A Rapid and Complete Photodegradation of Doxycycline Using rGO@CuO Nanocomposite Under Visible and Direct Sunlight: Mechanistic Insights and Real-Time Applicability
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Characterization
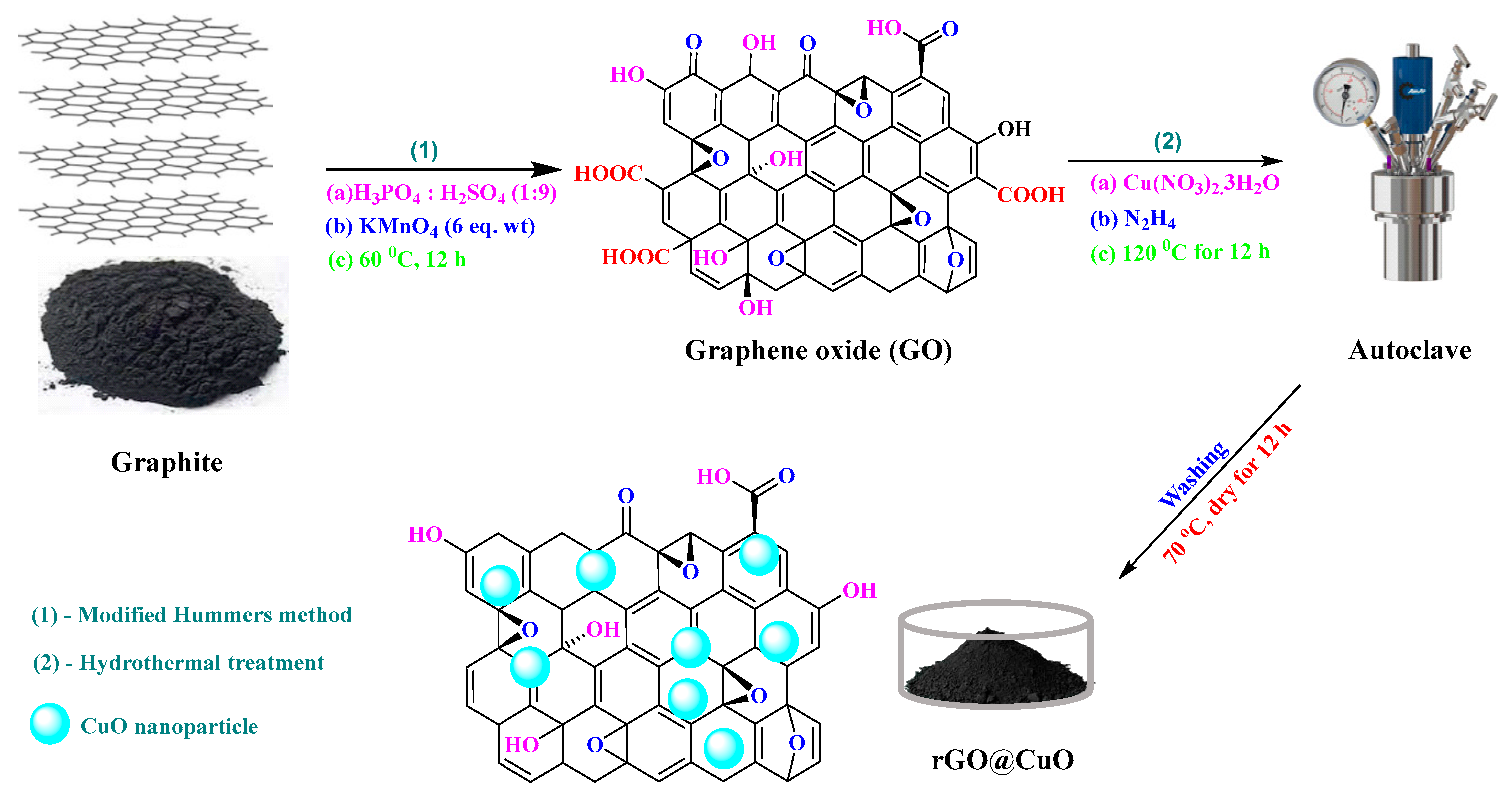
2.3. Synthesis of GO, rGO, CuO, and rGO@CuO
2.4. Photodegradation Studies
3. Results and Discussion
3.1. Characterization of Photocatalysts
3.2. DOX Adsorption–Photodegradation Studies
3.2.1. Effect of DOX Concentration and rGO@CuO Compositional Ratio
3.2.2. Influence of rGO@CuO1:1 Catalyst Dosage and pH on DOX Photodegradation
3.2.3. Effect of Photocatalyst Components on DOX Degradation
3.2.4. Effect of Sunlight on DOX Degradation
3.3. Real Sample and Reusability of the Photocatalyst
3.4. Isotherm Modeling and Kinetic Studies for DOX Degradation
3.5. Mechanism of DOX Photodegradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOX | Doxycycline |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| DDW | Double-distilled water |
| FT-IR | Fourier-transform infrared |
| p-XRD | Powder X-ray diffraction |
| HRTEM | High-resolution transmission electron microscope |
| XPS | X-ray photoelectron spectroscopy |
| TOC | Total organic carbon |
| TDS | Total dissolved solid |
| CB | Conduction band |
| VB | Valence band |
References
- Berendsen, B.J.A.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A.M. The Analysis of Animal Faeces as a Tool to Monitor Antibiotic Usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef]
- Navarro-Triviño, F.J.; Pérez-López, I.; Ruíz-Villaverde, R. Doxycycline, an Antibiotic or an Anti-Inflammatory Agent? The Most Common Uses in Dermatology. Actas Dermosifiliogr. 2020, 111, 561–566. [Google Scholar] [CrossRef]
- Molina, J.M.; Bercot, B.; Assoumou, L.; Rubenstein, E.; Algarte-Genin, M.; Pialoux, G.; Katlama, C.; Surgers, L.; Bébéar, C.; Dupin, N.; et al. Doxycycline Prophylaxis and Meningococcal Group B Vaccine to Prevent Bacterial Sexually Transmitted Infections in France (ANRS 174 DOXYVAC): A Multicentre, Open-Label, Randomised Trial with a 2 × 2 Factorial Design. Lancet. Infect. Dis. 2024, 24, 1093–1104. [Google Scholar] [CrossRef]
- Wilairatana, P.; Krudsood, S.; Treeprasertsuk, S.; Chalermrut, K.; Looareesuwan, S. The Future Outlook of Antimalarial Drugs and Recent Work on the Treatment of Malaria. Arch. Med. Res. 2002, 33, 416–421. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological Effects of Antibiotics on Natural Ecosystems: A Review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Sundararaman, S.; Kumar, J.A.; Deivasigamani, P.; Devarajan, Y. Emerging Pharma Residue Contaminants: Occurrence, Monitoring, Risk and Fate Assessment–A Challenge to Water Resource Management. Sci. Total Environ. 2022, 825, 153897. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Liu, S.; Xiao, Q.; Liang, W.; Song, Y. Contamination, Distribution, and Risk Assessment of Antibiotics in the Urban Surface Water of the Pearl River in Guangzhou, South China. Environ. Monit. Assess. 2021, 193, 98. [Google Scholar] [CrossRef]
