Phospholipid/HP-β-CD Hybrid Nanosystems Amplify Neohesperidin Bioavailability via Dual Enhancement of Solubility and Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PC-NH and NH-PC-CD Nanocomposites
2.3. Average Particle Size and Polydispersity Coefficient
2.4. Entrapment Efficiency of Neohesperidin
2.5. Characterization
2.5.1. Nanocomplex Characterization
2.5.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.5.3. Powder X-ray Diffraction (PXRD)
2.5.4. Differential Scanning Calorimetry (DSC)
2.6. Storage Stability
2.7. Antioxidant Activity
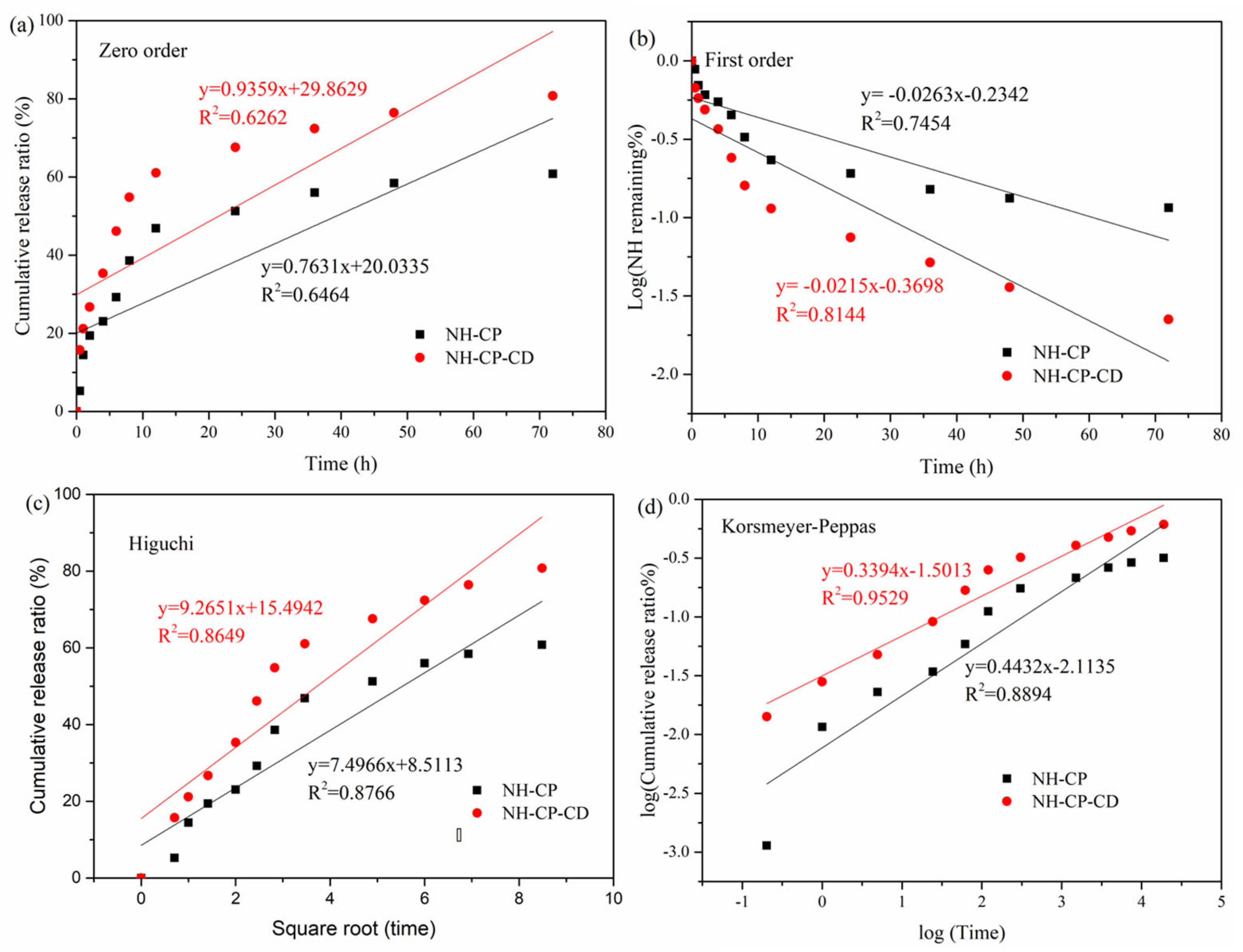
2.8. Research on In Vitro Release and Its Mechanism
2.9. Mucin Adhesion Study
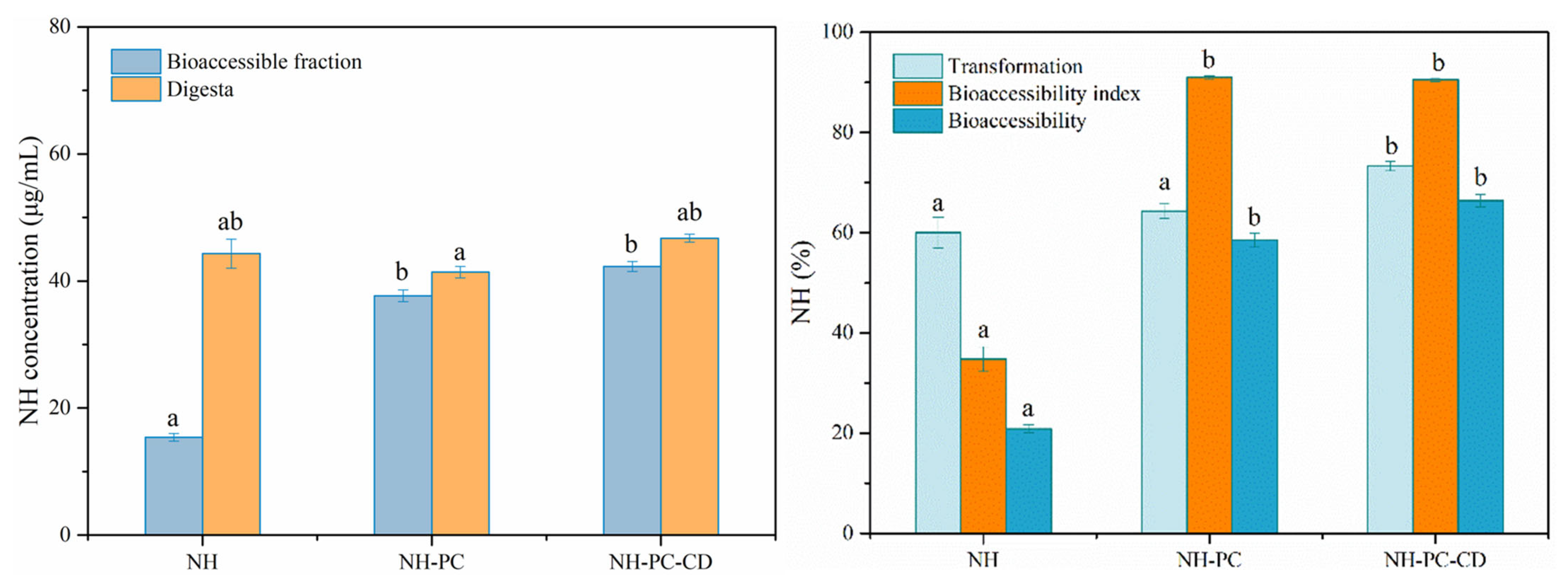
2.10. Simulate Gastrointestinal Digestion
Measurement of Neohesperidin Bioaccessibility
2.11. Molecular Simulations
2.12. Statistical Analyses
3. Results and Discussion
3.1. Characterization of Nano-Liposomes
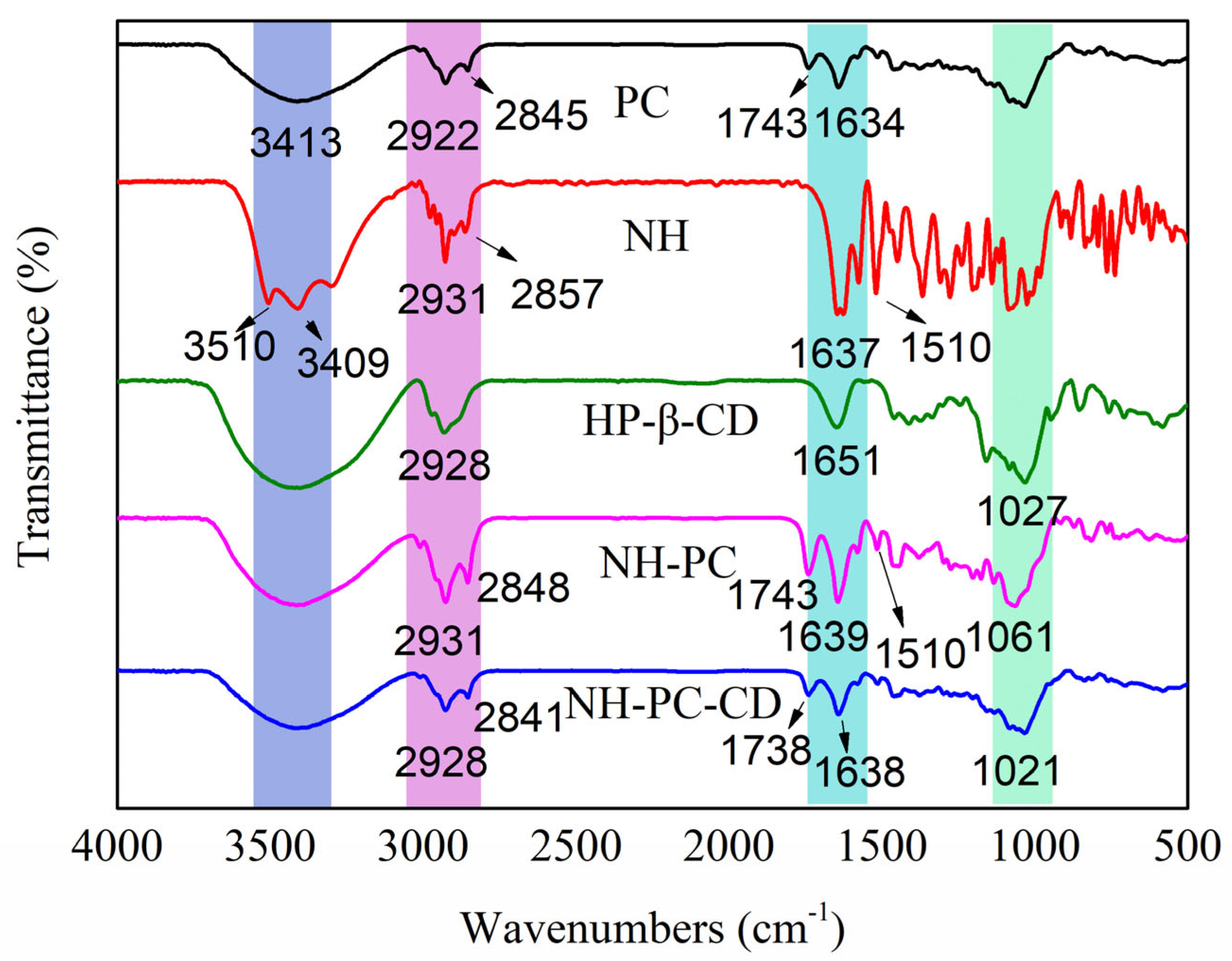
3.2. FT-IR
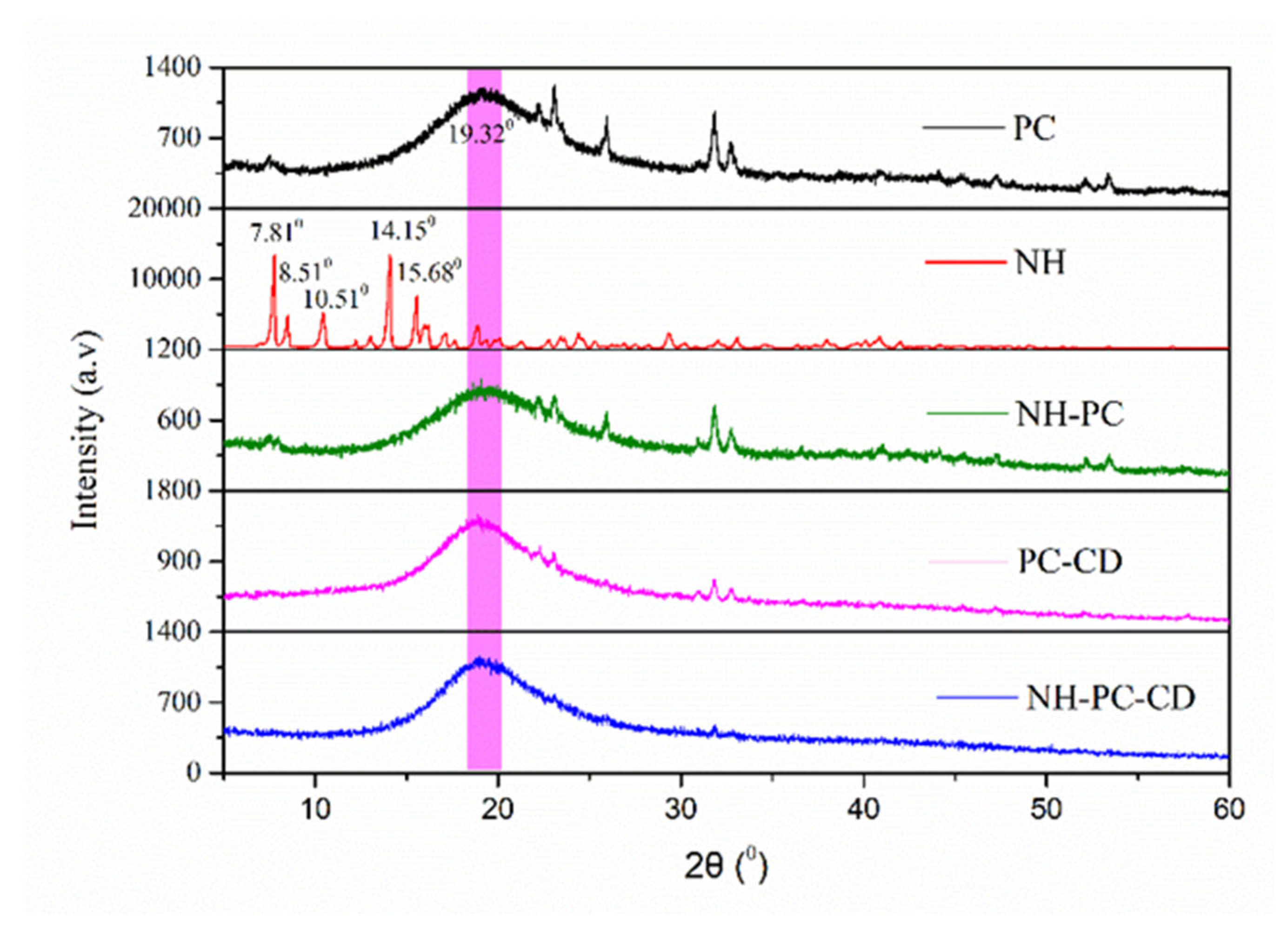
3.3. XRD
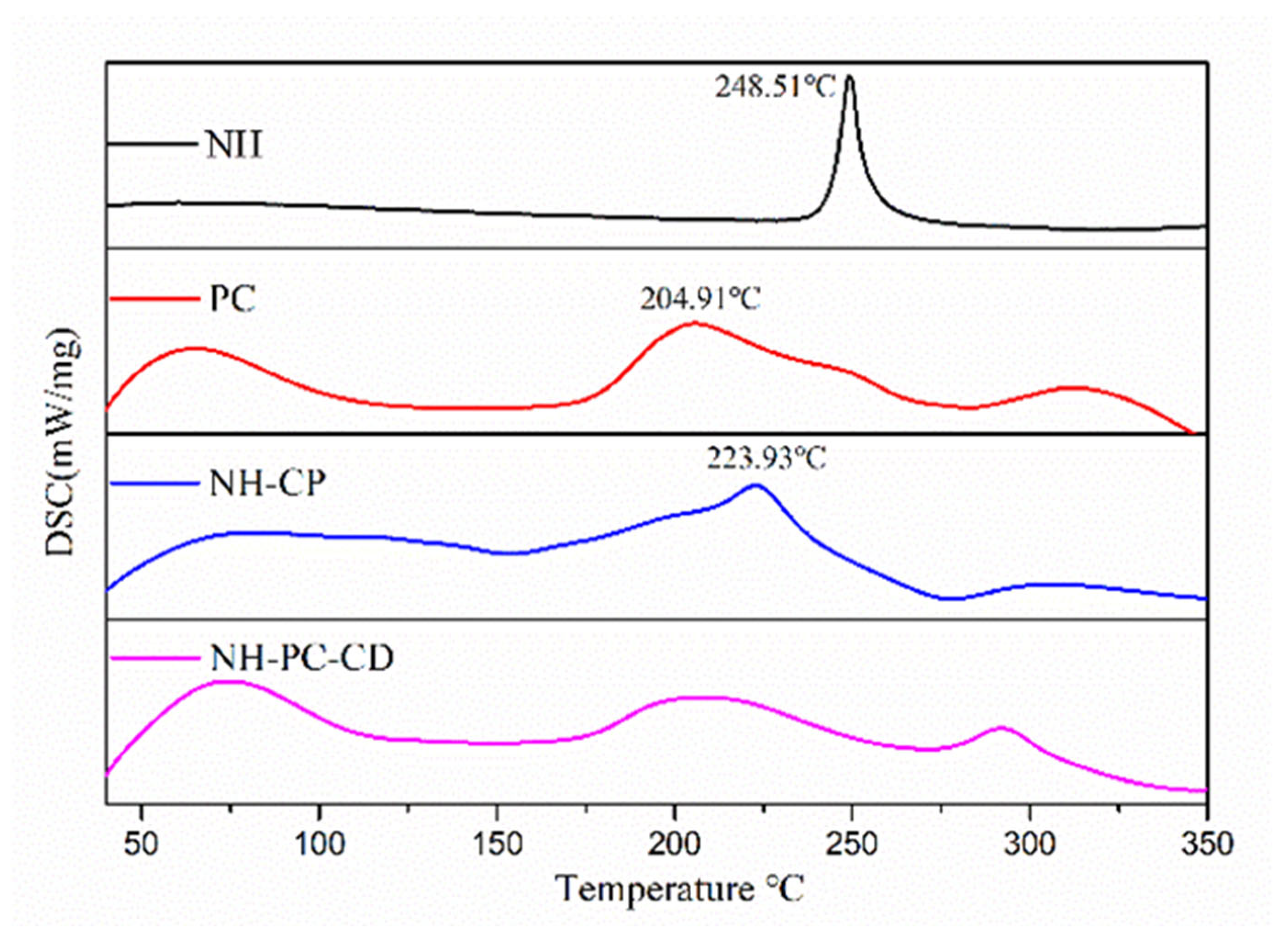
3.4. DSC
3.5. Storage Stability of Neohesperidin Liposomes
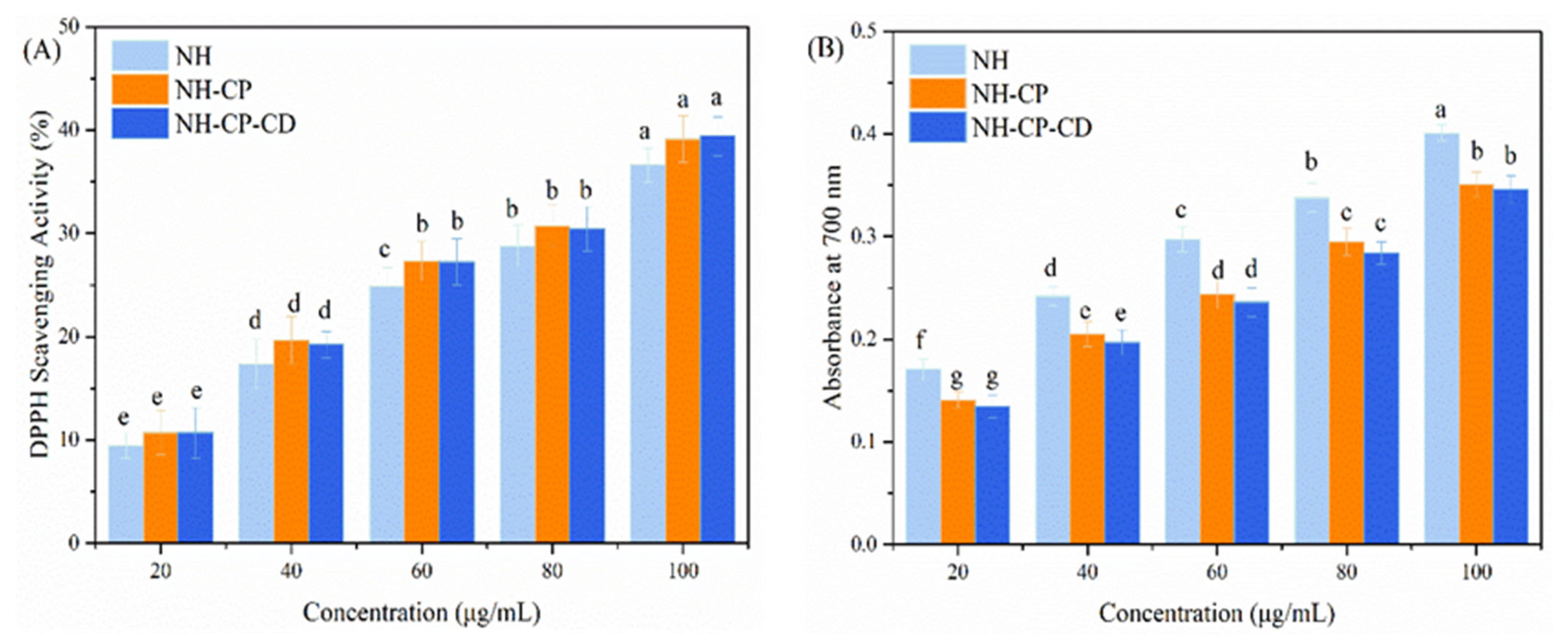
3.6. In Vitro Antioxidant Activity of Nano-Liposomes
3.7. In Vitro Release and Kinetic Studies
3.8. Mucin Adhesion Study
