Silver Nanoparticles-Decorated Porous Silicon Microcavity as a High-Performance SERS Substrate for Ultrasensitive Detection of Trace-Level Molecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Porous Silicon Substrates
2.3. Fabrication of SERS Substrates
2.4. Characterizations and SERS Measurement
3. Results and Discussion
3.1. Structure and Morphological Characteristics
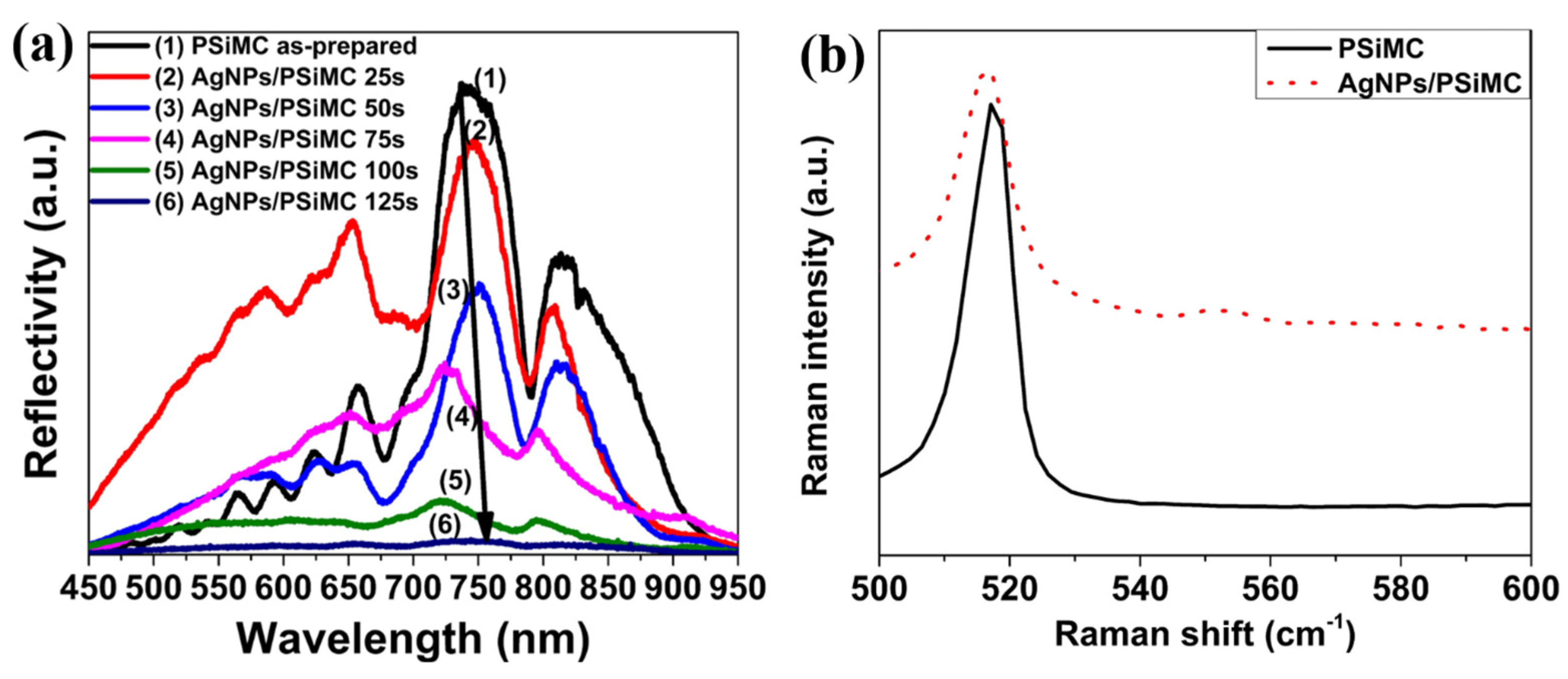
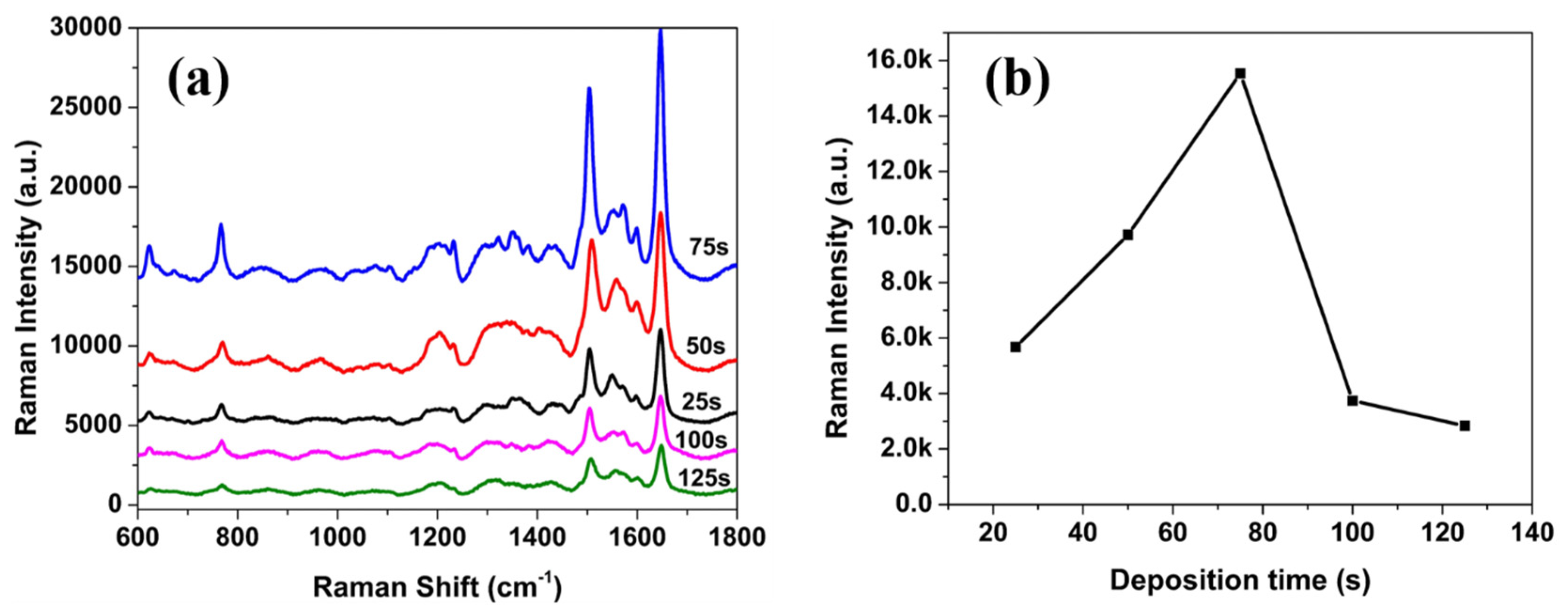
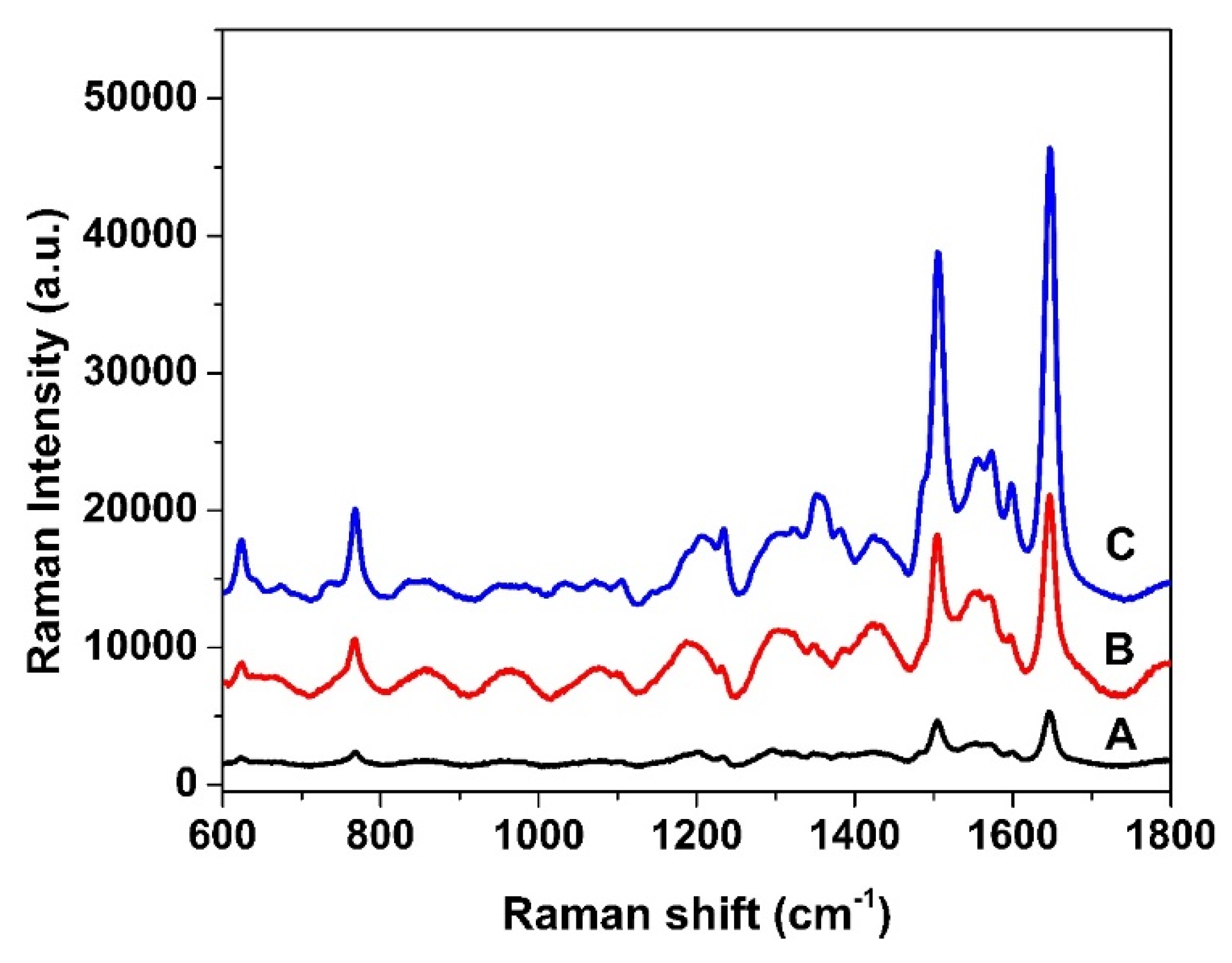
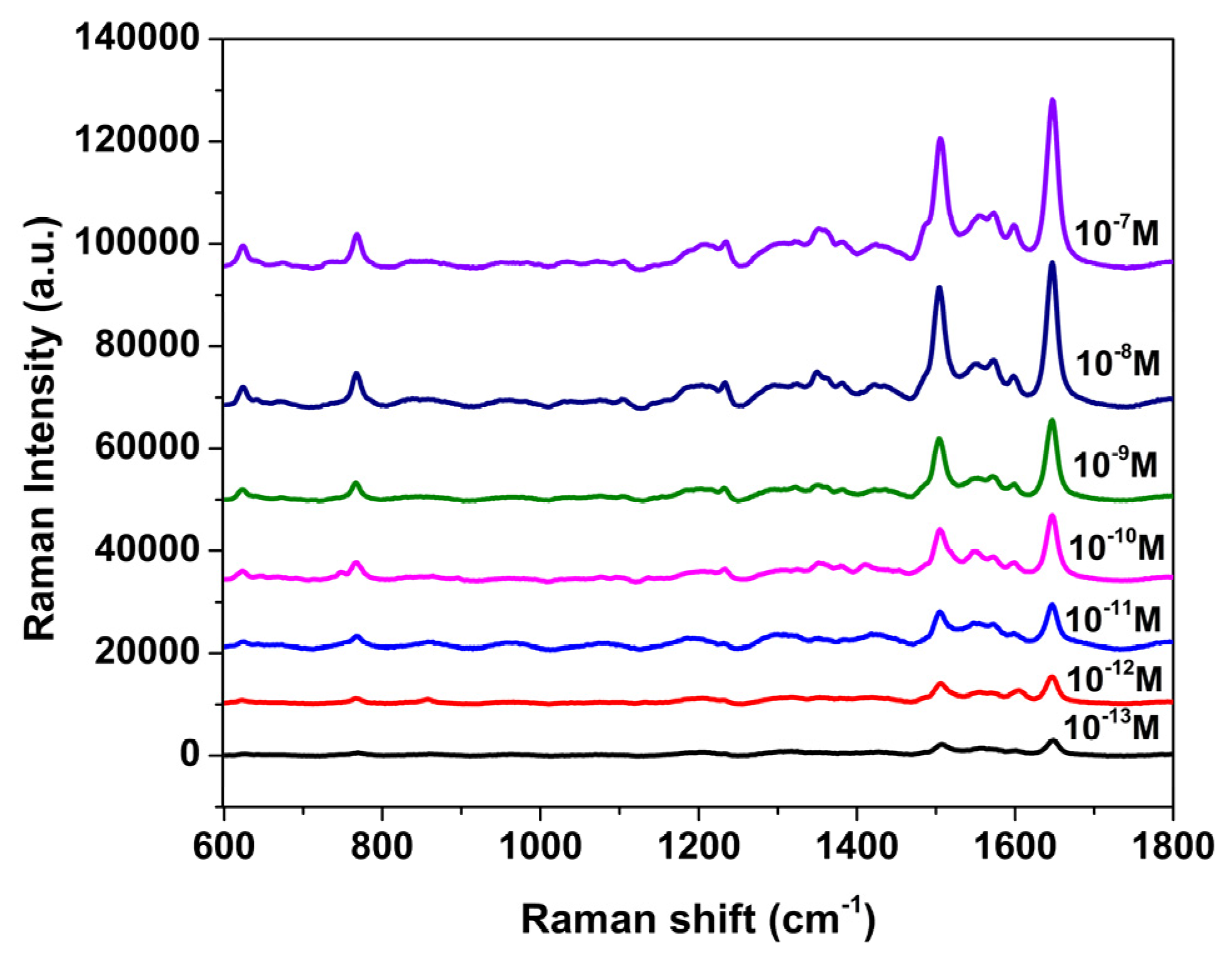
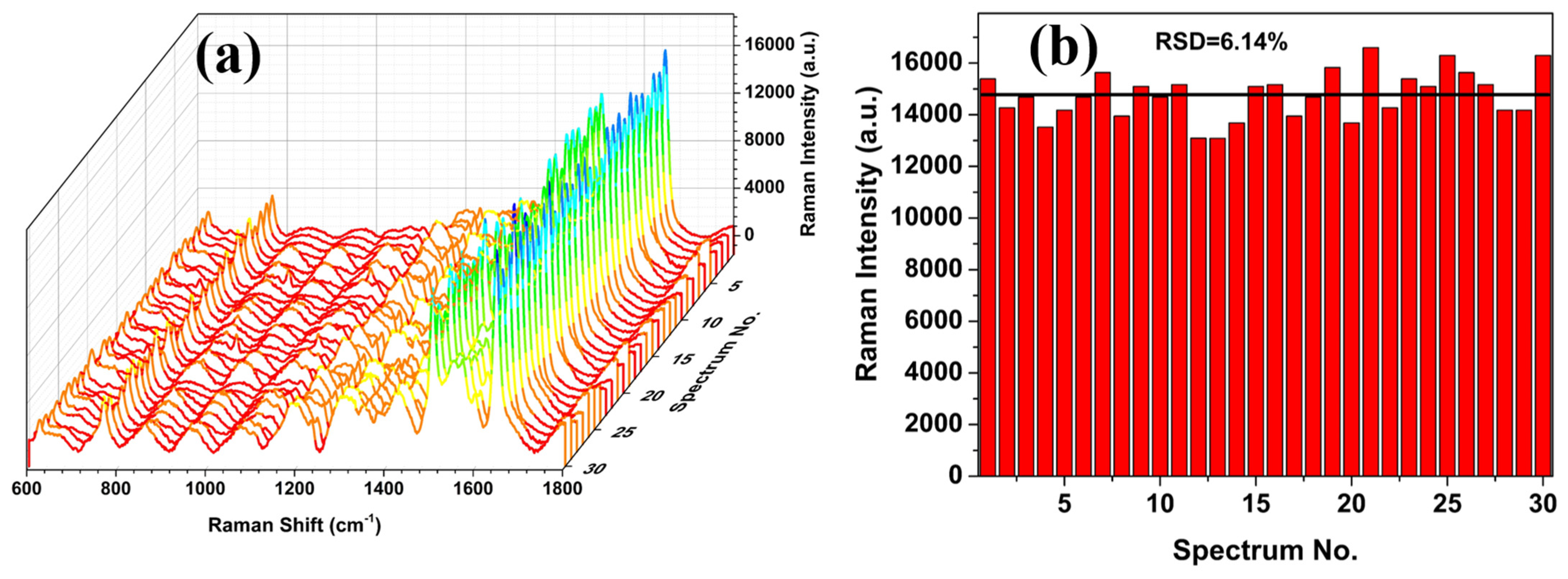
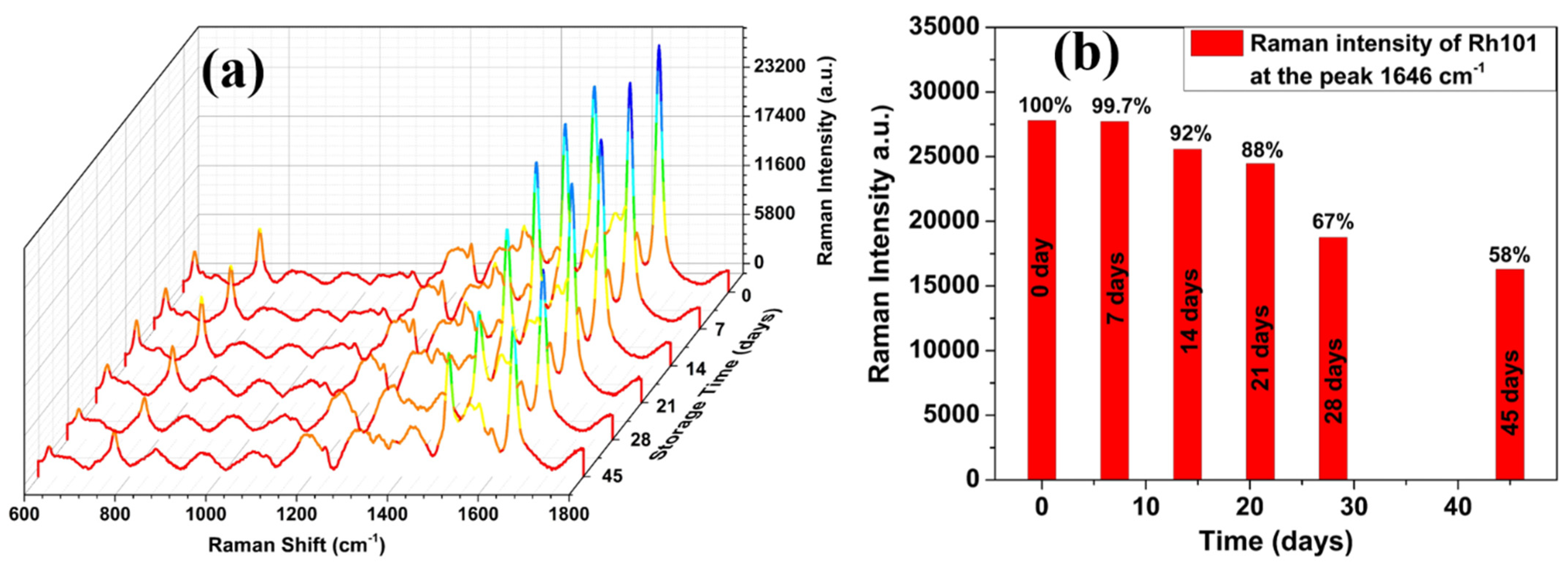
3.2. SERS Evaluation of AgNP-Coated PSiMC Active Substrates
3.3. Detection of Methyl Parathion on SERS Active Substrate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suleimenova, A.; Frasco, M.F.; Sales, M.G.F. An ultrasensitive paper-based SERS sensor for detection of nucleolin using silver-nanostars, plastic antibodies and natural antibodies. Talanta 2024, 279, 126543. [Google Scholar] [CrossRef] [PubMed]
- Serebrennikova, K.V.N.; Komova, N.S.; Zherdev, A.V.; Dzantiev, B.B. SERS Sensors with Bio-Derived Substrates Under the Way to Agricultural Monitoring of Pesticide Residues. Biosensors 2024, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Qi, X.; Yu, H.; Li, Y. Surface-enhanced Raman spectroscopy in pharmaceutical analysis: From component determination to mechanism research. Microchim. Acta 2025, 192, 211. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.; Dzhagan, V.; Kapush, O.; Isaieva, O.; Demydov, P.; Lytvyn, V.; Chegel, V.; Kuklaa, O.; Yukhymchuka, V. SERS of nitro group compounds for sensing of explosives. RSC Adv. 2025, 15, 252–260. [Google Scholar] [CrossRef]
- Mahadev, A.P.; Kavitha, C.; Perutil, J.R.; John, N.S. Synergistic SERS Enhancement of Highly Sensitive and Reusable rGO-Ag-ZrO2 Thin Film Nanocomposite to Detect Synthetic Textile Dyes. Langmuir 2025, 41, 2411–2426. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Hsu, Y.-K.; Chen, M.-H.; Chuang, C.-J.; Chen, Y.-C.; Lin, Y.-G. Silver-decorated hierarchical cuprous oxide micro/nanospheres as highly effective surface-enhanced Raman scattering substrates. Opt. Express 2014, 22, 14617–14624. [Google Scholar] [CrossRef][Green Version]