- Ali, M.; Wang, W.; Chaudhry, N.; Geng, Y. Hospital Waste Management in Developing Countries: A Mini Review. Waste Manag. Res. 2017, 35, 581–592. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. A Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modelling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of Antibiotic Resistance in China and Its Environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, I.S.; Oh, J.E. Human and Veterinary Pharmaceuticals in the Marine Environment Including Fish Farms in Korea. Sci. Total Environ. 2017, 579, 940–949. [Google Scholar] [CrossRef]
- Carpenter, L.; Miller, S.; Flynn, E.; Choo, J.M.; Collins, J.; Shoubridge, A.P.; Gordon, D.; Lynn, D.J.; Whitehead, C.; Leong, L.E.X.; et al. Exposure to Doxycycline Increases Risk of Carrying a Broad Range of Enteric Antimicrobial Resistance Determinants in an Elderly Cohort. J. Infect. 2024, 89, 106243. [Google Scholar] [CrossRef]
- Cheng, D.; Feng, Y.; Liu, Y.; Xue, J.; Li, Z. Dynamics of Oxytetracycline, Sulfamerazine, and Ciprofloxacin and Related Antibiotic Resistance Genes during Swine Manure Composting. J. Environ. Manag. 2019, 230, 102–109. [Google Scholar] [CrossRef]
- Bansal, O.P. Antibiotics in the Environment: A Review. Int. J. Front. Biol. Pharm. 2022, 2, 57–83. [Google Scholar] [CrossRef]
- Zaranyika, M.F.; Dzomba, P.; Kugara, J. Speciation and Persistence of Doxycycline in the Aquatic Environment: Characterization in Terms of Steady State Kinetics. J. Environ. Sci. Health B 2015, 50, 908–918. [Google Scholar] [CrossRef]
- Malek, A.E.; Granwehr, B.P.; Kontoyiannis, D.P. Doxycycline as a Potential Partner of COVID-19 Therapies. IDCases 2020, 21, e00864. [Google Scholar] [CrossRef]
- Carrillo, M.P.; Sevilla, M.; Casado, M.; Piña, B.; López, E.P.; Matamoros, V.; Vila-Costa, M.; Barata, C. Impact of the Antibiotic Doxycycline on the D. Magna Reproduction, Associated Microbiome and Antibiotic Resistance Genes in Treated Wastewater Conditions. Environ. Pollut. 2023, 334, 122188. [Google Scholar] [CrossRef]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A Review of the Influence of Treatment Strategies on Antibiotic Resistant Bacteria and Antibiotic Resistance Genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A Review on Industrial Wastewater Treatment via Electrocoagulation Processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.; Zou, Y.; Ma, B.; Wu, Y. No Evidential Correlation between Veterinary Antibiotic Degradation Ability and Resistance Genes in Microorganisms during the Biodegradation of Doxycycline. Ecotoxicol. Environ. Saf. 2018, 147, 759–766. [Google Scholar] [CrossRef]
- Spina-Cruz, M.; Maniero, M.G.; Guimarães, J.R. Advanced Oxidation Processes on Doxycycline Degradation: Monitoring of Antimicrobial Activity and Toxicity. Environ. Sci. Pollut. Res. 2019, 26, 27604–27619. [Google Scholar] [CrossRef]
- Zaidi, S.; Sivasankar, V.; Chaabane, T.; Alonzo, V.; Omine, K.; Maachi, R.; Darchen, A.; Prabhakaran, M. Separate and Simultaneous Removal of Doxycycline and Oxytetracycline Antibiotics by Electro-Generated Adsorbents (EGAs). J. Environ. Chem. Eng. 2019, 7, 102876. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, L.; Ge, Y.; Su, H.; Li, Z. Lignin Xanthate Resin–Bentonite Clay Composite as a Highly Effective and Low-Cost Adsorbent for the Removal of Doxycycline Hydrochloride Antibiotic and Mercury Ions in Water. J. Hazard. Mater. 2019, 368, 33–41. [Google Scholar] [CrossRef]