3.9. Bioaccessibility of Neohesperidin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, N.; Wan, W.; Zhu, S.; Liu, Q. Preparation of crystalline nanocellulose/hydroxypropyl β cyclodextrin/carboxymethyl cellulose polyelectrolyte complexes and their controlled release of neohesperidin-copper (II) in vitro. Int. J. Biol. Macromol. 2020, 163, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, J.; Wang, Y.; Liu, X.; Guan, L.; Chen, L. Protein-lipid composite nanoparticles for the oral delivery of vitamin B12: Impact of protein succinylation on nanoparticle physicochemical and biological properties. Food Hydrocoll. 2019, 92, 189–197. [Google Scholar] [CrossRef]
- Huang, S.; Yin, H.; Tian, F.; Wang, L.; Wu, W.; Li, X.; Zhao, Y.; Wang, X.; Li, Y.; Terp, M.; et al. Effects of mono-and di-glycerides/phospholipids (MDG/PL) on the bioavailability of lutein and DHA in rats: More effective for lutein. Food Biosci. 2025, 69, 106815. [Google Scholar] [CrossRef]
- Li, Z.-L.; Peng, S.; Chen, X.; Zhu, Y.; Zou, L.; Liu, W.; Liu, C. Pluronics modified liposomes for curcumin encapsulation: Sustained release, stability and bioaccessibility. Food Res. Int. 2018, 108, 246–253. [Google Scholar] [CrossRef]
- Drescher, S.; Blume, A. The 8th International Symposium on Phospholipids in Pharmaceutical Research–An Update on Current Research in Phospholipids presented at the biennial Symposium of the Phospholipid Research Center Heidelberg. Eur. J. Pharm. Sci. 2025, 210, 107126. [Google Scholar] [CrossRef]
