Phase Evolution of High-Entropy Stannate Pyrochlore Oxide Synthesized via Glycine-Assisted Sol–Gel Synthesis as a Thermal Barrier Coating Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of (Gd0.2Nd0.2La0.2Pr0.2Sm0.2)2Sn2O7 Oxide Nanoparticles
2.3. Characterization
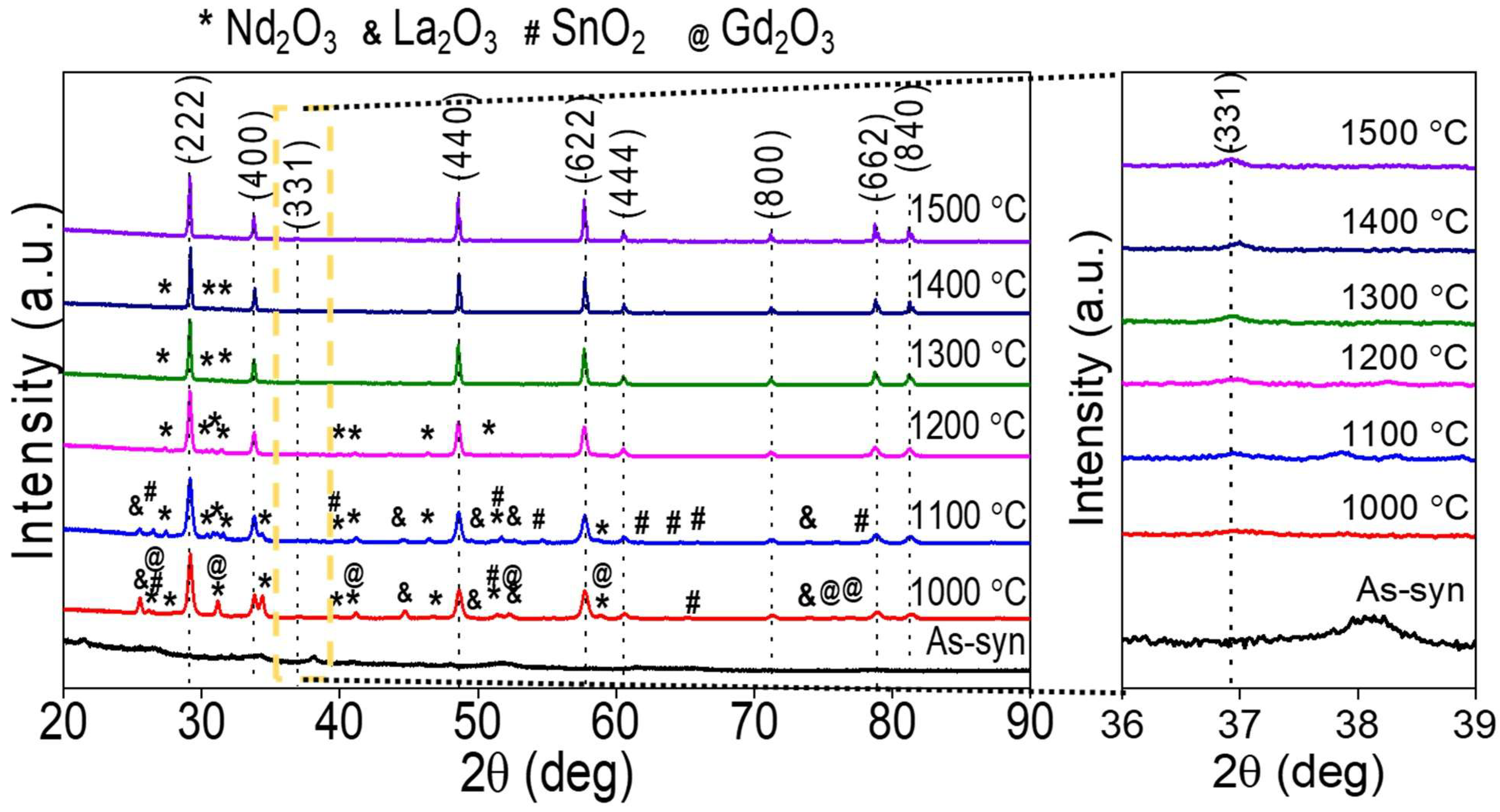
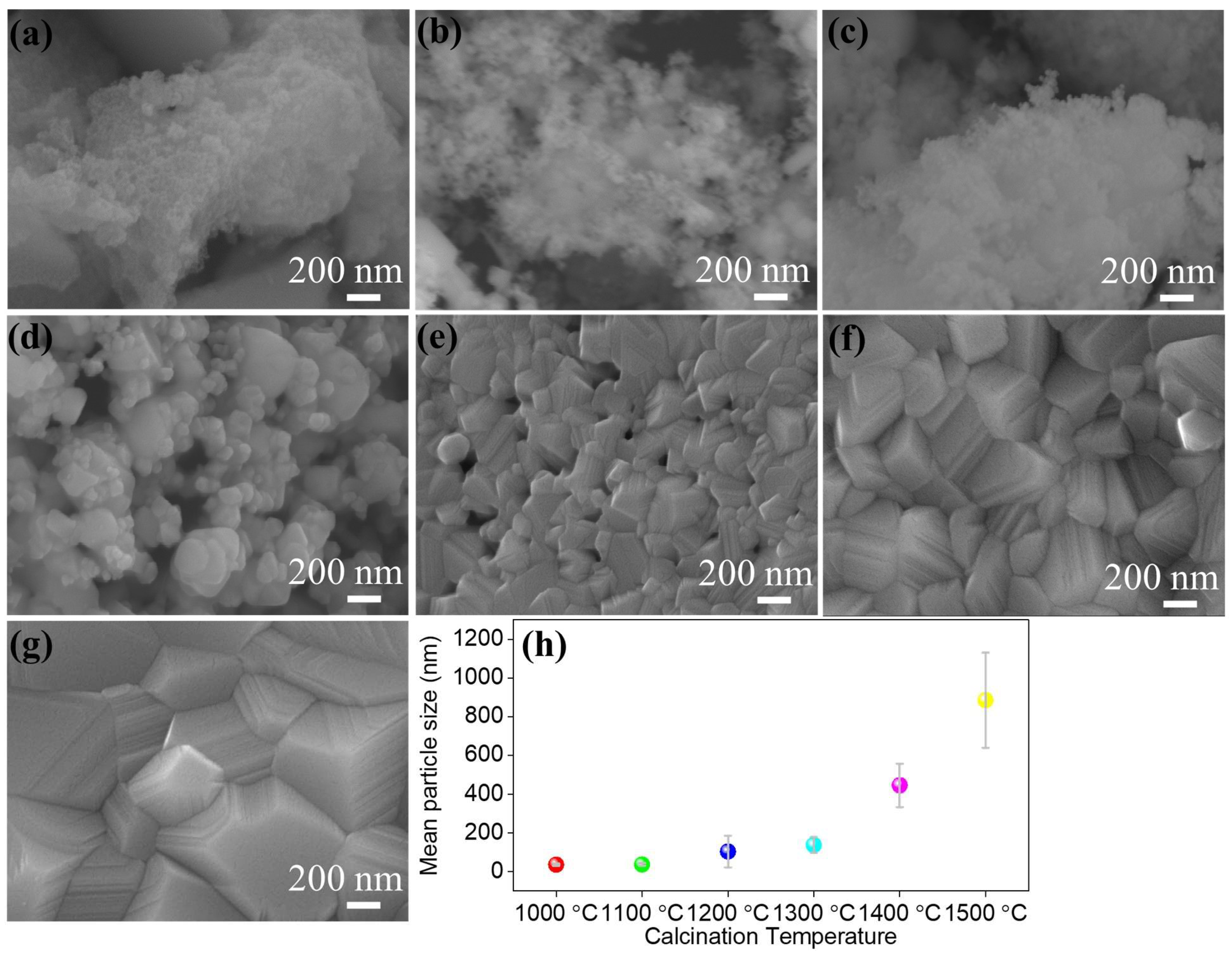
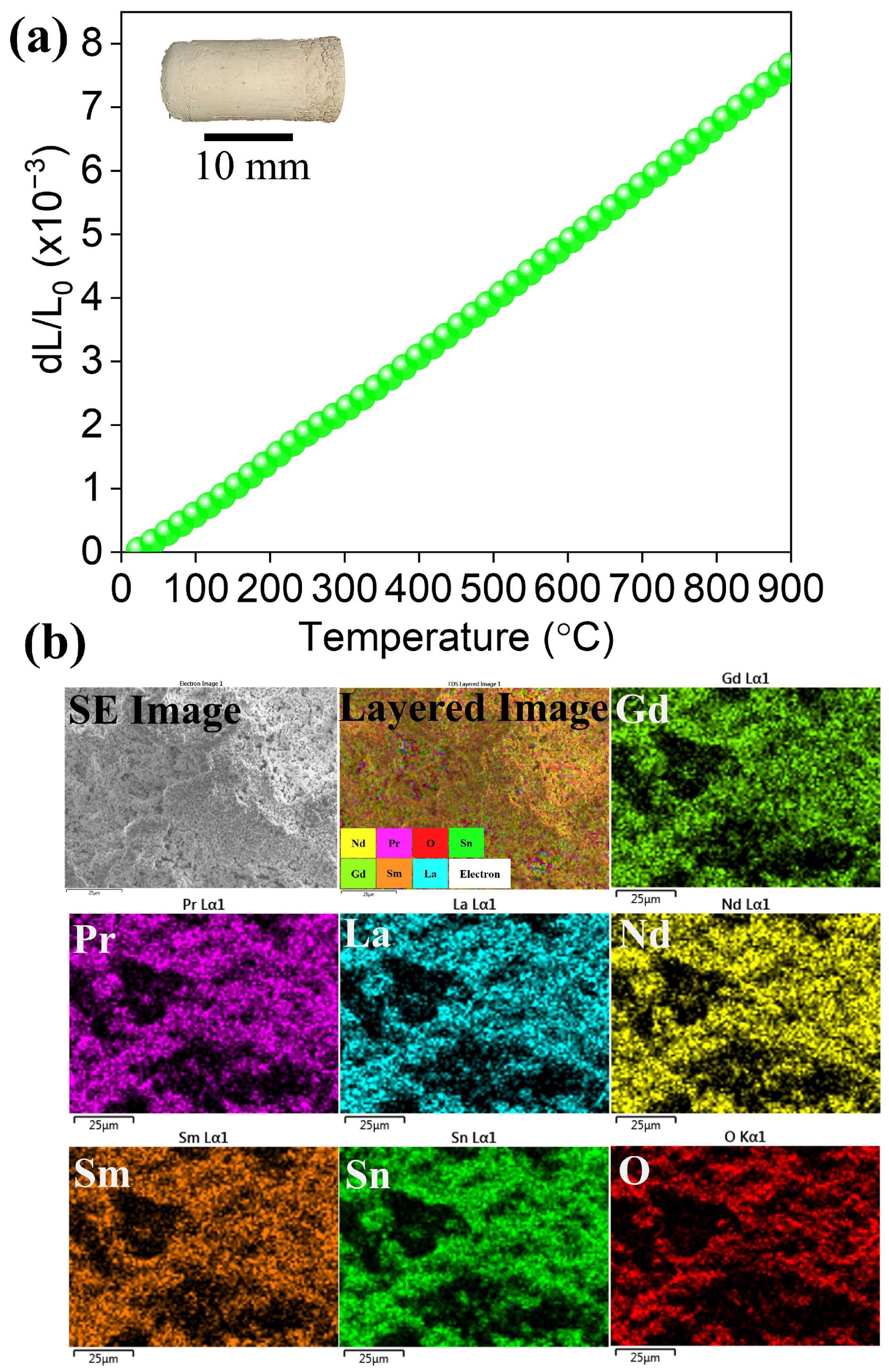
3. Results and Discussions
| S.No. | Composition | Thermal Expansion Coefficient ×10−6 K−1 | Reference |
|---|---|---|---|
| 1 | (Dy0.2Nd0.2Sm0.2Eu0.2Yb0.2)2Zr2O7 | 10.59 (1500 °C) | [12] |
| 2 | (La0.2Gd0.2Y0.2Sm0.2Ce0.2)2Zr2O7 | 11.1 (1000 °C) | [41] |
| 3 | (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Ce2O7 | 12 (1400 °C) | [11] |
| 4 | (La0.2Y0.2Sm0.2Eu0.2Gd0.2)2Zr2O7 | 11 (1200 °C) | [42] |
| 5 | (Y0.3Gd0.3Yb0.4)4Hf3O12 | 11 (1500 °C) | [43] |
| 6 | (La0.3Gd0.3Ca0.4)2(Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)2O7 | 9.0 (1200 °C) | [10] |
| 7 | Dy2(Ti0.2Zr0.2Hf0.2Ge0.2Sn0.2)2O7 | 10.3 | [30] |
| 8 | (La0.2Sm0.2Er0.2Yb 0.2Y0.2)2CexO3+2x (x = 4.4) | 13.12 (850 °C) | [39] |
| 9 | La2(Zr0.2Ce0.2Hf0.2Sn0.2Ti0.2)2O7 | 9.67 (1000 °C) | [44] |
| 10 | Er2(Y0.2Yb0.2Nb0.2Ta0.2Ce0.2)2O7 | 10.56 | [45] |
| 11 | La2(Zr0.2Ti0.2Y0.2YB0.2Nb0.2)2O7 | 9.374 (1000 °C) | [4] |
| 12 | (Gd0.2Nd0.2La0.2Pr0.2Sm0.2)2Sn2O7 | 8.702 (900 °C) | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDS | Energy Dispersive X-ray Spectroscopy |
| FESEM | Field-emission scanning electron microscopy |
| HEM | High-entropy materials |
| HEO | High-entropy oxide |
| TBC | Thermal barrier coating |
| W-H | Williamson–Hall |
| XRD | X-ray diffraction |
| YSZ | Yttrium-stabilized zirconium |
References
- Liu, L.; Wang, S.; Zhang, B.; Jiang, G.; Liu, H.; Yang, J.; Wang, J.; Liu, W. Present status and prospects of nanostructured thermal barrier coatings and their performance improvement strategies: A review. J. Manuf. Process. 2023, 97, 12–34. [Google Scholar] [CrossRef]
- Iqbal, A.; Moskal, G. Recent Development in Advance Ceramic Materials and Understanding the Mechanisms of Thermal Barrier Coatings Degradation. Arch. Comput. Methods Eng. 2023, 30, 4855–4896. [Google Scholar] [CrossRef]
