Core–Shell Engineering of One-Dimensional Cadmium Sulfide for Solar Energy Conversion
Abstract
1. Introduction
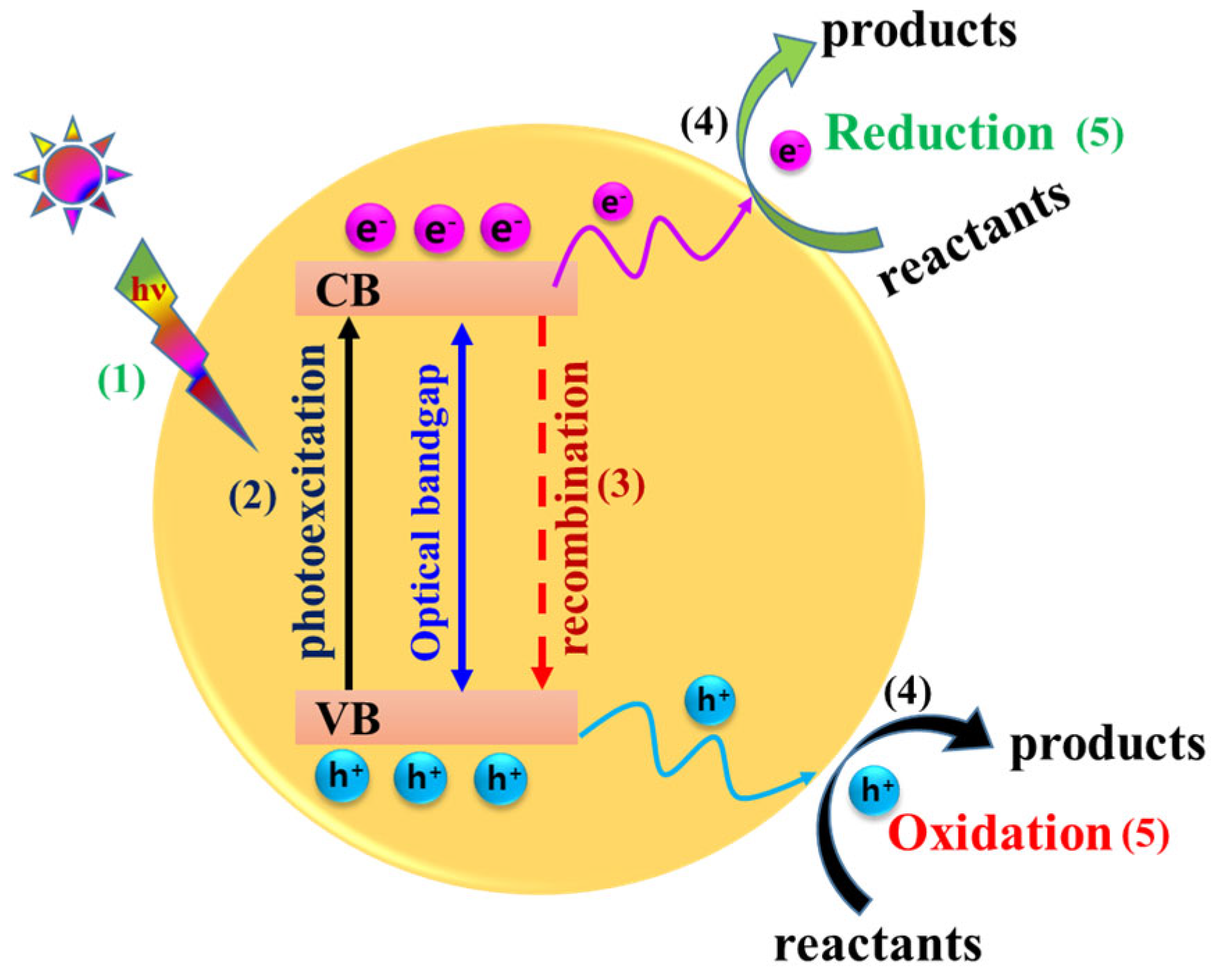
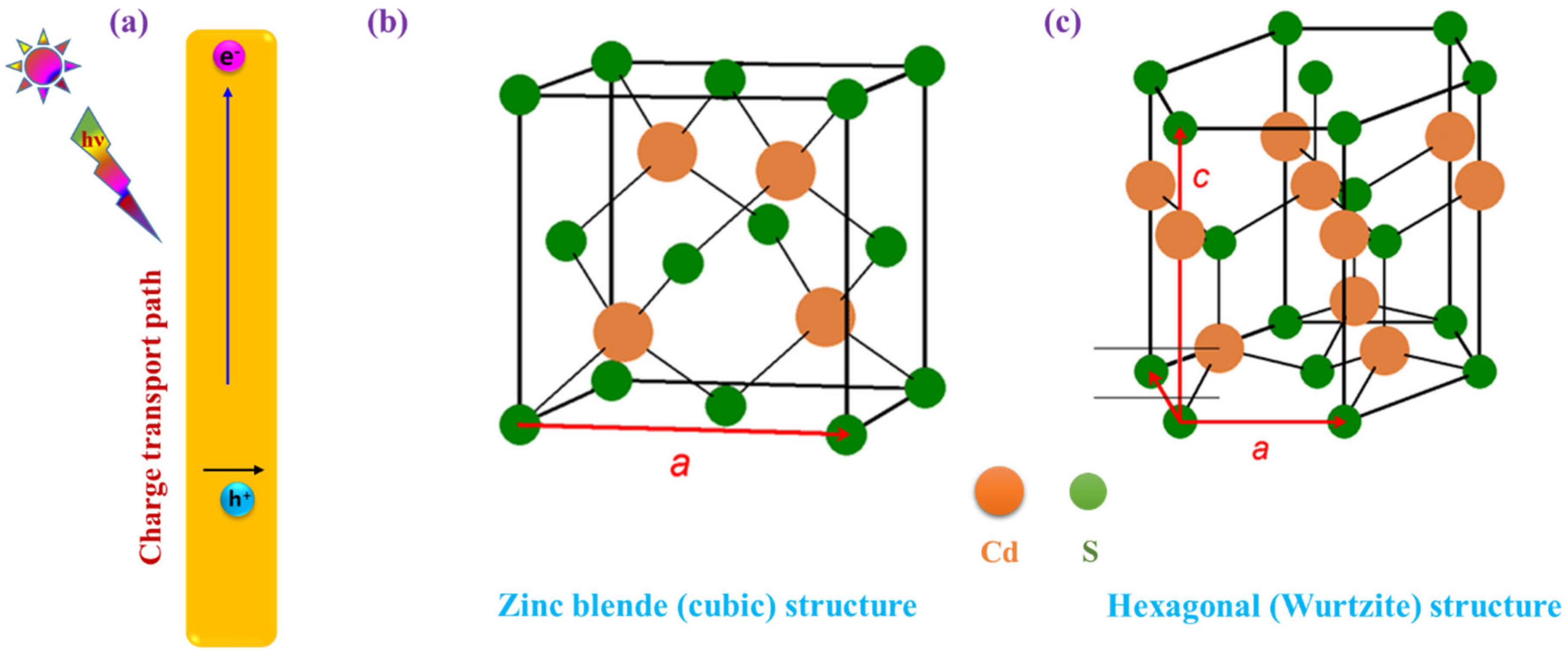
2. The Emergence of a 1D CdS Photocatalyst and Mechanistic Fundamentals
3. Strategies to Separate Charge Carriers in a 1D CdS Photocatalyst
3.1. Doping/Defect Engineering of 1D CdS
3.2. Metal Nanoparticle (NP) Loading onto 1D CdS
3.3. 1D CdS-Based Heterojunctioned Photocatalytic Systems
4. Core–Shell Heterostructured 1D CdS Photocatalysts
4.1. Metal Oxides Coated 1D CdS Core–Shell Heterostructures
4.2. Metal Sulfide-Coated 1D CdS Core–Shell Heterostructures
4.3. Carbon-Based Material-Coated 1D CdS Core–Shell Heterostructures
5. Conclusions and Future Challenges
- (i)
- The main feature in the photochemical reactions is the charge generation and migration phenomenon, which dictates the final activity of the designed photocatalysts. Therefore, understanding the photoinduced charge separation between the core and shell components is essential for optimizing the architecture and then enhancing the activity.
- (ii)
- Detailed investigations into morphology, crystal structure, defects, and reactive sites are crucial for achieving high-performance photocatalysts in several redox reactions. In this regard, precise control is essential in realizing the 1D CdS-based core–shell synergized nanostructures.
- (iii)
- To understand the synergetic interactions of the core and shell, extensive exploration of in situ characterizations, for example, in situ X-ray photoelectron spectroscopy (in situ XPS), Raman spectroscopy, X-ray absorption near edge structure (XANES), etc., is expected.