- Khan, J.; Alexander, A.; Ajazuddin; Saraf, S.; Saraf, S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J. Control. Release 2013, 168, 50–60. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, X.; Zhang, Q.; Wang, B.; Chen, Y.; Zang, C.; Wang, Y.; Dong, T.; Zhang, T. 20 (S)-Protopanaxadiol phospholipid complex: Process optimization, characterization, in vitro dissolution and molecular docking studies. Molecules 2016, 21, 1396. [Google Scholar] [CrossRef]
- Ma, J.; Teng, H.; Wang, J.; Zhang, Y.; Ren, T.; Tang, X.; Cai, C. A highly stable norcantharidin loaded lipid microspheres: Preparation, biodistribution and targeting evaluation. Int. J. Pharm. 2014, 473, 475–484. [Google Scholar] [CrossRef]
- Prasad, V.; Gundermann, S.; Drozdzik, M.; Gunderman, K. Essential phospholipids in fatty liver: A scientific update. Clin. Exp. Gastroenterol. 2016, 105–117. [Google Scholar] [CrossRef]
- Robert, C.; Couëdelo, L.; Vaysse, C.; Michalski, M. Vegetable lecithins: A review of their compositional diversity, impact on lipid metabolism and potential in cardiometabolic disease prevention. Biochimie 2020, 169, 121–132. [Google Scholar] [CrossRef]
- Dai, L.; Li, R.; Wei, Y.; Sun, C.; Mao, L.; Gao, Y. Fabrication of zein and rhamnolipid complex nanoparticles to enhance the stability and in vitro release of curcumin. Food Hydrocoll. 2018, 77, 617–628. [Google Scholar] [CrossRef]
- Takeuchi, H.; Tahara, K.; Onodera, R. Recent advances in particulate drug delivery systems: Oral, pulmonary, and ophthalmic administrations. Asian J. Pharm. Sci. 2016, 1, 3–4. [Google Scholar] [CrossRef][Green Version]
