Abstract
Visible-light-driven photocatalytic hydrogen production is one of the ideal green technologies for solar-to-chemical energy conversion. Carbon nitride (C3N4, CN) has been attracting extensive attention for its suitable band structure and stability, but the efficiency of photocatalytic hydrogen evolution is low due to insufficient visible-light absorption and rapid charge recombination. Herein, we develop a novel (F, K)-co-doped CN (FKCN) catalyst via a facile thermal polymerization approach using KOH-modified melamine and NH4F as the dopant precursors. The FKCN catalyst demonstrates broadened light absorption, significantly enhanced charge separation, and excellent cyclic stability. And the optimal F(0.15)K(6)CN catalyst achieves a hydrogen evolution rate of as high as 3101.5 μmol g−1 h−1 (12-fold that of pristine CN) under visible-light irradiation (λ ≥ 420 nm), which is among the best element-doped CN photocatalysts. This work highlights the effectiveness of a multi-element doping strategy in designing CN-based photocatalysts for efficient hydrogen evolution.
1. Introduction
In addressing the energy shortage crisis and environmental pollution, photocatalytic water splitting to generate hydrogen has emerged as one of the promising approaches for sustainable solar-to-chemical energy conversion [1,2], and the development of highly active and low-cost photocatalysts is of great significance [3]. Carbon nitride (C3N4, CN) has attracted significant attention in photocatalytic water splitting due to its unique sp2-hybridized conjugated structure and suitable band gap (~2.7 eV), which endows it with excellent photochemical stability and visible-light responsiveness [4,5,6]. However, the application of pristine CN in water splitting is limited by the restricted optical absorption edge (λ ≤ 460 nm), rapid recombination of photogenerated charge carriers, and smaller number of reactive surface sites [7,8,9].
To address these intrinsic drawbacks of pristine CN, various strategies have been developed, such as elemental doping [10], heterojunction construction [11], surface functional group modification [12], nanostructural design [13], etc. Among these approaches, elemental doping was used as an effective method to enhance the photocatalytic activity of CN, as it could tune the electronic structure, increase the active site density, and facilitate charge carrier separation/transfer [14,15]. Specifically, potassium (K) doping could be inserted into the CN interlayers to facilitate interlayer electron transfer and generate cyano groups to provide additional active sites [16]. For instance, Sun et al. synthesized K-doped CN ultrathin nanosheets via a KOH-assisted hydrothermal melamine method, leading to an improved hydrogen evolution performance, which was attributed to an increased specific surface area, upward-shifted conduction band edge for enhanced photoreduction ability, and prolonged charge carrier lifetime [17]. Wang et al. reported K-doped g-C3N4 using potassium bromide (KBr) as the dopant source through thermal polymerization, with the optimized K-CN-10 catalyst demonstrating a hydrogen evolution rate of 1337 μmol g−1 h−1 [18]. The enhancement was ascribed to a reduced band gap, improved light-harvesting capacity, and enhanced electron transport. Chang et al. introduced K atoms and cyano functional groups into CN via potassium thioacetate-mediated thermal polymerization, achieving a hydrogen evolution rate of 1319 μmol g−1 h−1 for the optimized K(0.05)-CN catalyst [19]. The improved performance was attributed to an increased electron density in heptazine ring delocalized π bonds from K doping and lone-pair electron delocalization by cyano groups.
To further improve the photocatalytic performance of K-doped CN, other elements were often co-doped with K to jointly modify the electronic structure of CN. For example, Guo et al. fabricated K/I-co-doped mesoporous CN using dicyandiamide and KI as precursors with SBA-15 as a hard template via thermal polymerization [20]. The optimized MP-CN-KI catalyst achieved a hydrogen evolution rate of 1611 μmol g−1 h−1, which was attributed to a prolonged carrier lifetime, improved electrical conductivity, and enhanced photocurrent density. Bi et al. synthesized S/K-co-doped CN through a condensation reaction of thiourea and dithiooxamide, with the optimized CN-0.20%Dx-25 catalyst demonstrating a hydrogen evolution rate of 1962 μmol g−1 h−1 [21]. This improvement was ascribed to K atoms creating charge transport channels between CN layers and synergistic effects of S/K co-doping that introduced additional electrons into the band gap, forming a metalloid-like band structure.
It is noteworthy that fluorine (F) has a smaller atomic radius and higher electronegativity compared to other dopant atoms, making it easier to be doped into CN and modifying its electronic structure. Herein, we report a (F, K)-co-doped CN (FKCN) photocatalyst synthesized through a novel thermal polymerization approach for enhanced photocatalytic H2 evolution. Synergistic integration of KOH-modified melamine with NH4F in a reaction system enables precise control over structural defects and electronic properties of FKCN. This synthetic route not only overcomes the limitations of conventional doping methods but also creates a new paradigm for designing high-efficiency photocatalytic materials with tailored energy band structures. This work demonstrates an innovative way via the integration of alkali metal and halogen co-doping of developing efficient photocatalysts with an enhanced light-harvesting capability and charge carrier separation efficiency.
2. Materials and Methods
2.1. Materials and Synthesis
Melamine (99%, Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China) was employed for the synthesis of the CN skeleton. Ammonium fluoride (98%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and potassium hydroxide (82%, Sinopharm Chemical Reagent Co., Ltd.) served as doping sources. Methanol (99.5%, Tianjin Anlong Bohua Medicinal Chemistry Co., Ltd., Tianjin, China) was utilized as the sacrificial agent. All reagents in the experiment were analytical-grade and used without further purification
Pristine CN was synthesized through thermal polymerization of melamine precursors. Specifically, 4.0 g of melamine was placed in a ceramic crucible and subjected to thermal treatment under an argon atmosphere (50 mL min−1). The temperature was heated to 823 K at a ramping rate of 5 K min−1 and maintained at this temperature for 4 h. The resulting pale-yellow product was obtained after natural cooling to an ambient temperature.
F-doped CN (F(x)CN) was fabricated via a solution impregnation–calcination strategy. In a typical procedure, 0.5 g of melamine and different amounts (x = 0.1, 0.125, 0.15, 0.175, 0.2 g) of ammonium fluoride (NH4F) were co-dissolved in 10 mL of deionized water. The homogeneous solution was continuously stirred at 353 K until complete solvent evaporation. The dried mixture was subsequently calcined under identical conditions to those for pristine CN synthesis. The obtained samples were named F(x)CN (x = 0.1, 0.125, 0.15, 0.175, 0.2).