- Segervald, J.; Malyshev, D.; Öberg, R.; Zäll, E.; Jia, X.; Wågberg, T.; Andersson, M. Ultra-Sensitive Detection of Bacterial Spores via SERS. ACS Sens. 2025, 10, 1237–1248. [Google Scholar] [CrossRef]
- Park, M.; Kim, Y.-S.; Kim, S.; Lim, J.Y. Ultra-sensitive, on-site pesticide detection for environmental and food safety monitoring using flexible cellulose nano fiber/Au nanorod@Ag SERS sensor. J. Hazard. Mater. 2025, 487, 137197. [Google Scholar] [CrossRef]
- Kang, R.H.; Baek, S.W.; Oh, C.-K.; Kim, Y.H.; Kim, D. Recent Advances of Macrostructural Porous Silicon for Biomedical Applications. ACS Appl. Mater. Interfaces 2025, 17, 5609–5626. [Google Scholar] [CrossRef]
- Mebed, A.M.; Abd-Elnaiem, A.M.; Malsche, W.D. Influence of Anodizing Parameters on the Electrochemical Characteristics and Morphology of Highly Doped P-type Porous Silicon. Silicon 2021, 13, 819–829. [Google Scholar] [CrossRef]
- Pyatilova, O.V.; Gavrilov, S.A.; Shilyaeva, Y.I.; Pavlov, A.A.; Shaman, Y.P.; Dudin, A.A. Influence of the doping type and level on the morphology of porous Si formed by galvanic etching. Semiconductors 2017, 51, 173–177. [Google Scholar] [CrossRef]
- Ghaderi, A.; Sabbaghzadeh, J.; Dejam, L.; Pour, G.B.; Moghimi, E.; Matos, R.S.; Filho, H.D.D.F.; Țălu, Ș.; Shayegan, A.S.; Aval, L.F.; et al. Nanoscale morphology, optical dynamics and gas sensor of porous silicon. Sci. Rep. 2024, 14, 3677. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, R.; Sharma, P.; Voelcker, N.H. Advancements in Porous Silicon Biosensors for Point of Care, Wearable, and Implantable Applications. ACS Appl. Mater. Interfaces 2025, 17, 2814–2843. [Google Scholar] [CrossRef]
- Lujan-Cabrera, I.A.; Anaya, E.K.R.; Sierra, J.A.R.; De Paz, J.P.Z.; Isaza, C.; Ramirez-Gutierrez, C.F. Thermal Stability and Optical Behavior of Porous Silicon and Porous Quartz Photonic Crystals for High-Temperature Applications. Photonics 2025, 12, 94. [Google Scholar] [CrossRef]
- Sampath, S.; Maydannik, P.; Ivanova, T.; Shestakova, M.; Homola, T.; Bryukvin, A.; Sillanpää, M.; Nagumothu, R.; Alagan, V. Efficient solar photocatalytic activity of TiO2 coated nano-porous silicon by atomic layer deposition. Superlattices Microstruct. 2016, 97, 155–166. [Google Scholar] [CrossRef]
