Experimental Evaluation and Thermodynamic Analysis of Magnetic Fe3O4@La-Zr-MOFs for Highly Efficient Fluoride and Phosphate Removal
Abstract
1. Introduction
2. Materials and Methods
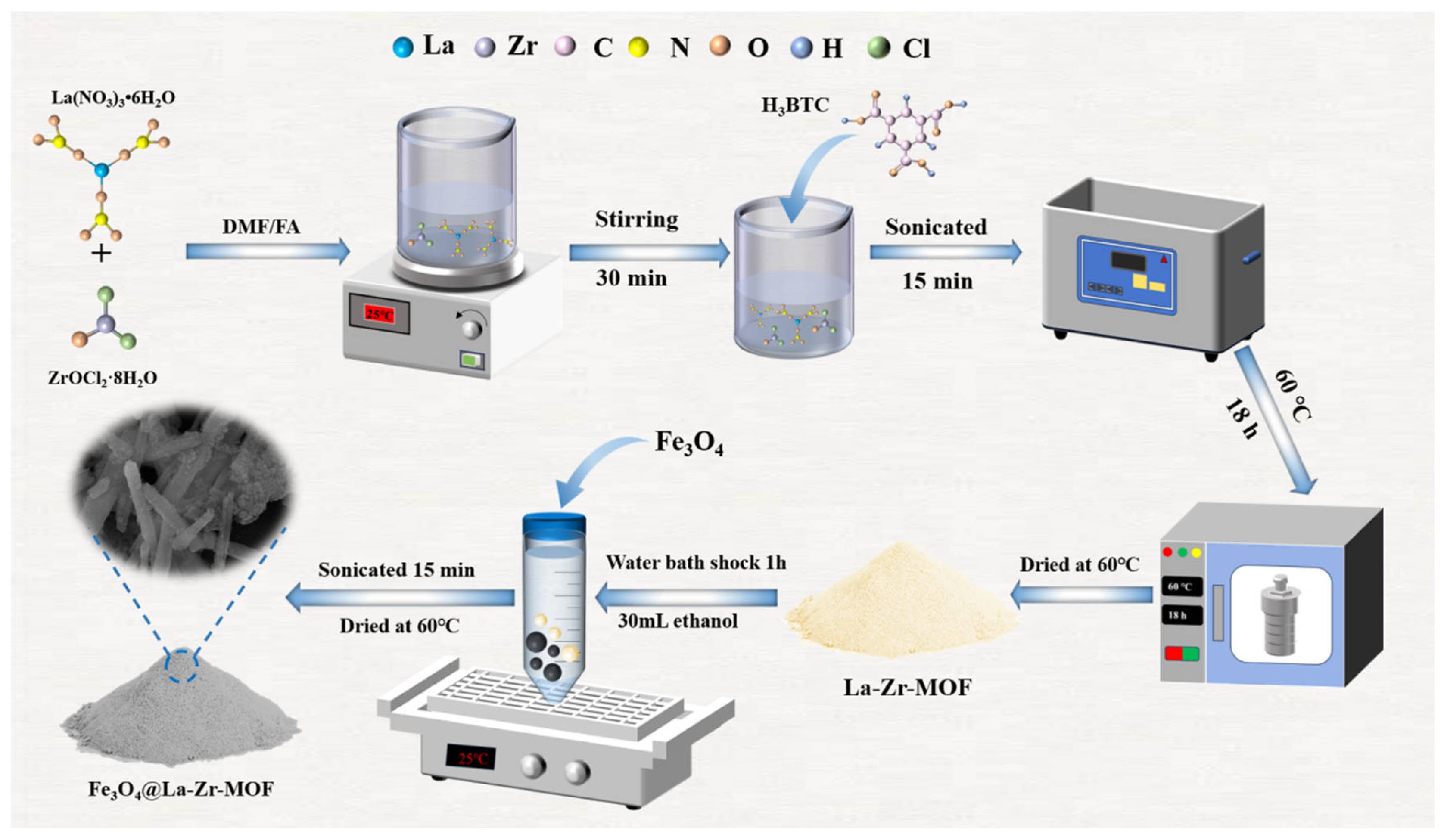
2.1. Material Synthesis
2.1.1. Synthesis of La-Zr-MOF
2.1.2. Synthesis of Fe3O4@La-Zr-MOFs
2.2. Static Adsorption Experiments
3. Results and Discussion
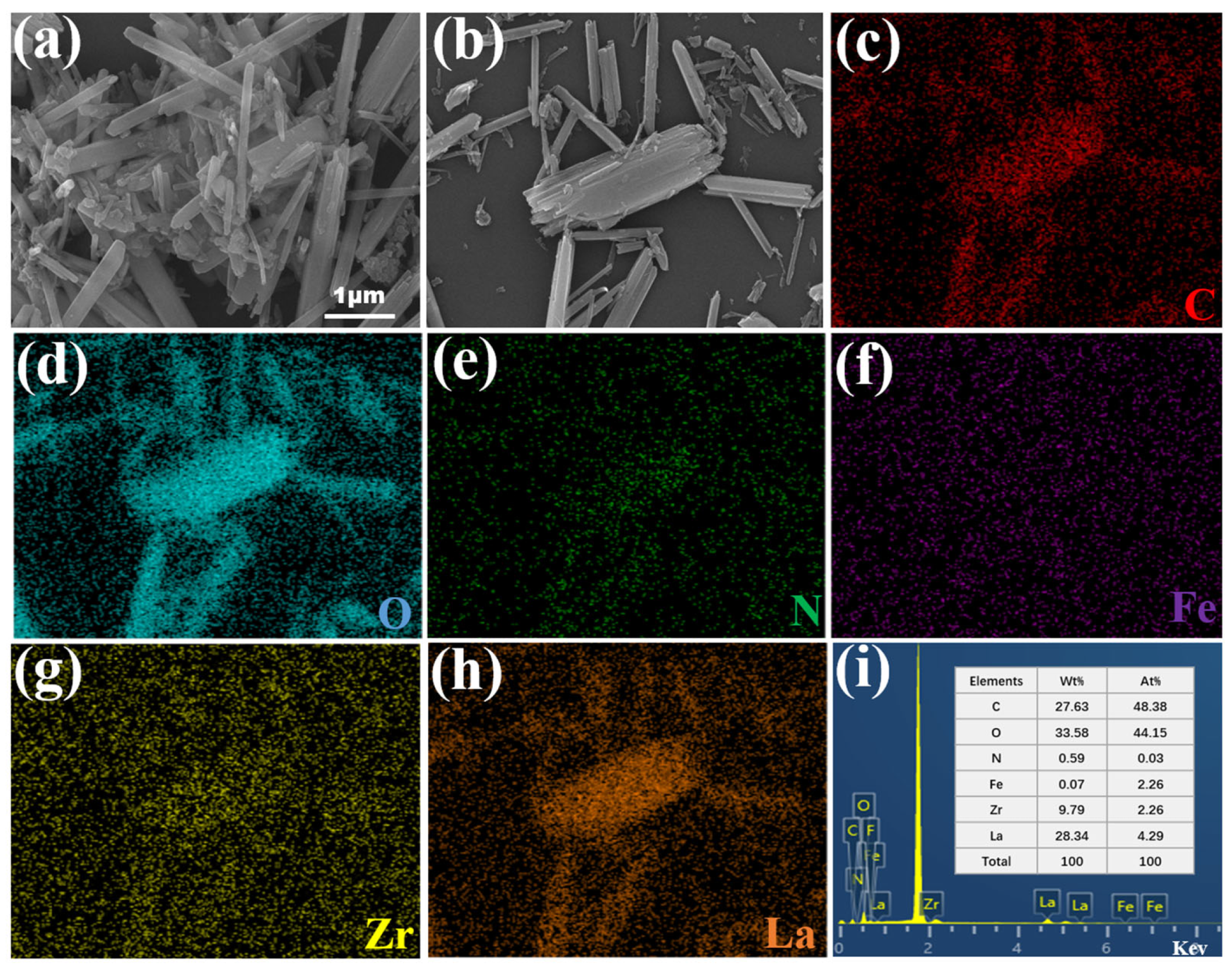
3.1. Morphological Analysis
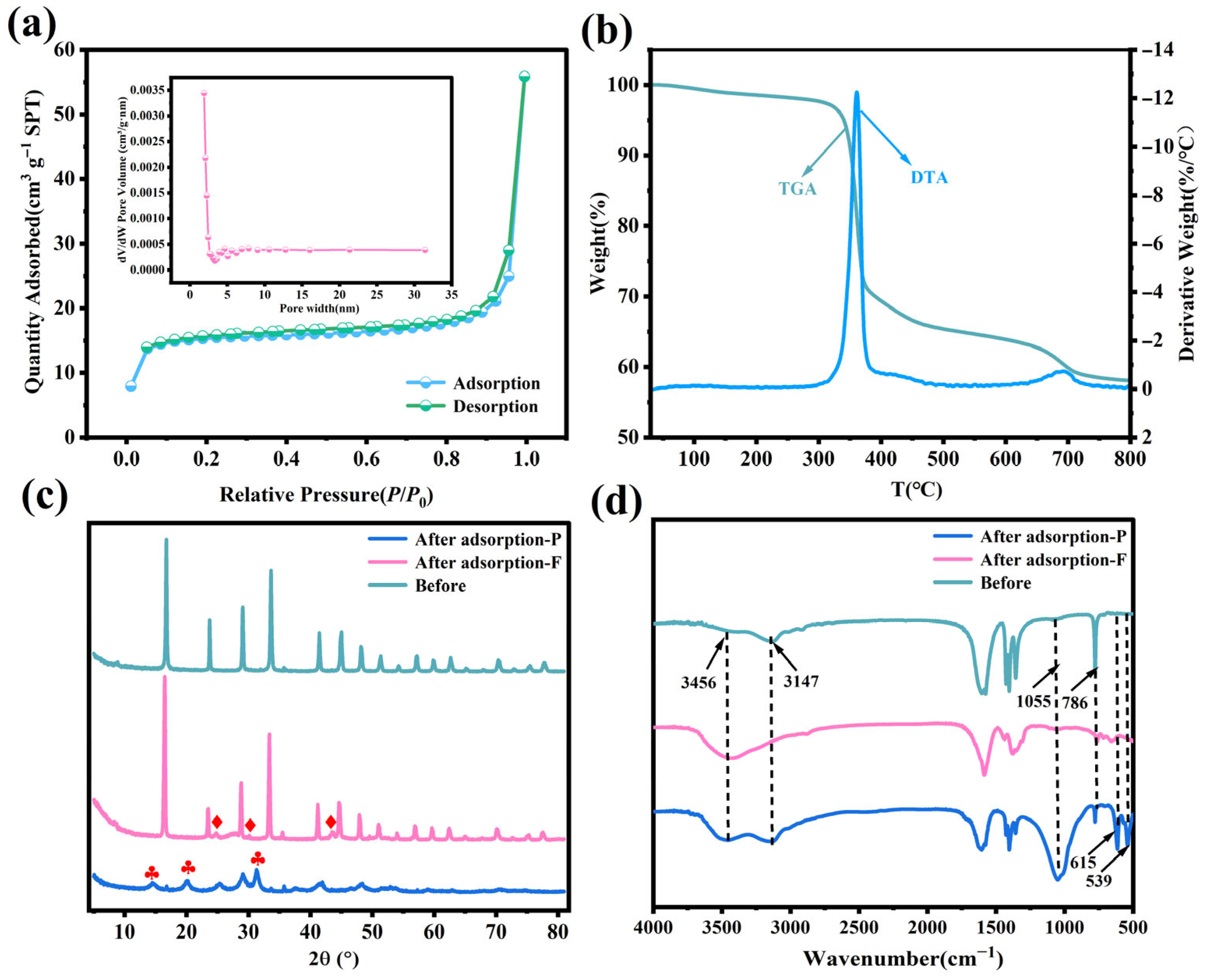
3.2. BET Analysis
3.3. Thermal Stability
3.4. XRD and FTIR
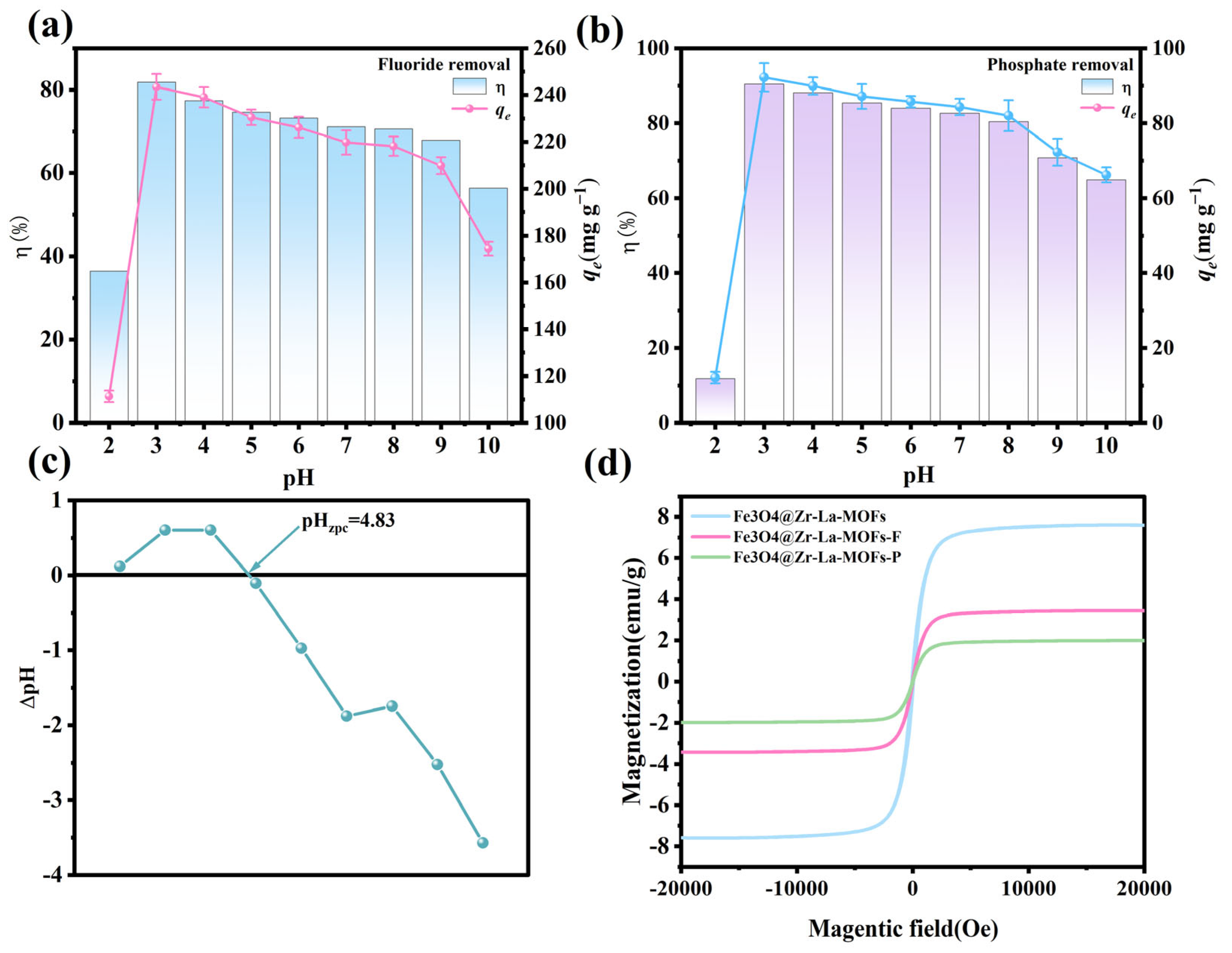
3.5. VSM
3.6. Effect of Solution pH
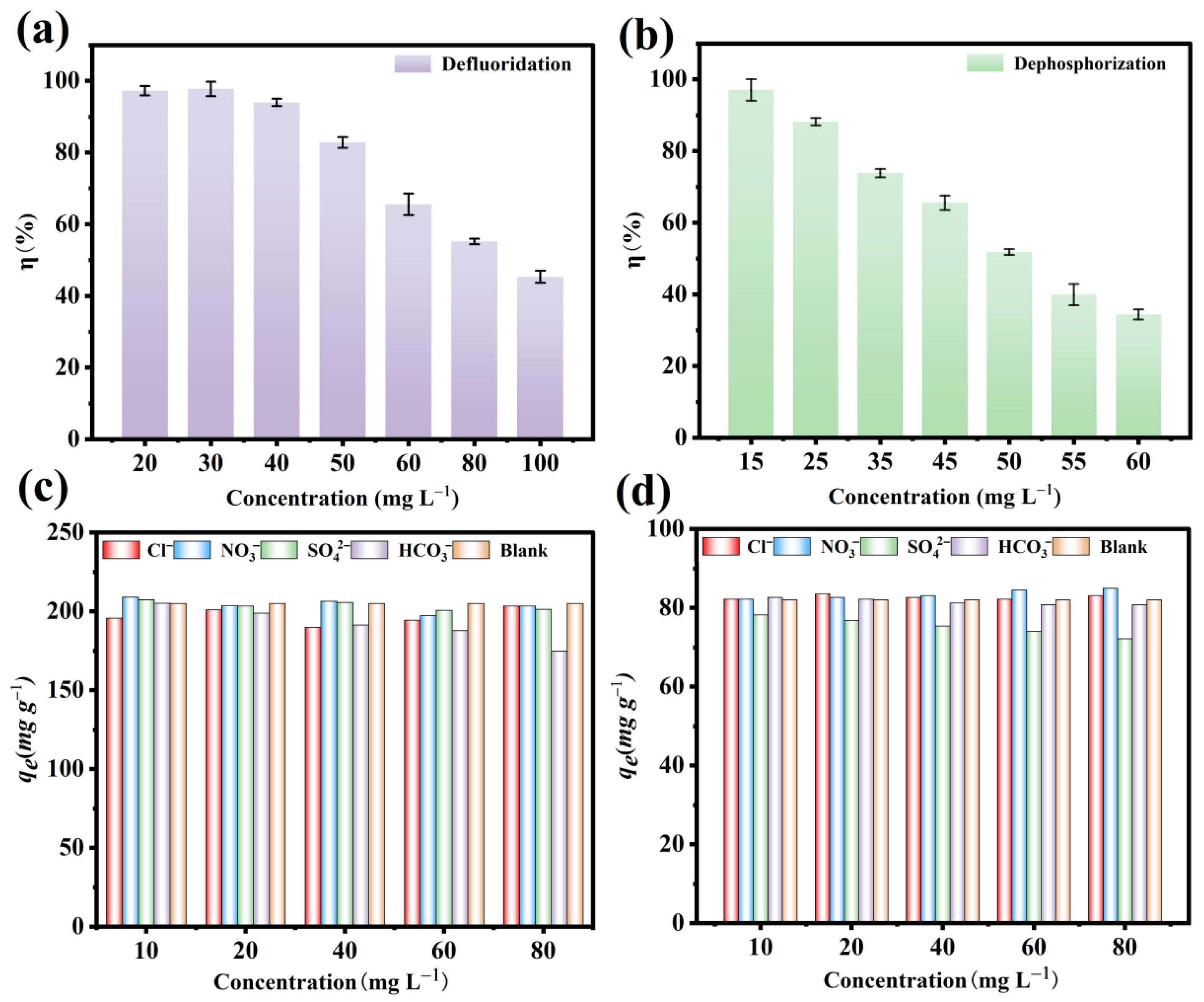
3.7. Effect of Initial Concentration
3.8. Effect of Coexisting Ions
3.9. Adsorption Kinetics
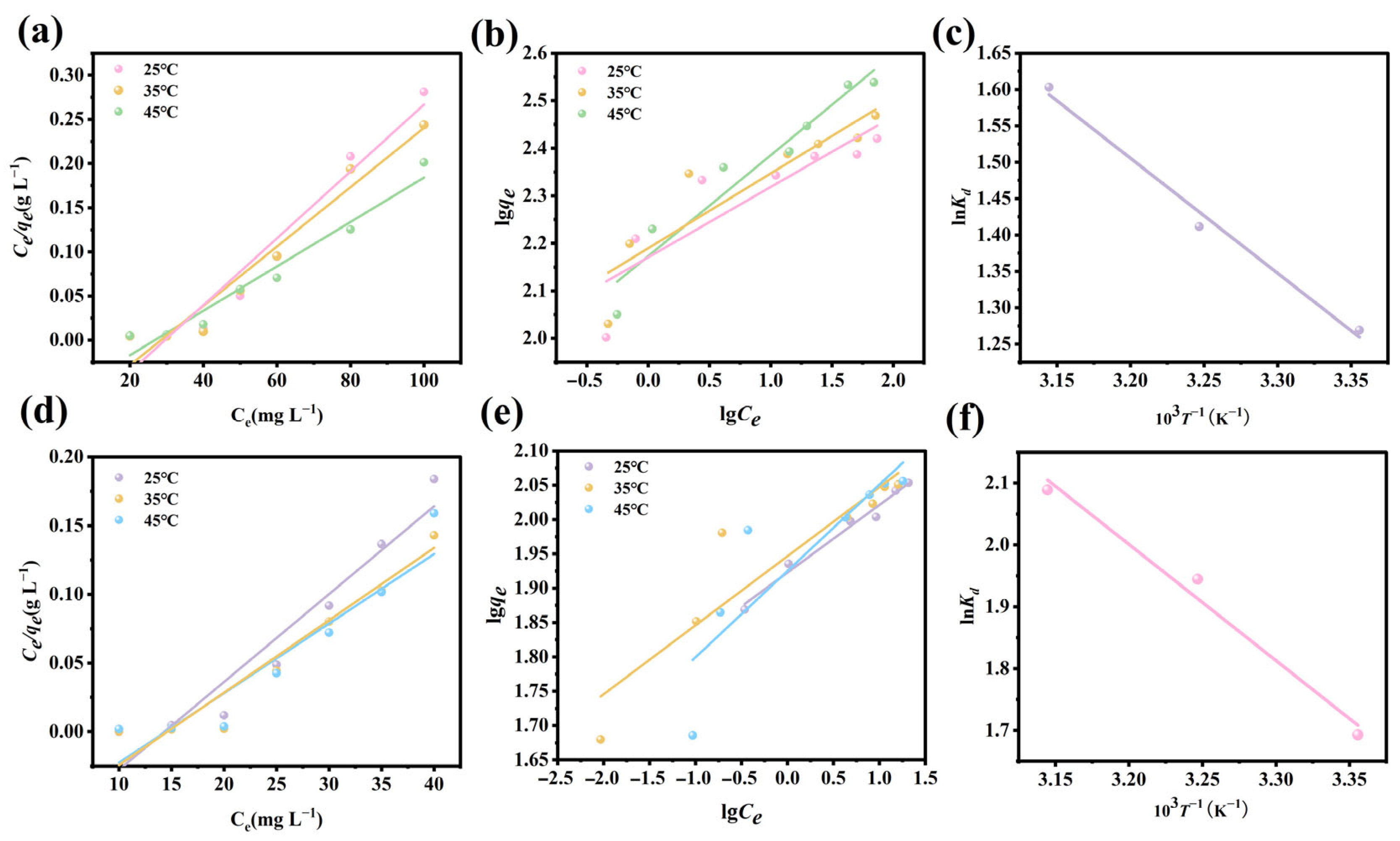
3.10. Adsorption Isotherms
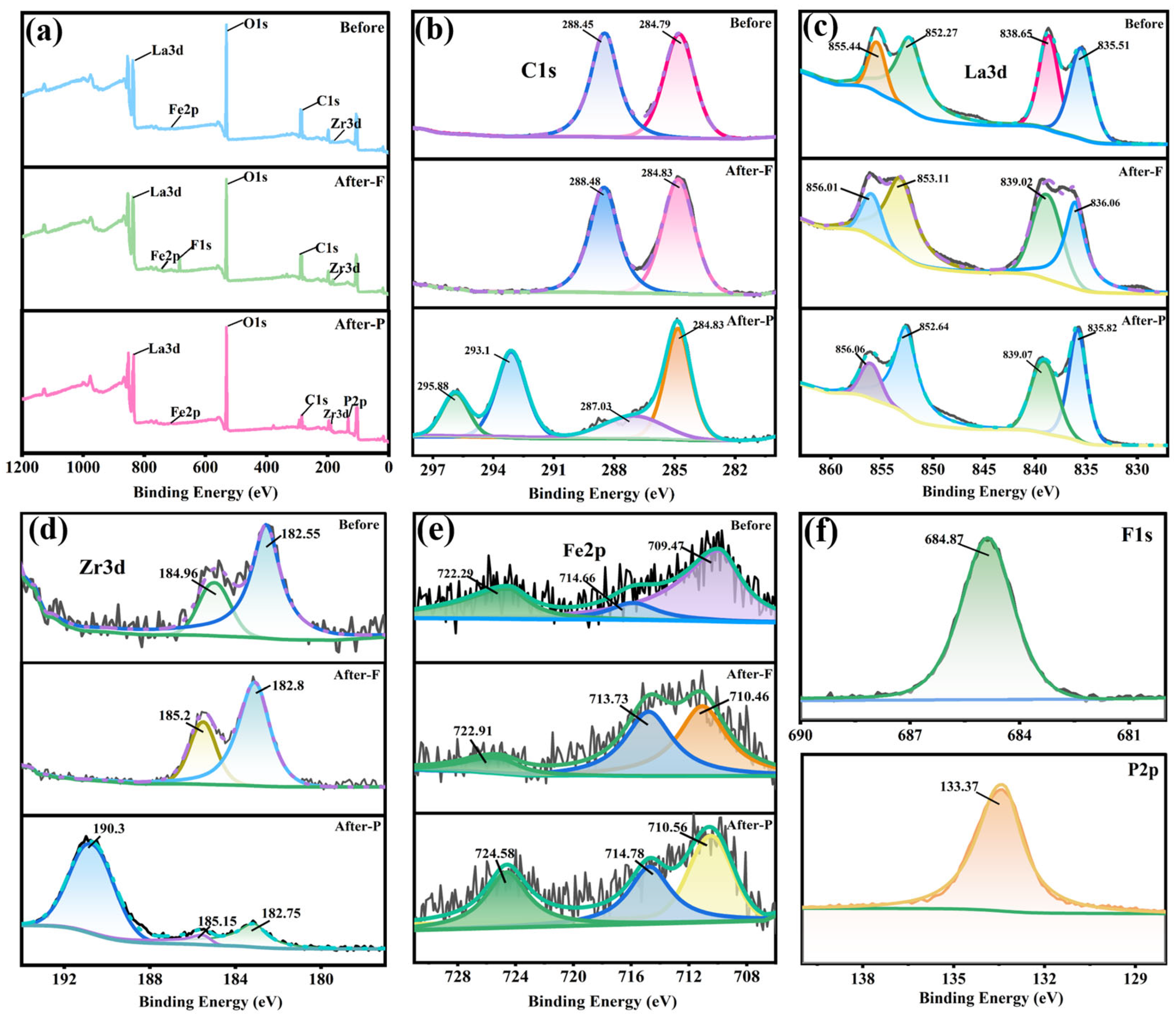
3.11. Adsorption Mechanism
3.12. Regeneration Properties of Adsorbents and Application in Real Wastewaters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, X.D.; Li, P.Y.; Ji, Y.J.; Wang, Y.H.; Su, Z.M.; Vetrimurugan, E. Groundwater Arsenic and Fluoride and Associated Arsenicosis and Fluorosis in China: Occurrence, Distribution and Management. Expo. Health 2020, 12, 355–368. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, B.; Yang, W.; Jia, Y.; Jiang, P.; Zhang, Q.; Kong, L.; Liu, J. Fluoride removal performance and mechanism of superparamagnetic Fe3O4 nanoparticles. Desalination Water Treat. 2021, 233, 281–291. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of fluoride removal from water environment by adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Kumar, I.A.; Viswanathan, N. A facile synthesis of magnetic particles sprayed gelatin embedded hydrotalcite composite for effective phosphate sorption. J. Environ. Chem. Eng. 2018, 6, 208–217. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Huo, S.; Li, F.; Li, K. Recent progress in metal-based composites toward adsorptive removal of phosphate: Mechanisms, behaviors, and prospects. Chem. Eng. J. 2022, 446, 137081. [Google Scholar] [CrossRef]
- Lu, N.C.; Liu, J.C. Removal of phosphate and fluoride from wastewater by a hybrid precipitation-microfiltration process. Sep. Purif. Technol. 2010, 74, 329–335. [Google Scholar] [CrossRef]
- Awual, M.R.; Jyo, A.; El-Safty, S.A.; Tamada, M.; Seko, N. A weak-base fibrous anion exchanger effective for rapid phosphate removal from water. J. Hazard. Mater. 2011, 188, 164–171. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Sozhan, G. Studies on a Mg-Al-Zn Alloy as an Anode for the Removal of Fluoride from Drinking Water in an Electrocoagulation Process. Clean-Soil Air Water 2009, 37, 372–378. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Benamor, A.; Hilal, N.; Leo, C.P. Hybrid coagulation-NF membrane process for brackish water treatment: Effect of antiscalant on water characteristics and membrane fouling. Desalination 2016, 393, 144–150. [Google Scholar] [CrossRef]
