Long-Range Interactions Between Neighboring Nanoparticles Tuned by Confining Membranes
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
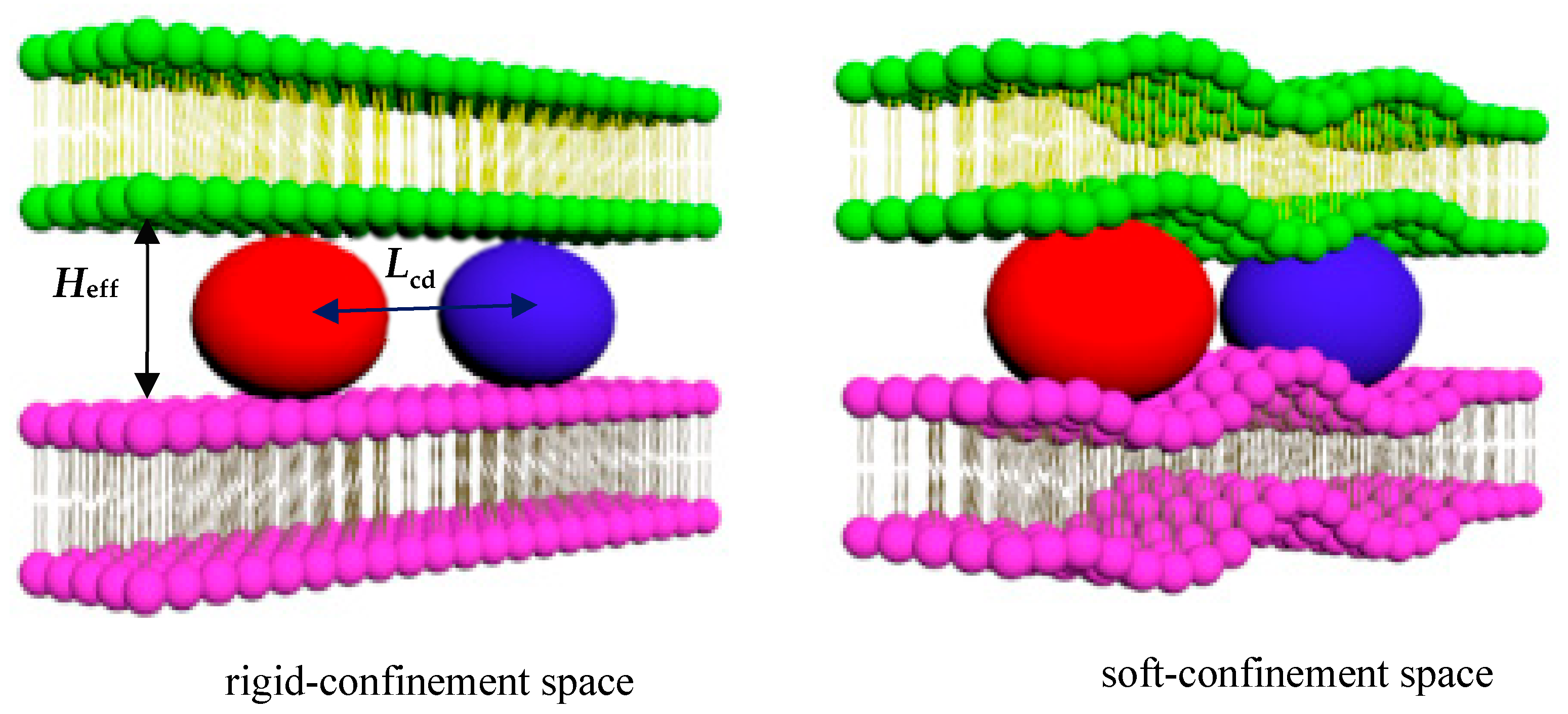
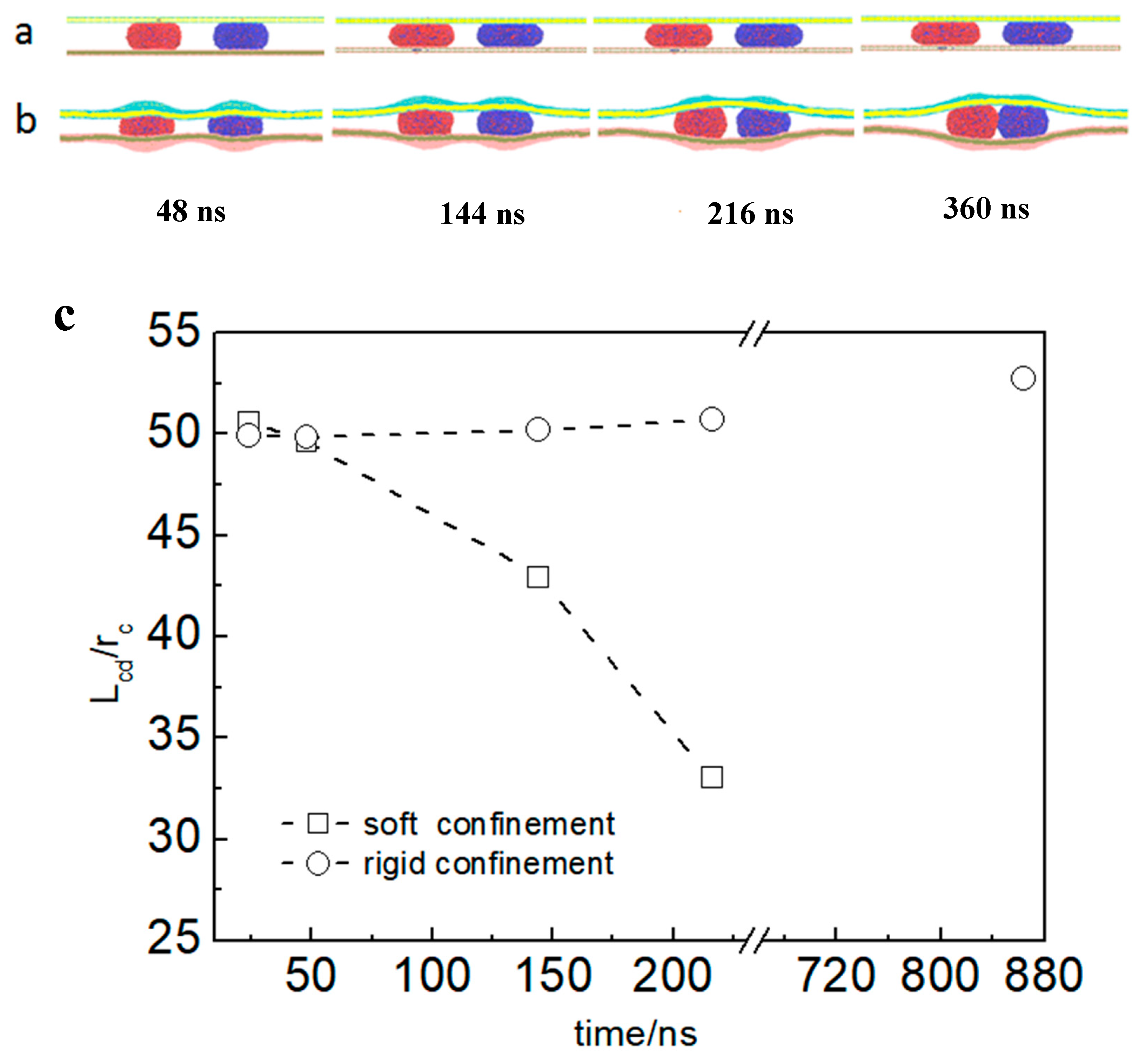
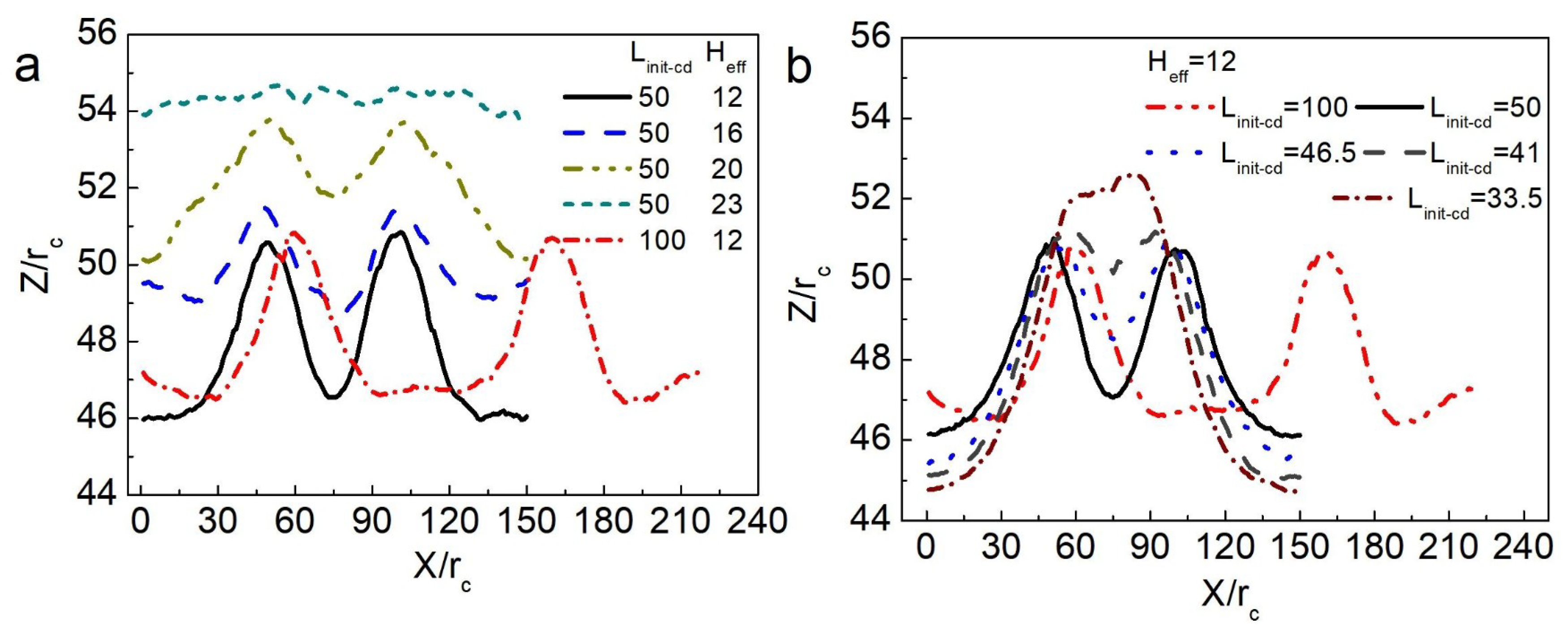
3.1. Directional Movement of Neighboring Vesicles Tuned by Confining Membranes
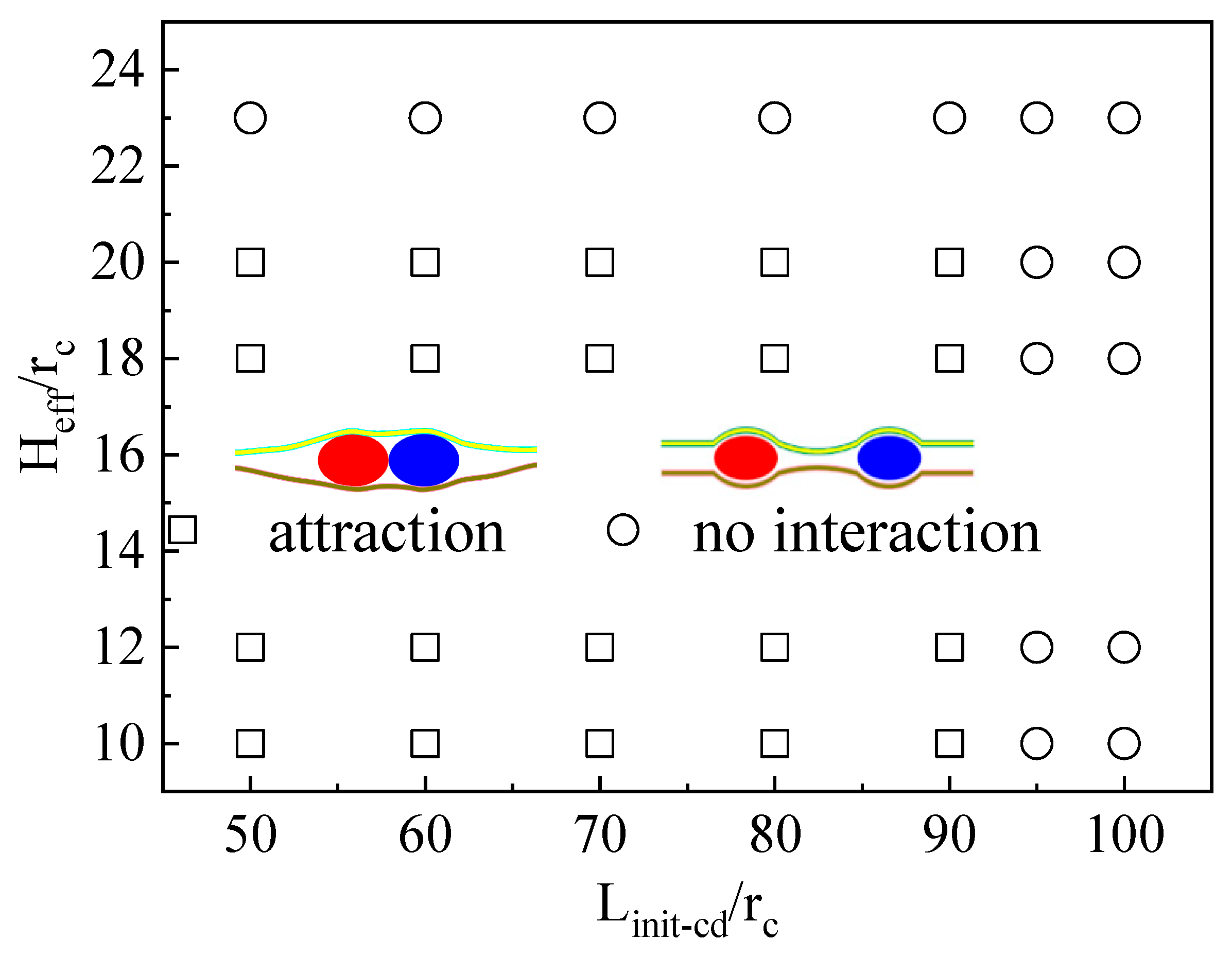
3.2. The Confinement of Asymmetric Deformation Causes Long-Range Interactions Between Neighboring Vesicles
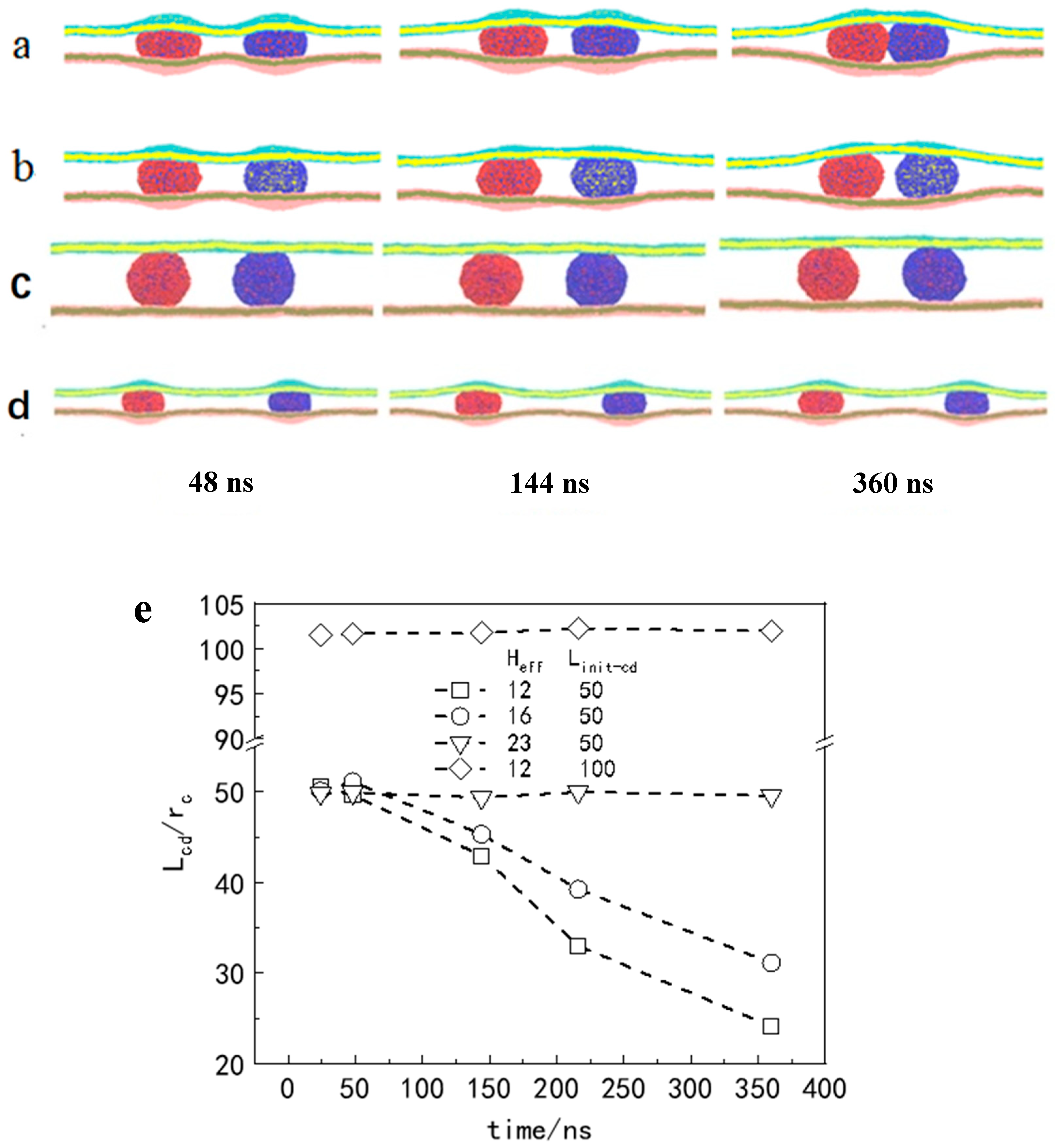
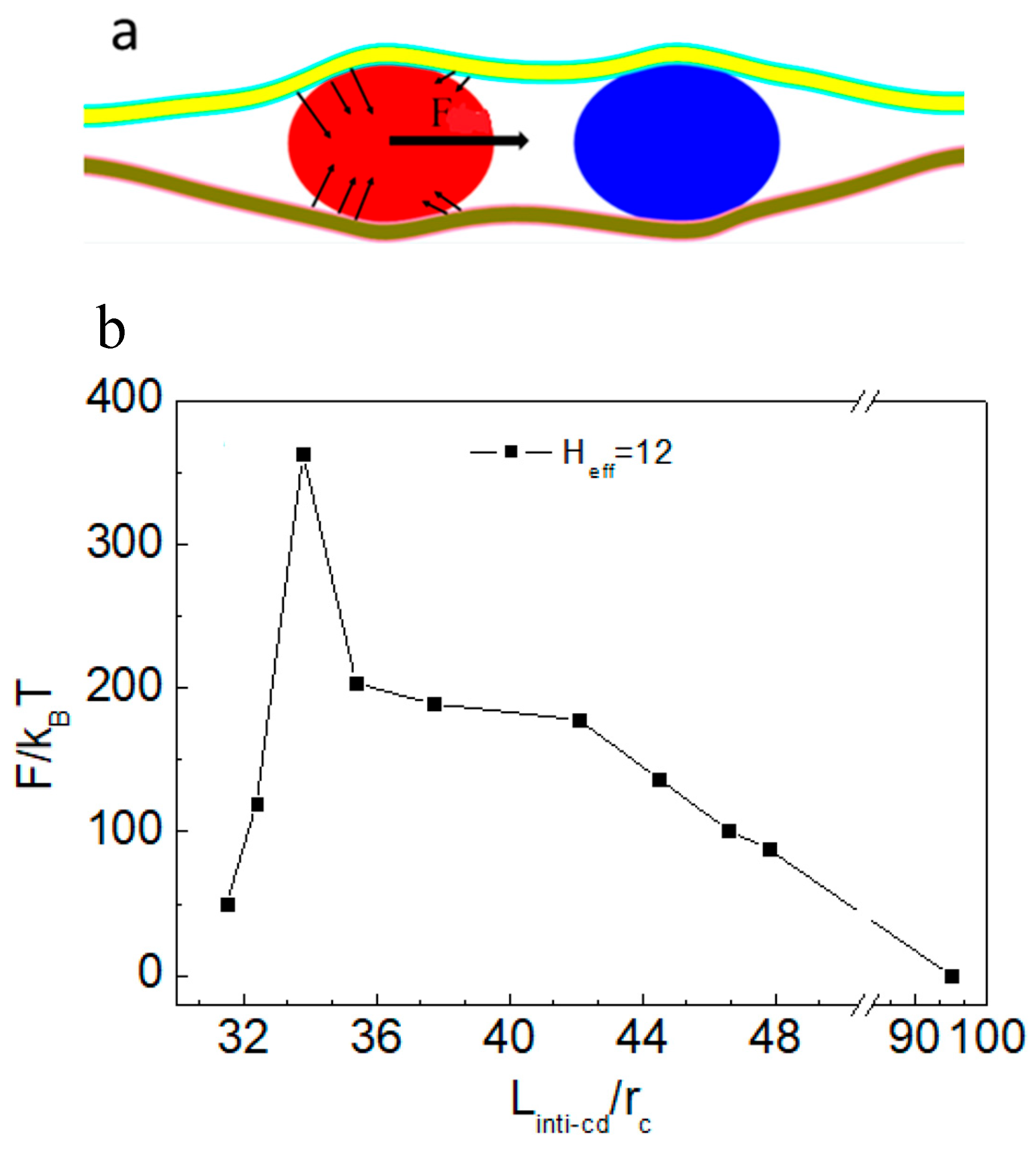
3.3. Force Analysis of Vesicles in Soft Confined Space
3.4. Motion of Multiple Particles in a Soft Confined Space
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Yue, H.; Zhai, X.; Huang, Z.; Ma, G.; Wei, W.; Yan, L. Transport of agraphene nanosheet sandwiched inside cell membranes. Sci. Adv. 2019, 5, eaaw3192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Liu, X.; Sun, H.; Yue, T.; Zhang, X.; Yuan, B.; Cao, D. Design