- Tsao, C.-W.; Zheng, Y.-S.; Sun, Y.-S.; Cheng, Y.-C. Surface-enhanced Raman scattering (SERS) spectroscopy on localized silver nanoparticle-decorated porous silicon substrate. Analyst 2021, 146, 7645–7652. [Google Scholar] [CrossRef]
- Kumar, K.; Shafeeq, M.M.; Kumar, P.; Munjal, R.; Mukhopadhyay, S.; Mondal, D.P.; Khan, M.A.; Vandana, V. Detection of water pollutants using super-hydrophobic porous silicon-based SERS substrates. Microchim. Acta 2024, 191, 357. [Google Scholar] [CrossRef]
- Novara, C.; Marta, S.D.; Virga, A.; Lamberti, A.; Angelini, A.; Chiadò, A.; Rivolo, P.; Geobaldo, F.; Sergo, V.; Bonifacio, A.; et al. SERS-Active Ag Nanoparticles on Porous Silicon and PDMS Substrates: A Comparative Study of Uniformity and Raman Efficiency. J. Phys. Chem. C 2016, 120, 16946–16953. [Google Scholar] [CrossRef]
- Ismail, N.F.; Lee, K.Y.; Mohd, N.S.H.; Ismail, L.N.; Rahim, A.F.A.; Abdullah, M.H.; Radzol, A.R.M. Nano Silver-Coated Porous Silicon-Based Surface-Enhanced Raman Spectroscopy Substrate for Low-Concentration Dengue NS1 Protein Detection. J. Appl. Spectrosc. 2024, 91, 480–488. [Google Scholar] [CrossRef]
- Mariani, S.; Paghi, A.; Mattina, A.A.L.; Debrassi, A.; Dähne, L.; Barillaro, G. Decoration of Porous Silicon with Gold Nanoparticles via Layer-by-Layer Nanoassembly for Interferometric and Hybrid Photonic/Plasmonic (Bio)sensing. ACS Appl. Mater. Interfaces 2019, 11, 43731–43740. [Google Scholar] [CrossRef]
- Al-Syadi, A.M.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsaiari, M. Immersion-plated palladium nanoparticles onto meso-porous silicon layer as novel SERS substrate for sensitive detection of imidacloprid pesticide. Sci. Rep. 2021, 11, 9174. [Google Scholar] [CrossRef] [PubMed]
- Al-Syadi, A.M.; Faisal, M.; El-Toni, A.M.; Khan, A.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A. Surface-enhanced Raman scattering (SERS) active substrate from gold nanoparticle-coated porous silicon for sensitive detection of horseradish peroxidase enzyme. Mater. Chem. Phys. 2022, 281, 125931. [Google Scholar] [CrossRef]