- Khatik, R.; Dwivedi, P.; Shukla, A.; Srivastava, P.; Rath, S.; Kumar Paliwal, S.; Dwivedi, A. Development, characterization and toxicological evaluations of phospholipids complexes of curcumin for effective drug delivery in cancer chemotherapy. Drug Deliv. 2016, 23, 1057–1068. [Google Scholar] [CrossRef]
- Singh, J.P.; Saini, G.; Tiwari, G.; Singh, B. Nano-Formulation Approaches to Enhance Transdermal Drug Delivery-An Updated Review of Nanovesicular Carrier “Transethosomes”. Pharm. Nanotechnol. 2024, 13, 739–757. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, S.; Chen, X.; Wu, M.; Wang, H.; Yin, H.; He, D.; Xiong, H.; Zhang, J. Design and evaluation of a novel evodiamine-phospholipid complex for improved oral bioavailability. AAPS PharmSciTech 2012, 13, 534–547. [Google Scholar] [CrossRef]
- Pujara, N.; Jambhrunkar, S.; Wong, K.; McGuckin, M.; Popat, A. Enhanced colloidal stability, solubility and rapid dissolution of resveratrol by nanocomplexation with soy protein isolate. J. Colloid Interface Sci. 2017, 488, 303–308. [Google Scholar] [CrossRef]
- Wang, C.; Xia, N.; Yu, M.; Zhu, S. Physicochemical properties and mechanism of solubilised neohesperidin system based on inclusion complex of hydroxypropyl-β-cyclodextrin. Int. J. Food Sci. Technol. 2023, 58, 107–115. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Qin, X.; Li, Z.L.; Gong, Y.C.; Li, Y.P. Synthesis, drug release and targeting behaviors of Novel Folated Pluronic F87/poly (lactic acid) block copolymer. Eur. Polym. J. 2015, 68, 233–242. [Google Scholar] [CrossRef]