of Small Nanoparticles Decorated with Amphiphilic Ligands: Self-Preservation Effect and Translocation into a Plasma Membrane. ACS Appl. Mater. Interfaces 2019, 11, 23822–23831. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Tajima, M.; Nakamura, H.; Ohsaki, S.; Watano, S. Effect of cholesterol on nanoparticle translocation across a lipid bilayer. Phys. Chem. Chem. Phys. 2024, 26, 21229–21239. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef]
- Davis, D.M.; Sowinski, S. Membrane nanotubes: Dynamic long-distance connections between animal cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 431–436. [Google Scholar] [CrossRef]
- Sharma, S.; Masud, M.K.; Kaneti, Y.V.; Rewatkar, P.; Koradia, A.; Hossain, M.S.A.; Yamauchi, Y.; Popat, A.; Salomon, C. Extracellular vesicle nanoarchitectonics for novel drug delivery applications. Small 2021, 17, 2102220. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Sedgwick, A.E.; D’Souza-Schorey, C. The biology of extracellular microvesicles. Traffic 2018, 19, 319–327. [Google Scholar] [CrossRef]
- Sowinski, S.; Jolly, C.; Berninghausen, O.; Purbhoo, M.A.; Chauveau, A.; Köhler, K.; Oddos, S.; Eissmann, P.; Brodsky, F.M.; Hopkins, C.; et al. Membrane Nanotubes Physically Connect T Cells over Long Distances Presenting a Novel Route for HIV-1 Transmission. Nat. Cell Biol. 2018, 10, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Liu, S.L.; Tian, Z.Q.; Zhang, Z.L.; Tang, H.W.; Pang, D.W. Myosin-Driven Intercellular Transportation of Wheat Germ Agglutinin Mediated by Membrane Nanotubes Between Human Lung Cancer Cells. ACS Nano 2012, 6, 10033–10041. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, A.; Aucher, A.; Eissmann, P.; Vivier, E.; Davis, D.M. Membrane Nanotubes Facilitate LongDistance Interactions Between Natural Killer Cells and Target Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5545–5550. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.; Evergren, E.; Golushko, I.; Prévost, C.; Renard, H.; Johannes, L.; McMahon, H.T.; Lorman, V.; Voth, G.A.; Bassereau, P. How curvature-generating proteins build scaffolds on membrane nanotubes. Proc. Natl. Acad. Sci. USA 2016, 113, 11226–11231. [Google Scholar] [CrossRef]
- Noguchi, H.; Tozzi, C.; Arroyo, M. Binding of anisotropic curvature-inducing proteins onto membrane tubes. Soft Matter 2022, 18, 3384–3394. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Yue, T.; Zhang, X.; Zhong, C. Exploring the shape deformation of biomembrane tubes with theoretical analysis and computer simulation. Soft Matter 2016, 12, 9077–9085. [Google Scholar] [CrossRef]
- Karal, M.A.S.; Billah, M.M.; Ahmed, M.; Ahamed, M.K. A review on the measurement of the bending rigidity of lipid membranes. Soft Matter 2023, 19, 8285–8304. [Google Scholar] [CrossRef]
- Lee, T.H.; Charchar, P.; Separovic, F.; Reid, G.E.; Yarovsky, I.; Aguilar, M.-I. The intricate link between membrane lipid structure and composition and membrane structural properties in bacterial membranes. Chem. Sci. 2024, 15, 3408–3427. [Google Scholar] [CrossRef]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef]
- Hurtig, J.; Chiu, D.T.; Önfelt, B. Intercellular nanotubes: Insights from imaging studies and beyond. WIREs Nanomed. Nanobiotechnol. 2010, 2, 260–276. [Google Scholar] [CrossRef]
- Epperla, C.P.; Mohan, N.; Chang, C.W.; Chen, C.-C.; Chang, H.C. Nanodiamond-mediated intercellular transport of proteins through membrane tunneling nanotubes. Small 2016, 11, 6097–6105. [Google Scholar] [CrossRef] [PubMed]
- Onfelt, B.; Davis, D.M. Can membrane nanotubes facilitate communication between immune cells? Biochem. Soc. Trans. 2004, 32, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Dubey, G.P.; Benyehuda, S. Intercellular nanotubes mediate bacterial communication. Cell 2011, 144, 590–600. [Google Scholar] [CrossRef]