- (iv)
- Even though the shell layer on 1D CdS offers significant physical protection from photocorrosion, the interactions between the 1D CdS core and shell should be stronger to restrict the leaching of Cd or S ions in the photocatalysts.
- (v)
- Synthesizing the 1D CdS core–shell nanostructures via a cost-effective approach and scaling up strategies also play a major role in utilizing them in sustainable energy technologies.
- (vi)
- In addition, advanced DFT calculation and simulation/modeling studies should also be adequately utilized to simulate and validate the growth formation and internal mechanisms of 1D CdS-based core–shell heterostructures in future research.
Author Contributions
Funding
Conflicts of Interest
References
- Low, J.X.; Yu, J.G.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, X.Y.; Shah, S.S.A.; Wang, C.; Li, X.Y.; Yan, Z.S.; Peng, L.S. Recent Advances in Single-Atom Catalysts for Photoelectrocatalytic Water Splitting. Carbon Energy 2025, 7, e695. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, J.J.; Yu, H.G.; Yu, J.G. Emerging S-Scheme Photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.B.; Chang, X.Y.; Wang, W.J.; Fan, H.Q. Graphitic Carbon Nitride for Photocatalytic Hydrogen Production from Water Splitting: Nano-Morphological Control and Electronic Band Tailoring. Nanomaterials 2025, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Fang, S.; Sun, H.D.; Chung, R.-J.; Fang, X.S.; He, H., Jr. Solar Hydrogen. Adv. Energy Mater. 2023, 13, 2203019. [Google Scholar] [CrossRef]
- Wang, Q.A.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges. And Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Martin, D.J.; Liu, G.G.; Moniz, S.J.A.; Bi, Y.P.; Beale, A.M.; Ye, J.H.; Tang, J.W. Efficient visible driven photocatalyst, silver phosphate: Performance, understanding and perspective. Chem. Soc. Rev. 2015, 44, 7808–7828. [Google Scholar] [CrossRef]
- Guo, C.F.; Chen, D.-L.; Hu, Y. Perspective on Defective Semiconductor Heterojunctions for CO2 Photoreduction. Langmuir 2022, 38, 6491–6498. [Google Scholar] [CrossRef]
- Tee, S.Y.; Kong, J.H.; Koh, J.J.Q.; Teng, C.P.; Wang, X.Z.; Wang, X.B.; Teo, S.L.; Thitsartarn, W.T.; Han, M.-Y.; She, Z.W. Structurally and surficially activated TiO2 nanomaterials for photochemical reactions. Nanoscale 2024, 16, 18165–18212. [Google Scholar] [CrossRef]
- Avcıoǧlu, C.; Avcıoǧlu, S.; Bekheet, M.F.; Gurlo, A. Photocatalytic Overall Water Splitting by SrTiO3: Progress Report and Design Strategies. ACS App. Energy Mater. 2023, 6, 1134–1154. [Google Scholar] [CrossRef]
- Ferrara, M.C.; Montechchi, M.; Mittiga, A.; Schioppa, M.; Mazzarelli, S.; Tapfer, L.; Lovergine, N.; Prete, P. Synthesis and annealing effects on microstructure and optical properties of wide-bandgap polycrystalline ferro-pseudobrookite FeTi2O5 sol-gel layers. Cer. Int. 2025, 51, 9669–9676. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A 2018, 555, 47–74. [Google Scholar] [CrossRef]
- Amirulsyafiee, A.; Khan, M.M.; Harunsani, M.H. Ag3PO4 and Ag3PO4–based visible light active photocatalysts: Recent progress, synthesis, and photocatalytic applications. Catalysts 2022, 12, 106556. [Google Scholar] [CrossRef]