- Liu, Y.; Ying, D.; Cai, Y.; Le, X. Improved antioxidant activity and physicochemical properties of curcumin by adding ovalbumin and its structural characterization. Food Hydrocoll. 2017, 72, 304–311. [Google Scholar] [CrossRef]
- Wan, K.; Sun, L.; Hu, X.; Zijun Yan, Z.; Zhang, Y.; Xue Zhang, X.; Zhang, J. Novel nanoemulsion based lipid nanosystems for favorable in vitro and in vivo characteristics of curcumin. Int. J. Pharm. 2016, 504, 80–88. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T.; Wei, S.; Zhou, C.; Lan, Y.; Cao, A.; Yang, J.; Wang, W. Mucus adhesion-and penetration-enhanced liposomes for paclitaxel oral delivery. Int. J. Pharm. 2018, 537, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res. Int. 2016, 81, 74–82. [Google Scholar] [CrossRef]
- Xia, N.; Wan, W.; Zhu, S.; Wang, H.; Ally, K. Synthesis and characterization of a novel soluble neohesperidin-copper (II) complex using Ion-exchange resin column. Polyhedron 2020, 188, 114694. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Karim, N.; Gowd, V.; Xie, J.; Zheng, X.; Chen, W. Pectin-chitosan conjugated nanoliposome as a promising delivery system for neohesperidin: Characterization, release behavior, cellular uptake, and antioxidant property. Food Hydrocoll. 