K-doped CN (K(y)CN) was prepared through a hydrothermal approach, where 8 g of melamine and different amounts (y = 2, 4, 6, 8 g) of potassium hydroxide (KOH) were dissolved in 70 mL deionized water and hydrothermally treated at 453 K for 12 h in a Teflon-lined autoclave. The resulting slurry was vacuum-filtered and dried at 333 K for 12 h. The obtained precursor was then calcined using the same protocol as for pristine CN. The obtained samples were named K(y)CN (y = 2, 4, 6, 8).
(F, K)-co-doped CN (F(x)K(6)CN) was prepared through a two-step modification approach, as illustrated in Figure 1. First, 8 g of melamine and 6 g of KOH were dispersed in 70 mL of deionized water and hydrothermally treated at 453 K for 12 h in a Teflon-lined autoclave. The resulting slurry was filtered and vacuum dried at 333 K for 12 h, obtaining K(6)CN. Then, 0.5 g of the pre-synthesized K(6)CN and different amounts (x = 0.1, 0.125, 0.15, 0.175, 0.2 g) of NH4F were added to 10 mL of aqueous solution under stirring and evaporated to dryness. Finally, the obtained precursor was then calcined using the same protocol as for pristine CN. The obtained samples were fully ground and denoted as F(x)K(6)CN (x = 0.1, 0.125, 0.15, 0.175, 0.2).
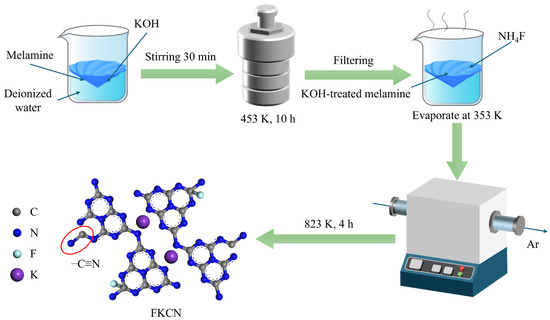
Figure 1.
Schematic illustration for the preparation of (F, K)-co-doped CN photocatalyst.
2.2. Characterization
The crystal structure was characterized by X-ray diffraction (XRD) on a D8 Advance diffractometer (Bruker Corporation, Karlsruhe, Germany) with monochromatic Cu Kα radiation (λ = 0.15418 nm), operating at a scanning range of 5–60°, a sampling interval of 0.01°, and a scanning speed of 10° min−1. Fourier transform infrared (FTIR) spectra were recorded on a Nicolet-6700 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) using the KBr pellet method for chemical bonding analysis, with a scanning range of 400–4000 cm−1, 32 scans, and a resolution of 8 cm−1. X-ray photoelectron spectroscopy (XPS) analysis was performed using an ESCALAB-250Xi spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) to determine the elemental composition chemical state. The microstructure was observed on a field emission scanning electron microscope (FE-SEM, Hitachi S-4800, Tokyo, Japan) and a field emission transmission electron microscope (FE-TEM, Thermo Fisher Scientific, Waltham, MA, USA). The specific surface area was quantitatively determined by nitrogen adsorption–desorption isotherms at 77 K using a Quantachrome Autosorb-iQ instrument (Thermo Fisher Scientific, Waltham, MA, USA), after the sample was degassed at 357 K for 6 h. The optical absorption property was investigated on a UV-3600 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Charge carrier dynamics were probed through both steady-state photoluminescence (PL) and time-resolved PL (TRPL) using a HORIBA Fluorolog-3 spectrofluorometer (Horiba Scientific, Irvine, CA, USA) at ambient temperature, with an excitation wavelength of 391 nm. Electron paramagnetic resonance (EPR) measurements were conducted at room temperature on a JES-FA200 spectrometer (Japan Electron Optics Laboratory Co., Ltd., Tokyo, Japan).
2.3. Photoelectrochemical Measurements
Photoelectrochemical (PEC) properties were measured using an electrochemical workstation (CHI660E, Shanghai Chenhua, Shanghai, China) with a standard three-electrode system. The as-prepared sample served as the working electrode (effective illumination area: ~1 cm2), while a platinum plate counter electrode and an Ag/AgCl reference electrode were immersed in a 0.5 M Na2SO4 aqueous electrolyte. A 300 W xenon lamp was employed as light irradiation source. Mott-Schottky analysis was conducted under a dark condition at three characteristic frequencies (1000, 900, and 800 kHz) with an AC amplitude of 5 mV versus the open circuit potential, and the capacitance–voltage relationships were recorded. Electrochemical impedance spectroscopy (EIS) was carried out at the open circuit potential with a 5 mV AC perturbation over a frequency range of 100 kHz to 1 Hz. Photocurrent response curves (i–t) were acquired under intermittent illumination with periodic light “on/off” pulses (20 s) at an applied bias of 0.6 V vs. RHE.
2.4. Hydrogen Evolution Reaction (HER) Measurements
Photocatalytic hydrogen evolution from water was carried out in a side irradiation Pyrex glass reaction cell of 330 mL connected to a closed circulation system with an inline evacuation system. In a standard procedure, 20 mg catalyst was uniformly dispersed by a magnetic stirrer in 270 mL aqueous solution containing methanol (10 vol%) as the sacrificial agent and H2PtCl6·6H2O (1 wt% Pt loading) as the cocatalyst precursor. Before reaction, the closed gas circulation system and the reaction cell were well evacuated and then introduced into ~2.5 kPa of argon gas. Prior to hydrogen collection, the suspension underwent 30 min of 300 W Xe lamp irradiation (λ ≥ 420 nm) to photodeposit platinum (Pt) nanoparticles as a cocatalyst onto the photocatalyst surface. After the closed gas circulation system and the reaction cell were evacuated and introduced into ~2.5 kPa of argon gas again, the photocatalytic hydrogen evolution reaction was initiated under visible-light irradiation from a 300 W Xe lamp (CEL-HXF300, CEAU-light Co., Ltd., Beijing, China) with a 420 nm long-pass optical filter. Evolved hydrogen was measured on an online gas chromatograph (GC-2014C, Shimadzu Corporation, Kyoto, Japan) equipped with a thermal conductivity detector (TCD) using argon carrier gas.