- Che, J.; Wang, X.; Liu, X.; Liang, G.; Zhang, S. Outstanding sintering resistance in pyrochlore-type La2(Zr0.7Ce0.3)2O7 for thermal barrier coatings material. Ceram. Int. 2021, 47, 6996–7004. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, X.; Song, R.; Wang, N.; Zhang, Y. An Investigation of a La2(Zr0.2Ti0.2Y0.2Yb0.2Nb0.2)2O7 High Entropy Oxide Coating. J. Mater. Eng. Perform. 2024, 33, 11309–11320. [Google Scholar] [CrossRef]
- Sang, W.; Zhang, H.; Liu, S.; Xie, W.; Hou, R.; Li, S.; Ma, H.; Zhang, H.; Chen, X.; Liu, X.; et al. Influence of Lu3+ addition on the structure, mechanical and thermophysical properties of Gd3TaO7 oxide. Ceram. Int. 2023, 49, 34958–34968. [Google Scholar] [CrossRef]
- Yang, J.; Han, Y.; Shahid, M.; Pan, W.; Zhao, M.; Wu, W.; Wan, C. A promising material for thermal barrier coating: Pyrochlore-related compound Sm2FeTaO7. Scr. Mater. 2018, 149, 49–52. [Google Scholar] [CrossRef]
- Li, F.; Zhou, L.; Liu, J.-X.; Liang, Y.; Zhang, G.-J. High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials. J. Adv. Ceram. 2019, 8, 576–582. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Wang, S.; Yao, J.; Zhao, Q.; Xie, E.; Chen, G.; Wang, Z.; Liu, Z.; Wang, Y.; et al. Improvement strategy on thermophysical properties of A2B2O7-type rare earth zirconates for thermal barrier coatings applications: A review. Int. J. Miner. Metall. Mater. 2024, 31, 1147–1165. [Google Scholar] [CrossRef]
- Keyvani, A.; Mahmoudinezhad, P.; Jahangiri, A.; Bahamirian, M. Synthesis and characterization of ((La1-xGdx)2Zr2O7; x = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 1) nanoparticles for advanced TBCs. J. Aust. Ceram. Soc. 2020, 56, 1543–1550. [Google Scholar] [CrossRef]
- Zhao, Z.; Ruan, Z.; Li, R.; Yan, S.; Sun, X.; Liu, C.; Zhang, D.; Xu, B.; Ren, Z.; Wang, M.; et al. High entropy pyrochlore (La0.3Gd0.3Ca0.4)2(Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)2O7 ceramic with amorphous-like thermal conductivity for environmental/thermal barrier coating applications. J. Mater. Sci. Technol. 2025, 205, 315–326. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, X.; An, Y.; Wang, Y.; Gao, M.; Zhou, H.; Chen, J. High-entropy (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Ce2O7: A potential thermal barrier material with improved thermo-physical properties. J. Adv. Ceram. 2022, 11, 615–628. [Google Scholar] [CrossRef]