- Lorenzo, E.; Oton, C.J.; Capuj, N.E.; Ghulinyan, M.; Navarro-Urrios, D.; Gaburro, Z.; Pavesi, L. Porous silicon-based rugate filters. Appl. Opt. 2005, 44, 5415–5421. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Nguyen, N.-T.; Straboni, A.; Lemiti, M. Structural and Optical Properties of Annealed Porous Silicon Bragg Reflector for Thin-Film Crystalline Silicon Solar Cells. Energy Procedia 2011, 10, 8–13. [Google Scholar] [CrossRef]
- Van, N.T.; Chinh, V.D.; Binh, P.T.; Hoi, P.V.; Huy, B.; Nga, P.T. Select and Rapid Detection of Methylene Blue on Porous Silicon Photonic Crystals Covered with Silver Nanoparticles by SERS. J. Electron. Mater. 2022, 51, 1946–1949. [Google Scholar] [CrossRef]
- Chen, N.; Ding, P.; Shi, Y.; Jin, T.; Su, Y.; Wang, H.; He, Y. Portable and Reliable Surface-Enhanced Raman Scattering Silicon Chip for Signal-On Detection of Trace Trinitrotoluene Explosive in Real Systems. Anal. Chem. 2017, 89, 5072–5078. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Vu, D.C.; Pham, T.S.; Bui, H.; Pham, T.B.; Hoang, T.H.C.; Pham, V.H.; Pham, V.H. Quasi-3D-Structured Surface-Enhanced Raman Scattering Substrates Based on Silver Nanoparticles/Mesoporous Silicon Hybrid. Phys. Status Solidi (A) Appl. Mater. Sci. 2022, 219, 2200128. [Google Scholar] [CrossRef]
- Wang, J.; Jia, Z.; Liu, Y. Improvement of SERS by the Optical Modulation of Photonic Crystal. IEEE Sens. J. 2019, 19, 11221–11227. [Google Scholar] [CrossRef]
- Zhong, F.; Wu, Z.; Guo, J.; Jia, D. Porous Silicon Photonic Crystals Coated with Ag Nanoparticles as Efficient Substrates for Detecting Trace Explosives Using SERS. Nanomaterials 2018, 8, 872. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Vu, D.C.; Pham, V.H.; Pham, T.B.; Pham, V.H.; Bui, H. Improvement of SERS for detection of ultra-low concentration of methyl orange by nanostructured silicon decorated with Ag nanoparticles. Optik 2021, 231, 166431. [Google Scholar] [CrossRef]
- Bai, Y.; Yan, L.; Wang, J.; Su, L.; Chen, N.; Tan, Z. Highly reproducible and uniform SERS substrates based on Ag nanoparticles with optimized size and gap. Photonics Nanostruct. Fundam. Appl. 2017, 23, 58–63. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, C.M.; Yang, L.; Chou, M.Y. Quantum Confinement and Electronic Properties of Silicon Nanowires. Phys. Rev. Lett. 2004, 92, 236805. [Google Scholar] [CrossRef] [PubMed]