- Wang, D.; Chen, N.; Yu, Y.; Hu, W.W.; Feng, C.P. Investigation on the adsorption of phosphorus by Fe-loaded ceramic adsorbent. J. Colloid Interface Sci. 2016, 464, 277–284. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and Recovery of Phosphate From Water Using Sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Nie, G.Z.; Wu, L.R.; Qiu, S.J.; Xu, Z.W.; Wang, H.L. Preferable phosphate sequestration using polymer-supported Mg/Al layered double hydroxide nanosheets. J. Colloid. Interface Sci. 2022, 614, 583–592. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, G.; Gao, M.; Huang, X.; Xu, D. Recent Advances and Applications of Magnetic Metal-Organic Frameworks in Adsorption and Enrichment Removal of Food and Environmental Pollutants. Crit. Rev. Anal. Chem. 2019, 50, 472–484. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.-W.; Jeon, B.-H. Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Fan, W.D.; Wang, X.; Xu, B.; Wang, Y.T.; Liu, D.D.; Zhang, M.; Shang, Y.Z.; Dai, F.N.; Zhang, L.L.; Sun, D.F. Amino-functionalized MOFs with high physicochemical stability for efficient gas storage/separation, dye adsorption and catalytic performance. J. Mater. Chem. A 2018, 6, 24486–24495. [Google Scholar] [CrossRef]
- Jeyaseelan, A.; Viswanathan, N. Design of Amino-Functionalized Benzene-1,4-Dicarboxylic Acid-Fabricated Lanthanum-Based Metal–Organic Frameworks for Defluoridation of Water. J. Chem. Eng. Data 2020, 65, 5328–5340. [Google Scholar] [CrossRef]
- Yin, C.; Li, S.; Liu, L.; Huang, Q.; Zhu, G.; Yang, X.; Wang, S. Structure-tunable trivalent Fe-Al-based bimetallic organic frameworks for arsenic removal from contaminated water. J. Mol. Liq. 2022, 346, 117101. [Google Scholar] [CrossRef]
- Hossien Saghi, M.; Chabot, B.; Rezania, S.; Sillanpää, M.; Akbar Mohammadi, A.; Shams, M.; Alahabadi, A. Water-stable zirconium and iron-based metal-organic frameworks (MOFs) as fluoride scavengers in aqueous medium. Sep. Purif. Technol. 2021, 270, 118645. [Google Scholar] [CrossRef]
- Song, J.Y.; Yu, Y.Y.; Zhang, Z.Y.; Yang, W.S.; Pan, W.B.; Jian, S.J.; Jiang, S.H.; Hu, J.P. Efficient Capture of Phosphate From Aqueous Media Using Bimetallic La-Zr-MOF. Appl. Organomet. Chem. 2025, 39, e7841. [Google Scholar] [CrossRef]
- Wang, J.; Wu, L.; Li, J.; Tang, D.; Zhang, G. Simultaneous and efficient removal of fluoride and phosphate by Fe-La composite: Adsorption kinetics and mechanism. J. Alloys Compd. 2018, 753, 422–432. [Google Scholar] [CrossRef]