- Luo, X.; Huang, S.; Huang, R.; Xu, C.; Hou, S.; Jin, H. Highly anti-sintering and toughened pyrochlore (Dy0.2Nd0.2Sm0.2Eu0.2Yb0.2)2Zr2O7 high-entropy ceramic for advanced thermal barrier coatings. Ceram. Int. 2023, 49, 23410–23416. [Google Scholar] [CrossRef]
- Fuentes, A.F.; Montemayor, S.M.; Maczka, M.; Lang, M.; Ewing, R.C.; Amador, U. A Critical Review of Existing Criteria for the Prediction of Pyrochlore Formation and Stability. Inorg. Chem. 2018, 57, 12093–12105. [Google Scholar] [CrossRef] [PubMed]
- Jitta, R.R.; Gundeboina, R.; Veldurthi, N.K.; Guje, R.; Muga, V. Defect pyrochlore oxides: As photocatalyst materials for environmental and energy applications—A review. J. Chem. Technol. Biotechnol. 2015, 90, 1937–1948. [Google Scholar] [CrossRef]
- Gayen, P.; Saha, S.; Ramani, V. Pyrochlores for Advanced Oxygen Electrocatalysis. Acc. Chem. Res. 2022, 55, 2191–2200. [Google Scholar] [CrossRef]
- Anantharaman, A.P.; Dasari, H.P. Potential of pyrochlore structure materials in solid oxide fuel cell applications. Ceram. Int. 2021, 47, 4367–4388. [Google Scholar] [CrossRef]
- Anandkumar, M.; Trofimov, E. Synthesis, Properties, and Applications of High-Entropy Oxide Ceramics: Current Progress and Future Perspectives. J. Alloys Compd. 2023, 960, 170690. [Google Scholar] [CrossRef]
- Jiao, Y.; Dai, J.; Fan, Z.; Cheng, J.; Zheng, G.; Grema, L.; Zhong, J.; Li, H.-F.; Wang, D. Overview of high-entropy oxide ceramics. Mater. Today 2024, 77, 92–117. [Google Scholar] [CrossRef]
- Fracchia, M.; Coduri, M.; Ghigna, P.; Anselmi-Tamburini, U. Phase stability of high entropy oxides: A critical review. J. Eur. Ceram. Soc. 2024, 44, 585–594. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Liao, M.; Weng, J.; Shen, J.; Chen, Y.; Du, Y. Novel nano spinel-type high-entropy oxide (HEO) catalyst for hydrogen production using ethanol steam reforming. Nanoscale 2023, 15, 8619–8632. [Google Scholar] [CrossRef]
