Chiral Perturbation Strategies for Circularly Polarized Thermally Activated Delayed-Fluorescence Small Molecules: Progress in the Application of Organic Light-Emitting Diodes
Abstract
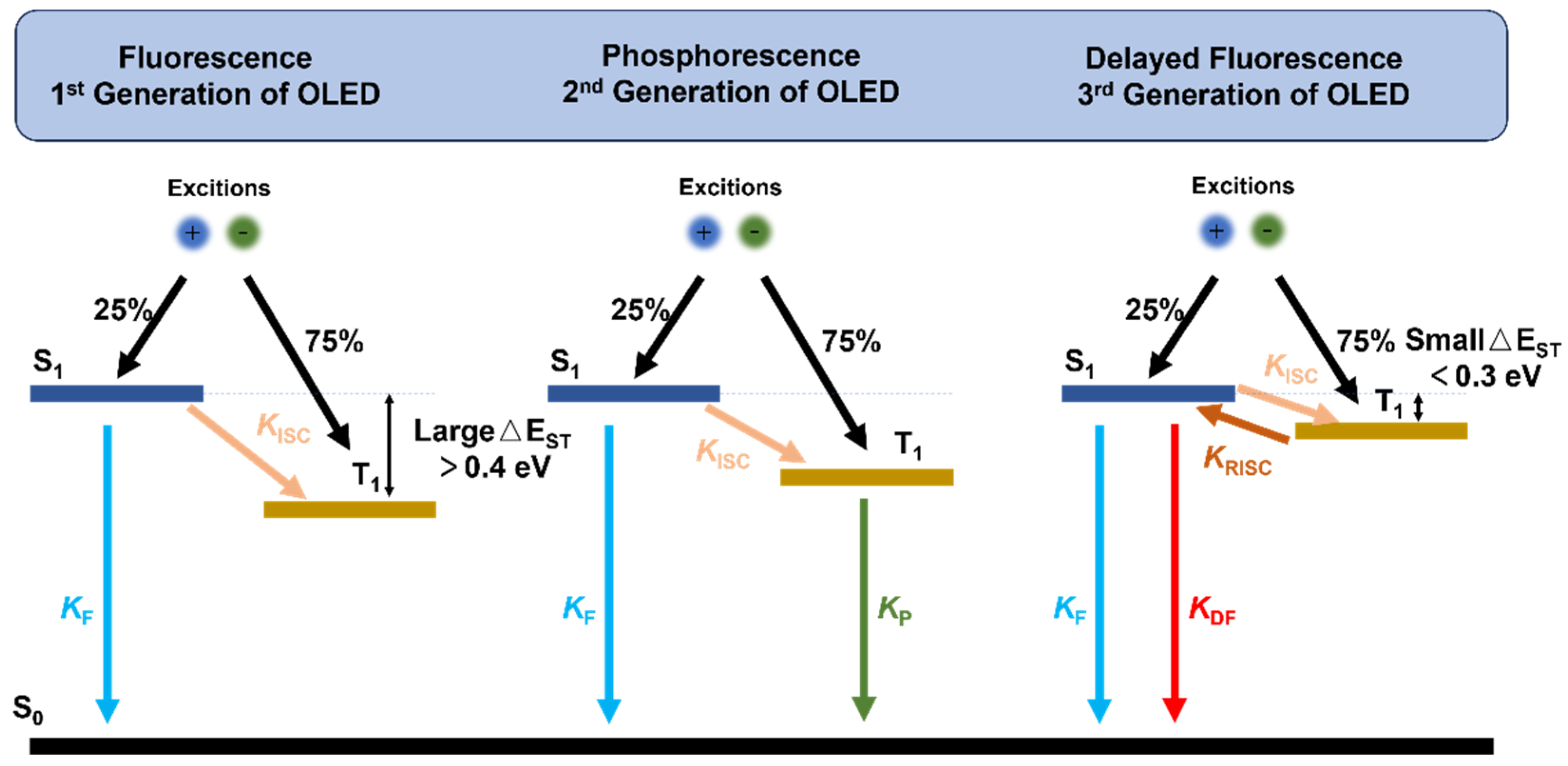
1. Introduction
2. Molecular Design of CP-TADF Materials and Performance of Electroluminescent Devices
2.1. Binaphthol
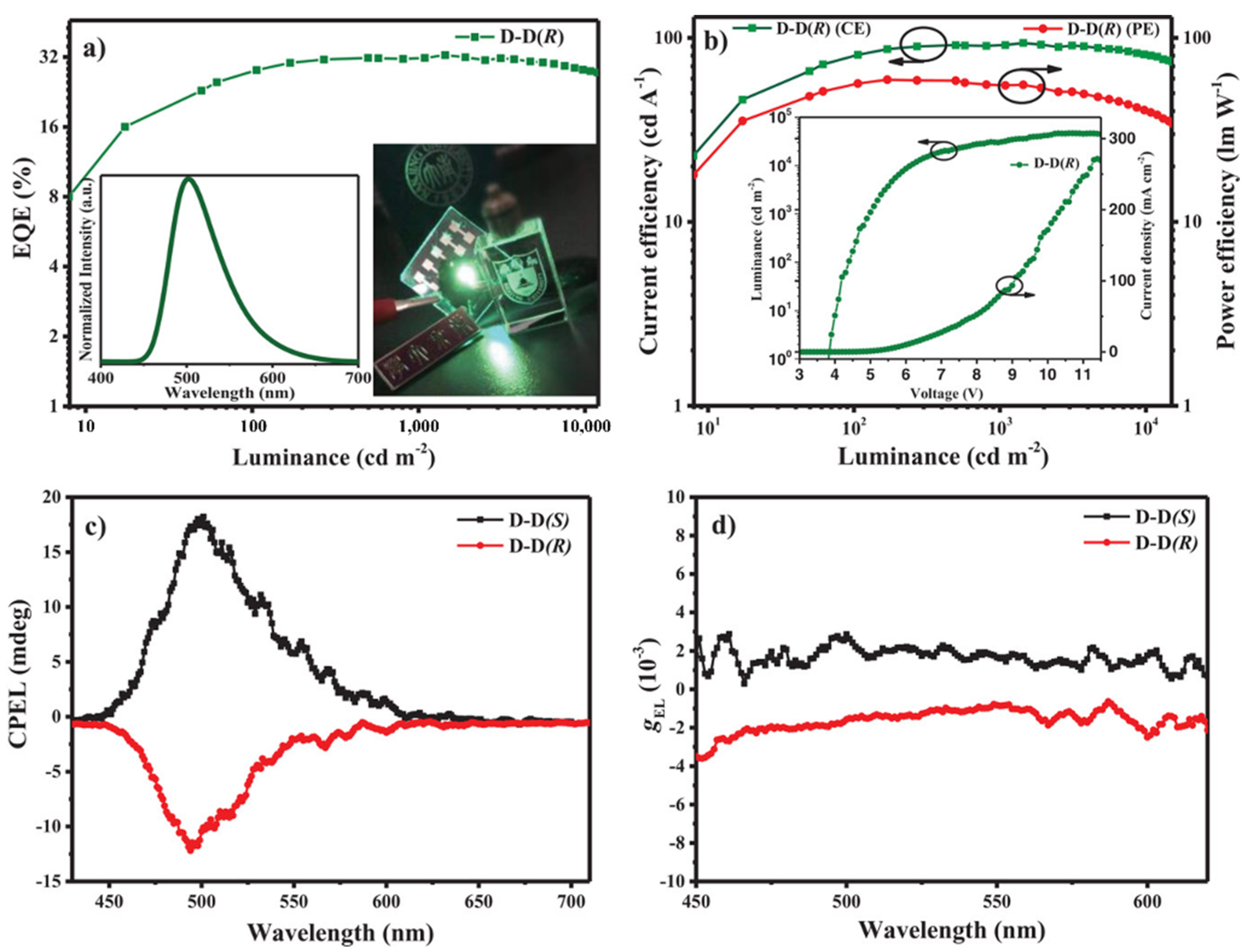
2.2. Octahydronaphthol
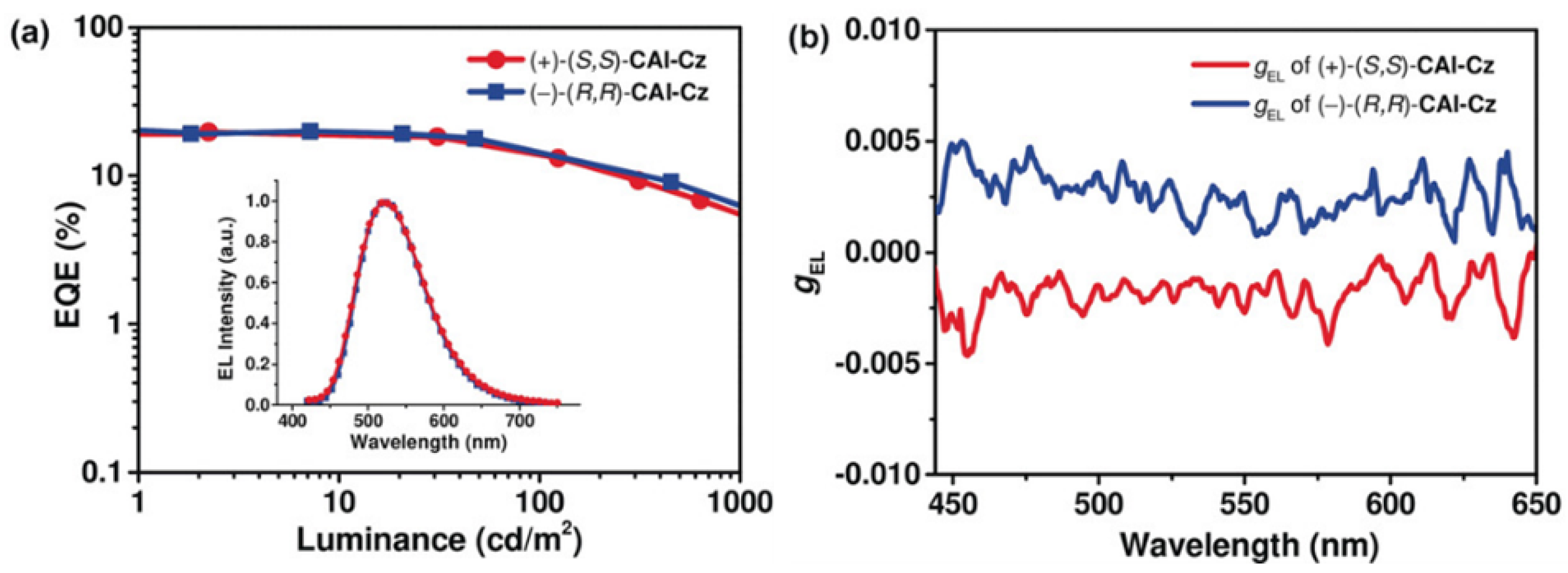
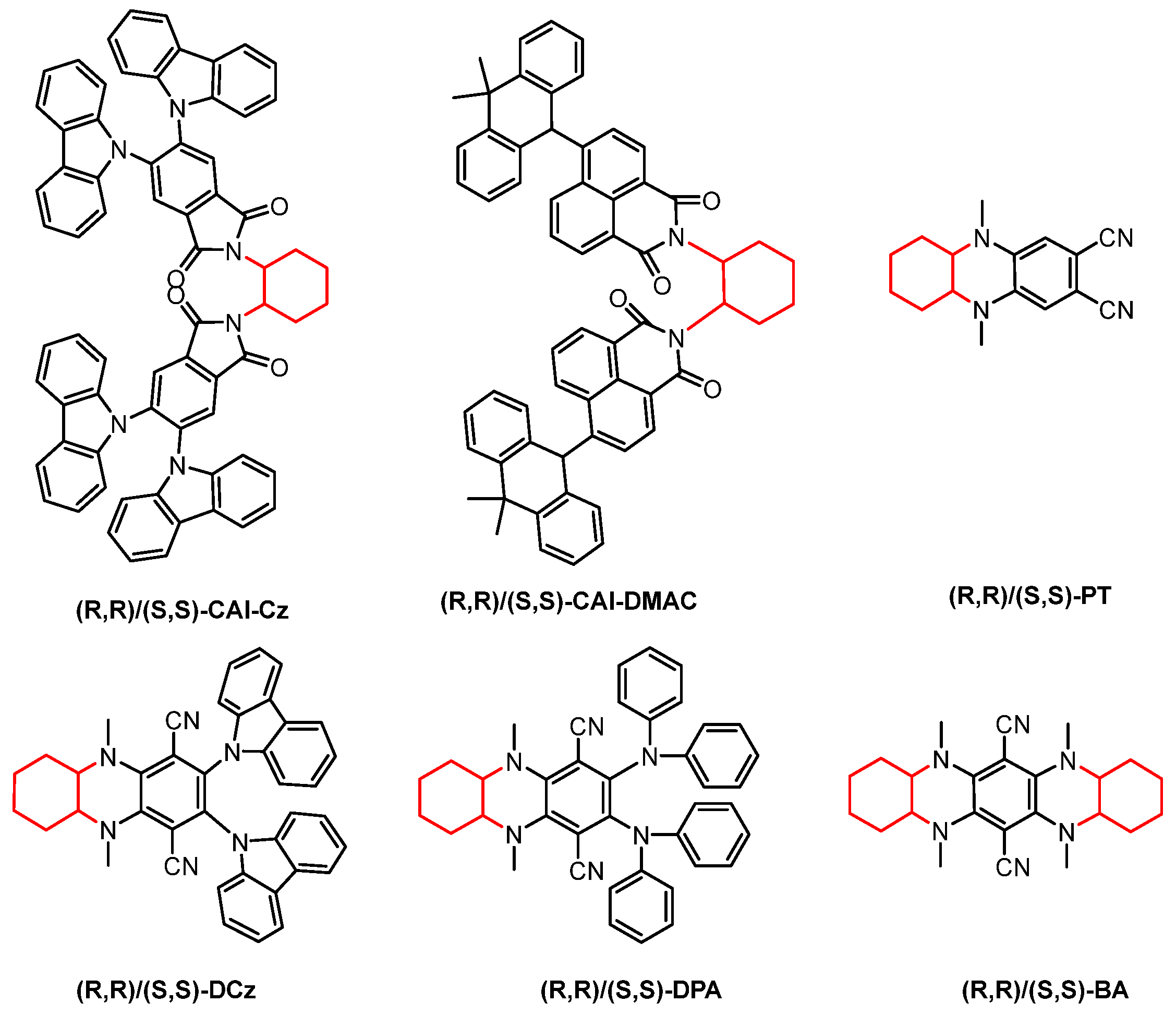
2.3. 1.2-Diaminocyclohexane
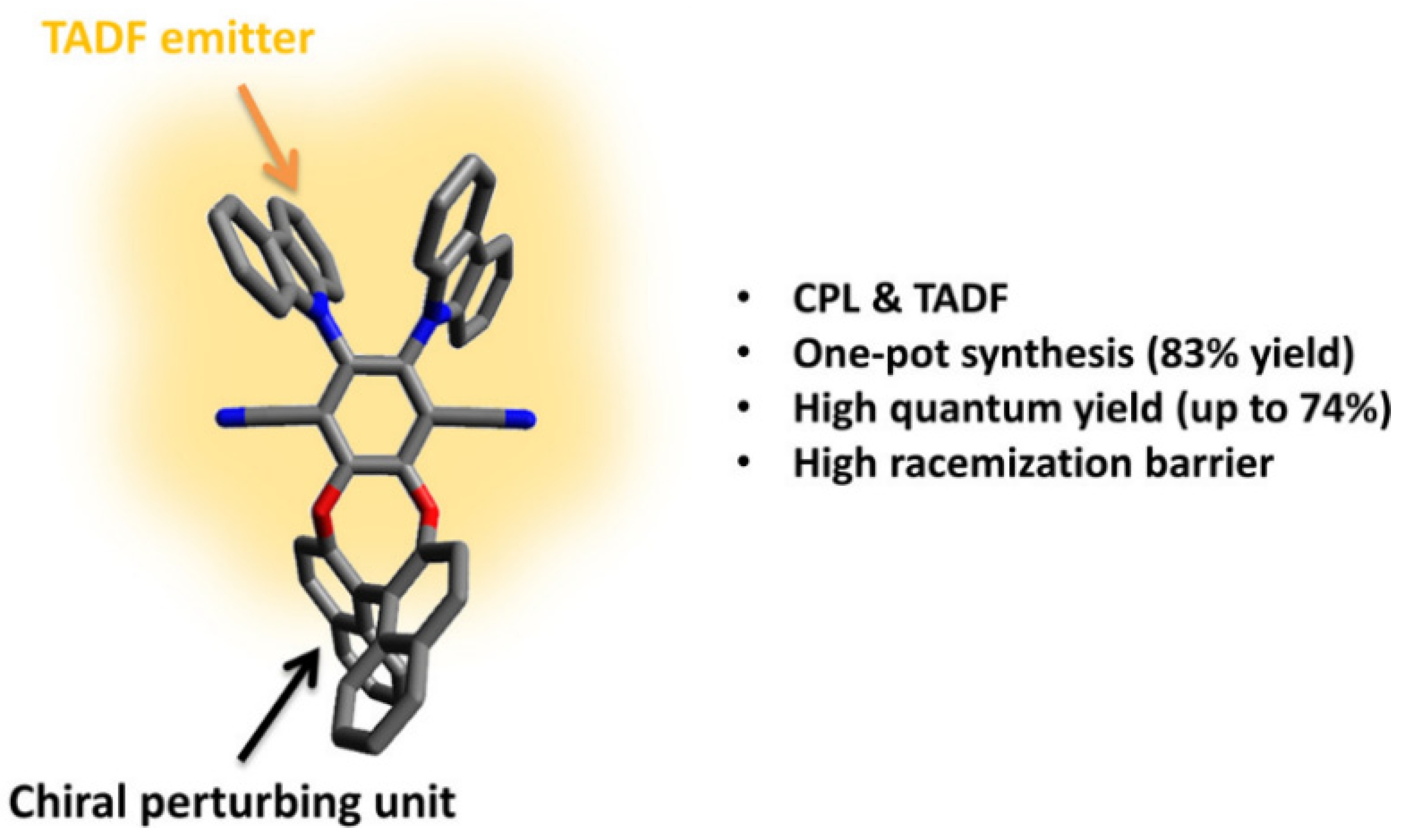
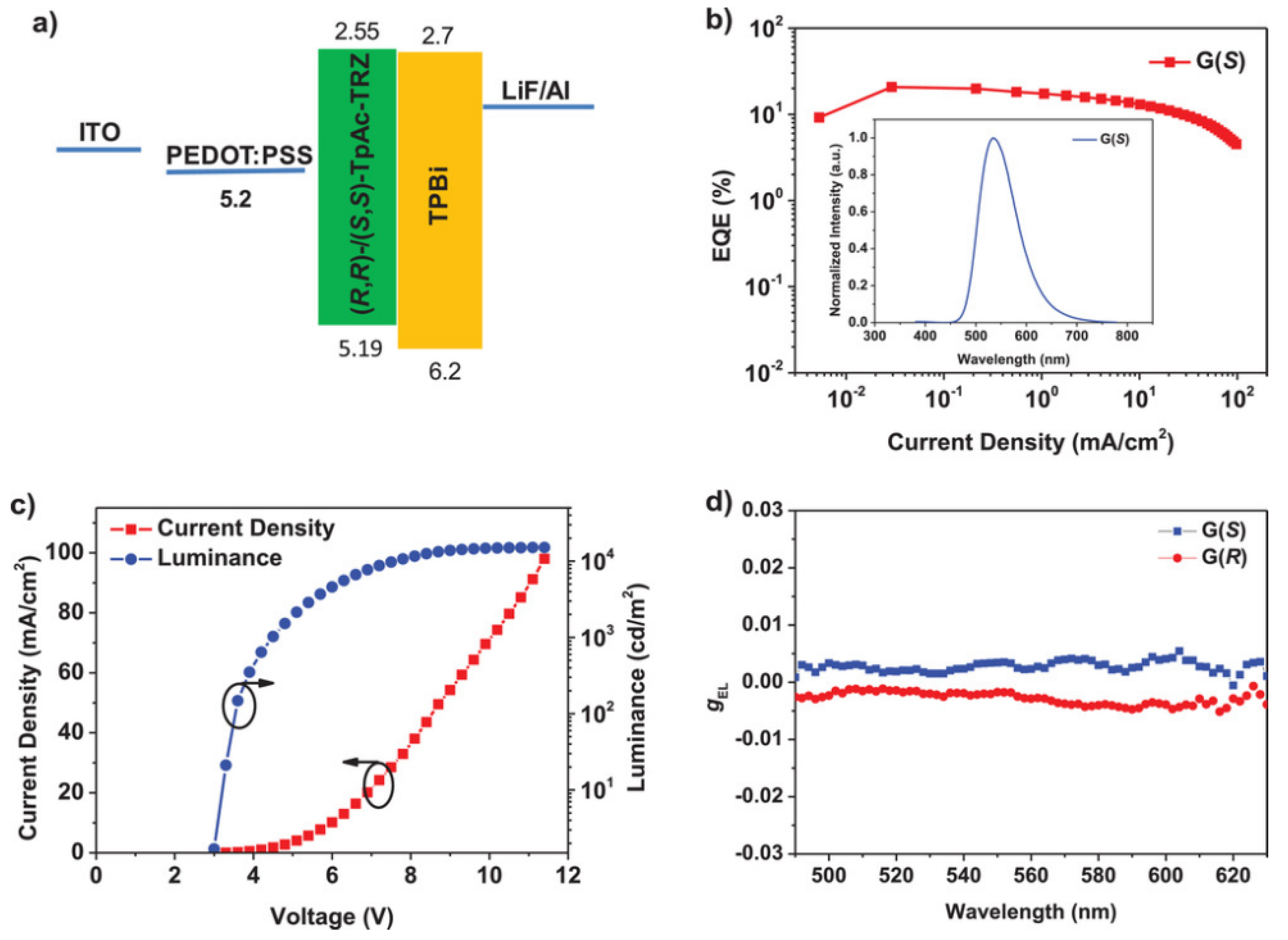
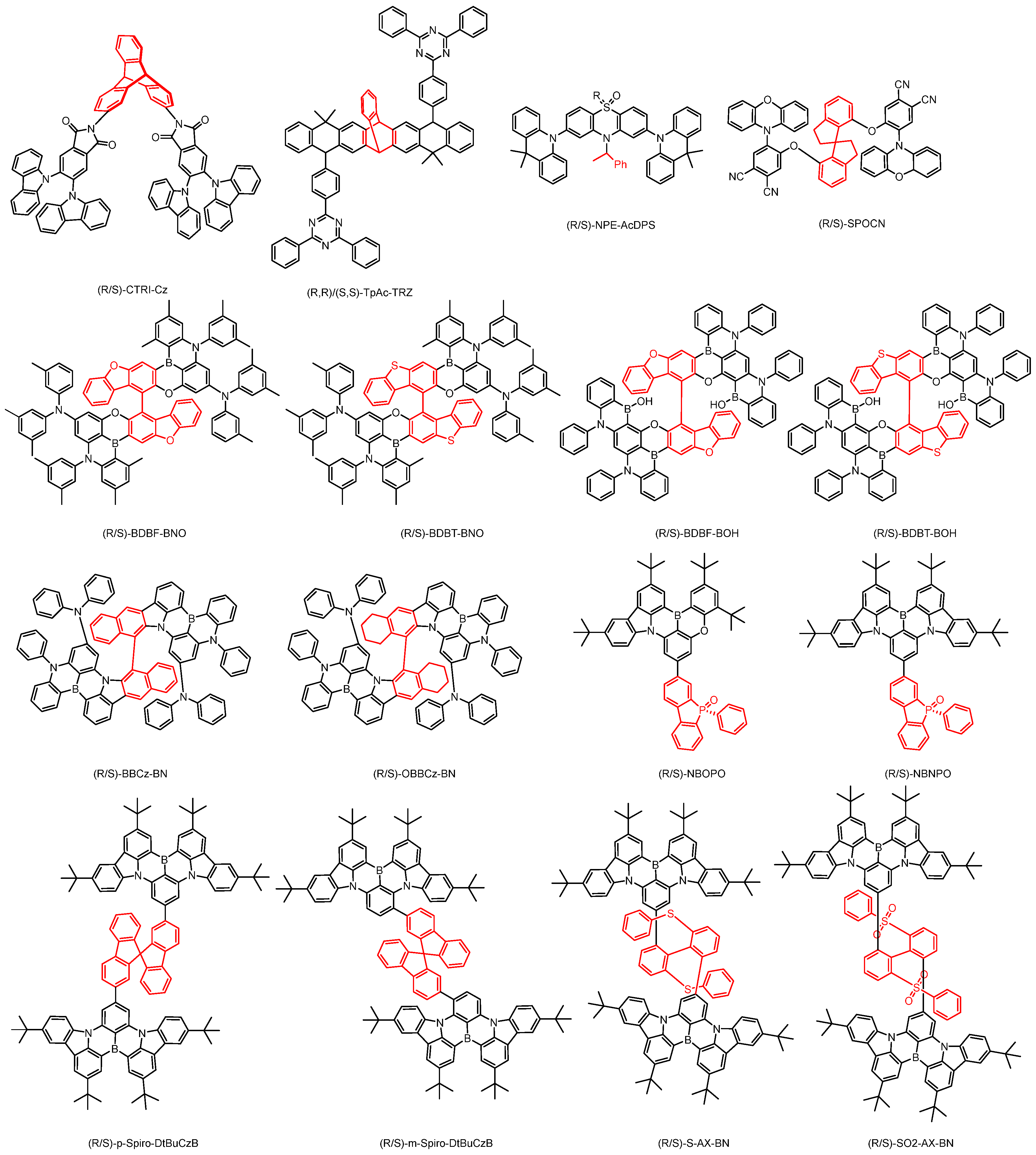
2.4. Other Chiral Units
3. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, F.; Qiu, C.; Liu, Z. Investigation of Autostereoscopic Displays Based on Various Display Technologies. Nanomaterials 2022, 12, 429. [Google Scholar] [CrossRef] [PubMed]
- Diesing, S.; Zhang, L.; Zysman-Colman, E.; Samuel, I.D.W. A Figure of Merit for Efficiency Roll-off in TADF-Based Organic LEDs. Nature 2024, 627, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S. A Brief History of OLEDs—Emitter Development and Industry Milestones. Adv. Mater. 2021, 33, e2005630. [Google Scholar] [CrossRef]
- Siddiqui, I.; Kumar, S.; Tsai, Y.-F.; Gautam, P.; Shahnawaz; Kesavan, K.; Lin, J.-T.; Khai, L.; Chou, K.-H.; Choudhury, A.; et al. Status and Challenges of Blue OLEDs: A Review. Nanomaterials 2023, 13, 2521. [Google Scholar] [CrossRef]
- Fang, H.; Li, J.; Gong, S.; Lin, J.; Xie, G. Inkjet Printing of High-Color-Purity Blue Organic Light-Emitting Diodes with Host-Free Inks. Molecules 2024, 29, 2147. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.W.; Vanslyke, S.A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913. [Google Scholar] [CrossRef]
- Shin, H.; Lee, S.; Kim, K.; Moon, C.; Yoo, S.; Lee, J.; Kim, J. Blue Phosphorescent Organic Light-Emitting Diodes Using an Exciplex Forming Co-host with the External Quantum Efficiency of Theoretical Limit. Adv. Mater. 2014, 26, 4730–4734. [Google Scholar] [CrossRef]