- Gu, Y.; Xie, D.; Ma, Y.; Qin, W.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Size Modulation of Zirconium-Based Metal Organic Frameworks for Highly Efficient Phosphate Remediation. ACS Appl. Mater. Interfaces 2017, 9, 32151–32160. [Google Scholar] [CrossRef]
- Su, S.; Zhang, R.; Rao, J.T.; Yu, J.H.; Jiang, X.; Wang, S.X.; Yang, X.J. Fabrication of lanthanum-modified MOF-808 for phosphate and arsenic(V) removal from wastewater. J. Environ. Chem. Eng. 2022, 10, 108527. [Google Scholar] [CrossRef]
- Min, X.Y.; Wu, X.; Shao, P.H.; Ren, Z.; Ding, L.; Luo, X.B. Ultra-high capacity of lanthanum-doped UiO-66 for phosphate capture: Unusual doping of lanthanum by the reduction of coordination number. Chem. Eng. J. 2019, 358, 321–330. [Google Scholar] [CrossRef]
- Li, K.; Zou, S.; Jin, G.; Yang, J.; Dou, M.; Qin, L.; Su, H.; Huang, F. Efficient removal of selenite in aqueous solution by MOF-801 and Fe3O4/MOF-801: Adsorptive behavior and mechanism study. Sep. Purif. Technol. 2022, 296, 101194. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Duan, P.; Jia, X.; Hou, X.; Xia, R.; Guo, Y.; Zhang, X. Characterization and Adsorption Properties of Magnetic Al-MOF Composite for Fluoride. Res. Environ. Sci. 2021, 34, 1139–1147. [Google Scholar]
- Xiong, G.; Chen, X.L.; You, L.X.; Ren, B.Y.; Ding, F.; Dragutan, I.; Dragutan, V.; Sun, Y.G. La-Metal-Organic Framework incorporating Fe3O4 nanoparticles, post-synthetically modified with Schiff base and Pd. A highly active, magnetically recoverable, recyclable catalyst for C-C cross-couplings at low Pd loadings. J. Catal. 2018, 361, 116–125. [Google Scholar] [CrossRef]
- Huang, P.; Qi, X.; Duan, X.; Jiang, W.; Yang, N.; Zhi, G.; Wang, J. Efficient arsenate capture using mixed-metal La/Zr-MOF internal complexation. New J. Chem. 2024, 48, 5311–5325. [Google Scholar] [CrossRef]
- Han, X.G.; Liu, Z.N.; Bakhtari, M.F.; Luo, J.N.; Deng, L.C. Preparation of novel Ce (IV)-based MOF/GO composite and its highly effective phosphate removal from aqueous solution. Environ. Eng. Res. 2023, 28, 220647. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhang, J.L.; Dai, Z.; Lei, Y.Y.; Liu, X.X.; Liu, G.X. Simple preparation and efficient fluoride removal of La anchored Zr-based metal-organic framework adsorbent. J. Environ. Chem. Eng. 2022, 10, 108807. [Google Scholar] [CrossRef]
- Quan, X.; Zhang, J. Phosphate Adsorption from Reclaimed Water via External Cage Expansion on CD-MOF Micro-Interface. Acs EST Water 2024, 4, 4793–4805. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, T.; Xiong, M.; Sun, A.; Xu, Y.; Wu, Y.; Shu, W.; Xu, Z. Tuning adsorption capacity of metal–organic frameworks with Al3+ for phosphorus removal: Kinetics, isotherm and regeneration. Inorg. Chem. Commun. 2021, 132, 108804. [Google Scholar] [CrossRef]