2019, 95, 432–444. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.; DeVoe, D.; Locascio, L.; Gaitan, M. Microfluidic directed formation of liposomes of controlled size. Langmuir 2007, 23, 6289–6293. [Google Scholar] [CrossRef]
- Isailović, B.D.; Kostić, I.T.; Zvonar, A.; Đorđević, V.B.; Gašperlin, M.; Nedović, V.A.; Bugarski, B.M. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Le, N.T.T.; Nguyen, D.T.D.; Nguyen, N.H.; Nguyen, C.K.; Nguyen, D.H. Methoxy polyethylene glycol–cholesterol modified soy lecithin liposomes for poorly water-soluble anticancer drug delivery. J. Appl. Polym. Sci. 2021, 138, 49858. [Google Scholar] [CrossRef]
- Londoño-Londoño, J.; Lima, V.R.D.; Jaramillo, C.; Creczynski-Pasa, T. Hesperidin and hesperetin membrane interaction: Understanding the role of 7-O-glycoside moiety in flavonoids. Arch. Biochem. Biophys. 2010, 499, 6–16. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Peres, I.; Rocha, S.; Gomes, J.; Morais, S.; Pereira, M.C.; Coelho, M. Preservation of catechin antioxidant properties loaded in carbohydrate nanoparticles. Carbohydr. Polym. 2011, 86, 147–153. [Google Scholar] [CrossRef]
- Niu, Y.; Ke, D.; Yang, Q.; Wang, X.; Chen, Z.; An, X.; Shen, W. Temperature-dependent stability and DPPH scavenging activity of liposomal curcumin at pH 7.0. Food Chem. 2012, 135, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Sessa, M.; Mediouni, H.; Mgaidi, A.; Ferrari, G. Encapsulation of bioactive compounds in nanoemulsion-based delivery systems. Procedia Food Sci. 2011, 1, 1666–1671. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Zhang, Y.; He, D.; Jiang, R.; Xie, X.; Yang, Q.; Li, K.; Xie, J.; Zhang, J. Phospholipid/hydroxypropyl-β-cyclodextrin supramolecular complexes are promising candidates for efficient oral delivery of curcuminoids. Int. J. Pharm. 2020, 582, 119301. [Google Scholar] [CrossRef]
- Bettini, R.; Catellani, P.L.; Santi, P.; Massimo, G.; Peppas, N.A.; Colombo, P. Translocation of drug particles in HPMC matrix gel layer: Effect of drug solubility and influence on release rate. J. Control. Release 2001, 70, 383–391. [Google Scholar] [CrossRef]
- Fathi, M.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Methotrexate-conjugated chitosan-grafted pH-and thermo-responsive magnetic nanoparticles for targeted therapy of ovarian cancer. Int. J. Biol. Macromol. 2020, 154, 1175–1184. [Google Scholar] [CrossRef]





| Liposomes | NH-PC | NH-PC-CD | ||
|---|---|---|---|---|
| Size | EE% | Size | EE% | |
| Day 0 | 87.7 ± 1.3 a | 96.5 ± 2.2 a | 120.7 ± 1.7 a | 95.6 ± 1.2 a |
| Day 10 | 93.0 ± 1.6 b | 91.4 ± 1.9 b | 121.1 ± 2.1 a | 94.2 ± 1.0 a |
| Day 20 | 98.9 ± 2.1 c | 87.7 ± 1.3 c | 122.2 ± 1.9 a | 93.8 ± 1.1 a |
| Day 30 | 103.0 ± 1.0 d | 84.1 ± 2.1 d | 122.9 ± 1.8 a | 92.3 ± 1.2 a |