- Anandkumar, M.; Kannan, P.K.; Sudarsan, S.; Uchaev, D.A.; Trofimov, E.A. Reusable high-entropy oxide environmental photocatalyst towards toxic Cr(VI) reduction with tailored bandgap via solution combustion synthesis. Adv. Powder Technol. 2024, 35, 104429. [Google Scholar] [CrossRef]
- Akrami, S.; Murakami, Y.; Watanabe, M.; Ishihara, T.; Arita, M.; Fuji, M.; Edalati, K. Defective high-entropy oxide photocatalyst with high activity for CO2 conversion. Appl. Catal. B Environ. 2022, 303, 120896. [Google Scholar] [CrossRef]
- Anandkumar, M.; Kannan, P.K.; Sudarsan, S.; Trofimov, E.A. High-entropy oxide (CeGdHfPrZr)O2 nanoparticles as reusable photocatalyst for wastewater remediation. Surf. Interfaces 2024, 51, 104815. [Google Scholar] [CrossRef]
- Kante, M.V.; Weber, M.L.; Ni, S.; van den Bosch, I.C.G.; van der Minne, E.; Heymann, L.; Falling, L.J.; Gauquelin, N.; Tsvetanova, M.; Cunha, D.M.; et al. A High-Entropy Oxide as High-Activity Electrocatalyst for Water Oxidation. ACS Nano 2023, 17, 5329–5339. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.X.; Liao, Y.-C.; Lin, C.-C.; Su, Y.-H.; Ting, J.-M. Advanced High Entropy Perovskite Oxide Electrocatalyst for Oxygen Evolution Reaction. Adv. Funct. Mater. 2021, 31, 2101632. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Duan, C.; Mao, J.; Dong, Y.; Dong, K.; Wang, Z.; Luo, S.; Liu, Y.; Qi, X. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. J. Alloys Compd. 2020, 844, 156158. [Google Scholar] [CrossRef]
- Anandkumar, M.; Kannan, P.K.; Morozov, R.S.; Zaitseva, O.V.; Sudarsan, S.; Trofimov, E.A. Electrochemical detection of p-nitrophenol using glassy carbon electrode modified using high-entropy oxide nanoparticles. Ceram. Int. 2025, 51, 2770–2778. [Google Scholar] [CrossRef]
- Jiang, T.; Wu, F.; Ren, Y.; Qiu, J.; Chen, Z. Pyrochlore phase (Y,Dy,Ce,Nd,La)2Sn2O7 as a superb anode material for lithium-ion batteries. J. Solid State Electrochem. 2023, 27, 763–772. [Google Scholar] [CrossRef]
- Trofimov, E.; Ostovari Moghaddam, A.; Litvinyuk, K.; Anandkumar, M.; Efimova, M.; Mikhailov, D.; Zaitseva, O. Synthesis and characterization of the RE2A2OTr oxides with an ultrahigh-entropy sublattice occupied by rare-earth elements. Mater. Lett. 2025, 379, 137668. [Google Scholar] [CrossRef]