- Hu, J.; Song, J.; Han, X.; Wen, Q.; Yang, W.; Pan, W.; Jian, S.; Jiang, S. Fabrication of Ce-La-MOFs for defluoridation in aquatic systems: A kinetics, thermodynamics and mechanisms study. Sep. Purif. Technol. 2023, 314, 123562. [Google Scholar] [CrossRef]
- Gong, Z.; Dai, Z.-K.; Dong, Z.-Y.; Liu, Q.-X.; Milichko, V.A.; Liu, H.-J.; Liu, J.; Niu, R.; Gong, J. Green synthesis of luminescent La-MOF nanoparticle from waste poly(ethylene terephthalate) for high-performance in Fe(III) detection. Rare Met. 2024, 43, 3833–3843. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, L.; Chen, J.P. Adsorption of fluoride by Fe-Mg-La triple-metal composite: Adsorbent preparation, illustration of performance and study of mechanisms. Chem. Eng. J. 2015, 262, 839–846. [Google Scholar] [CrossRef]
- Jian, S.; Shi, F.; Hu, R.; Liu, Y.; Chen, Y.; Jiang, W.; Yuan, X.; Hu, J.; Zhang, K.; Jiang, S.; et al. Electrospun magnetic La2O3–CeO2–Fe3O4 composite nanofibers for removal of fluoride from aqueous solution. Compos. Commun. 2022, 33, 101194. [Google Scholar] [CrossRef]
- Huo, S.H.; Yan, X.P. Metal-organic framework MIL-100(Fe) for the adsorption of malachite green from aqueous solution. J. Mater. Chem. 2012, 22, 7449–7455. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhou, Q.; Chen, J.H.; Zhang, L.; Chang, N. Phosphate adsorption on hydroxyl-iron-lanthanum doped activated carbon fiber. Chem. Eng. J. 2013, 215, 859–867. [Google Scholar] [CrossRef]
- He, Q.; Zhao, H.; Teng, Z.; Wang, Y.; Sun, W.; Guo, Y.; Ji, X.; Hu, W.; Shiung Lam, S.; Li, M. Efficient recovery of phosphate by Fe3O4/La-MOF: An insight of adsorption performance and mechanism from electrochemical properties. Sep. Purif. Technol. 2023, 314, 123529. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Lu, J.; Cui, Y.; Wang, Y.; Wang, X.; Xue, J.; Cao, H. Advanced magnetic adsorbents for enhanced phosphorus and fluoride removal from wastewater: Mechanistic insights and applications. Sep. Purif. Technol. 2025, 353, 128195. [Google Scholar] [CrossRef]
- Deng, Y.; Nordstrom, D.K.; Blaine McCleskey, R. Fluoride geochemistry of thermal waters in Yellowstone National Park: I. Aqueous fluoride speciation. Geochim. Cosmochim. Acta 2011, 75, 4476–4489. [Google Scholar] [CrossRef]
- Yu, Y.; Song, J.; Zhang, Z.; Yang, W.; Pan, W.; Jian, S.; Hu, J.; Luo, Q. Efficient recovery of phosphate by La-MOFs: An excellent adsorption performance and mechanism. Prog. Nat. Sci. Mater. Int. 2023, 33, 854–863. [Google Scholar] [CrossRef]
- Deng, H.; Yu, X. Adsorption of fluoride, arsenate and phosphate in aqueous solution by cerium impregnated fibrous protein. Chem. Eng. J. 2012, 184, 205–212. [Google Scholar] [CrossRef]