| Mathematical Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Zero Order Model | First Order Model | Higuchi Model | Korsmeyer–Peppas Model | ||||||
| K0 | R02 | K1 | R12 | KH | RH2 | KK | Rk2 | n | |
| 37 °C | |||||||||
| NH-PC | 0.763 | 0.646 | 0.0263 | 0.745 | 7.50 | 0.877 | 2.11 | 0.889 | 0.443 |
| NH-PC-CD | 0.936 | 0.626 | 0.0215 | 0.814 | 9.27 | 0.865 | 1.50 | 0.953 | 0.339 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, N.; Zhou, Q.; Liu, Y.; Gao, D.; Zhu, S.; Feng, Z. Phospholipid/HP-β-CD Hybrid Nanosystems Amplify Neohesperidin Bioavailability via Dual Enhancement of Solubility and Stability. Nanomaterials 2025, 15, 862. https://doi.org/10.3390/nano15110862
Xia N, Zhou Q, Liu Y, Gao D, Zhu S, Feng Z. Phospholipid/HP-β-CD Hybrid Nanosystems Amplify Neohesperidin Bioavailability via Dual Enhancement of Solubility and Stability. Nanomaterials. 2025; 15(11):862. https://doi.org/10.3390/nano15110862
Chicago/Turabian StyleXia, Na, Qian Zhou, Yanquan Liu, Dan Gao, Siming Zhu, and Zuoshan Feng. 2025. "Phospholipid/HP-β-CD Hybrid Nanosystems Amplify Neohesperidin Bioavailability via Dual Enhancement of Solubility and Stability" Nanomaterials 15, no. 11: 862. https://doi.org/10.3390/nano15110862
APA StyleXia, N., Zhou, Q., Liu, Y., Gao, D., Zhu, S., & Feng, Z. (2025). Phospholipid/HP-β-CD Hybrid Nanosystems Amplify Neohesperidin Bioavailability via Dual Enhancement of Solubility and Stability. Nanomaterials, 15(11), 862. https://doi.org/10.3390/nano15110862