The apparent quantum yield (AQY) of F0.15K6CN was calculated using the following equation:
where Ne is total number of electrons transferred in the reaction, Np is the total number of incident photons, rH is the hydrogen evolution rate (mol/s), NA is Avogadro’s constant (6.02 × 1023 mol−1), h is Planck’s constant (6.62 × 10−34 J·s), c is the speed of light (3 × 108 m s−1), A is the illumination area (m2), and λ is the incident light wavelength (m).
3. Results and Discussion
3.1. Structure, Morphology, and Optical Properties of Catalysts
The crystal structures of catalysts were analyzed by powder X-ray diffraction (XRD) (Figure 2a and Figure S1). The CN exhibited two typical diffraction peaks at 12.9° and 27.4°, corresponding to the (210) in-planar repeated heptazine units and the (002) interlayer packing structure, respectively [22]. The diffraction peak intensities of K(6)CN and F(0.15)CN were weaker than that of CN and decreased with the increase in doping amount, indicating that the introduction of K and F atoms might alter the growth of the CN crystal planes [23]. Moreover, F(0.15)K(6)CN showed weaker diffraction peaks’ intensity than K(6)CN and F(0.15)CN, signifying a more significant structural transformation after (K, F) co-doping. More importantly, the (002) diffraction peak of K(6)CN and F(0.15)K(6)CN shifted to lower diffraction angles than CN, indicating that the K+ intercalation in the CN interlayer increased the stacking distance between the nanosheets, which was favorable for the rapid interlayer transfer of the photogenerated charge carrier [24]. The functional groups of the obtained samples were examined using Fourier transform infrared (FTIR) spectroscopy (Figure 2b and Figure S2). All samples exhibited similar characteristic peaks at 805, 1127–1684, 3063–3343, and 3435 cm−1, which were ascribed to the bending vibration of the heptazine unit and stretching vibrations of N−C=N, N−H, and O−H, respectively, indicating that K and/or F element doping did not change the basic framework of CN [25]. From the zoomed display in Figure 2b, a new characteristic peak at 2175 cm−1 assigned to the asymmetric stretching vibration of cyano groups (−C≡N) was observed in K(6)CN and F(0.15)K(6)CN. The peak intensity increased with the increase in K doping amount, indicating that K doping led to the opening of the heptazine ring [26]. The strong electron-withdrawing property of −C≡N was conducive to charge separation [27]. In addition, no characteristic peaks related to K or F were detected in K(6)CN, F(0.15)CN, and F(0.15)K(6)CN, due to the too low doping amounts of these elements.
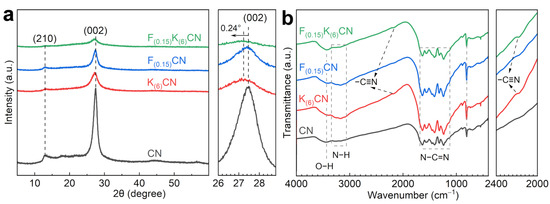
Figure 2.
(a) XRD patterns and (b) FTIR spectra of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN.
The SEM image of pristine CN showed a roughly aggregated nanostructure (Figure 3a). After the introduction of K atoms, K(6)CN maintained the same morphology as CN (Figure 3b). However, F(0.15)CN exhibited a large number of nanosheet structures with a relatively smooth surface (Figure 3c). Interestingly, after (K, F) co-doping, F(0.15)K(6)CN was composed of a roughly aggregated nanostructure and smooth-surfaced nanosheets (Figure 3d). TEM images also showed a similar layered stacking structure for all samples (Figure S3). Specifically, K(6)CN featured a relatively loose and rough surface, F(0.15)CN had a smoother surface, and F(0.15)K(6)CN presented a morphology in between K(6)CN and F(0.15)CN. The specific surface area and pore size of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN were measured by N2 adsorption-desorption isotherms (Figure S4a). All samples exhibited type IV isotherms with H3 hysteresis loops, demonstrating that these nanosheets were mesoporous materials. The specific surface areas of F(0.15)K(6)CN (23.2 m2 g−1), K(6)CN (22.0 m2 g−1), and F(0.15)CN (22.9 m2 g−1) were similar but higher than that of CN (17.9 m2 g−1), consistent with the SEM observations. Furthermore, the number of pore sizes within the range of 3–10 nm in F(0.15)K(6)CN was also similar to those in K(6)CN and F(0.15)CN but higher than that in CN (Figure S4b), indicating that K and/or F doping resulted in the formation of more mesopores. Importantly, the increased BET surface area and mesopores might provide more active sites for improved photocatalytic activity [28].

Figure 3.
SEM images: (a) CN, (b) K(6)CN, (c) F(0.15)CN, and (d) F(0.15)K(6)CN.
The surface compositions and chemical states of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN were investigated by X-ray photoelectron spectroscopy (XPS). As shown in Figure 4a, the C 1s high-resolution XPS spectrum of CN could be deconvoluted into three peaks at binding energies of 284.8, 286.2, and 288.2 eV, corresponding to graphitic carbon (C−C/C=C), C−NHx on the edges of heptazine units, and sp2-hybridized carbon (N=C−N), respectively [29]. In the N 1s high-resolution XPS spectrum of CN (Figure 4b), four distinct peaks appeared at binding energies of 398.7, 399.9, 401.2, and 404.6 eV, which were attributed to sp2-hybridized nitrogen (C=N−C), the amino functional group (N−Hx), tertiary nitrogen (C−N3), and the charging effect, respectively [30,31]. The C 1s and N 1s XPS spectra of both K(6)CN and F(0.15)CN were similar to that of CN. However, the peak positions of N=C−N in the C 1s spectrum and C=N−C in the N 1s spectrum of F(0.15)K(6)CN shifted to a higher binding energy than those of CN. It indicated that (K, F) co-doping was more likely to modify the electronic structure of CN compared to sole doping with K or F, resulting in the generation of more dissociated electrons. For the O 1s high-resolution XPS spectra (Figure S5), all samples exhibited a similar characteristic peak of adsorbed water at 532.4 eV, indicating that the K or F doping did not introduce additional oxygen elements [32]. The K 2p high-resolution XPS spectra of K(6)CN and F(0.15)K(6)CN exhibited two prominent peaks at 293.4 and 295.5 eV (Figure 4c), corresponding to K 2p3/2 and K 2p1/2, respectively [33]. As shown in Figure 4d, a clear peak at 685.9 eV in the F 1s high-resolution XPS spectra was observed for F(0.15)CN and F(0.15)K(6)CN, which was assigned to the coordination of the C−F bond [34]. We need to note that due to the intercalation of K+ into the interlayers of CN, the interlayer spacing was expanded, allowing a large amount of F− with a smaller ionic radius to be incorporated into CN. As a result, F(0.15)K(6)CN exhibited a more intense peak than F(0.15)CN, the latter of which showed a decrease in the signal-to-noise ratio, an increase in baseline noise of the peak, and a “diffuse” peak shape possibly caused by statistical fluctuations. Similar phenomena have been observed in previous reports [35,36]. The above results indicated that K atoms were effectively doped into the CN interlayer, while F atoms were incorporated into the CN framework through the formation of C−F bonds.