- Li, C.; Duan, C.; Han, C.; Xu, H. Secondary Acceptor Optimization for Full-Exciton Radiation: Toward Sky-Blue Thermally Activated Delayed Fluorescence Diodes with External Quantum Efficiency of ≈30%. Adv. Mater. 2018, 30, e1804228. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Li, Z.; Wang, Q.; Cai, X.; Wei, J.; Wang, Y. Constructing Charge-Transfer Excited States Based on Frontier Molecular Orbital Engineering: Narrowband Green Electroluminescence with High Color Purity and Efficiency. Angew. Chem. Int. Ed. Engl. 2020, 59, 17442–17446. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Liu, H.; Zhuang, Z.; Wang, J.; Yu, M.; Yang, D.; Ma, D.; Zhao, Z.; Tang, B.Z. Creating Efficient Delayed Fluorescence Luminogens with Acridine-Based Spiro Donors to Improve Horizontal Dipole Orientation for High-performance OLEDs. Chem. Eng. J. 2022, 435, 134934. [Google Scholar] [CrossRef]
- Yang, S.; Qu, Y.; Liao, L.; Jiang, Z.; Lee, S. Research Progress of Intramolecular π-Stacked Small Molecules for Device Applications. Adv. Mater. 2021, 34, 2104125. [Google Scholar] [CrossRef] [PubMed]
- Rui, J.; Pu, J.; Chen, Z.; Tang, H.; Wang, L.; Su, S.-J.; Cao, D. Single-molecule White Organic Light-Emitting Diodes Based on Dual-Conformation Diindolophenthiazine Derivatives. J. Mater. Chem. C 2024, 12, 9182–9188. [Google Scholar] [CrossRef]
- Jang, E.-B.; Choi, G.-S.; Bae, E.-J.; Ju, B.-K.; Park, Y.-W. Doping-Free Phosphorescent and Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes with an Ultra-Thin Emission Layer. Nanomaterials 2023, 13, 2366. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-L.; Lv, X.; Meng, L.-Y.; Chen, X.-L.; Lu, C.-Z. Tröger’s Base-Derived Thermally Activated Delayed Fluorescence Dopant for Efficient Deep-Blue Organic Light-Emitting Diodes. Molecules 2023, 28, 4832. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Shizu, K.; Huang, S.; Hirata, S.; Miyazaki, H.; Adachi, C. Design of Efficient Thermally Activated Delayed Fluorescence Materials for Pure Blue Organic Light Emitting Diodes. J. Am. Chem. Soc. 2012, 134, 14706–14709. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, C.; Liang, J.; Zhuang, X.; Liu, Y.; Wang, Y. High-Efficiency and Narrowband Green Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes Based on Two Diverse Boron Multi-Resonant Skeletons. Molecules 2024, 29, 841. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, M.; Zhuang, Y.; Liu, S.; Huang, W.; Zhao, Q. Circularly Polarized Luminescence from Organic Micro-/Nano-Structures. Light. Sci. Appl. 2021, 10, 1–18. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Li, Z. The Progress of Circularly Polarized Luminescence in Chiral Purely Organic Materials. Adv. Photon- Res. 2020, 2, 2000136. [Google Scholar] [CrossRef]