- Piscanec, S.; Cantoro, M.; Ferrari, A.C.; Zapien, J.A.; Lifshitz, Y.; Lee, S.T.; Hofmann, S.; Robertson, J. Raman spectroscopy of silicon nanowires. Phys. Rev. B 2003, 68, 241312. [Google Scholar] [CrossRef]
- Virga, A.; Rivolo, P.; Descrovi, E.; Chiolerio, A.; Digregorio, G.; Frascella, F.; Soster, M.; Bussolino, F.; Marchiò, S.; Geobaldo, F.; et al. SERS active Ag nanoparticles in mesoporous silicon: Detection of organic molecules and peptide–antibody assays. J. Raman Spectrosc. 2012, 43, 730–736. [Google Scholar] [CrossRef]
- Li-lin, J.; Wei-long, L.; Yun-fei, S.; Xing, H.; Yang, W.; Chang, W.; Hong-lin, W.; Fang, Y.; Yan-qiang, Y. Photoinduced intermolecular electron transfer and off-resonance Raman characteristics of Rhodamine 101/N,N-diethylaniline. Chem. Phys. 2014, 429, 12–19. [Google Scholar] [CrossRef]
- Kundu, A.; Rani, R.; Hazra, K.S. Controlled nanofabrication of metal-free SERS substrate on few layered black phosphorus by low power focused laser irradiation. Nanoscale 2019, 11, 16245–16252. [Google Scholar] [CrossRef]
- He, X.N.; Gao, Y.; Mahjouri-Samani, M.; Black, P.N.; Allen, J.; Mitchell, M.; Xiong, W.; Zhou, Y.S.; Jiang, L.; Lu, Y.F. Surface-enhanced Raman spectroscopy using gold-coated horizontally aligned carbon nanotubes. Nanotechnology 2012, 23, 205702. [Google Scholar] [CrossRef]
- Mamichev, D.A.; Gonchar, K.A.; Timoshenko, V.Y.; Mussabek, G.K.; Nikulin, V.E.; Taurbaev, T.I. Enhanced Raman scattering in multilayer structures of porous silicon. J. Raman Spectrosc. 2011, 42, 1392–1395. [Google Scholar] [CrossRef]
- Delfan, A.; Liscidini, M.; Sipe, J.E. Surface enhanced Raman scattering in the presence of multilayer dielectric structures. J. Opt. Soc. Am. B 2012, 29, 1863–1874. [Google Scholar] [CrossRef]
- Huang, S.H.; Jiang, X.; Peng, B.; Janisch, C.; Cocking, A.; Özdemir, Ş.K.; Liu, Z.; Yang, L. Surface-enhanced Raman scattering on dielectric microspheres with whispering gallery mode resonance. Photonics Res. 2018, 6, 346–356. [Google Scholar] [CrossRef]