- Vayer, F.; Decorse, C.; Bérardan, D.; Dragoe, N. New entropy-stabilized oxide with pyrochlore structure: Dy2(Ti0.2Zr0.2Hf0.2Ge0.2Sn0.2)2O7. J. Alloys Compd. 2021, 883, 160773. [Google Scholar] [CrossRef]
- Anandkumar, M.; Kesavan, K.P.; Sudarsan, S.; Zaitseva, O.V.; Ostovari Moghaddam, A.; Iarushina, D.V.; Trofimov, E.A. Band-Gap Engineering of High-Entropy Fluorite Metal Oxide Nanoparticles Facilitated by Pr3+ Incorporation by Gel Combustion Synthesis. Gels 2025, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Matović, B.; Zagorac, D.; Cvijović-Alagić, I.; Zagorac, J.; Butulija, S.; Erčić, J.; Hanzel, O.; Sedlák, R.; Lisnichuk, M.; Tatarko, P. Fabrication and characterization of high entropy pyrochlore ceramics. Boletín Soc. Española Cerámica Vidr. 2023, 62, 66–76. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, K.; Li, Y.; Jiang, X. Preparation and properties of high-entropy pyrochlore A2Ti2O7 with multi-elements at A site. Ceram. Int. 2024, 50, 1–8. [Google Scholar] [CrossRef]
- He, J.; He, G.; Wang, P.; Xu, L.; Liu, J.; Tao, J. Pyrochlore–fluorite dual-phase high-entropy RE2(Ce0.2Zr0.2Hf0.2Sn0.2Ti0.2)2O7 (RE2HE2O7, RE = La, Nd, Sm, Eu, Gd, Dy) ceramics with glass-like thermal conductivity. J. Mater. Sci. 2022, 57, 17563–17576. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, F.; Du, H.; Jin, L.; Yan, L.; Hu, Q.; Yu, Y.; Qu, S.; Wei, X.; Xu, Z.; et al. Grain size engineered lead-free ceramics with both large energy storage density and ultrahigh mechanical properties. Nano Energy 2019, 58, 768–777. [Google Scholar] [CrossRef]
- Mou, H.; Zhao, H.; Tian, H.; Ma, G.; Liu, M.; Wang, H.; Xie, F.; Cai, Z. Effects of hBN content and particle size on microstructure, mechanical and tribological properties of NiCr-Cr3C2-hBN coatings. Surf. Coat. Technol. 2024, 478, 130330. [Google Scholar] [CrossRef]
- Xiao, Y.; Cheng, D.; Li, G.; Yin, R.; Li, P.; Gao, Z. Preparation of MgO ceramics by low temperature sintering with MgF2 and Al2O3 as sintering additives. J. Electroceramics 2025, in press. [Google Scholar] [CrossRef]
- Tong, M.; Hou, W.; Shi, X.; Han, D.; Feng, T.; Fu, Q. The effect of Hf6Ta2O17 self-sintering on the cyclic ablation and mechanical performances of C/Hf-Ta-Si-C composites with a PyC-SiC bilayer interphase. Compos. Part B Eng. 2025, 294, 112149. [Google Scholar] [CrossRef]