- Wu, S.; Lin, Y.; Hu, Y.H. Strategies of Tuning Catalysts for Efficient Photodegradation of Antibiotics in Water Environments: A Review. J. Mater. Chem. A Mater. 2021, 9, 2592–2611. [Google Scholar] [CrossRef]
- Wei, L.; Li, H.; Lu, J. Algae-Induced Photodegradation of Antibiotics: A Review. Environ. Pollut. 2021, 272, 115589. [Google Scholar] [CrossRef]
- Zaidi, S.; Chaabane, T.; Sivasankar, V.; Darchen, A.; Maachi, R.; Msagati, T.A.M. Electro-Coagulation Coupled Electro-Flotation Process: Feasible Choice in Doxycycline Removal from Pharmaceutical Effluents. Arab. J. Chem. 2019, 12, 2798–2809. [Google Scholar] [CrossRef]
- He, T.; Bao, J.; Leng, Y.; Snow, D.; Kong, S.; Wang, T.; Li, X. Biotransformation of Doxycycline by Brevundimonas Naejangsanensis and Sphingobacterium Mizutaii Strains. J. Hazard. Mater. 2021, 411, 125126. [Google Scholar] [CrossRef]
- Yu, F.; Tian, F.; Zou, H.; Ye, Z.; Peng, C.; Huang, J.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wei, X. ZnO/Biochar Nanocomposites via Solvent Free Ball Milling for Enhanced Adsorption and Photocatalytic Degradation of Methylene Blue. J. Hazard. Mater. 2021, 415, 125511. [Google Scholar] [CrossRef]
- Khan, F.; Khan, M.S.; Kamal, S.; Arshad, M.; Ahmad, S.I.; Nami, S.A.A. Recent Advances in Graphene Oxide and Reduced Graphene Oxide Based Nanocomposites for the Photodegradation of Dyes. J. Mater. Chem. C Mater. 2020, 8, 15940–15955. [Google Scholar] [CrossRef]
- Mondal, A.; Prabhakaran, A.; Gupta, S.; Subramanian, V.R. Boosting Photocatalytic Activity Using Reduced Graphene Oxide (RGO)/Semiconductor Nanocomposites: Issues and Future Scope. ACS Omega 2021, 6, 8734–8743. [Google Scholar] [CrossRef]
- Albero, J.; Mateo, D.; García, H. Graphene-Based Materials as Efficient Photocatalysts for Water Splitting. Molecules 2019, 24, 906. [Google Scholar] [CrossRef]
- Alwan, S.H.; Salem, K.H.; Alshamsi, H.A. Visible Light-Driven Photocatalytic Degradation of Rhodamine B Dye onto TiO2/RGO Nanocomposites. Mater. Today Commun. 2022, 33, 104558. [Google Scholar] [CrossRef]
- Elumalai, N.; Prabhu, S.; Selvaraj, M.; Silambarasan, A.; Navaneethan, M.; Harish, S.; Ramu, P.; Ramesh, R. Enhanced Photocatalytic Activity of ZnO Hexagonal Tube/r-GO Composite on Degradation of Organic Aqueous Pollutant and Study of Charge Transport Properties. Chemosphere 2022, 291, 132782. [Google Scholar] [CrossRef]
- Heng, S.; Song, Q.; Liu, S.; Guo, H.; Pang, J.; Qu, X.; Bai, Y.; Li, L.; Dang, D. Construction of 2D Polyoxoniobate/RGO Heterojunction Photocatalysts for the Enhanced Photodegradation of Tetracycline. Appl. Surf. Sci. 2021, 553, 149505. [Google Scholar] [CrossRef]
- Anandan, S.; Lee, G.-J.; Wu, J.J. Sonochemical Synthesis of CuO Nanostructures with Different Morphology. Ultrason. Sonochem. 2012, 19, 682–686. [Google Scholar] [CrossRef]
- Hsieh, S.-H.; Ting, J.-M. Characterization and Photocatalytic Performance of Ternary Cu-Doped ZnO/Graphene Materials. Appl. Surf. Sci. 2018, 427, 465–475. [Google Scholar] [CrossRef]
- Arai, T.; Yanagida, M.; Konishi, Y.; Iwasaki, Y.; Sugihara, H.; Sayama, K. Promotion Effect of CuO Co-Catalyst on WO3-Catalyzed Photodegradation of Organic Substances. Catal. Commun. 2008, 9, 1254–1258. [Google Scholar] [CrossRef]