- Nehra, M.; Dilbaghi, N.; Singhal, N.K.; Hassan, A.A.; Kim, K.H.; Kumar, S. Metal organic frameworks MIL-100(Fe) as an efficient adsorptive material for phosphate management. Environ. Res. 2019, 169, 229–236. [Google Scholar] [CrossRef]
- Zhang, N.; Lin, L.-S.; Gang, D. Adsorptive selenite removal from water using iron-coated GAC adsorbents. Water Res. 2008, 42, 3809–3816. [Google Scholar] [CrossRef]
- Hao, H.T.; Wang, Y.L.; Shi, B.Y. NaLa (CO3)2 hybridized with Fe3O4 for efficient phosphate removal: Synthesis and adsorption mechanistic study. Water Res. 2019, 155, 1–11. [Google Scholar] [CrossRef]
- Song, J.; Yu, Y.; Han, X.; Yang, W.; Pan, W.; Jian, S.; Duan, G.; Jiang, S.; Hu, J. Novel MOF(Zr)-on-MOF(Ce) adsorbent for elimination of excess fluoride from aqueous solution. J. Hazard. Mater. 2024, 463, 132843. [Google Scholar] [CrossRef]
- Yang, L.; Shan, X.; Zhao, Y.; Xiao, Z.; An, Q.; Zhai, S. Efficient phosphate capture of Fe3O4/UiO-66-NH2/CeO2 in wide pH spectrum. Microporous Mesoporous Mater. 2022, 331, 111653. [Google Scholar] [CrossRef]
- Liu, R.T.; Chi, L.N.; Wang, X.Z.; Wang, Y.; Sui, Y.M.; Xie, T.T.; Arandiyan, H. Effective and selective adsorption of phosphate from aqueous solution via trivalent-metals-based amino-MIL-101 MOFs. Chem. Eng. J. 2019, 357, 159–168. [Google Scholar] [CrossRef]
- Kim, H.; Cha, B.; Nam, S.-N.; Yoon, Y.; Hong, H.-J.; Elanchezhiyan, S.S.; Park, C.M. Synthesis of zirconium-based metal-organic framework/gelatin aerogel for removing phosphate and fluoride from aqueous solutions. Int. J. Biol. Macromol. 2025, 297, 139749. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Tang, N.; Wang, Y.; Yang, X.; Wang, S. Highly efficient capture of phosphate from water via cerium-doped metal-organic frameworks. J. Clean. Prod. 2020, 265, 121782. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Q.; Ji, F.; Jiang, L.; Liu, C.; Shen, Q.; Liu, Q. Phosphate removal performances of layered double hydroxides (LDH) embedded polyvinyl alcohol/lanthanum alginate hydrogels. Chem. Eng. J. 2022, 430, 132754. [Google Scholar] [CrossRef]
- Kong, L.C.; Tian, Y.; Pang, Z.; Huang, X.H.; Li, M.; Yang, R.C.; Li, N.; Zhang, J.; Zuo, W. Synchronous phosphate and fluoride removal from water by 3D rice-like lanthanum-doped La@MgAl nanocomposites. Chem. Eng. J. 2019, 371, 893–902. [Google Scholar] [CrossRef]
- Zhao, M.X.; Zhou, X.M.; Li, J.F.; Li, F.; Li, X.D.; Yu, J.X.; Guo, L.; Song, G.P.; Xiao, C.Q.; Zhou, F.; et al. Efficient removal of phosphate and fluoride from phosphogypsum leachate by lanthanum-modified zeolite: Synchronous adsorption behavior and mechanism. J. Environ. Chem. Eng. 2024, 12, 113294. [Google Scholar] [CrossRef]
