70 eV ↓ 2.79 eV | 12.5 m2/g ↓ 59.4 m2/g |  | Photocatalytic degradation |
| References | Synthesis of Bulk g-C3N4 | Thermal Oxidation Treatment | Assistant Method | Results | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mbulk g-C3N4 | Temperature | Treated Time | Band Gap | BET | Morphology | Application | |||
| [69] | Dicyandiamide 550 °C for 4 h in static air 2.3 °C/min | 0.4 g | 500 °C 5 °C/min | 2 h in static air | - | 2.77 eV ↓ 2.97 eV | 50 m2/g ↓ 306 m2/g |  | Photocatalytic hydrogen evolution |
| [79] | Thiourea 550 °C for 2 h 15 °C/min | - | 550 °C | 2 h in air | - | 2.42 eV ↓ 2.86 eV | 27 m2/g ↓ 151 m2/g |  | Visible light photocatalytic removal of NOx |
| [80] | Melamine 520 °C for 4 h 5 °C min−1 in static air | 1.0 g | 800 °C 600 °C min−1 | 15 min | cooled by circulation cooling water (15 °C) | 2.64 eV ↓ 2.81 eV | 7.38 m2/g ↓ 60.51 m2/g |  | Photocatalytic hydrogen evolution |
| [73] | Dicyandiamide 550 °C for 4 h 2 °C/min in static air | 0.5 g | 500 °C 5 °C/min | 2 h | put quickly into liquid nitrogen | 2.68 eV ↓ 2.57 eV | 15.6 m2/g ↓ 142.8 m2/g |  | Photocatalytic degradation |
| [74] | Urea 550 °C for 9 h 10 °C/min in air | - | - | - | A single continuous heating process | - | 40.22 m2/g ↓ 117.27 m2/g |  | Photocatalytic hydrogen evolution |
| [81] | Melamine 520 °C for 4 h in air 5 °C/min | 0.5 g | 520 °C 5 °C/min | 4 h in air | 0.5 g 200 mL water ultrasonication 2 h | 2.64 eV ↓ 2.72 eV | 10.94 m2/g ↓ 99.73 m2/g |  | Photocatalytic hydrogen evolution |
| [77] | Melamine 773 K for 2 h 2 K min −1 793 K for 2 h | 1 g | 793 K 2 K min −1 | 6 h | Increased calcination time | 2.67 eV ↓ 2.81 eV | 10.89 m2/g ↓ 277.98 m2/g |  | Photocatalytic hydrogen evolution |
| [82] | Melamine 550 °C for 4 h 2 °C min−1 | 0.4 g | 550 °C | 30 min | wice | - | - | - | Lithium-sulfur batteries |
| [83] | Dicyandiamide 550 °C for 4 h 5 °C min−1 in air | - | 600 °C | 2 h | H2 atmosphere | 2.78 eV ↓ 1.82 eV | 7 m2/g ↓ 114 m2/g |  | Photoeletrocatalytic Degradation of 4-Chlorophenol |
| [84] | Dicyandiamide 550 °C for 4 h | 0.3 g | 510 °C | 1 h | NH3 atmosphere | 2.59 eV ↓ 2.90 eV | 6 m2/g ↓ 196 m2/g |  | Photocatalytic hydrogen evolution |
| [83] | Melamine 550 °C for 4 h 5 °C/min in N2 gas | - | 300 °C | - | Self-producted NH3 atmosphere | 2.78 eV ↓ 3.00 eV | 6.57 m2/g ↓ 38.51 m2/g |  | Photocatalytic degradation |
| [86] | Melamine 550 °C for 4 h 10 °C min−1 | 6 g | 300 °C 2 °C min−1 | 1 h | Mass ratio of CN (B): KOH is 1:2 in 50 mL H2O | 2.55 eV ↓ 2.66 eV | 10.3 m2/g ↓ 265.2 m2/g |  | Visible-light-driven water splitting |
| [87] | Melamine 550 °C for 2 h in static air 5 °C min−1 | 0.1 g | 350 °C 5 °C min−1 | 1.5 h in static air | 0.1 g of bulk g-C3N4, 0.2 g of KOH and 5 mL of H2O | 2.53 eV ↓ 2.75 eV | 219 m2/g |  | Photocatalytic hydrogen evolution |
| References | Synthesis of Bulk g-C3N4 | Post-Hydrothermal | Assistant Method | Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mbulk g-C3N4 | Type of Solution | Treated Temperature | Treated Time | Band Gap | BET | Morphology | Application | |||