- Alessandri, I. Enhancing Raman Scattering without Plasmons: Unprecedented Sensitivity Achieved by TiO2 Shell-Based Resonators. J. Am. Chem. Soc. 2013, 135, 5541–5544. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Jehlička, J.; Vítek, P.; Edwards, H.G.M. On the definition of Raman spectroscopic detection limits for the analysis of biomarkers in solid matrices. Planet. Space Sci. 2012, 62, 48–54. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. About Estimating the Limit of Detection by the Signal to Noise Approach. Pharm. Anal. Acta. 2015, 6, 1000355. [Google Scholar] [CrossRef]
- Thayer, E.; Turner, W.; Blama, S.; Devadas, M.S.; Hondrogiannis, E.M. Signal detection limit of a portable Raman spectrometer for the SERS detection of gunshot residue. MRS Commun. 2019, 9, 948–955. [Google Scholar] [CrossRef]
- Xie, J.; Li, L.; Khan, I.M.; Wang, Z.; Ma, X. Flexible paper-based SERS substrate strategy for rapid detection of methyl parathion on the surface of fruit. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118104. [Google Scholar] [CrossRef]
- Li, Z.; Meng, G.; Huang, Q.; Hu, X.; He, X.; Tang, H.; Wang, Z.; Li, F. Ag Nanoparticle-Grafted PAN-Nanohump Array Films with 3D High-Density Hot Spots as Flexible and Reliable SERS Substrates. Small 2015, 11, 5452–5459. [Google Scholar] [CrossRef]
- Wu, H.; Luo, Y.; Huang, Y.; Dong, Q.; Huo, C.; Huo, D.; Zhao, J.; Lei, Y. A Simple SERS-Based Trace Sensing Platform Enabled by AuNPs-Analyte/AuNPs Double-Decker Structure on Wax-Coated Hydrophobic Surface. Front. Chem. 2018, 6, 482. [Google Scholar] [CrossRef]
- Zhu, C.; Hu, X.; Wang, X. Silver nanocubes/graphene oxide hybrid film on a hydrophobic surface for effective molecule concentration and sensitive SERS detection. Appl. Surf. Sci. 2019, 470, 423–429. [Google Scholar] [CrossRef]
- Wu, H.; Luo, Y.; Hou, C.; Huo, D.; Wang, W.; Zhao, J.; Lei, Y. Rapid and fingerprinted monitoring of pesticide methyl parathion on the surface of fruits/leaves as well as in surface water enabled by gold nanorods based casting-and-sensing SERS platform. Talanta 2019, 200, 84–90. [Google Scholar] [CrossRef]