- Saman, F.; Se Ling, C.H.; Ayub, A.; Rafeny, N.H.B.; Mahadi, A.H.; Subagyo, R.; Nugraha, R.E.; Prasetyoko, D.; Bahruji, H. Review on synthesis and modification of g-C3N4 for photocatalytic H2 production. Int. J. Hydrog. Energy 2024, 77, 1090–1116. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Pan, G.; Wang, N.; Xing, Y.; Zhang, Z. Recent progress in CdS-based S-scheme photocatalysts. J. Mater. Sci. Technol. 2024, 171, 162–184. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A Mini-Review on ZnIn2S4-Based Photocatalysts for Energy and Environmental Application. Green Energy Environ. 2022, 7, 176–204. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, H.; Peng, W. 2D Transition Metal Dichalcogenides and Graphene-Based Ternary Composites for Photocatalytic Hydrogen Evolution and Pollutants Degradation. Nanomaterials 2017, 7, 62. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, P.; Foo, J.J.; Zhang, J.; Qian, W.; Chen, C.; Ong, W.-J. Dimensionality-Dependent MoS2 toward Efficient Photocatalytic Hydrogen Evolution: From Synthesis to Modifications in Doping, Surface and Heterojunction Engineering. Mater. Today Nano 2022, 18, 100191. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C.; Zhang, Y.; Chen, S.; Lin, Z.; Peng, F.; Zhang, P. Recent Advances in Metal Sulfides: From Controlled Fabrication to Electrocatalytic, Photocatalytic and Photoelectrochemical Water Splitting and Beyond. Chem. Soc. Rev. 2019, 48, 4178–4280. [Google Scholar] [CrossRef]
- Di, J.; Jiang, W. Recent Progress of Low-Dimensional Metal Sulfides Photocatalysts for Energy and Environmental Applications. Mater. Today Catal. 2023, 1, 100001. [Google Scholar] [CrossRef]
- Wang, J.; Lin, S.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Nanostructured Metal Sulfides: Classification, Modification Strategy, and Solar-Driven CO2 Reduction Application. Adv. Funct. Mater. 2021, 31, 2008008. [Google Scholar] [CrossRef]
- Pakiari, A.H.; Jamshidi, Z. Nature and Strength of M−S Bonds (M = Au. Ag. and Cu) in Binary Alloy Gold Clusters. J. Phys. Chem. A 2010, 114, 9212–9221. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, L. Metal Sulphide Semiconductors for Photocatalytic Hydrogen Production. Catal. Sci. Technol. 2013, 3, 1672–1690. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Fan, X.; Singh, D.J.; Zheng, W.T. Recent Progress of TMD Nanomaterials: Phase Transitions and Applications. Nanoscale 2020, 12, 1247–1268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, Y.; Zhao, Z.; Yuan, C.; Lou, X.W.D. A Ternary Fe1−xS@Porous Carbon Nanowires/Reduced Graphene Oxide Hybrid Film Electrode with Superior Volumetric and Gravimetric Capacities for Flexible Sodium Ion Batteries. Adv. Energy Mater. 2019, 9, 1803052. [Google Scholar] [CrossRef]
- Lovergine, N.; Cingolani, R.; Mancini, A.M.; Ferrara, M. Photoluminescence of CVD grown CdS epilayers on CdTe substrates. J. Cryst. Growth 1992, 118, 304–308. [Google Scholar] [CrossRef]
- Chatterjee, B.; Bandyopadhyay, A. Review on the Synthesis of Metal Sulfides Gas Sensors and Their Performances at Room Temperature. Mater. Sci. Eng. B 2023, 297, 116781. [Google Scholar] [CrossRef]
- Wang, F.; Huang, F.; Yu, F.; Kang, X.; Wang, Q.; Liu, Y. Metal-Sulfide Photocatalysts for Solar-Fuel Generation across the Solar Spectrum. Cell Rep. Phys. Sci. 2023, 4, 101450. [Google Scholar] [CrossRef]
- Wei, R.-B.; Huang, Z.-L.; Gu, G.-H.; Wang, Z.; Zeng, L.; Chen, Y.; Liu, Z.-Q. Dual-Cocatalysts Decorated Rimous CdS Spheres Advancing Highly-Efficient Visible-Light Photocatalytic Hydrogen Production. Appl. Catal. B 2018, 231, 101–107. [Google Scholar] [CrossRef]