| [75] | Dicyandiamide 550 °C for 4 h 2.9 °C min−1 | 1.0 g | 90 mL NaOH 0.12 M | 120 °C | 18 h | - | 2.75 eV ↓ 2.67 eV | 29.7 m2/g ↓ 64.7 m2/g |  | Photocatalytic oxidation of gaseous NO |
| [76] | Melamine 550 °C for 2 h 10 °C min−1 | 1.0 g | 90 mL NaOH 0.1 M | 130 °C | 18 h | - | - | 7.7 m2/g ↓ 53.7 m2/g |  | Photocatalytic NO oxidation in gas phase |
| [70] | Dicyandiamide 550 °C for 4 h 4 °C min−1 in air | 0.2 g | (50-X) mL distilled water -ammonium hydroxides | 120 °C | 12 h | - | 2.79 eV ↓ 2.91 eV | 7.43 m2/g ↓ 42.78 m2/g |  | Photocatalytic hydrogen generation |
| [90] | Melamine 550 °C for 3 h 10 °C/min | 0.5 g | 35 mL ammonium hydroxide (mass fraction = 5%) | 160 °C | 4 h | - | 2.76 eV ↓ 2.86 eV | 14.6 m2/g ↓ 44.8 m2/g |  | Photocatalytic hydrogen generation |
| [91] | Melamine 550 °C for 4 h 2.5 °C/min in N2 | 0.23 g | 60 mL distilled water | 180 °C | 6 h | Porous g-C3N4 ---sealed condensation | 2.68 eV ↓ 2.07 eV | 1.59 m2/g ↓ 65.6 m2/g |  | Overall water splitting |
| [92] | Polycondensation of urea | 0.5 g | 10 mL 0.1 M KOH | 150 °C | 12 h | Carbon thermal reduction | 2.72 eV ↓ 2.57 eV | 38.7 m2/g ↓ 197.0 m2/g |  | Photocatalytic hydrogen evolution |
| References | Materials | Morphology | Synthesis | BET | Application |
|---|---|---|---|---|---|
| [21] | g-C3N4 |  | Pre-treatment | 81.58 m2/g | H2 production activity and degradation rate |
| [32] | g-C3N4 |  | Pre-treatment | 345 m2/g | Photocatalytic activity for NO removal |
| [33] | g-C3N4 |  | Pre-treatment | 26.2 m2/g | Photocatalytic degradation |
| [37] | g-C3N4 |  | Pre-treatment | 669.15 m2/g | Photocatalytic degradation |
| [43] | g-C3N4 |  | In-process | 128 m2/g | Photocatalytic degradation |
| [39] | g-C3N4 |  | Pre-treatment | 67.5 m2/g | Photocatalytic activity for NO removal |
| [49] | g-C3N4 |  | In-process | 50.1 m2/g | Photocatalytic degradation of fluoroquinolone antibiotics |
| [62] | g-C3N4 |  | Post-treatment | 25.7 m2/g | Photo-reduction of p-nitrophenol |
| [79] | g-C3N4 |  | Post-treatment | 151 m2/g | Visible light photocatalytic removal of NOx |
| [73] | g-C3N4 |  | Post-treatment | 142.8 m2/g | Photocatalytic degradation |
| [83] | g-C3N4 |  | Post-treatment | 114 m2/g | Photoeletrocatalytic Degradation of 4-Chlorophenol |
| [93] | g-C3N4 |  | In-process | 241.4 m2/g | Photocatalytic degradation |
| [94] | Mn@g-C3N4 /PANI/wood-derived carbon |  | Composite materials | - | Photocatalytic degradation |
| [95] | g-C3N4 | - | Pre-treatment | - | Photoelectrocatalytic degradation |
| [96] | g-C3N4@biogenic FeS |  | Composite materials | - | Photocatalytic degradation |
| [97] | NaYF4@g-C3N4 |  | Composite materials | 14.10 m2/g | Photocatalytic degradation |
| References | Materials | Morphology | Synthesis | BET | Application |
|---|---|---|---|---|---|
| [28] | g-C3N4 |  | Pre-treatment | 42.2 m2/g | Photocatalytic hydrogen evolution |
| [25] | g-C3N4 |  | Pre-treatment | 81.4 m2/g | Photocatalytic hydrogen evolution |
| [26] | g-C3N4 |  | Pre-treatment | 181.74 m2/g | Photocatalytic hydrogen evolution |
| [36] | g-C3N4 |  | Pre-treatment | 228.4 m2/g | Photocatalytic hydrogen evolution |
| [38] | g-C3N4 |  | Pre-treatment | 127.8 m2/g | Photocatalytic hydrogen evolution |