- Tang, F.; Zhang, M.; Li, Z.; Du, Z.; Chen, B.; He, X.; Zhao, S. Hexagonally arranged arrays of urchin-like Ag-nanoparticle decorated ZnO-nanorods grafted on PAN-nanopillars as surface-enhanced Raman scattering substrates. CrystEngComm 2018, 20, 3550–3558. [Google Scholar] [CrossRef]
- Luo, W.; Chen, M.; Hao, N.; Huang, X.; Zhao, X.; Zhu, Y.; Yang, H.; Chen, X. In situ synthesis of gold nanoparticles on pseudo-paper films as flexible SERS substrate for sensitive detection of surface organic residues. Talanta 2019, 197, 225–233. [Google Scholar] [CrossRef]
- Yaseen, T.; Pu, H.; Sun, D.-W. Rapid detection of multiple organophosphorus pesticides (triazophos and parathion-methyl) residues in peach by SERS based on core-shell bimetallic Au@Ag NPs. Food Addit. Contam. Part A 2019, 36, 762–778. [Google Scholar] [CrossRef]
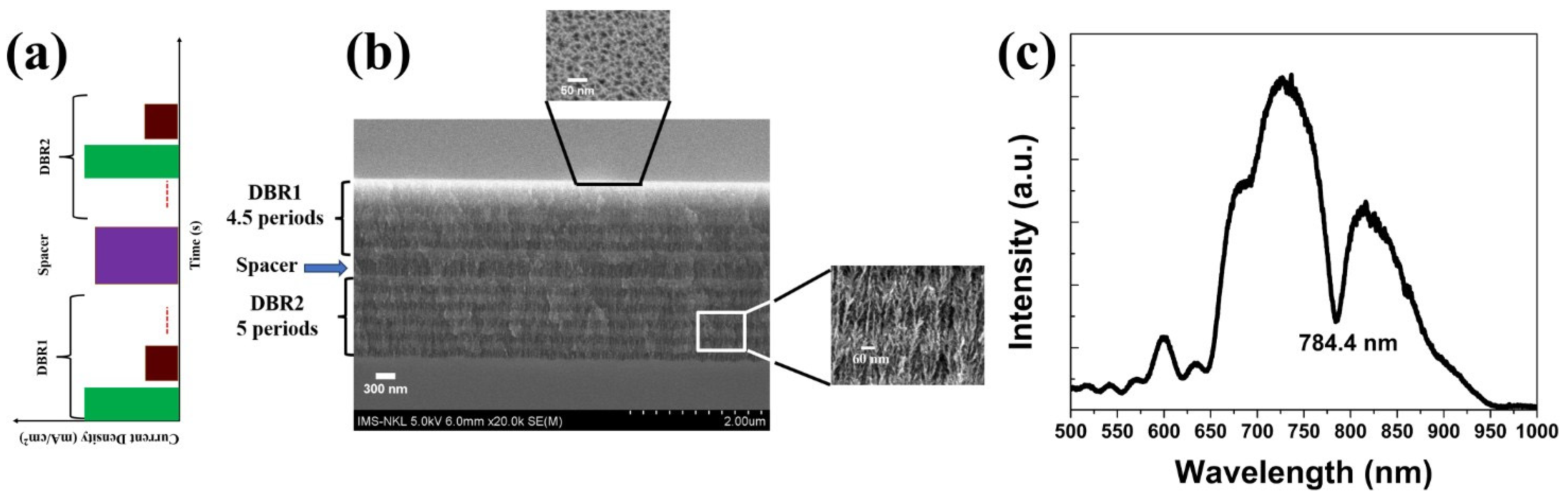
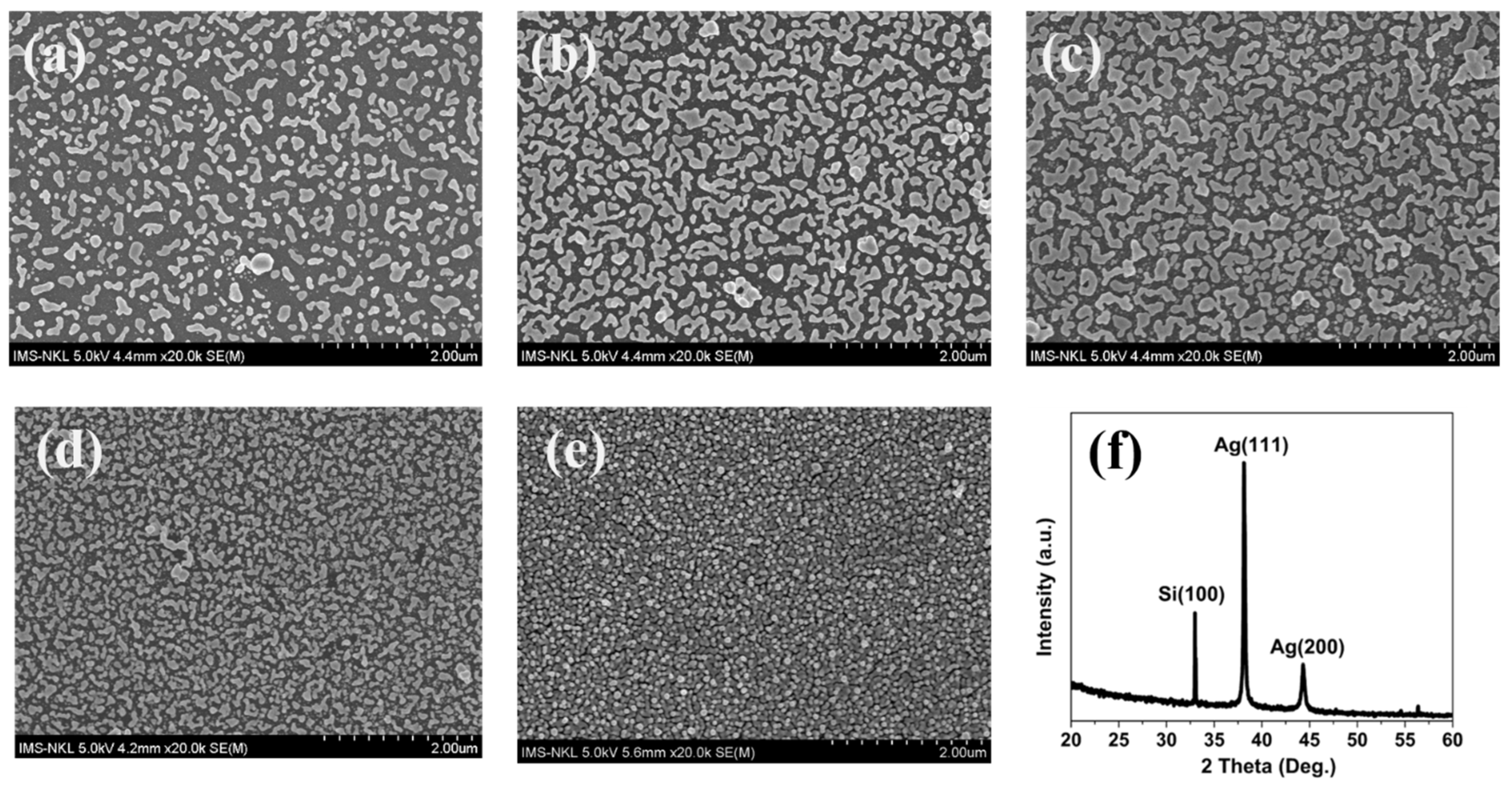

| Sample | Current Density (mA/cm2) | Time (sec) | Thickness | |
|---|---|---|---|---|
| Single layer | Low index layer | 50 | 93 | 2.9 µm |
| DBR (12 periods) | High index layer | 15 | 8.4 | 126.6 nm |
| Low index layer | 50 | 3.2 | 112.6 nm | |
| PSiMC | Top DBR (4.5 periods) | 15 | 8.4 | 126.6 nm |
| 50 | 3.2 | 112.6 nm | ||
| Spacer | 50 | 6.4 | 225.2 nm | |
| Bottom DBR (5 periods) | 15 | 8.4 | 126.6 nm | |
| 50 | 3.2 | 112.6 nm |
| Raman Shift (cm−1) | Assignment | Raman Shift (cm−1) | Assignment |
|---|---|---|---|
| 622 | C-C-C ring in-plane vibration | 1232 | CH2 + CH wag + C-O-C stretch |
| 673 | C-C-C ring out-of-plane vibration | 1347 | C-O-C stretch + C-C-C ring stretch |
| 768 | C-H out-of-plane bending | 1423 | |
| 846 | CH2 out-of-plane wag | 1504 | CH2 scissoring + C-N stretch |
| 962 | CH2 + CH wag + C-C-C ring vibration + C-O-C stretch | 1570 | Aromatic C-C stretching |
| 1061 | CH2 + CH wag + C-C-C ring vibration | 1597 | Xanthene ring stretch |
| 1203 | CH2 + CH wag + C-O-C stretch | 1646 | Aromatic C-C stretching |
| SERS Substrate Type | Raman Instrumentation | Excitation Wavelength (nm) | Reported Detection Limit | Reference |
|---|---|---|---|---|
| Ag nanocube/graphene oxide composite | Renishaw confocal microprobe Raman (India) | 532 | 2.0 × 10−12 M | [48] |