- Mishra, S.R.; Ahmaruzzaman, M. CuO and CuO-Based Nanocomposites: Synthesis and Applications in Environment and Energy. Sustain. Mater. Technol. 2022, 33, e00463. [Google Scholar] [CrossRef]
- Sakib, A.A.M.; Masum, S.M.; Hoinkis, J.; Islam, R.; Molla, M.A.I. Synthesis of CuO/ZnO Nanocomposites and Their Application in Photodegradation of Toxic Textile Dye. J. Compos. Sci. 2019, 3, 91. [Google Scholar] [CrossRef]
- Ranjeh, M.; Beshkar, F.; Amiri, O.; Salavati-Niasari, M.; Moayedi, H. Pechini Sol-Gel Synthesis of Cu2O/Li3BO3 and CuO/Li3BO3 Nanocomposites for Visible Light-Driven Photocatalytic Degradation of Dye Pollutant. J. Alloys Compd. 2020, 815, 152451. [Google Scholar] [CrossRef]
- Raha, S.; Mohanta, D.; Ahmaruzzaman, M. Novel CuO/Mn3O4/ZnO Nanocomposite with Superior Photocatalytic Activity for Removal of Rabeprazole from Water. Sci. Rep. 2021, 11, 15187. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Patial, S.; Hasija, V.; Khan, A.A.P.; Singh, P.; Gautam, S.; Kaur, M.; Nguyen, V.-H. Engineering Nanostructures of CuO-Based Photocatalysts for Water Treatment: Current Progress and Future Challenges. Arab. J. Chem. 2020, 13, 8424–8457. [Google Scholar] [CrossRef]
- Muniyalakshmi, M.; Sethuraman, K.; Silambarasan, D. Synthesis and Characterization of Graphene Oxide Nanosheets. Mater. Today Proc. 2020, 21, 408–410. [Google Scholar] [CrossRef]
- Shah, R.M.; Yunus, R.M.; Masdar, M.S.M.; Minggu, L.J.; Wong, W.Y.; Salehmin, M.N.I. High Photoelectrochemical Performance of a P-Type Reduced Graphene Oxide-Copper Oxide/Cu Foil (RGO-CuO/Cu) Photoelectrode Prepared by a One-Pot Hydrothermal Method. Int. J. Energy Res. 2021, 45, 13865–13877. [Google Scholar] [CrossRef]
- Saadi, H.; Khaldi, O.; Pina, J.; Costa, T.; Seixas de Melo, J.S.; Vilarinho, P.; Benzarti, Z. Effect of Co Doping on the Physical Properties and Organic Pollutant Photodegradation Efficiency of ZnO Nanoparticles for Environmental Applications. Nanomaterials 2024, 14, 122. [Google Scholar] [CrossRef]
- Tumampos, S.B.; Ensano, B.M.B.; Pingul-Ong, S.M.B.; Ong, D.C.; Kan, C.-C.; Yee, J.-J.; de Luna, M.D.G. Isotherm, Kinetics and Thermodynamics of Cu (II) and Pb (II) Adsorption on Groundwater Treatment Sludge-Derived Manganese Dioxide for Wastewater Treatment Applications. Int. J. Environ. Res. Public Health 2021, 18, 3050. [Google Scholar] [CrossRef]
- Fang, Y.; Luo, B.; Jia, Y.; Li, X.; Wang, B.; Song, Q.; Kang, F.; Zhi, L. Renewing Functionalized Graphene as Electrodes for High-Performance Supercapacitors. Adv. Mater. 2012, 24, 6348–6355. [Google Scholar] [CrossRef]
- Wang, G.; Wang, B.; Park, J.; Yang, J.; Shen, X.; Yao, J. Synthesis of Enhanced Hydrophilic and Hydrophobic Graphene Oxide Nanosheets by a Solvothermal Method. Carbon 2009, 47, 68–72. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Green Reduction of Graphene Oxide by Aqueous Phytoextracts. Carbon 2012, 50, 5331–5339. [Google Scholar] [CrossRef]
- Jose, P.P.A.; Kala, M.S.; Kalarikkal, N.; Thomas, S. Reduced Graphene Oxide Produced by Chemical and Hydrothermal Methods. Mater. Today Proc. 2018, 5, 16306–16312. [Google Scholar] [CrossRef]
- Alves, D.C.B.; Silva, R.; Voiry, D.; Asefa, T.; Chhowalla, M. Copper Nanoparticles Stabilized by Reduced Graphene Oxide for CO2 Reduction Reaction. Mater. Renew. Sustain. Energy 2015, 4, 2. [Google Scholar] [CrossRef]