- Xu, L.; Su, L.; Wang, H.; Gao, H.; Lu, D.; Peng, K.; Niu, M.; Cai, Z. Tuning stoichiometry of high-entropy oxides for tailorable thermal expansion coefficients and low thermal conductivity. J. Am. Ceram. Soc. 2022, 105, 1548–1557. [Google Scholar] [CrossRef]
- Zhang, D.; Liao, K.; Yu, Y.; Tian, Z.; Cao, Y. Microstructure and thermal & mechanical properties of La2Zr2O7@YSZ composite ceramic. Ceram. Int. 2020, 46, 4737–4747. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, N.; Song, R.; Zhou, M.; Tang, X.; Zhang, Y. A new TBC material: (La0.2Gd0.2Y0.2Sm0.2Ce0.2)2Zr2O7 high-entropy oxide. Ceram. Int. 2024, 50, 2490–2500. [Google Scholar] [CrossRef]
- Fu, S.; Jia, Z.; Wan, D.; Bao, Y. Synthesis, microstructure and thermophysical properties of (La0.2Y0.2Sm0.2Eu0.2Gd0.2)2Zr2O7 high-entropy oxide ceramic. Ceram. Int. 2024, 50, 5510–5515. [Google Scholar] [CrossRef]
- Ye, F.; Luo, T.; Meng, F.; Guo, L. Structure, thermal and mechanical properties of mid-entropy thermal barrier ceramic (Y0.3Gd0.3Yb0.4)4Hf3O12 prepared by ultrafast high-temperature sintering. Ceram. Int. 2024, 50, 181–187. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, Y.; Feng, X.; Tian, Z.; Song, R. Thermal barrier coatings with high-entropy oxide as a top coat. Ceram. Int. 2022, 48, 1349–1359. [Google Scholar] [CrossRef]
- Xu, L.; Su, L.; Wang, H.; Niu, M.; Zhuang, L.; Peng, K.; Fan, X.; Gao, H.; Lu, D. Phase evolution and thermophysical properties of high-entropy RE2(Y0.2Yb0.2Nb0.2Ta0.2Ce0.2)2O7 oxides. J. Am. Ceram. Soc. 2022, 105, 5490–5500. [Google Scholar] [CrossRef]
- Weber, W.J.; Kinsler-Fedon, C.; Keppens, V.; Zhang, Y.; Mir, A.H. Temperature dependence of irradiation-induced amorphization in a high-entropy titanate pyrochlore. MRS Commun. 2024, 14, 1364–1370. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, X.; Jiang, M.; Liu, X.; Qian, Z.; Zhang, Q.; Qiao, Y. Design and synthesis of high-entropy A2B2O7-type pyrochlore ceramics for the immobilization of molten salt radwastes. Ceram. Int. 2024, 50, 52640–52648. [Google Scholar] [CrossRef]