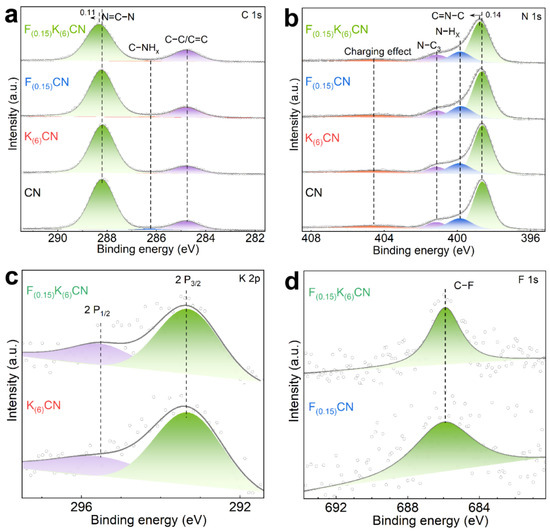
Figure 4.
(a) C 1s and (b) N 1s high-resolution XPS spectra of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN. (c) K 2p high-resolution XPS spectra of K(6)CN and F(0.15)K(6)CN. (d) F 1s high-resolution XPS spectra of F(0.15)CN and F(0.15)K(6)CN.
The effects of K and F co-doping on the optical properties of CN were analyzed using UV–vis diffuse reflectance spectroscopy. As shown in Figure 5a, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN exhibited an increased light capture capability compared to CN over the full wavelength range of 250–800 nm. More importantly, the light absorption capability of F(0.15)K(6)CN was significantly higher than that of K(6)CN or F(0.15)CN, revealing that (K, F) co-doping could more easily optimize the electronic structure of CN, which was consistent with the XPS results. Furthermore, the optical band gaps (Eg) of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN were determined by the Tauc plot from the Kubelka–Munk function conversion to be 2.74, 2.72, 2.70, and 2.66 eV, respectively (Figure 5b). In order to clarify the band structure of samples, the flat band potentials were calculated according to the Mott−Schottky plots (Figure S6). Notably, the obtained positive slopes confirmed that all samples were typical of an n-type semiconductor. Therefore, the conduction band potential (ECB) values of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN were approximately equal to their flat band potentials of −1.11, −1.16, −1.01, and −1.05 eV, respectively [37]. The corresponding valence band potential (EVB) values of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN were calculated using the equation EVB = Eg + ECB to be 1.63, 1.56, 1.69, and 1.61 eV, respectively [38]. On the basis of the above results, the band structures of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN are illustrated in Figure 5c, which satisfied the requirements for photocatalytic water splitting to produce H2.
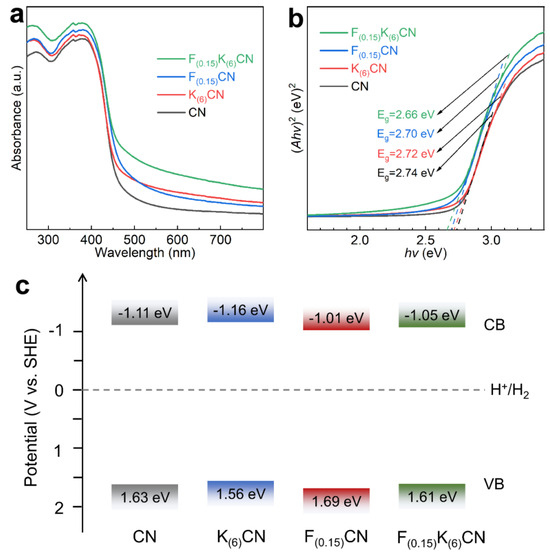
Figure 5.
(a) UV-vis diffuse reflectance spectra, (b) corresponding Tauc plot, and (c) band structure alignments of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN.
3.2. Photocatalytic H2 Evolution Performance
Photocatalytic hydrogen production was carried out using methanol as a hole scavenger under visible-light irradiation (λ ≥ 420 nm). As shown in Figure 6a, the photocatalytic activities of K(y)CN (y = 2, 4, 6, 8) initially increased and then decreased when increasing the K doping concentration, and K(6)CN exhibited the highest H2 production rate. For the (K, F)-co-doped samples F(x)K(6)CN (x = 0.1, 0.125, 0.15, 0.175, 0.2), the optimal F(0.15)K(6)CN exhibited the highest H2 production rate of 3101.5 μmol g−1 h−1 (Figure 6b). In addition, Figure 6c shows that the H2 production rates of K(6)CN (575 μmol g−1 h−1) and F(0.15)CN (680 μmol g−1 h−1) were approximately 2.3 and 2.7 times that of CN (255 μmol g−1 h−1), respectively. Notably, the H2 production rate of F(0.15)K(6)CN was about 12.2 times that of CN, indicating a significant synergistic effect of (K, F) co-doping in enhancing photocatalytic activity. Moreover, the H2 production rate of F(0.15)K(6)CN could be increased from 3101.5 to 15,124 μmol g−1 h−1 under full-spectrum-light irradiation (Figure 6d). It is worth noting that the photocatalytic H2 production performance of F(0.15)K(6)CN surpassed those of most recently reported element-doped CN photocatalysts (Table S1). We also conducted additional photocatalytic activity tests using Cu, Fe, and Ni as the cocatalysts, which showed apparently improved activities but not as good as that when using Pt as the cocatalyst (Figure S7).