- Gong, Z.; Li, Z.; Zhong, Y. Circularly Polarized Luminescence of Coordination Aggregates. Aggregate 2022, 3, e177. [Google Scholar] [CrossRef]
- Imai, Y.; Amasaki, R.; Yanagibashi, Y.; Suzuki, S.; Shikura, R.; Yagi, S. Magnetically Induced Near-Infrared Circularly Polarized Electroluminescence from an Achiral Perovskite Light-Emitting Diode. Magnetochemistry 2024, 10, 39. [Google Scholar] [CrossRef]
- Zhao, W.; Tan, K.; Guo, W.; Guo, C.; Li, M.; Chen, C. Acceptor Copolymerized Axially Chiral Conjugated Polymers with TADF Properties for Efficient Circularly Polarized Electroluminescence. Adv. Sci. 2024, 11, e2309031. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, S.; Zhou, Y.; Shi, C.; Xu, L.; Liao, X.; Sun, N.; Zheng, Y.-X.; Ding, L.; Ding, J. Chiral perturbation in D-O-A organic Phosphors towards Efficient Circularly Polarized Electroluminescence. Sci. China Mater. 2024, 68, 1351–1358. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Teng, J.; Zhou, H.; Zhao, W.; Chen, C. Chiral TADF-Active Polymers for High-Efficiency Circularly Polarized Organic Light-Emitting Diodes. Angew. Chem. Int. Ed. Engl. 2021, 60, 23619–23624. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Xi, J.; Yan, Z.; Hu, J.; Wang, Y.; Yuan, L.; Song, S.; Zuo, J.; Zheng, Y. Efficient Circularly Polarized Electroluminescence Based on Chiral MR-TADF Materials with Innovative Planar Structure Design Strategy. Adv. Funct. Mater. 2025. [Google Scholar] [CrossRef]
- Imagawa, T.; Hirata, S.; Totani, K.; Watanabe, T.; Vacha, M. Thermally Activated Delayed Fluorescence with Circularly Polarized Luminescence Characteristics. Chem. Commun. 2015, 51, 13268–13271. [Google Scholar] [CrossRef]
- Feuillastre, S.; Pauton, M.; Gao, L.; Desmarchelier, A.; Riives, A.J.; Prim, D.; Tondelier, D.; Geffroy, B.; Muller, G.; Clavier, G.; et al. Design and Synthesis of New Circularly Polarized Thermally Activated Delayed Fluorescence Emitters. J. Am. Chem. Soc. 2016, 138, 3990–3993. [Google Scholar] [CrossRef]
- Song, F.; Xu, Z.; Zhang, Q.; Zhao, Z.; Zhang, H.; Zhao, W.; Qiu, Z.; Qi, C.; Zhang, H.; Sung, H.H.Y.; et al. Highly Efficient Circularly Polarized Electroluminescence from Aggregation-Induced Emission Luminogens with Amplified Chirality and Delayed Fluorescence. Adv. Funct. Mater. 2018, 28, 1800051. [Google Scholar] [CrossRef]