- Vinodha, G.; Shima, P.D.; Cindrella, L. Mesoporous Magnetite Nanoparticle-Decorated Graphene Oxide Nanosheets for Efficient Electrochemical Detection of Hydrazine. J. Mater. Sci. 2019, 54, 4073–4088. [Google Scholar] [CrossRef]
- Tran, D.-T.; Nguyen, T.-H.; Vu, P.-T.; Dang, V.-Q.; Van, H.-T. Cu-ZnO/CdS/RGO Tertiary Heterojunction for Improved Photocatalytic Degradation of Synthetic Dyes Using Visible Light. J. Water Process Eng. 2024, 57, 104687. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Weldegebrieal, G.K.; Garg, S.; Oh, W.C.; Hamizi, N.A.; Johan, M.R. Enhanced Photocatalytic Activity of Rgo-Cuo Nanocomposites for the Degradation of Organic Pollutants. Catalysts 2021, 11, 1008. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and Safe Graphene Preparation on Solution Based Platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zhang, Y.; Liu, J.; Duan, Y. Facile Synthesis of Hierarchical Nanocomposites of Aligned Polyaniline Nanorods on Reduced Graphene Oxide Nanosheets for Microwave Absorbing Materials. RSC Adv. 2017, 7, 54031–54038. [Google Scholar] [CrossRef]
- Ranjbar-Azad, M.; Behpour, M. Facile in Situ Co-Precipitation Synthesis of CuO–NiO/RGO Nanocomposite for Lithium-Ion Battery Anodes. J. Mater. Sci. Mater. Electron. 2021, 32, 18043–18056. [Google Scholar] [CrossRef]
- Verma, P.; Das, T.; Kumar, P.; Das, S. Surface-Passivated RGO@ CuO/6A5N2TU Colloidal Heterostructures for Efficient Removal of Ofloxacin from Contaminated Water through Dual-Mode Complexation: Insights into Kinetics and Adsorption Isotherm Model Study. Appl. Nanosci. 2023, 13, 4555–4572. [Google Scholar] [CrossRef]
- Saxena, S.; Tyson, T.A.; Shukla, S.; Negusse, E.; Chen, H.; Bai, J. Investigation of Structural and Electronic Properties of Graphene Oxide. Appl. Phys. Lett. 2011, 99, 013104. [Google Scholar] [CrossRef]
- Zheng, F.; Xu, W.-L.; Jin, H.-D.; Hao, X.-T.; Ghiggino, K.P. Charge Transfer from Poly(3-Hexylthiophene) to Graphene Oxide and Reduced Graphene Oxide. RSC Adv. 2015, 5, 89515–89520. [Google Scholar] [CrossRef]
- Zhang, D.; Qin, J.; Wei, D.; Yang, S.; Wang, S.; Hu, C. Enhancing the CO Preferential Oxidation (CO-PROX) of CuO–CeO2/Reduced Graphene Oxide (RGO) by Conductive RGO-Wrapping Based on the Interfacial Charge Transfer. Catal. Lett. 2018, 148, 3454–3466. [Google Scholar] [CrossRef]
- Dhiman, P.; Rana, G.; Alshgari, R.A.; Kumar, A.; Sharma, G.; Naushad, M.; ALOthman, Z.A. Magnetic Ni–Zn Ferrite Anchored on g-C3N4 as Nano-Photocatalyst for Efficient Photo-Degradation of Doxycycline from Water. Environ. Res. 2023, 216, 114665. [Google Scholar] [CrossRef]
- Yin, L.; Wang, H.; Li, L.; Li, H.; Chen, D.; Zhang, R. Microwave-Assisted Preparation of Hierarchical CuO@rGO Nanostructures and Their Enhanced Low-Temperature H2S-Sensing Performance. Appl. Surf. Sci. 2019, 476, 107–114. [Google Scholar] [CrossRef]
- Huang, Q.; Kang, F.; Liu, H.; Li, Q.; Xiao, X. Highly Aligned Cu2O/CuO/TiO2 Core/Shell Nanowire Arrays as Photocathodes for Water Photoelectrolysis. J. Mater. Chem. A 2013, 1, 2418–2425. [Google Scholar] [CrossRef]
- Carley, A.F.; Roberts, M.W.; Santra, A.K. Interaction of Oxygen and Carbon Monoxide with CsAu Surfaces. J. Phys. Chem. B 1997, 101, 9978–9983. [Google Scholar] [CrossRef]