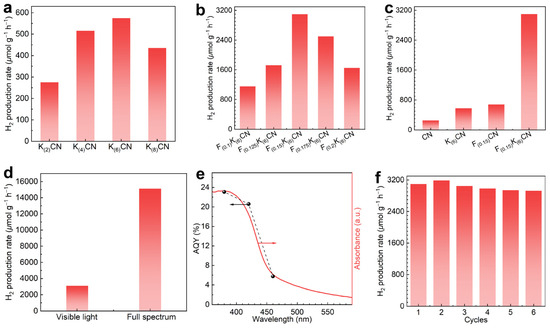
Figure 6.
The H2 production rates over (a) K(y)CN (y = 2, 4, 6, 8), (b) F(x)K(6)CN (x = 0.1, 0.125, 0.15, 0.175, 0.2), and (c) CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN under visible-light irradiation. (d) Comparison of H2 production rates over F(0.15)K(6)CN under visible-light and full-spectrum-light irradiation. (e) The wavelength-dependent AQY of F(0.15)K(6)CN for photocatalytic H2 production. (f) The cycling experiment of F(0.15)K(6)CN.
The apparent quantum yields (AQYs) of F(0.15)K(6)CN for photocatalytic H2 production were measured under various forms of monochromatic light irradiation. As shown in Figure 6e, the AQY values at wavelengths of 380, 420, and 460 nm were determined to be 23.1, 20.6, and 5.8%, respectively, consistent with the absorption spectrum. This result strongly suggested the nature of photocatalytic H2 production over F(0.15)K(6)CN. The stability of the photocatalytic H2 production of F(0.15)K(6)CN was evaluated through six cycling tests. As shown in Figure 6f, the H2 production rate of F(0.15)K(6)CN exhibited no significant decline, indicating its excellent stability in photocatalytic activity. In addition, the robust structural stability of F(0.15)K(6)CN was confirmed through the nearly unchanged XRD patterns (Figure S8a) and FTIR spectra (Figure S8b) before and after the cycling tests.
3.3. Mechanistic Investigation of the Enhanced Photocatalytic H2 Production over F(0.15)K(6)CN
In order to obtain evidence of the electronic structural changes caused by (K, F) co-doping, the electron paramagnetic resonance (EPR) spectra were measured to detect the spin state of unpaired electrons (Figure S9). All samples displayed a single Lorentzian line with an essentially same g value of 2.004, corresponding to unpaired electrons from the sp2-carbon atoms of π-conjugated aromatic rings [39]. Clearly, the EPR signal intensity of F(0.15)K(6)CN was significantly higher than those of CN, K(6)CN, and F(0.15)CN, indicating that F(0.15)K(6)CN had a higher concentration of unpaired electrons. This was probably because the insertion of K atoms and the formation of C−F bonds altered the symmetrical electronic structure of the catalyst, which was beneficial for the separation and migration of photogenerated charge carriers.
To investigate the separation and transfer behaviors of charge carriers in photocatalysts, steady-state and time-resolved PL spectra were measured. As shown in Figure 7a, the PL emission peak at approximately 465 nm for CN originated from the radiative recombination during the decay of excited electrons in heptazine rings [40]. The PL emission peak positions of F(0.15)CN and F(0.15)K(6)CN were red shifted relative to that of CN, in accordance with the narrowed band gap. In addition, the PL intensity of K(6)CN and F(0.15)CN was much lower than that of CN, indicating that K or F doping could act as a trapping site to suppress the recombination of photogenerated electron-hole pairs [41]. With (K, F) co-doping, the PL intensity of F(0.15)K(6)CN was further reduced, indicating that the introduction of K and F dual sites significantly increased the separation efficiency of a photogenerated charge carrier. Meanwhile, fitting the time-resolved PL spectra revealed that F(0.15)K(6)CN exhibited a shorter average radiative lifetime (τa, 2.93 ns) than CN (3.71 ns), K(6)CN (3.70 ns), and F(0.15)CN (3.14 ns), indicating enhanced photoexciton dissociation and nonradiative energy transfer efficiency due to (K, F) co-doping (Figure 7b).
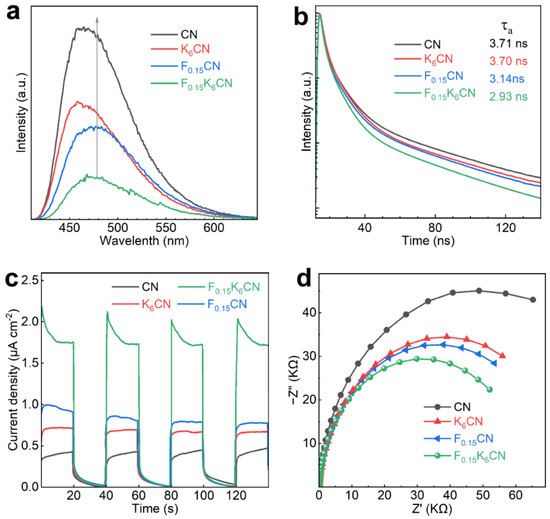
Figure 7.
(a) Steady-state PL spectra, (b) time-resolved PL spectra, (c) photocurrent response curves, and (d) EIS Nyquist plots of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN.
The separation and transfer ability of photogenerated charge carriers was further characterized by photoelectrochemical tests. Figure 7c shows the photocurrent response curves of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN under on/off visible-light illumination (λ ≥ 420 nm). Clearly, F0.15K6CN exhibited the highest photocurrent density (1.72 μA cm−2) compared to CN (0.43 μA cm−2), K(6)CN (0.67 μA cm−2), and F(0.15)CN (0.79 μA cm−2), indicating that F(0.15)K(6)CN had an efficient photogenerated electron-hole pair separation ability. Moreover, the electrochemical impedance spectroscopy (EIS) spectra of the samples were measured. As shown in Figure 7d, the EIS Nyquist plot arc radius of F(0.15)K(6)CN was smaller than those of CN, K(6)CN, and F(0.15)CN, suggesting that (K, F) co-doping reduced the charge transfer resistance at the photocatalyst interface, which benefited the migration of charge carriers [42]. The above results proved that (K, F) co-doping effectively accelerated the charge separation efficiency and transport rate, thereby enhancing the photocatalytic H2 production activity of F(0.15)K(6)CN.