- Karlsson, A.; Karlsson, R.; Karlsson, M.; Cans, A.S.; Strömberg, A.; Ryttsén, F.; Orwar, O. Molecular engineering: Networks of nanotubes and containers. Nature 1999, 409, 150–152. [Google Scholar] [CrossRef]
- Khan, F.A.; Albalawi, R.; Pottoo, F.H. Trends in targeted delivery of nanomaterials in colon cancer diagnosis and treatment. Med. Res. Rev. 2022, 42, 227–258. [Google Scholar] [CrossRef]
- Marquez, C.A.; Oh, C.I.; Ahn, G.; Shin, W.R.; Kim, Y.H.; Ahn, J.Y. Synergistic vesicle-vector systems for targeted delivery. J. Nanobiotechnol. 2024, 22, 6. [Google Scholar] [CrossRef]
- Zhao, S.; Di, Y.; Fan, H.; Xu, C.; Li, H.; Wang, Y.; Wang, W.; Li, C.; Wang, J. Targeted delivery of extracellular vesicles: The mechanisms, techniques and therapeutic applications. Mol. Biomed. 2024, 5, 60. [Google Scholar] [CrossRef]
- Xiong, K.; Zhao, J.; Yang, D.; Cheng, Q.; Wang, J.; Ji, H. Cooperative wrapping of nanoparticles of various sizes and shapes by lipid membranes. Soft Matter 2017, 13, 4644–4652. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Yue, T.; Zhang, X.; Zhong, C. Pulling force and surface tension drive membrane fusion. J. Chem. Phys. 2017, 147, 194703. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Sreekumari, A.; Lipowsky, R. Lipids with bulky head groups generate large membrane curvatures by small compositional asymmetries. J. Chem. Phys. 2018, 149, 084901. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, Y.; Lin, H.; Zhang, C.; Liu, Z.; Yu, L.; Wang, X.; Lin, Y. Novel multiscale simulations on the membrane formation via hybrid induced phase separation process based on dissipative particle dynamics. Sep. Purif. Technol. 2023, 314, 123614. [Google Scholar] [CrossRef]