- He, M.; Zhang, P.; Huo, S.; Zhang, X.; Gong, A.; Zhang, W.; Li, K. Remarkable phosphate electrosorption/desorption by bimetallic MOF-derived hierarchically porous carbon electrode: In-situ creation of multiple active centers and boosting electrochemical activities. Chem. Eng. J. 2022, 446, 137396. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, B.; Ali Khan, N.; Zhi, X.; Zhang, Y.; Xie, H.; Zhu, H. Synergy in Nano-Fe3O4 and MOF-808 composites enriches their applications in sludge anaerobic fermentation. Chem. Eng. J. 2024, 493, 152471. [Google Scholar] [CrossRef]
- Chiou, C.-S.; Lin, Y.-F.; Chen, H.-W.; Chang, C.-C.; Chang, S.-H. Adsorption of Phosphate in Aqueous Solution by Magnetite Modified with Diethylenetriamine. J. Nanosci. Nanotechnol. 2015, 15, 2850–2857. [Google Scholar] [CrossRef]
- Luengo, C.V.; Volpe, M.A.; Avena, M.J. High sorption of phosphate on Mg-Al layered double hydroxides: Kinetics and equilibrium. J. Environ. Chem. Eng. 2017, 5, 4656–4662. [Google Scholar] [CrossRef]
- Wan, K.L.; Huang, L.; Yan, J.; Ma, B.Y.; Huang, X.J.; Luo, Z.X.; Zhang, H.G.; Xiao, T.F. Removal of fluoride from industrial wastewater by using different adsorbents: A review. Sci. Total Environ. 2021, 773, 145535. [Google Scholar] [CrossRef]
- Jian, S.; Cheng, Y.; Ma, X.; Guo, H.; Hu, J.; Zhang, K.; Jiang, S.; Yang, W.; Duan, G. Excellent fluoride removal performance by electrospun La-Mn bimetal oxide nanofibers. New J. Chem. 2022, 46, 490–497. [Google Scholar] [CrossRef]
- Lin, S.; Wei, W.; Wu, X.; Zhou, T.; Mao, J.; Yun, Y.-S. Selective recovery of Pd(II) from extremely acidic solution using ion-imprinted chitosan fiber: Adsorption performance and mechanisms. J. Hazard. Mater. 2015, 299, 10–17. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Pu, Z.; Zhao, Y.; Jia, D.; Chen, H.; Wen, T.; Hu, B.; Alsaedi, A.; Hayat, T.; et al. Synthesis of magnetic Fe3O4/CFA composites for the efficient removal of U(VI) from wastewater. Chem. Eng. J. 2017, 320, 448–457. [Google Scholar] [CrossRef]
- Cai, J.G.; Li, A.M.; Shi, H.Y.; Fei, Z.H.; Long, C.; Zhang, Q.X. Adsorption characteristics of aniline and 4-methylaniline onto bifunctional polymeric adsorbent modified by sulfonic groups. J. Hazard. Mater. 2005, 124, 173–180. [Google Scholar] [CrossRef]
- Zhang, R.D.; Zhang, J.H.; Zhang, X.N.; Dou, C.C.; Han, R.P. Adsorption of Congo red from aqueous solutions using cationic surfactant modified wheat straw in batch mode: Kinetic and equilibrium study. J. Taiwan Inst. Chem. Eng. 2014, 45, 2578–2583. [Google Scholar] [CrossRef]