4. Conclusions
In summary, we successfully developed a (F, K)-co-doped carbon nitride (FKCN) catalyst via thermal polymerization of KOH-assisted hydrothermally modified melamine and NH4F. Characterizations revealed that K doping intercalated into CN interlayers, facilitating rapid charge carrier transport and generating cyano groups as additional active sites, while F doping formed C−F bonds, modulating the electronic structure to significantly enhance photogenerated charge separation. These synergistic effects from (F, K) doping resulted in an optimized band structure and expanded visible-light absorption. The optimal F(0.15)K(6)CN photocatalyst achieved a high hydrogen evolution rate of 3101.5 μmol g−1 h−1, which surpassed most of reported element-doped CN photocatalysts. This work demonstrates that multi-element doping is a viable strategy to design high-performance CN-based photocatalysts for sustainable hydrogen production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15131021/s1, Figure S1 XRD patterns of (a) CN and K(y)CN (y = 2, 4, 6, 8); (b) CN and F(x)K(6)CN (x = 0.1, 0.125, 0.15, 0.175, 0.2); Figure S2 FTIR spectra of (a) CN and K(y)CN (y = 2, 4, 6, 8); (b) CN and F(x)K(6)CN (x = 0.1, 0.125, 0.15, 0.175, 0.2); Figure S3 TEM images of (a) CN, (b) K(6)CN, (c) F(0.15)CN, and (d) F(0.15)K(6)CN; Figure S4 (a) N2 sorption isotherms and (b) pore size (D) distribution curves of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN; Figure S5 High-resolution O 1s XPS spectra of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN; Figure S6 Mott–Schottky plots of (a) CN, (b) K(6)CN, (c) F(0.15)CN, and (d) F(0.15)K(6)CN; Figure S7 Photocatalytic hydrogen production activities of F(0.15)K(6)CN without cocatalyst loading, and loaded with 1 wt% of Cu, Fe, and Ni; Figure S8 (a) XRD patterns and (b) FTIR spectra of F(0.15)K(6)CN before and after the cyclic experiments; Figure S9 EPR spectra of CN, K(6)CN, F(0.15)CN, and F(0.15)K(6)CN; Table S1 Photocatalytic H2 production performance comparison between F(0.15)K(6)CN and other reported element-doped CN photocatalysts. Refs. [17,18,19,20,21,43,44,45,46,47,48,49,50,51] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, F.B., G.B., and D.W.; methodology and investigation, F.B., G.B., J.Y. (Junbo Yu), H.H., and D.W.; data curation, F.B.; writing—original draft preparation, F.B. and G.B.; writing—review and editing, D.W.; supervision, D.W.; funding acquisition and resources, H.H., J.Y. (Jinhua Ye), and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the National Natural Science Foundation of China (51572191).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kuspanov, Z.; Bakbolat, B.; Baimenov, A.; Issadykov, A.; Yeleuov, M.; Daulbayev, C. Photocatalysts for a sustainable future: Innovations in large-scale environmental and energy applications. Sci. Total Environ. 2023, 885, 163914. [Google Scholar] [CrossRef] [PubMed]
- Falope, T.; Lao, L.; Hanak, D.; Huo, D. Hybrid energy system integration and management for solar energy: A review. Energ. Convers. Manag. X 2024, 21, 100527. [Google Scholar] [CrossRef]
- Gunawan, D.; Zhang, J.; Li, Q.; Toe, C.Y.; Scott, J.; Antonietti, M.; Guo, J.; Amal, R. Materials Advances in Photocatalytic Solar Hydrogen Production: Integrating Systems and Economics for a Sustainable Future. Adv. Mater. 2024, 36, e2404618. [Google Scholar] [CrossRef] [PubMed]
- Kyriakos, P.; Hristoforou, E.; Belessiotis, G.V. Graphitic Carbon Nitride (g-C3N4) in Photocatalytic Hydrogen Production: Critical Overview and Recent Advances. Energies 2024, 17, 3159. [Google Scholar] [CrossRef]
- Ohnishi, A.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Extension of π-conjugated aromatic structure in g-C3N4 nanosheets and their applications into bisphenol E decomposition. J. Solid State Electrochem. 2024, 28, 4527–4549. [Google Scholar] [CrossRef]
- Mengesha, D.N.; Shiferraw, B.T.; Kim, H. Modification of the electronic structure of g-C3N4 using urea to enhance the visible light-assisted degradation of organic pollutants. Environ. Sci. Pollut. Res. Int. 2023, 30, 102910–102926. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Su, L.; Zhao, J. π-π stacking derived from graphene-like biochar/g-C3N4 with tunable band structure for photocatalytic antibiotics degradation via peroxymonosulfate activation. J. Hazard. Mater. 2022, 423, 126944. [Google Scholar] [CrossRef]
- Saman, F.; Se Ling, C.H.; Ayub, A.; Rafeny, N.H.B.; Mahadi, A.H.; Subagyo, R.; Nugraha, R.E.; Prasetyoko, D.; Bahruji, H. Review on synthesis and modification of g-C3N4 for photocatalytic H2 production. Int. J. Hydrogen Energy 2024, 77, 1090–1116. [Google Scholar] [CrossRef]
- Phoon, B.L.; Yang, T.C.K.; Leo, B.F.; Lai, C.W.; Phang, S.W.; Juan, J.C. Mesoporous semi-ionic F-doped g-C3N4 as efficient photocatalyst for tetracycline removal under visible light. Environ. Technol. Innov. 2023, 32, 103303. [Google Scholar] [CrossRef]
- Yue, J.; Yang, H.; Zhou, L.; Liu, C.; Wang, S.; Kang, X. At Least Five: Benefit Origins of Potassium and Sodium Co-Doping on Carbon Nitride for Integrating Pharmaceuticals Degradation and Hydrogen Peroxide Production. Appl. Catal. B Environ. Energy 2025, 361, 124599. [Google Scholar] [CrossRef]