- Sun, S.; Wang, J.; Chen, L.; Chen, R.; Jin, J.; Chen, C.; Chen, S.; Xie, G.; Zheng, C.; Huang, W. Thermally Activated Delayed Fluorescence Enantiomers for Solution-Processed Circularly Polarized Electroluminescence. J. Mater. Chem. C 2019, 7, 14511–14516. [Google Scholar] [CrossRef]
- Frédéric, L.; Desmarchelier, A.; Plais, R.; Lavnevich, L.; Muller, G.; Villafuerte, C.; Clavier, G.; Quesnel, E.; Racine, B.; Meunier-Della-Gatta, S.; et al. Maximizing Chiral Perturbation on Thermally Activated Delayed Fluorescence Emitters and Elaboration of the First Top-Emission Circularly Polarized OLED. Adv. Funct. Mater. 2020, 30, 2004838. [Google Scholar] [CrossRef]
- Yan, Z.P.; Liu, T.T.; Wu, R.; Liang, X.; Li, Z.Q.; Zhou, L.; Zheng, Y.X.; Zuo, J.L. Chiral Thermally Activated Delayed Fluorescence Materials Based on R/S-N2,N2′-Diphenyl-[1,1′-binaphthalene]-2,2′-diamine Donor with Narrow Emission Spectra for Highly Efficient Circularly Polarized Electroluminescence. Adv. Funct. Mater. 2021, 31, 2103875. [Google Scholar] [CrossRef]
- Liang, J.; Hu, J.; Huo, Z.; Yan, Z.; Yuan, L.; Zhong, X.; Wei, Y.; Song, S.; Liu, Q.; Song, Y.; et al. Two Different Chiral Groups Based Thermally Activated Delayed Fluorescence Materials for Circularly Polarized OLEDs. Chem.-Asian J. 2024, 19, e202400664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Quan, Y.; Ye, S.; Cheng, Y. Solution-Processed White Circularly Polarized Organic Light-Emitting Diodes Based on Chiral Binaphthyl Emitters. Chem.-A Eur. J. 2020, 27, 589–593. [Google Scholar] [CrossRef]
- Xue, P.; Wang, X.; Wang, W.; Zhang, J.; Wang, Z.; Jin, J.; Zheng, C.; Li, P.; Xie, G.; Chen, R. Solution-Processable Chiral Boron Complexes for Circularly Polarized Red Thermally Activated Delayed Fluorescent Devices. ACS Appl. Mater. Interfaces 2021, 13, 47826–47834. [Google Scholar] [CrossRef]
- Zhou, L.; Ni, F.; Li, N.; Wang, K.; Xie, G.; Yang, C. Tetracoordinate Boron-Based Multifunctional Chiral Thermally Activated Delayed Fluorescence Emitters. Angew. Chem. Int. Ed. Engl. 2022, 61, e202203844. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, D.; Wu, K.; Xie, G.; Zhao, Z.; Tang, B.Z. Delayed Fluorescence and Amplified Chirality via Modified Substitution Position for Deep-red Circularly Polarized Organic Light Emitting-diodes. Chem. Res. Chin. Univ. 2024, 40, 1–7. [Google Scholar] [CrossRef]