- Chava, R.K.; Son, N.; Kim, Y.S.; Kang, M. Controlled Growth and Bandstructure Properties of One Dimensional Cadmium Sulfide Nanorods for Visible Photocatalytic Hydrogen Evolution Reaction. Nanomaterials 2020, 10, 619. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, C.; Lin, Z.; Jiang, S.; Song, S. Low-Energy Facets on CdS Allomorph Junctions with Optimal Phase Ratio to Boost Charge Directional Transfer for Photocatalytic H2 Fuel Evolution. Small 2020, 16, 2000944. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, B.; Qu, F.; Wu, X. Synthesis of Self-Assembled CdS Nanospheres and Their Photocatalytic Activities by Photodegradation of Organic Dye Molecules. Chem. Eng. J. 2014, 258, 203–209. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, H.; Li, Y.; Lu, K. Photocatalytic Activity of CdS Nanoparticles Enhanced by the Interaction between Piezotronic Effect and Phase Junction. J. Alloys Compd. 2020, 815, 152494. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, W.; Xu, R.; Shi, Y.; Zhang, B. Synthesis of Ultrathin CdS Nanosheets as Efficient Visible-Light-Driven Water Splitting Photocatalysts for Hydrogen Evolution. Chem. Commun. 2013, 49, 9803–9805. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Xi, B.; Qian, Y. CdS Hierarchical Nanostructures with Tunable Morphologies: Preparation and Photocatalytic Properties. J. Phys. Chem. C 2010, 114, 14029–14035. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Yang, C.; Li, Y. Controlled Synthesis of CdS Nanorods and Hexagonal Nano-Crystals. J. Mater. Chem. 2003, 13, 2641–2648. [Google Scholar] [CrossRef]
- Kumar, D.P.; Hong, S.Y.; Reddy, D.A.; Kim, T.K. Noble metal-free ultrathin MoS2 nanosheet-decorated CdS nanorods as an efficient photocatalyst for spectacular hydrogen evolution under solar light irradiation. J. Mater. Chem. A 2016, 4, 18551–18558. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, L.; Wang, C.; Wang, W.; Ling, T.; Yang, J.; Dong, C.; Lin, F.; Du, X.-W. Zinc-Blende CdS Nanocubes with Coordinated Facets for Photocatalytic Water Splitting. ACS Catal. 2017, 7, 1470–1477. [Google Scholar] [CrossRef]
- Widness, J.K.; Enny, D.G.; McFarlane-Connelly, K.S.; Miedenbauer, M.T.; Krauss, T.D.; Weix, D.J. CdS Quantum Dots as Potent Photoreductants for Organic Chemistry Enabled by Auger Processes. J. Am. Chem. Soc. 2022, 144, 12229–12246. [Google Scholar] [CrossRef]
- Vaquero, F.; Fierro, J.L.G.; Navarro Yerga, R.M. From Nanorods to Nanowires of CdS Synthesized by a Solvothermal Method: Influence of the Morphology on the Photoactivity for Hydrogen Evolution from Water. Molecules 2016, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Barzgar Vishlaghi, M.; Balkan, T.; Rehman, Z.U.; Kaya, S. Scaling-Up Photocatalytic Activity of CdS from Nanorods to Nanowires for the MB Degradation. Inorg. Chem. Commun. 2021, 130, 108744. [Google Scholar] [CrossRef]
- Zhai, T.; Fang, X.; Li, L.; Bando, Y.; Golberg, D. One-Dimensional CdS Nanostructures: Synthesis, Properties, and Applications. Nanoscale 2010, 2, 168–187. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Han, B.; Han, C.; Xu, Y.-J. One-Dimensional CdS-Based Materials for Artificial Photoredox Reactions. J. Mater. Chem. A 2017, 5, 2387–2410. [Google Scholar] [CrossRef]
- Wang, W.; Xue, J.; Liu, J. Recent Advances in CdS Heterojunctions: Morphology, Synthesis, Performances and Prospects. J. Mater. Chem. A 2024, 12, 10659–10675. [Google Scholar] [CrossRef]
- Yuan, J.L.; Wen, J.Q.; Gao, Q.Z.; Chen, S.C.; Li, J.M.; Li, X.; Fang, Y.P. Amorphous Co3O4 modified CdS nanorods with enhanced visible-light photocatalytic H2-production activity. Dalton. Trans. 2015, 44, 1680–1689. [Google Scholar] [CrossRef]
- Fang, X.S.; Zhang, L.D. One-Dimensional (1D) ZnS Nanomaterials and Nanostructures. J. Mater. Sci. Technol. 2006, 22, 721–736. Available online: https://www.jmst.org/EN/Y2006/V22/I06/721 (accessed on 14 May 2025).