- Peng, D.; Mao, L.; Sun, J.; Li, X.; Shi, H.; Su, Z. S-Scheme Graphitic Carbon Nitride/Nickel Titanate (G-C3N4/NiTiO3) Heterojunction as Bifunctional Photocatalysts for Hydrogen Production and Pollutants Degradation. Int. J. Hydrogen Energy 2025, 114, 60–70. [Google Scholar] [CrossRef]
- Huang, M.; Xu, L.; Jiang, M.; Wang, B. Graphitic Carbon Nitride Modified with 1, 2, 3-Tribromopropane for Visible-Light-Driven Photocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2025, 103, 624–632. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Yang, Q.; Zhang, Z.; Fang, X. Mesoporous G-C3N4 Nanosheets Prepared by Calcining a Novel Supramolecular Precursor for High-Efficiency Photocatalytic Hydrogen Evolution. Appl. Surf. Sci. 2018, 450, 46–56. [Google Scholar] [CrossRef]
- Tai, M.; Che, G.; Zhou, T.; Teng, H.; Liu, C.; Hu, B. Tailoring C-Defect O-Doping and N-Π* Transition Awakened Porous Ultra-Thin Carbon Nitride for Efficient Peroxymonosulfate Activation: Performances and Mechanism Insight. J. Environ. Sci. 2025, 152, 353–367. [Google Scholar] [CrossRef]
- Quan, Y.; Li, R.; Li, X.; Chen, R.; Ng, Y.H.; Huang, J.; Hu, J.; Lai, Y. S-Modified Graphitic Carbon Nitride with Double Defect Sites for Efficient Photocatalytic Hydrogen Evolution. Small 2024, 20, 2406576. [Google Scholar] [CrossRef]
- Li, Y.; Shi, L.; Mao, Y.; Zhang, Y.; Wang, H. Efficient Reduction of Uranyl under Aerobic Conditions by Sodium and Potassium Co-Doped Carbon Nitride. Chem. Eng. J. 2022, 446, 136872. [Google Scholar] [CrossRef]
- Sun, S.; Li, J.; Cui, J.; Gou, X.; Yang, Q.; Jiang, Y.; Liang, S.; Yang, Z. Simultaneously Engineering K-Doping and Exfoliation into Graphitic Carbon Nitride (G-C3N4) for Enhanced Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 778–787. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Zhang, Y.; Fang, J.; Zhou, Y.; Yuan, S.; Zhang, C.; Chen, W. One-Pot Synthesis of K-Doped G-C3N4 Nanosheets with Enhanced Photocatalytic Hydrogen Production under Visible-Light Irradiation. Appl. Surf. Sci. 2018, 440, 258–265. [Google Scholar] [CrossRef]
- Chang, X.; Fan, H.; Zhu, S.; Lei, L.; Wu, X.; Feng, C.; Wang, W.; Ma, L. Engineering Doping and Defect in Graphitic Carbon Nitride by One-Pot Method for Enhanced Photocatalytic Hydrogen Evolution. Ceram. Int. 2023, 49, 6729–6738. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Q.; Li, Z.; Zhang, Z.; Fang, X. Enhanced Photocatalytic Hydrogen Evolution Performance of Mesoporous Graphitic Carbon Nitride Co-Doped with Potassium and Iodine. Appl. Catal. B Environ. 2018, 221, 362–370. [Google Scholar] [CrossRef]
- Bi, J.; Zhu, L.; Wu, J.; Xu, Y.; Wang, Z.; Zhang, X.; Han, Y. Optimizing Electronic Structure and Charge Transport of Sulfur/Potassium Co-Doped Graphitic Carbon Nitride with Efficient Photocatalytic Hydrogen Evolution Performance. Appl. Organomet. Chem. 2019, 33, e5163. [Google Scholar] [CrossRef]
- Wu, C.J.; He, S.C.; Kuo, T.C.; Wu, J.J. Fluid Mechanical and Visible-Light-Driven Piezophotocatalysis in MoS2/Carbon-Rich Carbon Nitride Heterostructures for Enhanced Green Energy Production and Environmental Remediation. ACS Appl. Mater. Interfaces 2025, 17, 15544. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, J.; Hong, M.; Sun, R. Potassium and Sulfur Dual Sites on Highly Crystalline Carbon Nitride for Photocatalytic Biorefinery and CO2 Reduction. ACS Catal. 2023, 13, 2106–2117. [Google Scholar] [CrossRef]
- Xiong, T.; Cen, W.; Zhang, Y.; Dong, F. Bridging the G-C3N4 Interlayers for Enhanced Photocatalysis. ACS Catal. 2016, 6, 2462–2472. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, J.; Chen, Q.; Luo, H.; Chen, J.; Wang, R. Enhanced Directional Transfer of Charge Carriers and Optimized Electronic Structure in Fluorine Doped Polymeric Carbon Nitride Nanosheets for Efficient Photocatalytic Water Splitting. Nanoscale 2025, 17, 6004–6016. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, A.; Cheng, R.; Chen, F.; Kannan, P.; Molochas, C.; Tsiakaras, P. Bi, K Co-Doped Graphitic Phase Carbon Nitride for Efficient Photocatalytic H2O2 Production. Chem. Eng. J. 2024, 489, 151145. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Zhang, S.; Liu, Y.; Wang, G.; Sun, C.; Zhao, H. Potassium-Ion-Assisted Regeneration of Active Cyano Groups in Carbon Nitride Nanoribbons: Visible-Light-Driven Photocatalytic Nitrogen Reduction. Angew. Chem. Int. Ed. 2019, 58, 16644–16650. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Pei, J.; Zhou, X.; Chen, S.; Sun, F. In Situ Analysis Photogenerated Electron Transport Behavior of C Self-Doped Carbon Nitride for Photocatalytic H2 Production. ACS Catal. 2024, 14, 12093–12101. [Google Scholar] [CrossRef]
- Cruz, D.; Żółtowska, S.; Savateev, O.; Antonietti, M.; Giusto, P. Carbon Nitride Caught in the Act of Artificial Photosynthesis. Nat. Commun. 2025, 16, 374. [Google Scholar] [CrossRef]
- Huang, J.; Klahn, M.; Tian, X.; Dai, X.; Rabeah, J.; Aladin, V.; Corzilius, B.; Bartling, S.; Lund, H.; Steinfeldt, N. Exfoliated Polymeric Carbon Nitride Nanosheets for Photocatalytic Applications. ACS Appl. Nano Mater. 2024, 7, 7442–7452. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Shi, W.X.; Zhuang, G.L.; Zhao, Q.P.; Ren, J.; Zhang, P.; Yin, H.Q.; Lu, T.B.; Zhang, Z.M. W Single-Atom Catalyst for CH4 Photooxidation in Water Vapor. Adv. Mater. 2022, 34, 2204448. [Google Scholar] [CrossRef]