- Bai, H.; Guo, H.; Wang, J.; Dong, Y.; Liu, B.; Xie, Z.; Guo, F.; Chen, D.; Zhang, R.; Zheng, Y. A Room-Temperature NO2 Gas Sensor Based on CuO Nanoflakes Modified with RGO Nanosheets. Sens. Actuators B Chem. 2021, 337, 129783. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Yan, X.; Qian, J.; Pei, X.; Zhou, L.; Ma, R.; Zhang, M.; Du, Y.; Bai, L. Enhanced Photodegradation of Doxycycline (DOX) in the Sustainable NiFe2O4/MWCNTs/BiOI System under UV Light Irradiation. Environ. Res. 2021, 199, 111264. [Google Scholar] [CrossRef]
- Wang, W.; Han, Q.; Zhu, Z.; Zhang, L.; Zhong, S.; Liu, B. Enhanced Photocatalytic Degradation Performance of Organic Contaminants by Heterojunction Photocatalyst BiVO4/TiO2/RGO and Its Compatibility on Four Different Tetracycline Antibiotics. Adv. Powder Technol. 2019, 30, 1882–1896. [Google Scholar] [CrossRef]
- Mohammadi, M.; Rezaee Roknabadi, M.; Behdani, M.; Kompany, A. Enhancement of Visible and UV Light Photocatalytic Activity of RGO-TiO2 Nanocomposites: The Effect of TiO2/Graphene Oxide Weight Ratio. Ceram. Int. 2019, 45, 12625–12634. [Google Scholar] [CrossRef]
- Gao, J.; Gao, Y.; Sui, Z.; Dong, Z.; Wang, S.; Zou, D. Hydrothermal Synthesis of BiOBr/FeWO4 Composite Photocatalysts and their Photocatalytic Degradation of Doxycycline. J. Alloys Compd. 2018, 732, 43–51. [Google Scholar] [CrossRef]
- Deng, C.; Li, S.; Wang, J.; Yang, Q.; Chen, H.; Tang, F.; Yang, X. Fabrication of α-Fe2O3/TiO2 Heterojunction for Photocatalytic Degradation of Doxycycline Hydrochloride. J. Mol. Liq. 2025, 417, 126618. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, J.L.; Altaee, A.; Ahmed, M.B.; Johir, M.A.H.; Ren, J.; Li, X. Improved Photocatalysis of Perfluorooctanoic Acid in Water and Wastewater by Ga2O3/UV System Assisted by Peroxymonosulfate. Chemosphere 2020, 239, 124722. [Google Scholar] [CrossRef]
- Zhao, K.; Lu, Z.-H.; Zhao, P.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Modified Tri–Axial Electrospun Functional Core–Shell Nanofibrous Membranes for Natural Photodegradation of Antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Park, K.; Ali, I.; Kim, J.-O. Photodegradation of Perfluorooctanoic Acid by Graphene Oxide-Deposited TiO2 Nanotube Arrays in Aqueous Phase. J. Environ. Manag. 2018, 218, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Prabagar, J.S.; Yashas, S.R.; Gurupadayya, B.; Wantala, K.; Diganta, D.B.; Shivaraju, H.P. Degradation of Doxycycline Antibiotics Using Lanthanum Copper Oxide Microspheres under Simulated Sunlight. Environ. Sci. Pollut. Res. 2022, 29, 57204–57214. [Google Scholar] [CrossRef]
- Le, T.-H.; Tran, D.-T.; Nghiem, L.D. A Novel Tertiary Magnetic ZnFe2O4/BiOBr/RGO Nanocomposite Catalyst for Photodegrading Organic Contaminants by Visible Light. Sci. Total Environ. 2023, 891, 164358. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Song, C.; Wu, S.; Cheng, M.; Tao, P.; Shao, M.; Gao, G. Adsorption Studies of Coconut Shell Carbons Prepared by KOH Activation for Removal of Lead (II) from Aqueous Solutions. Sustainability 2013, 6, 86–98. [Google Scholar] [CrossRef]