- Matović, B.; Belozerova, N.M.; Kozlenko, D.P.; Zel, I.Y.; Maletaškić, J.; Zagorac, D.; Butulija, S.; Cvijović-Alagić, I. High-pressure behavior of high-entropy A2B2O7 pyrochlore. Ceram. Int. 2024, 50, 52649–52654. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, L.; Miao, X.; Yang, L.; Zhou, S. A new-type high-entropy electrocatalyst with a pyrochlore structure for acid-water oxidation. J. Mater. Chem. A 2024, 12, 12785–12794. [Google Scholar] [CrossRef]
- Teng, Z.; Wang, P.; Zeng, S.; Feng, W.; Chen, C.; Jia, P.; Tan, Y.; Peng, S. Reactive spark plasma sintering of high-entropy (La1/7Nd1/7Sm1/7Eu1/7Gd1/7Dy1/7Ho1/7)2Zr2O7 pyrochlore ceramic. Ceram. Int. 2024, 50, 6892–6897. [Google Scholar] [CrossRef]
- Guo, D.; Zhou, F.; Xu, B.; Wang, Y.; Wang, Y. High-entropy (La0.2Nd0.2Sm0.2Gd0.2Yb0.2)2(Zr0.75Ce0.25)2O7 thermal barrier coating material with significantly enhanced fracture toughness. Chin. J. Aeronaut. 2023, 36, 556–564. [Google Scholar] [CrossRef]
- Li, Y.; Lei, Y.; Zhao, S.; Xiao, H.; Liu, H.; Wang, Y.; Luo, Y.; Zhang, J.; Wang, J.; Ewing, R.C.; et al. Phase transformation and radiation resistance of B-site high entropy pyrochlores. Scr. Mater. 2023, 229, 115367. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, R.; Feng, S.; Fu, J.; Yang, Y.; Wang, H.; Hao, Z.; Li, J. High-entropy (Ho0.2Y0.2Dy0.2Gd0.2Eu0.2)2Ti2O7/TiO2 composites with excellent mechanical and thermal properties. J. Eur. Ceram. Soc. 2023, 43, 6398–6406. [Google Scholar] [CrossRef]
- Zhao, G.; Cai, S.; Zhang, Y.; Gu, H.; Xu, C. Reactive flash sintering of high-entropy oxide (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7: Microstructural evolution and aqueous durability. J. Eur. Ceram. Soc. 2023, 43, 2593–2600. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, J.; Ye, S.; Yang, K.; Li, M.; Wang, H.; He, J. High-entropy rare earth titanates with low thermal conductivity designed by lattice distortion. J. Am. Ceram. Soc. 2023, 106, 6279–6291. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, X.; Zhang, P.; Li, Z.; Xu, J.; Reece, M.J.; Gao, F. Dual-phase rare-earth-zirconate high-entropy ceramics with glass-like thermal conductivity. J. Eur. Ceram. Soc. 2021, 41, 2861–2869. [Google Scholar] [CrossRef]