- Tong, J.; Wang, P.; Liao, X.; Wang, Y.; Zheng, Y.; Pan, Y. Chiral Sulfonyl Binaphthalene-Based Thermally Activated Delayed Fluorescence Materials for Circularly Polarized Electroluminescence. Adv. Opt. Mater. 2024, 12, 2302730. [Google Scholar] [CrossRef]
- Xing, S.; Zhong, X.; Liao, X.; Wang, Y.; Yuan, L.; Ni, H.; Zheng, Y. Axially Chiral Multiple Resonance Thermally Activated Delayed Fluorescence Enantiomers for Efficient Circularly Polarized Electroluminescence. Adv. Opt. Mater. 2024, 12, 2400685. [Google Scholar] [CrossRef]
- Gong, M.; Guo, X.; Yuan, L.; Jiang, H.; Zeng, G.; Zheng, W.-H.; Zheng, Y.-X. Axially Chiral Biphenoxazine-Based Multi-Resonance Thermally Activated Delayed Fluorescence Materials for Circularly Polarized Electroluminescence. Chem. Eng. J. 2025, 505, 159719. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Peng, X.; Liu, D.; Chen, Z.; Xie, W.; Liu, K.; Su, S. Excited-State Engineering of Chalcogen-Bridged Chiral Molecules for Efficient OLEDs with Diverse Luminescence Mechanisms. Angew. Chem. Int. Ed. Engl. 2025, 64, e202420474. [Google Scholar] [CrossRef]
- Wu, Z.-G.; Yan, Z.-P.; Luo, X.-F.; Yuan, L.; Liang, W.-Q.; Wang, Y.; Zheng, Y.-X.; Zuo, J.-L.; Pan, Y. Non-Doped and Doped Circularly Polarized Organic Light-Emitting Diodes with High Performances Based on Chiral Octahydro-Binaphthyl Delayed Fluorescent Luminophores. J. Mater. Chem. C 2019, 7, 7045–7052. [Google Scholar] [CrossRef]
- Wu, Z.; Han, H.; Yan, Z.; Luo, X.; Wang, Y.; Zheng, Y.; Zuo, J.; Pan, Y. Chiral Octahydro-Binaphthol Compound-Based Thermally Activated Delayed Fluorescence Materials for Circularly Polarized Electroluminescence with Superior EQE of 32.6% and Extremely Low Efficiency Roll-Off. Adv. Mater. 2019, 31, e1900524. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhou, J.; Zeng, X.; An, Z.; Li, Y.; Han, D.; Duan, P.; Wu, Z.; Zheng, Y.; Tang, J. Efficient Circularly Polarized Electroluminescence from Chiral Thermally Activated Delayed Fluorescence Emitters Featuring Symmetrical and Rigid Coplanar Acceptors. Adv. Opt. Mater. 2021, 9, 2100017. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Cai, X.; Li, C.; Wang, Y. Highly Efficient Electroluminescence from Narrowband Green Circularly Polarized Multiple Resonance Thermally Activated Delayed Fluorescence Enantiomers. Adv. Mater. 