- Zhang, J.; Wageh, S.; Al-Ghamdi, A.; Yu, J. New Understanding on the Different Photocatalytic Activity of Wurtzite and Zinc-Blende CdS. Appl. Catal. B Environ. 2016, 192, 101–107. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Z.-R.; Sun, Y.; Colmenares, J.C.; Xu, Y.-J. One-Dimension-Based Spatially Ordered Architectures for Solar Energy Conversion. Chem. Soc. Rev. 2015, 44, 5053–5075. [Google Scholar] [CrossRef]
- Hu, J.; Odom, T.W.; Lieber, C.M. Chemistry and Physics in One Dimension: Synthesis and Properties of Nanowires and Nanotubes. Acc. Chem. Res. 1999, 32, 435–445. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sakata, T.; Mori, H.; Yoneyama, H. Preparation of Monodisperse CdS Nanocrystals by Size Selective Photocorrosion. J. Phys. Chem. 1996, 100, 13781–13785. [Google Scholar] [CrossRef]
- Ning, X.; Lu, G. Photocorrosion Inhibition of CdS-Based Catalysts for Photocatalytic Overall Water Splitting. Nanoscale 2020, 12, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Jie, L.; Gao, X.; Cao, X.; Wu, S.; Long, X.; Ma, Q.; Su, J. A Review of CdS Photocatalytic Nanomaterials: Morphology, Synthesis Methods. And Applications. Mater. Sci. Semicond. Process. 2024, 176, 108288. [Google Scholar] [CrossRef]
- Ullah, H.; Haneef, Z.; Ahmad, A.; Butler, I.S.; Dara, R.N.; Rehman, Z. MoS2 and CdS Photocatalysts for Water Decontamination: A Review. Inorg. Chem. Commun. 2023, 153, 110775. [Google Scholar] [CrossRef]
- Fang, W.; Yan, J.; Wei, Z.; Liu, J.; Guo, W.; Jiang, Z.; Shangguan, W. Account of Doping Photocatalyst for Water Splitting. Chin. J. Catal. 2024, 60, 1–24. [Google Scholar] [CrossRef]
- Ng, B.-J.; Putri, L.K.; Kong, X.Y.; Pasbakhsh, P.; Chai, S.-P. Overall Pure Water Splitting Using One-Dimensional P-Doped Twinned Zn0.5Cd0.5S1-x Nanorods via Synergetic Combination of Long-Range Ordered Homojunctions and Interstitial S Vacancies with Prolonged Carrier Lifetime. Appl. Catal. B 2020, 262, 118309. [Google Scholar] [CrossRef]
- Chen, W.; Duan, G.-R.; Liu, T.-Y.; Jia, Z.-M.; Liu, X.-H.; Chen, S.-M.; Yang, X.-J. Synthesis of Homogeneous One-Dimensional NixCd1−xS Nanorods with Enhanced Visible-Light Response by Ethanediamine-Assisted Decomposition of Complex Precursors. J. Mater. Sci. 2015, 50, 3920–3928. [Google Scholar] [CrossRef]
- Chava, R.K.; Son, N.G.; Kang, M.S. Controllable Oxygen Doping and Sulfur Vacancies in One Dimensional CdS Nanorods for Boosted Hydrogen Evolution Reaction. J. Alloys Compd. 2021, 873, 159797. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, Challenge and Perspective of Heterogeneous Photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef]
- Jin, J.A.; Yu, J.G.; Liu, G.; Wong, P.K. Single Crystal CdS Nanowires with High Visible-Light Photocatalytic H2-Production Performance. J. Mater. Chem. A 2013, 1, 10927–10934. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.Y.; Kang, M.S. Smart Hybridization of Au Coupled CdS Nanorods with Few Layered MoS2 Nanosheets for High Performance Photocatalytic Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 6445–6457. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, L.; Liu, F.; Si, Z.; Huo, M.; Li, Z.; Chen, Z. Metal Ni Nanoparticles In-Situ Anchored on CdS Nanowires as Effective Cocatalyst for Boosting the Photocatalytic H2 Production and Degradation Activity. J. Alloys Compd. 2024, 973, 172747. [Google Scholar] [CrossRef]
- Chava, R.K.; Son, N.G.; Kang, M.S. Bismuth Quantum Dots Anchored One-Dimensional CdS as Plasmonic Photocatalyst for Pharmaceutical Tetracycline Hydrochloride Pollutant Degradation. Chemosphere 2022, 300, 134570. [Google Scholar] [CrossRef]