- Abdelfatah, A.M.; El-Maghrabi, N.; Mahmoud, A.E.D.; Fawzy, M. Synergetic Effect of Green Synthesized Reduced Graphene Oxide and Nano-Zero Valent Iron Composite for the Removal of Doxycycline Antibiotic from Water. Sci. Rep. 2022, 12, 19372. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, K.; Xu, W.; Chen, D.; Fang, J.; Zhu, X.; Sun, J.; Liang, Y.; Hu, X.; Li, R. Adsorption-Photocatalysis Synergistic Removal of Contaminants under Antibiotic and Cr (VI) Coexistence Environment Using Non-Metal g-C3N4 Based Nanomaterial Obtained by Supramolecular Self-Assembly Method. J. Hazard. Mater. 2021, 404, 124171. [Google Scholar] [CrossRef]
- Yang, K.; Ye, J.; Zhao, Y.; Ge, K.; Cao, J.; Wang, S.; Zhang, Z.; Zhang, Y.; Yang, Y. IO-TiO2/PCN-222 Heterostructure with a Tightly Connected Interface and Its Photocatalytic Activity. ChemistrySelect 2021, 6, 4238–4246. [Google Scholar] [CrossRef]
- Wu, Q.; Song, Y. Enhanced Interfacial Charge Migration through Fabrication of p-n Junction in ZnIn2S4/NiFe2O4/Biochar Composite for Photocatalytic Doxycycline Hydrochloride Degradation. Chem. Eng. J. 2023, 453, 139745. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Zhou, J. Facile Fabrication of Multi-Walled Carbon Nanotubes (MWCNTs)/α-Bi2O3 Nanosheets Composite with Enhanced Photocatalytic Activity for Doxycycline Degradation under Visible Light Irradiation. J. Mater. Sci. 2019, 54, 3294–3308. [Google Scholar] [CrossRef]
- Sruthi, L.; Okla, M.K.; Janani, B.; Abdel-Maksoud, M.A.; Saleh, I.A.; Abu-Harirah, H.A.; Khan, S.S. Construction of RGO-Bi2Sn2O7-NiFe2O4 Nanoheterojunction System for the Enhanced Photodegradation of Doxycycline: A Brief Insight on Degradation Kinetics and Toxicological Evaluation on Allium Cepa. J. Clean. Prod. 2024, 434, 139936. [Google Scholar] [CrossRef]
- Hong, P.; Li, Y.; He, J.; Saeed, A.; Zhang, K.; Wang, C.; Kong, L.; Liu, J. Rapid Degradation of Aqueous Doxycycline by Surface CoFe2O4/H2O2 System: Behaviors, Mechanisms, Pathways and DFT Calculation. Appl. Surf. Sci. 2020, 526, 146557. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Hu, Z. Nano-Sized g-C3N4 Thin Layer@CeO2 Sphere Core-Shell Photocatalyst Combined with H2O2 to Degrade Doxycycline in Water under Visible Light Irradiation. Sep. Purif. Technol. 2019, 227, 115665. [Google Scholar] [CrossRef]
- Yan, M.; Hua, Y.; Zhu, F.; Gu, W.; Jiang, J.; Shen, H.; Shi, W. Fabrication of Nitrogen Doped Graphene Quantum Dots-BiOI/MnNb2O6 p-n Junction Photocatalysts with Enhanced Visible Light Efficiency in Photocatalytic Degradation of Antibiotics. Appl. Catal. B 2017, 202, 518–527. [Google Scholar] [CrossRef]
- Klauson, D.; Šakarašvili, M.; Pronina, N.; Krichevskaya, M.; Kärber, E.; Mikli, V. Aqueous Photocatalytic Degradation of Selected Micropollutants by Pd-Modified Titanium Dioxide in Three Photoreactor Types. Environ. Technol. 2017, 38, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, D.; Xu, W.; Fang, J.; Sun, J.; Liu, Z.; Chen, Y.; Liang, Y.; Fang, Z. Synergistic Adsorption-Photocatalytic Degradation of Different Antibiotics in Seawater by a Porous g-C3N4/Calcined-LDH and Its Application in Synthetic Mariculture Wastewater. J. Hazard. Mater. 2021, 416, 126183. [Google Scholar] [CrossRef] [PubMed]
- Swetha, S.; Abdel-Maksoud, M.A.; Okla, M.K.; Janani, B.; Dawoud, T.M.; El-Tayeb, M.A.; Khan, S.S. Triple-Mechanism Driven Fe-Doped n-n Hetero-Architecture of Pr6O11-MoO3 Decorated g-C3N4 for Doxycycline Degradation and Bacterial Photoinactivation. Chem. Eng. J. 2023, 461, 141806. [Google Scholar] [CrossRef]