2021, 33, 2100652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Liu, X.; Zhu, Y.; Li, M.; Chen, C.-F. Aromatic-Imide-Based TADF Enantiomers for Efficient Circularly Polarized Electroluminescence. J. Mater. Chem. C 2022, 10, 4805–4812. [Google Scholar] [CrossRef]
- Liu, T.-T.; Yan, Z.-P.; Hu, J.-J.; Yuan, L.; Luo, X.-F.; Tu, Z.-L.; Zheng, Y.-X. Chiral Thermally Activated Delayed Fluorescence Emitters-Based Efficient Circularly Polarized Organic Light-Emitting Diodes Featuring Low Efficiency Roll-Off. ACS Appl. Mater. Interfaces 2021, 13, 56413–56419. [Google Scholar] [CrossRef]
- Yan, Z.; Yuan, L.; Zhang, Y.; Mao, M.; Liao, X.; Ni, H.; Wang, Z.; An, Z.; Zheng, Y.; Zuo, J. A Chiral Dual-Core Organoboron Structure Realizes Dual-Channel Enhanced Ultrapure Blue Emission and Highly Efficient Circularly Polarized Electroluminescence. Adv. Mater. 2022, 34, e2204253. [Google Scholar] [CrossRef]
- Sun, B.; Ding, L.; Wang, X.; Tu, Z.-L.; Fan, J. Circularly Polarized Thermally Activated Delayed Fluorescence OLEDs with Nearly BT.2020 Red Emission. Chem. Eng. J. 2023, 476, 146511. [Google Scholar] [CrossRef]
- Liang, N.; Liu, J.; Lin, Y.; Xie, Z.; Cui, B.; Gong, Z.; Gan, Q.; Zhong, Y.; Feng, Y.; Yao, C. Construction of Circular Polarized Luminescence Molecules for Intense Near Infrared OLEDs. Adv. Opt. Mater. 2024, 12, 2303155. [Google Scholar] [CrossRef]
- Qu, L.; Xiao, H.; Zhang, B.; Yang, Q.; Song, J.; Zhou, X.; Xu, Z.-X.; Xiang, H. Axially Chiral Biphenoxazine-Based Thermally Activated Delayed Fluorescence Materials for Solution-Processed Circularly Polarized Organic Light-Emitting Diodes. Chem. Eng. J. 2023, 471, 144709. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Zhang, D.; Cai, M.; Duan, L.; Fung, M.; Chen, C. Stable Enantiomers Displaying Thermally Activated Delayed Fluorescence: Efficient OLEDs with Circularly Polarized Electroluminescence. Angew. Chem. Int. Ed. Engl. 2018, 57, 2889–2893. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lu, H.-Y.; Chen, C.; Li, M.; Chen, C.-F. 1,8-Naphthalimide-Based Circularly Polarized TADF Enantiomers as The Emitters for Efficient Orange-Red OLEDs. Org. Electron. 2019, 70, 71–77. [Google Scholar] [CrossRef]