- Wang, B.; Chen, C.X.; Jiang, Y.Y.; Ni, P.J.; Zhang, C.H.; Yang, Y.; Lu, Y.Z.; Liu, P. Rational designing 0D/1D Z-scheme heterojunction on CdS nanorods for efficient visible-light-driven photocatalytic H2 evolution. Chem. Eng. J. 2021, 412, 128690. [Google Scholar] [CrossRef]
- Sudrajat, H.; Nobatova, M. Heterojunction Photocatalysts: Where Are They Headed? RSC Adv. Interfaces 2025, 2, 599–619. [Google Scholar] [CrossRef]
- Lin, M.; Chen, H.; Zhang, Z.; Wang, X. Engineering Interface Structures for Heterojunction Photocatalysts. Phys. Chem. Chem. Phys. 2023, 25, 4388–4407. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, F.; Lang, D. Hierarchical Layered WS2/Graphene-Modified CdS Nanorods for Efficient Photocatalytic Hydrogen Evolution. ChemSusChem 2016, 9, 996–1002. [Google Scholar] [CrossRef]
- Rangappa, A.P.; Kumar, D.P.; Gopannagari, M.; Reddy, D.A.; Hong, Y.; Kim, Y.; Kim, T.K. Highly efficient hydrogen generation in water using 1D CdS nanorods integrated with 2D SnS2 nanosheets under solar light irradiation. Appl. Surf. Sci. 2020, 508, 144803. [Google Scholar] [CrossRef]
- Chava, R.K.; Son, N.G.; Kang, M.S. Band structure alignment transitioning strategy for the fabrication of efficient photocatalysts for solar fuel generation and environmental remediation applications. J. Colloid Interface Sci. 2022, 627, 247–260. [Google Scholar] [CrossRef]
- He, B.; Bie, C.; Fei, X.; Cheng, B.; Yu, J.; Ho, W.K.; Al-Ghamdi, A.A.; Wageh, S. Enhancement in the photocatalytic H2 production activity of CdS NRs by Ag2S and NiS dual cocatalysts. Appl. Catal. B 2021, 288, 119994. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, X.; Liu, J.; Fu, X.; Yang, Y.; Han, H.; Fan, Y.; Zhang, S.; Meng, S.; Chen, S. Construction of NiPx/MoS2/NiS/CdS composite to promote photocatalytic H2 production from glucose solution. J. Am. Ceram. Soc. 2021, 104, 5307–5316. [Google Scholar] [CrossRef]
- Paranthaman, V.; Devi, K.S.S.; Bhojanaa, K.B.; Aravindan, V.; Raman, G.; Kumar, R.S.; Doroody, C.; Rajamony, R.K.; Krishnan, P.S. Experimental and theoretical insights into enhanced light harvesting in dye-sensitized solar cells via Au@TiO2 core-shell and BaTiO3 nanoparticles. J. Taiwan Ins. Chem. Eng. 2024, 165, 105778. [Google Scholar] [CrossRef]
- Chava, R.K.; Oh, S.-Y.; Yu, Y.-T. Enhanced H2 gas sensing properties of Au@In2O3 core–shell hybrid metal–semiconductor heteronanostructures. CrystEngComm 2016, 18, 3655–3666. [Google Scholar] [CrossRef]
- Sundarapandi, M.; Pandikumar, A.; Rameshkumar, P.; Ramaraj, R. Tailoring the shell structures in core-shell metal nanostructures for improved catalytic reduction of nitroaromatics. Nano Trends 2025, 9, 100083. [Google Scholar] [CrossRef]
- Liu, B.-T.; Pan, X.-H.; Zhang, D.-Y.; Wang, R.; Chen, J.-Y.; Fang, H.-R.; Liu, T.-F. Construction of Function-Oriented Core–Shell Nanostructures in Hydrogen-Bonded Organic Frameworks for Near-Infrared-Responsive Bacterial Inhibition. Angew. Chem. Int. Ed. 2021, 60, 25701–25707. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, H.; Jiang, Y.; Zhang, W.; Zhang, J.; Wu, X.; Liu, Z.C.; Deng, W. Hierarchical Sb2S3/ZnIn2S4 core–shell heterostructure for highly efficient photocatalytic hydrogen production and pollutant degradation. J. Colloid Interface Sci. 2022, 623, 109–123. [Google Scholar] [CrossRef]
- Dong, W.; Pan, F.; Xu, L.; Zheng, M.; Sow, C.H.; Wu, K.; Xu, G.Q.; Chen, W. Facile synthesis of CdS@TiO2 core–shell nanorods with controllable shell thickness and enhanced photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 2015, 349, 279–386. [Google Scholar] [CrossRef]