| Au nanorods sensor | Ocean Optics handheld Raman | 785 | 1.0 × 10−6 M | [49] |
| Ag–ZnO nanorods with PAN-nanopillar architecture | Renishaw inVia confocal micro-Raman | 532 | 1.0 × 10−8 M | [50] |
| AuNP-based pseudo-paper film (APPF) | Portable Raman (B&W Tek) | 785 | ~5.1 × 10−11 M | [51] |
| Au@Ag core–shell nanoparticles | Confocal Raman system | 633 | ~3.8 × 10−9 M | [52] |
| AuNP–analyte/AuNP double-decker structure | Portable Raman spectrometer (QE Pro, Ocean Optics) | 785 | 1.0 × 10−8 M | [47] |
| Filter paper coated with AgNPs | B&W Tek portable Raman | 785 | ~5.5 × 10−8 M | [45] |
| AgNP/PSi microcavity (PSiMC) | Confocal Raman microscope (Horiba LabRAM) | 785 | 1.0 × 10−10 M | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, M.T.; Bui, H.; Hoang, T.H.C.; Pham, V.H.; Loan, N.T.; Le, L.V.; Pham, T.B.; Duc, C.V.; Do, T.C.; Kim, T.J.; et al. Silver Nanoparticles-Decorated Porous Silicon Microcavity as a High-Performance SERS Substrate for Ultrasensitive Detection of Trace-Level Molecules. Nanomaterials 2025, 15, 1007. https://doi.org/10.3390/nano15131007
Hoang MT, Bui H, Hoang THC, Pham VH, Loan NT, Le LV, Pham TB, Duc CV, Do TC, Kim TJ, et al. Silver Nanoparticles-Decorated Porous Silicon Microcavity as a High-Performance SERS Substrate for Ultrasensitive Detection of Trace-Level Molecules. Nanomaterials. 2025; 15(13):1007. https://doi.org/10.3390/nano15131007
Chicago/Turabian StyleHoang, Manh Trung, Huy Bui, Thi Hong Cam Hoang, Van Hai Pham, Nguyen Thu Loan, Long Van Le, Thanh Binh Pham, Chinh Vu Duc, Thuy Chi Do, Tae Jung Kim, and et al. 2025. "Silver Nanoparticles-Decorated Porous Silicon Microcavity as a High-Performance SERS Substrate for Ultrasensitive Detection of Trace-Level Molecules" Nanomaterials 15, no. 13: 1007. https://doi.org/10.3390/nano15131007
APA StyleHoang, M. T., Bui, H., Hoang, T. H. C., Pham, V. H., Loan, N. T., Le, L. V., Pham, T. B., Duc, C. V., Do, T. C., Kim, T. J., Pham, V. H., & Nguyen, T. V. (2025). Silver Nanoparticles-Decorated Porous Silicon Microcavity as a High-Performance SERS Substrate for Ultrasensitive Detection of Trace-Level Molecules. Nanomaterials, 15(13), 1007. https://doi.org/10.3390/nano15131007