- Luo, X.-F.; Han, H.-B.; Yan, Z.-P.; Wu, Z.-G.; Su, J.; Zou, J.-W.; Zhu, Z.-Q.; Zheng, Y.-X.; Zuo, J.-L. Multicolor Circularly Polarized Photoluminescence and Electroluminescence with 1,2-Diaminecyclohexane Enantiomers. ACS Appl. Mater. Interfaces 2020, 12, 23172–23180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Teng, J.; Zhou, H.; Chen, C. High-Performance Solution-Processed Nondoped Circularly Polarized OLEDs with Chiral Triptycene Scaffold-Based TADF Emitters Realizing Over 20% External Quantum Efficiency. Adv. Funct. Mater. 2021, 31, 2106418. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Chen, C.; Cui, L.; Teng, J.-M.; Li, M.; Lu, H.-Y.; Chen, C.-F. Triptycene-Derived TADF Enantiomers Displaying Circularly Polarized Luminescence and High-efficiency Electroluminescence. Org. Electron. 2021, 99, 106355. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, C.-W.; Tang, Y.-K.; Xiao, Z.; Li, N.; Hua, T.; Cao, X.; Zhou, C.; Wu, C.-C.; Yang, C. Chiral Thermally Activated Delayed Fluorescence Emitters for Circularly Polarized Luminescence and Efficient Deep Blue OLEDs. Dye. Pigment. 2022, 197, 109860. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, M.; Song, S.; Wang, Y.; Zheng, Y.; Zuo, J.; Pan, Y. Circularly Polarized White Organic Light-Emitting Diodes Based on Spiro-Type Thermally Activated Delayed Fluorescence Materials. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, X.; Luo, X.; Song, S.; Li, S.; Wang, Y.; Mao, Z.; Xu, W.; Zheng, Y.; Zuo, J.; et al. Chiral Spiro-Axis Induced Blue Thermally Activated Delayed Fluorescence Material for Efficient Circularly Polarized OLEDs with Low Efficiency Roll-Off. Angew. Chem. Int. Ed. Engl. 2021, 60, 8435–8440. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, Y.; Yan, Z.; Hu, J.; Mao, D.; Ni, H.; Zheng, Y. Circularly Polarized Electroluminescence from Intrinsically Axial Chiral Materials Based on Bidibenzo[b,d]furan/bidibenzo[b,d]thiophene. Adv. Funct. Mater. 2024, 34. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, J.; Yan, Z.; Yang, Y.; Mao, D.; Hu, J.; Ni, H.; Li, C.; Zuo, J.; Zheng, Y. Tetraborated Intrinsically Axial Chiral Multi-resonance Thermally Activated Delayed Fluorescence Materials. Angew. Chem. Int. Ed. Engl. 2024, 63, e202407277. [Google Scholar] [CrossRef]