- Sun, G.; Xiao, B.; Zheng, H.; Shi, J.-W.; Mao, S.; He, C.; Li, Z.; Cheng, Y. Ascorbic acid functionalized CdS–ZnO core–shell nanorods with hydrogen spillover for greatly enhanced photocatalytic H2 evolution and outstanding photostability. J. Mater. Chem. A 2021, 9, 9735–9744. [Google Scholar] [CrossRef]
- Babu, B.; Harish, V.V.N.; Koutavarapu, R.; Shim, J.; Yoo, K. Enhanced visible-light-active photocatalytic performance using CdS nanorods decorated with colloidal SnO2 quantum dots: Optimization of core–shell nanostructure. J. Ind. Eng. Chem. 2019, 76, 476–487. [Google Scholar] [CrossRef]
- Chava, R.K.; Son, N.; Kim, Y.S.; Kang, M. Integration of perovskite type Bi2MoO6 nanosheets onto one dimensional CdS: A type-II heterostructured photocatalytic system for efficient charge separation in the hydrogen evolution reaction. Inorg. Chem. Front. 2020, 7, 2818–2832. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, S.; Shi, Y.; Liu, X.; Wu, Z.; Jiang, Q.; Zhou, T.; Liu, N.; Hu, J. Construction of CdS/CoOx core-shell nanorods for efficient photocatalytic H2 evolution. Appl. Catal. B 2018, 234, 109–116. [Google Scholar] [CrossRef]
- Zhang, S.; Ou, X.; Xiang, Q.; Carabineiro, S.A.C.; Fan, J.; Lv, K. Research progress in metal sulfides for photocatalysis: From activity to stability. Chemosphere 2022, 303, 135085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, J.; Ding, Z.; Zhang, J.; Wang, X. Optimal synthesis of platinum-free 1D/2D CdS/MoS2 (CM) heterojunctions with improved photocatalytic hydrogen production performance. J. Alloys Compd. 2020, 813, 152234. [Google Scholar] [CrossRef]
- Wang, T.; Chai, Y.; Ma, D.; Chen, W.; Zheng, W.; Huang, S. Multidimensional CdS nanowire/CdIn2S4 nanosheet heterostructure for photocatalytic and photoelectrochemical applications. Nano Res. 2017, 10, 2699–2711. [Google Scholar] [CrossRef]
- Fu, W.; Wang, J.; Zhou, S.; Li, R.; Peng, T. Controllable Fabrication of Regular Hexagon-Shaped SnS2 Nanoplates and Their Enhanced Visible-Light-Driven H2 Production Activity. ACS Appl. Nano Mater. 2018, 1, 2923–2933. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.Y.; Kang, M. Enhanced photoexcited carrier separation in CdS–SnS2 heteronanostructures: A new 1D–0D visible-light photocatalytic system for the hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 13614–13628. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, M.; Wang, F. Oxygen-controlled photo-reforming of biopolyols to CO over Z-scheme CdS@g-C3N4. Chem 2022, 8, 465–479. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jin, J.; Zhang, J.; Lin, Z.; Huang, F.; Yu, J. Efficient Visible-Light Photocatalytic Hydrogen Evolution and Enhanced Photostability of Core/Shell CdS/g-C3N4 Nanowires. ACS Appl. Mater. Interfaces 2013, 5, 10317–10324. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chava, R.K.; Kang, M. Core–Shell Engineering of One-Dimensional Cadmium Sulfide for Solar Energy Conversion. Nanomaterials 2025, 15, 1000. https://doi.org/10.3390/nano15131000
Chava RK, Kang M. Core–Shell Engineering of One-Dimensional Cadmium Sulfide for Solar Energy Conversion. Nanomaterials. 2025; 15(13):1000. https://doi.org/10.3390/nano15131000
Chicago/Turabian StyleChava, Rama Krishna, and Misook Kang. 2025. "Core–Shell Engineering of One-Dimensional Cadmium Sulfide for Solar Energy Conversion" Nanomaterials 15, no. 13: 1000. https://doi.org/10.3390/nano15131000
APA StyleChava, R. K., & Kang, M. (2025). Core–Shell Engineering of One-Dimensional Cadmium Sulfide for Solar Energy Conversion. Nanomaterials, 15(13), 1000. https://doi.org/10.3390/nano15131000