- Moussa, H.; Girot, E.; Mozet, K.; Alem, H.; Medjahdi, G.; Schneider, R. ZnO Rods/Reduced Graphene Oxide Composites Prepared via a Solvothermal Reaction for Efficient Sunlight-Driven Photocatalysis. Appl. Catal. B 2016, 185, 11–21. [Google Scholar] [CrossRef]
- Mahrsi, M.I.; Chouchene, B.; Gries, T.; Carré, V.; Medjahdi, G.; Ayari, F.; Balan, L.; Schneider, R. 0D/1D CuO-Cu2O/ZnO Pn Heterojunction with High Photocatalytic Activity for the Degradation of Dyes and Naproxen. J. Environ. Chem. Eng. 2024, 12, 113072. [Google Scholar] [CrossRef]
- Tatykayev, B.; Donat, F.; Alem, H.; Balan, L.; Medjahdi, G.; Uralbekov, B.; Schneider, R. Synthesis of Core/Shell ZnO/RGO Nanoparticles by Calcination of ZIF-8/RGO Composites and Their Photocatalytic Activity. ACS Omega 2017, 2, 4946–4954. [Google Scholar] [CrossRef]











| Isotherm Model | Parameters | ||
|---|---|---|---|
| Langmuir model | Qm | KL | R2 |
| 89.686 | 0.065 | 0.99 | |
| Temkin model | BT | KT | R2 |
| 0.046 | 1.047 | 0.919 | |
| Freundlich model | 1/n | KF | R2 |
| 110 | 84.23 | 0.98 | |
| Photocatalyst | DOX Conc. (ppm) | Dosage (mg/mL) | Light Source | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|
| P25 | 25 | 0.1 | 300 W Xe lamp | 80 | 26 | [85] |
| ZnIn2S4/NiFe2O4/biochar | 30 | 0.05 | Visible light | 120 | 66 | [86] |
| MWCNTs/α-Bi2O3 | 10 | 0.05 | 150 W Xe lamp | 120 | 91 | [87] |
| rGO-Bi2Sn2O7-NiFe2O4 | 10 | 50 | Visible light | 270 | 97 | [88] |
| BiOBr/FeWO4 | N/A | 1 | Visible light 300 W | 60 | 90 | [73] |
| CoFe2O4/H2O2 | 20 | 60 | N/A | 60 | 90 | [89] |
| 5% g-C3N4@CeO2 | 10 | Visible light 150 W | 60 | 84 | [90] | |
| (NGQDs)-BiOI/MnNb2O6 | 10 | 250 W Xe lamp | 60 | 87 | [91] | |
| Pd–TiO2 | 25 | UVA (15 W) | 100 | [92] | ||
| Modified g-C3N4/MgZnA | 10 | 50 | Visible light | 120 | 91 | [93] |
| Fe/Pr6O11–MoO3@gC3N4 NCs | 50 | 50 | Visible light | 220 | 98 | [94] |
| rGO@CuO1:1 | 70 | 1 | 10 W LED | 30 | 100 | This work |
| rGO@CuO1:1 | 70 | 1 | Sunlight | 30 | 94 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, P.; Das, S.; Raj, S.; Schneider, R. A Rapid and Complete Photodegradation of Doxycycline Using rGO@CuO Nanocomposite Under Visible and Direct Sunlight: Mechanistic Insights and Real-Time Applicability. Nanomaterials 2025, 15, 953. https://doi.org/10.3390/nano15130953
Verma P, Das S, Raj S, Schneider R. A Rapid and Complete Photodegradation of Doxycycline Using rGO@CuO Nanocomposite Under Visible and Direct Sunlight: Mechanistic Insights and Real-Time Applicability. Nanomaterials. 2025; 15(13):953. https://doi.org/10.3390/nano15130953
Chicago/Turabian StyleVerma, Panchraj, Subrata Das, Shubham Raj, and Raphaël Schneider. 2025. "A Rapid and Complete Photodegradation of Doxycycline Using rGO@CuO Nanocomposite Under Visible and Direct Sunlight: Mechanistic Insights and Real-Time Applicability" Nanomaterials 15, no. 13: 953. https://doi.org/10.3390/nano15130953
APA StyleVerma, P., Das, S., Raj, S., & Schneider, R. (2025). A Rapid and Complete Photodegradation of Doxycycline Using rGO@CuO Nanocomposite Under Visible and Direct Sunlight: Mechanistic Insights and Real-Time Applicability. Nanomaterials, 15(13), 953. https://doi.org/10.3390/nano15130953