- Yuan, L.; Mao, D.; Yan, Z.; Hu, J.; Ni, H.; Hong, X.; Zuo, J.; Zheng, Y. Controlling of Circularly Polarized Luminescence via Modulating the Angle Between Transition Electric and Magnetic Dipole Moments. Adv. Funct. Mater. 2025, 35, 2421020. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Z.-Y.; Chen, Z.-X.; Xing, S.; Huo, Z.-Z.; Hong, X.-F.; Yuan, L.; Li, W.; Zheng, Y.-X. Multiple-Resonance Thermally Activated Delayed Fluorescence Materials Based on Phosphorus Central Chirality for Efficient Circularly Polarized Electroluminescence. Mater. Horizons 2024, 11, 4722–4729. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-Q.; Han, X.; Huo, Z.-Z.; Yip, C.-F.; Hong, X.-F.; Ding, M.-N.; Zheng, Y.-X. Chiral Multiple-Resonance Thermally Activated Delayed Fluorescence Materials Based on Chiral Spiro-Axis Skeleton for Efficient Circularly Polarized Electroluminescence. Sci. China Chem. 2024, 67, 2257–2264. [Google Scholar] [CrossRef]
- Wang, X.; Xing, S.; Xiao, X.; Yuan, L.; Hou, Z.; Zheng, Y. Axial Chiral Biphenyl MR-TADF Enantiomers for Efficient Narrowband Circularly Polarized Electroluminescence. Adv. Funct. Mater. 2024, 35, 2412044. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, T.; Xu, L.; Tang, H.; Wang, L.; Cao, D. Chiral Perturbation Strategies for Circularly Polarized Thermally Activated Delayed-Fluorescence Small Molecules: Progress in the Application of Organic Light-Emitting Diodes. Nanomaterials 2025, 15, 1053. https://doi.org/10.3390/nano15131053
Fan T, Xu L, Tang H, Wang L, Cao D. Chiral Perturbation Strategies for Circularly Polarized Thermally Activated Delayed-Fluorescence Small Molecules: Progress in the Application of Organic Light-Emitting Diodes. Nanomaterials. 2025; 15(13):1053. https://doi.org/10.3390/nano15131053
Chicago/Turabian StyleFan, Tianwen, Linxian Xu, Hao Tang, Lingyun Wang, and Derong Cao. 2025. "Chiral Perturbation Strategies for Circularly Polarized Thermally Activated Delayed-Fluorescence Small Molecules: Progress in the Application of Organic Light-Emitting Diodes" Nanomaterials 15, no. 13: 1053. https://doi.org/10.3390/nano15131053
APA StyleFan, T., Xu, L., Tang, H., Wang, L., & Cao, D. (2025). Chiral Perturbation Strategies for Circularly Polarized Thermally Activated Delayed-Fluorescence Small Molecules: Progress in the Application of Organic Light-Emitting Diodes. Nanomaterials, 15(13), 1053. https://doi.org/10.3390/nano15131053