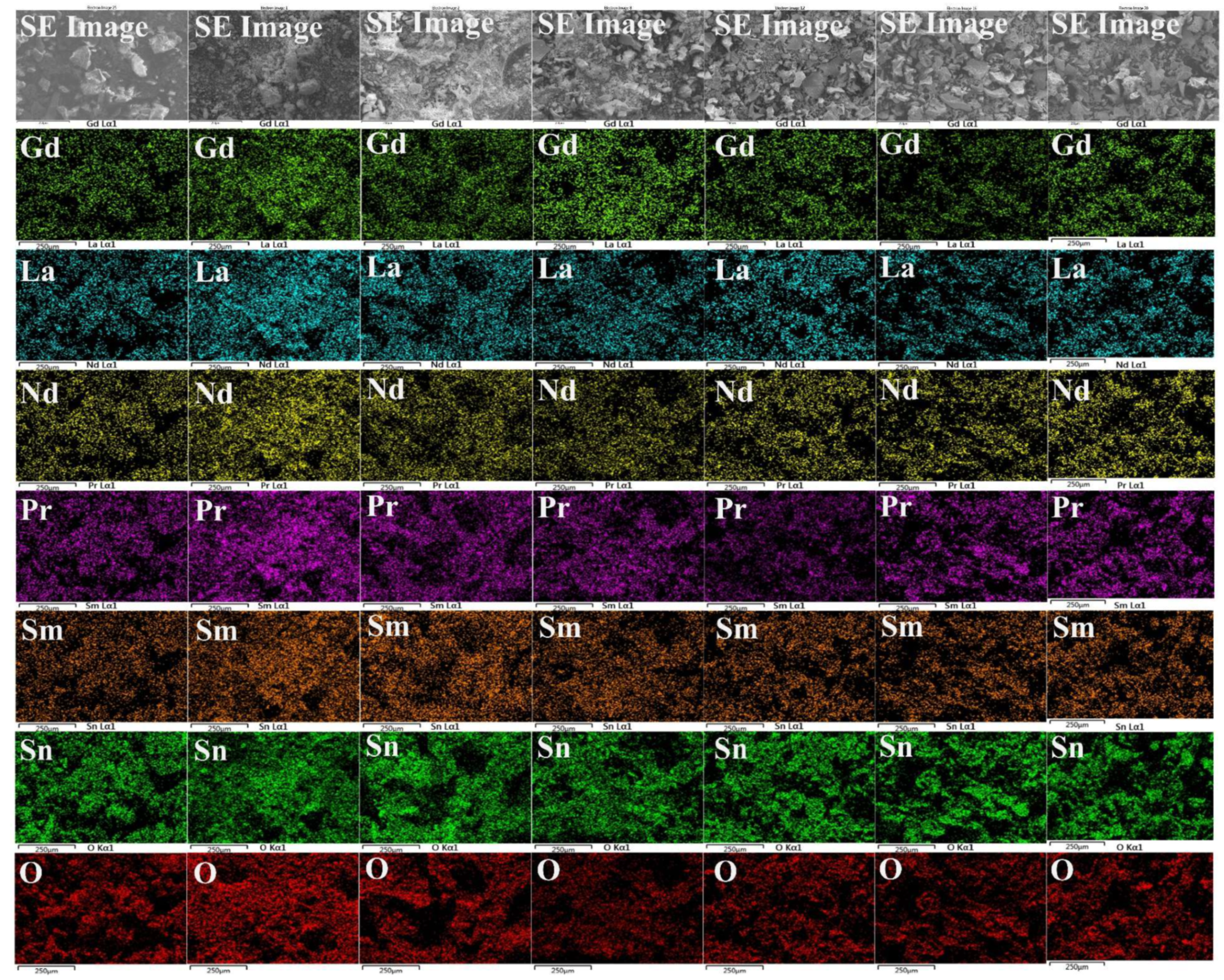
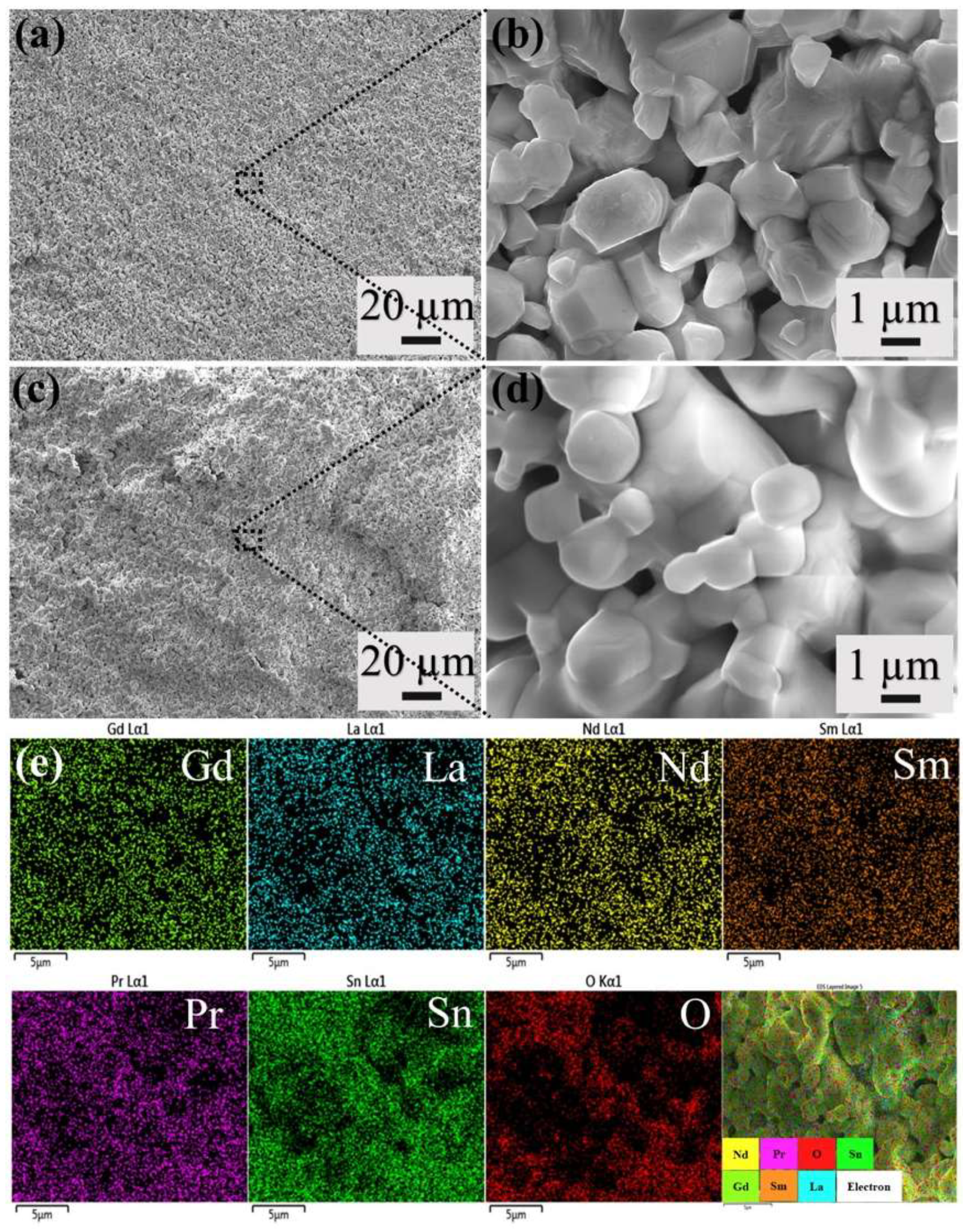
| Calcination Temperature (°C) | Element (at %) | |||||
|---|---|---|---|---|---|---|
| Sn | La | Pr | Nd | Sm | Gd | |
| As-synthesized | 38.92 | 13.81 | 11.68 | 12.47 | 11.68 | 11.45 |
| 1000 | 41.44 | 12.90 | 11.93 | 11.06 | 11.26 | 11.41 |
| 1100 | 43.37 | 11.29 | 11.31 | 11.12 | 11.56 | 11.35 |
| 1200 | 47.72 | 11.34 | 9.92 | 9.18 | 11.01 | 10.82 |
| 1300 | 48.60 | 11.98 | 9.77 | 8.86 | 10.13 | 10.65 |
| 1400 | 49.59 | 10.30 | 10.02 | 9.12 | 10.35 | 10.62 |
| 1500 | 50.18 | 10.97 | 9.38 | 9.83 | 9.64 | 9.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anandkumar, M.; Kesavan, K.P.; Sudarsan, S.; Zhivulin, D.E.; Shaburova, N.A.; Ostovari Moghaddam, A.; Litvinyuk, K.S.; Trofimov, E.A. Phase Evolution of High-Entropy Stannate Pyrochlore Oxide Synthesized via Glycine-Assisted Sol–Gel Synthesis as a Thermal Barrier Coating Material. Nanomaterials 2025, 15, 939. https://doi.org/10.3390/nano15120939
Anandkumar M, Kesavan KP, Sudarsan S, Zhivulin DE, Shaburova NA, Ostovari Moghaddam A, Litvinyuk KS, Trofimov EA. Phase Evolution of High-Entropy Stannate Pyrochlore Oxide Synthesized via Glycine-Assisted Sol–Gel Synthesis as a Thermal Barrier Coating Material. Nanomaterials. 2025; 15(12):939. https://doi.org/10.3390/nano15120939
Chicago/Turabian StyleAnandkumar, Mariappan, Kannan Pidugu Kesavan, Shanmugavel Sudarsan, Dmitry Evgenievich Zhivulin, Natalia Aleksandrovna Shaburova, Ahmad Ostovari Moghaddam, Ksenia Sergeevna Litvinyuk, and Evgeny Alekseevich Trofimov. 2025. "Phase Evolution of High-Entropy Stannate Pyrochlore Oxide Synthesized via Glycine-Assisted Sol–Gel Synthesis as a Thermal Barrier Coating Material" Nanomaterials 15, no. 12: 939. https://doi.org/10.3390/nano15120939
APA StyleAnandkumar, M., Kesavan, K. P., Sudarsan, S., Zhivulin, D. E., Shaburova, N. A., Ostovari Moghaddam, A., Litvinyuk, K. S., & Trofimov, E. A. (2025). Phase Evolution of High-Entropy Stannate Pyrochlore Oxide Synthesized via Glycine-Assisted Sol–Gel Synthesis as a Thermal Barrier Coating Material. Nanomaterials, 15(12), 939. https://doi.org/10.3390/nano15120939