| [46] | g-C3N4 |  | In-process | 239 m2/g | Photocatalytic hydrogen evolution |
| [51] | g-C3N4 |  | In-process | 131 m2/g | Photocatalytic hydrogen evolution |
| [53] | g-C3N4 |  | In-process | 310.7 m2/g | Photocatalytic hydrogen evolution |
| [54] | g-C3N4 |  | In-process | 132.26 m2/g | Photocatalytic hydrogen evolution |
| [55] | g-C3N4 |  | In-process | 135.1 m2/g | Photocatalytic hydrogen evolution |
| [58] | g-C3N4 |  | In-process | 160 m2/g | Photocatalytic hydrogen evolution |
| [60] | g-C3N4 |  | Post-treatment | 55.4 m2/g | Photocatalytic hydrogen evolution and CO2 conversion |
| [65] | g-C3N4 |  | Post-treatment | 205.8 m2/g | Photocatalytic hydrogen evolution |
| [71] | g-C3N4 |  | Post-treatment | 384 m2/g | Hydrogen evolution |
| [69] | g-C3N4 |  | Post-treatment | 306 m2/g | Photocatalytic hydrogen evolution |
| [74] | g-C3N4 |  | Post-treatment | 117.27 m2/g | Photocatalytic hydrogen evolution |
| [81] | g-C3N4 | 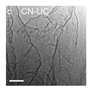 | Post-treatment | 99.73 m2/g | Photocatalytic hydrogen evolution |
| [77] | g-C3N4 |  | Post-treatment | 277.98 m2/g | Photocatalytic hydrogen evolution |
| [82] | g-C3N4 | - | Post-treatment | - | Lithium–sulfur batteries |
| [84] | g-C3N4 |  | Post-treatment | 196 m2/g | Photocatalytic hydrogen evolution |
| [114] | g-C3N4/Fe2TiO5 |  | Composite materials | 20.28 m2/g | CO2 Photoreduction |
| [115] | CN-Nv-C3N4 |  | Post-treatment | - | Lithium metal batteries |
| [116] | g-C3N4 S-Scheme Homojunction |  | Post-treatment | 122.04 m2/g | CO2 photoreduction |
| [117] | Pt NP decorated C3N4 |  | Composite materials | 106.19 m2/g | Photocatalytic hydrogen evolution |
| [118] | CK-CNC |  | In-process | 158.65 m2/g | Low-temperature sodium-ion batteries |
| [119] | Li-C3N4 |  | In-process | - | Oxygen reduction reactions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Zhao, M.; Yang, Q.; Bao, L.; Chen, L.; Liu, W.; Feng, J. A Review on Pre-, In-Process, and Post-Synthetic Strategies to Break the Surface Area Barrier in g-C3N4 for Energy Conversion and Environmental Remediation. Nanomaterials 2025, 15, 956. https://doi.org/10.3390/nano15130956
Gao M, Zhao M, Yang Q, Bao L, Chen L, Liu W, Feng J. A Review on Pre-, In-Process, and Post-Synthetic Strategies to Break the Surface Area Barrier in g-C3N4 for Energy Conversion and Environmental Remediation. Nanomaterials. 2025; 15(13):956. https://doi.org/10.3390/nano15130956
Chicago/Turabian StyleGao, Mingming, Minghao Zhao, Qianqian Yang, Lan Bao, Liwei Chen, Wei Liu, and Jing Feng. 2025. "A Review on Pre-, In-Process, and Post-Synthetic Strategies to Break the Surface Area Barrier in g-C3N4 for Energy Conversion and Environmental Remediation" Nanomaterials 15, no. 13: 956. https://doi.org/10.3390/nano15130956
APA StyleGao, M., Zhao, M., Yang, Q., Bao, L., Chen, L., Liu, W., & Feng, J. (2025). A Review on Pre-, In-Process, and Post-Synthetic Strategies to Break the Surface Area Barrier in g-C3N4 for Energy Conversion and Environmental Remediation. Nanomaterials, 15(13), 956. https://doi.org/10.3390/nano15130956