- Huang, G.; Xiao, B.; Bao, L.; Wang, D.; Luo, Y.; Yan, S.; Gao, H. Crystalline Oxygen-Bridged Carbon Nitride from Self-Assembled Supramolecular Intermediate for Efficient Photocatalytic H2 Evolution. J. Mater. Chem. A 2024, 12, 3480–3488. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, J.; Wang, A.; Kannan, P.; Jing, S.; Chen, F.; Tsiakaras, P. Synthesis of Novel Nanoflowers-Like P, K Co-Doped Graphitic Carbon Nitride for Efficient H2O2 Photoproduction. J. Colloid Interface Sci. 2025, 677, 729–739. [Google Scholar] [CrossRef]
- Yang, D.; Ye, Q.; Qu, C.; Meng, F.; Wang, L.; Li, Y. Visible-Light-Driven F/C Co-Doping G-C3N4 Nanosheets for Efficient Hydrogen Evolution: Charge Redistribution on C4 Delocalized Large Π Bond. Appl. Catal. B Environ. Energy 2025, 361, 124637. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Y.; Feng, F.; Wang, W.; Shi, W.; Zhang, W.; Li, Y.; Lou, H.; Cui, C. A Recyclable Molten-Salt Synthesis of B and K Co-doped g-C3N4 for Photocatalysis of Overall Water Vapor Splitting. Appl. Surf. Sci. 2021, 537, 148014. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Hao, H.; He, H.; Cai, H.; Shang, F.; An, B.; Li, X.; Yang, S. The Construction of Phosphorus-Doped g-C3N4/Rh-Doped SrTiO3 with Type-II Band Alignment for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 4428. [Google Scholar] [CrossRef]
- Ren, M.; Meng, J.; Yang, Y.; Zhang, X.; Yang, G.; Qin, L.; Guo, Y. Synergy between Palladium Single Atoms and Small Nanoparticles Co-Anchored on Carbon Atom Self-Doped Graphitic Carbon Nitride Boosting Photocatalytic H2 Generation. Appl. Catal. B Environ. Energy 2024, 345, 123680. [Google Scholar] [CrossRef]
- Cao, S.; Yang, H.; Zeng, F.; Lu, Y.; Chen, H.; Jiang, F. Self-Assembly Synthesis of Oxygen and Sulfur Co-Doped Porous Graphitic Carbon Nitride Nanosheets for Boosting CO2 Photoreduction. ChemSusChem 2025, 18, e202401570. [Google Scholar] [CrossRef]
- You, Q.; Zhang, C.; Cao, M.; Wang, B.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Defects Controlling, Elements Doping, and Crystallinity Improving Triple-Strategy Modified Carbon Nitride for Efficient Photocatalytic Diclofenac Degradation and H2O2 Production. Appl. Catal. B Environ. 2023, 321, 121941. [Google Scholar] [CrossRef]
- Huang, Q.-S.; Li, Q.; Chu, C.; Liu, Q.; Li, Z.; Mao, S. Synergetic Regulation of Electronic Structure of Graphitic Carbon Nitride through Phosphorus and Carbon Co-Doping for Enhanced Photocatalytic CO2 Reduction. Chem. Eng. J. 2024, 482, 149155. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, D.; Li, J.; Wang, X.; Zhu, J.; Francis, P.S.; Zheng, Y. Sulfur and Potassium Co-Doped Graphitic Carbon Nitride for Highly Enhanced Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2020, 273, 119050. [Google Scholar] [CrossRef]
- Zandipak, R.; Bahramifar, N.; Torabi, M.; Calero, M.; Muñoz-Batista, M.J.; Solís, R.R. Synergistic Effect of Graphitic-Like Carbon Nitride and Sulfur-Based Thiazole-Linked Organic Polymer Heterostructures for Boosting the Photocatalytic Degradation of Pharmaceuticals in Water. Chem. Eng. J. 2024, 494, 152843. [Google Scholar] [CrossRef]
- Xia, X.; Xie, C.; Xu, B.; Ji, X.; Gao, G.; Yang, P. Role of B-doping in g-C3N4 nanosheets for enhanced photocatalytic NO removal and H2 generation. J. Ind. Eng. Chem. 2022, 105, 303–312. [Google Scholar] [CrossRef]
- Miao, Z.; Xu, F.; Zhao, B.; Song, Y.; Sun, P.; Wu, G.; Xu, K.; Yan, P.; Mo, Z.; Xu, H. P-doped and cyano-modified carbon nitride nanotubes for photocatalytic hydrogen evolution coupled with bisphenol A degradation. J. Colloid Interface Sci. 2025, 686, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Khan, M.Q.; Alsalme, A.; Kim, H. Sulfur-doped graphitic-carbon nitride (S@g-C3N4) as bi-functional catalysts for hydrazine sensing and hydrogen production applications. Synth. Met. 2022, 288, 117100. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Z.; Chen, Y.; Huang, H.; Hu, J.; Wen, B. In situ synthesis of 2D ultrathin cobalt doped g-C3N4 nanosheets enhances photocatalytic performance by accelerating charge transfer. J. Alloys Compd. 2021, 859, 157754. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Huang, Z.; Guan, X.; Zong, S.; Cheng, C.; Zheng, B.; Guo, L. Synchronous construction of CoS2 in-situ loading and S doping for g-C3N4: Enhanced photocatalytic H2-evolution activity and mechanism insight. Chem. Eng. J. 2020, 401, 126135. [Google Scholar] [CrossRef]
- Cao, J.; Jin, X.; Ma, Z.; Wang, H.; Xu, Y.; Guo, Y.; Xie, H.; Zhang, J. One-step synthesis of C quantum dots/C doped g-C3N4 photocatalysts for visible-light-driven H2 production from water splitting. J. Phys. D Appl. Phys. 2022, 55, 444008. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Hasan, I. Improved photocatalytic hydrogen evolution using sulfur-doped graphite-like carbon nitride (S-G-C3N4) photocatalyst. ChemistrySelect 2023, 8, e202302369. [Google Scholar] [CrossRef]
- Liu, R.-Y.; Ding, L.; Yang, G.-D.; Zhang, J.-Y.; Jiao, R.; Sun, H.-Z. Hollow Mo2C nanospheres modified B-doped g-C3N4 for high efficient photocatalysts. J. Phys. D Appl. Phys. 2022, 55, 454001. [Google Scholar] [CrossRef]
- Liu, F.; Li, W.; Wang, L.; Rao, X.; Zheng, S.; Zhang, Y. Sulfur-and strontium-doped graphitic carbon nitride for efficient photocatalytic hydrogen evolution. ACS Appl. Energy Mater. 2022, 5, 15834–15843. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).