- Mitsuhashi, H.; Morikawa, R.; Noguchi, Y.; Takasu, M. Dissipative particle dynamics simulations for shape change of growing lipid bilayer vesicles. Life 2023, 13, 306. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Tian, F.; Yue, T.; Yang, K.; Zhang, X. Long-Range Interactions Between Neighboring Nanoparticles Tuned by Confining Membranes. Nanomaterials 2025, 15, 912. https://doi.org/10.3390/nano15120912
Liu X, Tian F, Yue T, Yang K, Zhang X. Long-Range Interactions Between Neighboring Nanoparticles Tuned by Confining Membranes. Nanomaterials. 2025; 15(12):912. https://doi.org/10.3390/nano15120912
Chicago/Turabian StyleLiu, Xuejuan, Falin Tian, Tongtao Yue, Kai Yang, and Xianren Zhang. 2025. "Long-Range Interactions Between Neighboring Nanoparticles Tuned by Confining Membranes" Nanomaterials 15, no. 12: 912. https://doi.org/10.3390/nano15120912
APA StyleLiu, X., Tian, F., Yue, T., Yang, K., & Zhang, X. (2025). Long-Range Interactions Between Neighboring Nanoparticles Tuned by Confining Membranes. Nanomaterials, 15(12), 912. https://doi.org/10.3390/nano15120912