- Sahu, S.; Mallik, L.; Pahi, S.; Barik, B.; Sahu, U.K.; Sillanpää, M.; Patel, R.K. Facile synthesis of poly o-toluidine modified lanthanum phosphate nanocomposite as a superior adsorbent for selective fluoride removal: A mechanistic and kinetic study. Chemosphere 2020, 252, 126551. [Google Scholar] [CrossRef]
- Li, B.; Zheng, J.-Q.; Guo, J.-Z.; Dai, C.-Q. A novel route to synthesize MOFs-derived mesoporous dawsonite and application in elimination of Cu(II) from wastewater. Chem. Eng. J. 2020, 383, 123174. [Google Scholar] [CrossRef]
- Yang, H.; Fu, P.; Zhang, G.; Zhang, Q.; Li, X. Adsorption Removal of Phosphate and Fluoride from Aqueous Solution Using Oily Sludge-based Pyrolysis Residue. Environ. Process. 2021, 8, 1517–1531. [Google Scholar] [CrossRef]
- Belaye, M.; Taddesse, A.M.; Teju, E.; Sanchez-Sanchez, M.; Yassin, J.M. Preparation and Adsorption Behavior of Ce(III)-MOF for Phosphate and Fluoride Ion Removal from Aqueous Solutions. ACS Omega 2023, 8, 23860–23869. [Google Scholar] [CrossRef]
- Yin, C.; Huang, Q.; Zhu, G.; Liu, L.; Li, S.; Yang, X.; Wang, S. High-performance lanthanum-based metal–organic framework with ligand tuning of the microstructures for removal of fluoride from water. J. Colloid Interface Sci. 2022, 607, 1762–1775. [Google Scholar] [CrossRef]
- Han, M.; Zhang, J.; Hu, Y.; Han, R. Preparation of Novel Magnetic Microspheres with the La and Ce-Bimetal Oxide Shell for Excellent Adsorption of Fluoride and Phosphate from Solution. J. Chem. Eng. Data 2019, 64, 3641–3651. [Google Scholar] [CrossRef]
- Pandi, K.; Viswanathan, N. Enhanced defluoridation and facile separation of magnetic nano-hydroxyapatite/alginate composite. Int. J. Biol. Macromol. 2015, 80, 341–349. [Google Scholar] [CrossRef]
- Legese, W.; Taddesse, A.M.; Teju, E. Al2O3/Fe3O4/ZrO2 ternary oxide sorbent: Synthesis, characterization and sorption behavior to fluoride and phosphate ions from aqueous solution. Bull. Chem. Soc. Ethiop. 2022, 36, 555–569. [Google Scholar] [CrossRef]
- Ma, C.H.; Zhang, X.T.; Wen, K.; Wang, R.; Han, R.P. Facile synthesis of polyethyleneimine@Fe3O4 loaded with zirconium for enhanced phosphate adsorption: Performance and adsorption mechanism. Korean J. Chem. Eng. 2021, 38, 135–143. [Google Scholar] [CrossRef]
- Rahdar, A.; Ahmadi, S.; Fu, J.; Rahdar, S. Iron oxide nanoparticle preparation and its use for the removal of fluoride from aqueous solution: Application of isotherm, kinetic, and thermodynamics. Desalination Water Treat. 2019, 137, 174–182. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Chen, J.; Xu, H.; Zhu, Y.; Chen, Z.; Shen, M.; Yang, W. Simultaneous removal of phosphate and fluoride from acid wastewater by Ce-La bimetal oxides: Performance and mechanism. China Environ. Sci. 2023, 43, 5148–5156. [Google Scholar]
- Du, Y.; Wang, X.; Nie, G.; Xu, L.; Hu, Y. Enhanced phosphate removal by using La-Zr binary metal oxide nanoparticles confined in millimeter-sized anion exchanger. J. Colloid Interface Sci. 2020, 580, 234–244. [Google Scholar] [CrossRef]
- Li, F.; Jin, J.; Shen, Z.; Ji, H.; Yang, M.; Yin, Y. Removal and recovery of phosphate and fluoride from water with reusable mesoporous Fe3O4@mSiO2@mLDH composites as sorbents. J. Hazard. Mater. 2020, 388, 121734. [Google Scholar] [CrossRef]
- Rego, R.M.; Sriram, G.; Ajeya, K.V.; Jung, H.-Y.; Kurkuri, M.D.; Kigga, M. Cerium based UiO-66 MOF as a multipollutant adsorbent for universal water purification. J. Hazard. Mater. 2021, 416, 125941. [Google Scholar] [CrossRef]
- Song, J.; Yang, W.; Han, X.; Jiang, S.; Zhang, C.; Pan, W.; Jian, S.; Hu, J. Performance of Rod-Shaped Ce Metal–Organic Frameworks for Defluoridation. Molecules 2023, 28, 3492. [Google Scholar] [CrossRef]
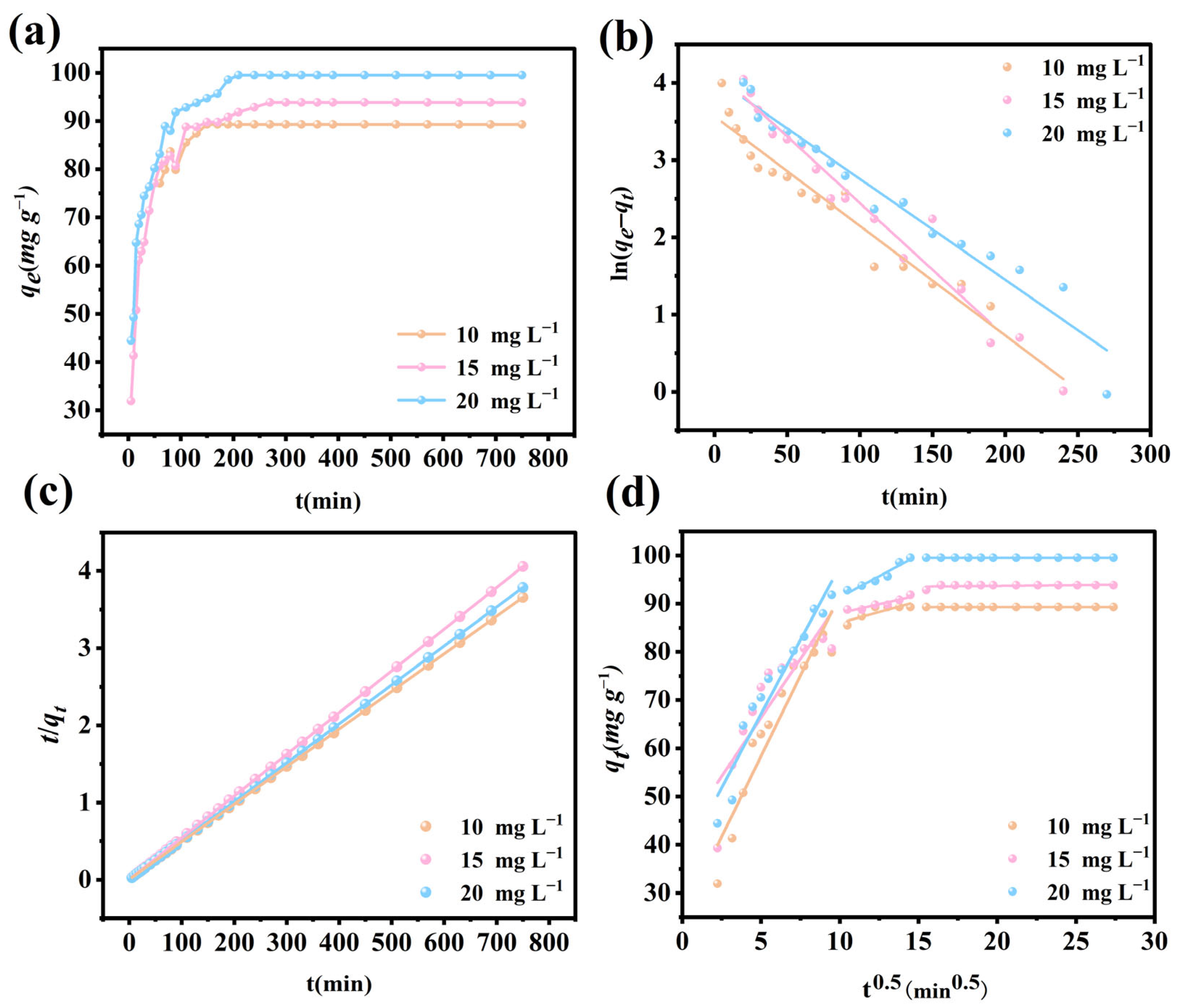
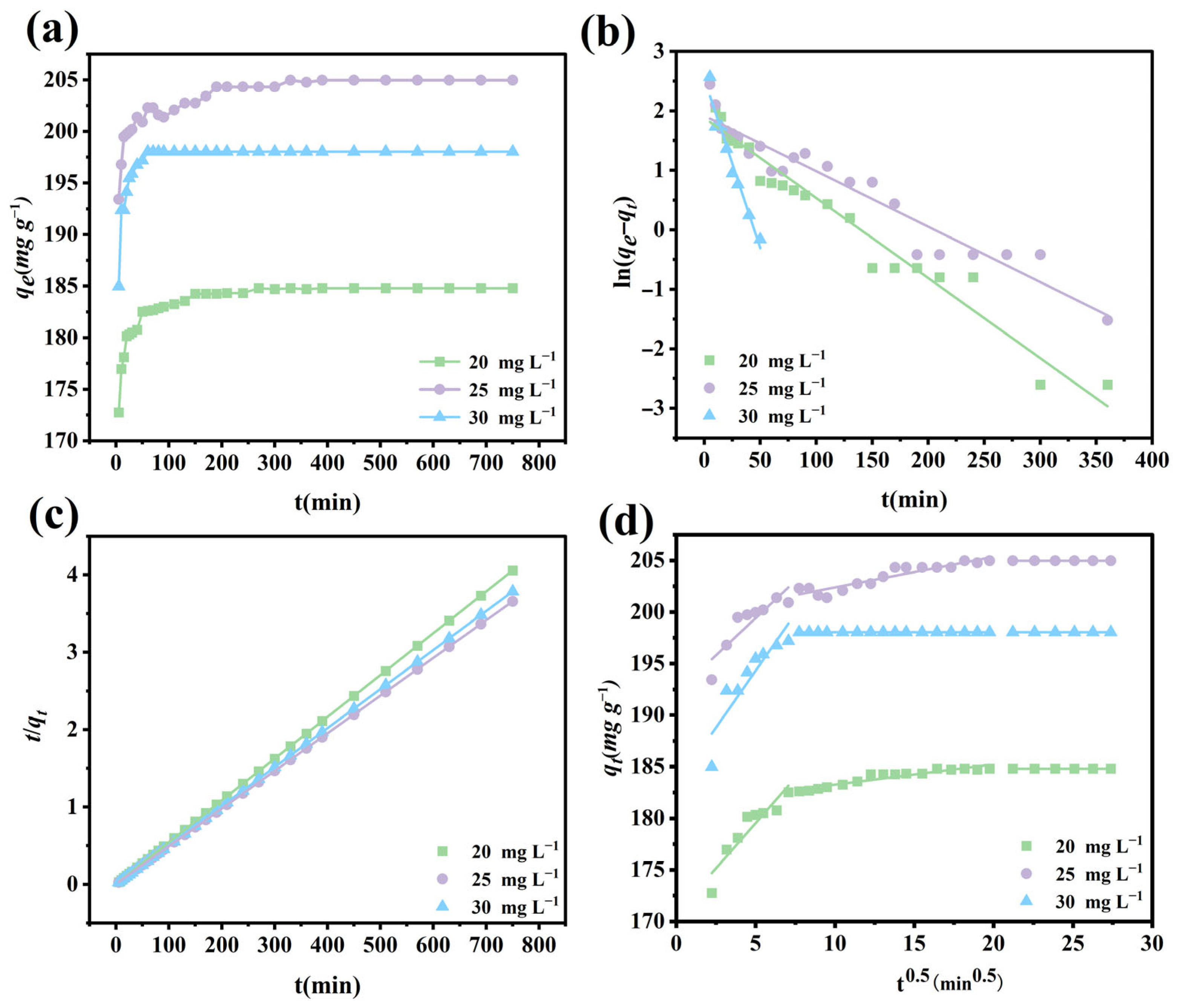
| Model | C0 (mg L–1) | k | qe (mg g–1) | R2 |
|---|---|---|---|---|
| Pseudo-first order | 10 | 0.0141 | 35.4864 | 0.9590 |
| 15 | 0.0172 | 64.9105 | 0.9415 | |
| 20 | 0.0131 | 58.2800 | 0.9549 | |
| Pseudo-second order | 10 | 0.0013 | 90.7441 | 0.9997 |
| 15 | 0.0011 | 95.3288 | 0.9998 | |
| 20 | 0.0010 | 101.3171 | 0.9998 |
| Model | C0 (mg L–1) | k | qe (mg g–1) | R2 |
|---|---|---|---|---|
| Pseudo-first order | 20 | 0.0134 | 6.5727 | 0.9514 |
| 25 | 0.0093 | 6.8037 | 0.9241 | |
| 30 | 0.0568 | 12.6405 | 0.9608 | |
| Pseudo-second order | 20 | 0.0076 | 185.1851 | 1 |
| 25 | 0.0284 | 198.0198 | 1 | |
| 30 | 0.0027 | 205.3388 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Chen, X.; Yu, Y.; Pan, W.; Liu, R.; Song, J.; Hu, J. Experimental Evaluation and Thermodynamic Analysis of Magnetic Fe3O4@La-Zr-MOFs for Highly Efficient Fluoride and Phosphate Removal. Nanomaterials 2025, 15, 1043. https://doi.org/10.3390/nano15131043
Zhang Z, Chen X, Yu Y, Pan W, Liu R, Song J, Hu J. Experimental Evaluation and Thermodynamic Analysis of Magnetic Fe3O4@La-Zr-MOFs for Highly Efficient Fluoride and Phosphate Removal. Nanomaterials. 2025; 15(13):1043. https://doi.org/10.3390/nano15131043
Chicago/Turabian StyleZhang, Ziyi, Xinyun Chen, Yongyi Yu, Wenbin Pan, Ruilai Liu, Jiangyan Song, and Jiapeng Hu. 2025. "Experimental Evaluation and Thermodynamic Analysis of Magnetic Fe3O4@La-Zr-MOFs for Highly Efficient Fluoride and Phosphate Removal" Nanomaterials 15, no. 13: 1043. https://doi.org/10.3390/nano15131043
APA StyleZhang, Z., Chen, X., Yu, Y., Pan, W., Liu, R., Song, J., & Hu, J. (2025). Experimental Evaluation and Thermodynamic Analysis of Magnetic Fe3O4@La-Zr-MOFs for Highly Efficient Fluoride and Phosphate Removal. Nanomaterials, 15(13), 1043. https://doi.org/10.3390/nano15131043







