Synergistic Regulation of Combustion Behavior and Safety Characteristics of Graphene Modified Core–Shell Al@AP Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Morphological and Structural Characterisation
2.3. Thermal Decomposition Characterisation
2.4. Laser Ignition Test for Characterising the Combustion Characteristics
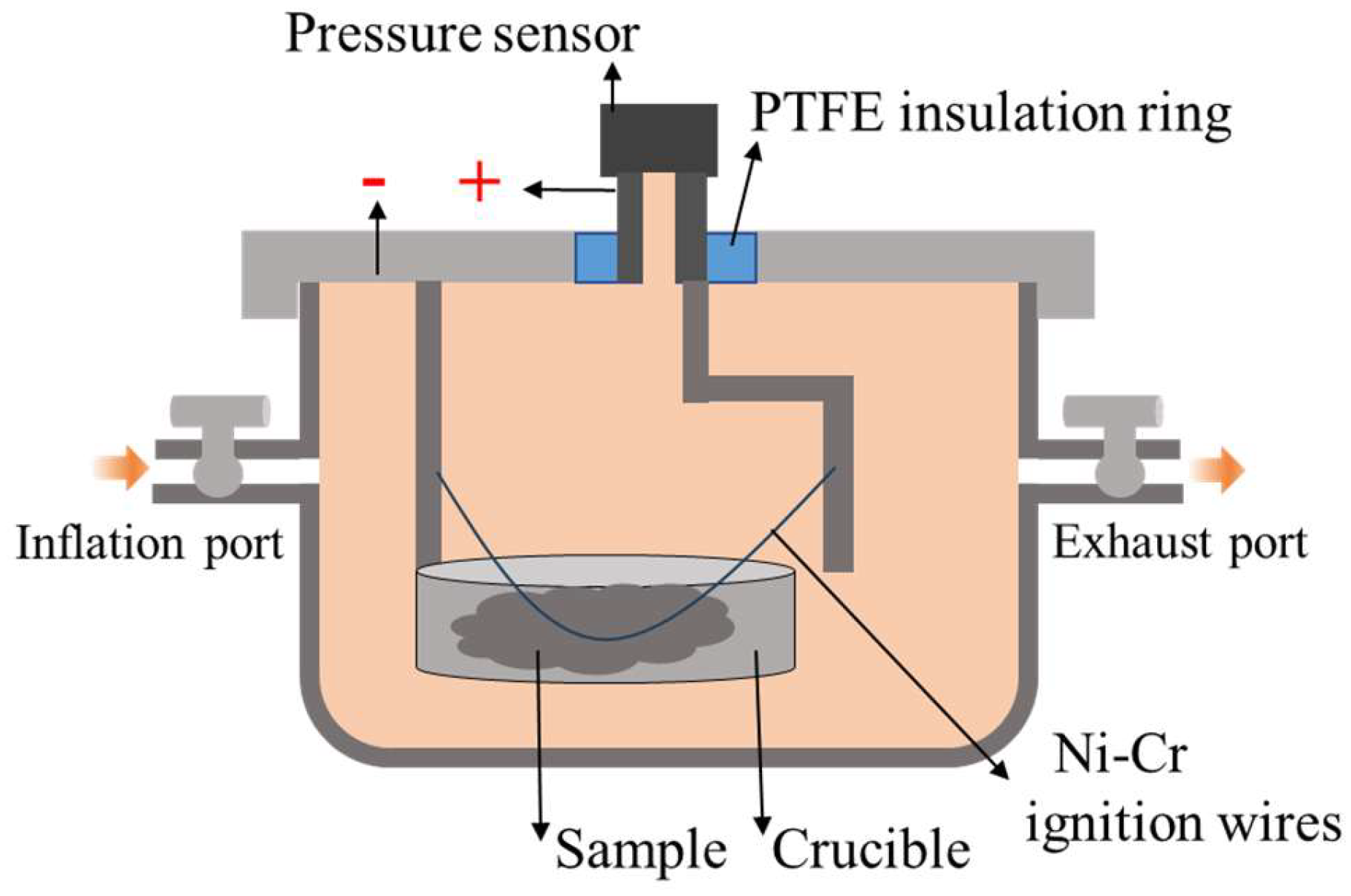
2.5. Closed-Bomb Testing
2.6. Impact and Friction Sensitivity Testing
3. Results and Discussion
3.1. Surface Morphology and Structural Analysis
3.2. Analysis of the Thermal Decomposition Characteristics
3.3. Analysis of Closed Combustion Pressure
3.4. Analysis of Combustion Characteristics
3.5. Impact and Friction Sensitivities
3.6. Comprehensive Effect of Graphene on Al@AP Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP | ammonium perchlorate |
| DMF | Dimethylformamide |
| LTD | low-temperature decomposition |
| HTD | high-temperature decomposition |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| TG-DSC-FTIR | Thermogravimetric analysis-differential scanning calorimetry-Fourier transform infrared spectroscopy |
| XRD | X-ray diffraction |
References
- Zhang, X.-X.; Yang, S.-L.; Xue, Z.-H.; Chen, S.; Yan, Q.-L. Multi-scale modified nitramine crystals with conjugated structure intercalation and thin-layer catalyst coating for well-controlled energy release rate Chem. Eng. J. 2022, 448, 137730. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Solid propellants: AP/HTPB composite propellants. Arabian J. Chem. 2019, 12, 2061–2068. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Trache, D.; Tarchoun, A.F.; Boukeciat, H.; Kadri, D.E.; Hassam, H.; Ouahioune, S.; Sahnoun, N.; Thakur, S.; Klapötke, T.M. Application of co-crystallization method for the production of ammonium perchlorate/ammonium nitrate oxidizer for solid rocket propellants. Chem. Eng. J. 2024, 487, 150654. [Google Scholar] [CrossRef]
- Trache, D.; Klapötke, T.M.; Maiz, L.; Abd-Elghany, M.; DeLuca, L.T. Recent advances in new oxidizers for solid rocket propulsion. Green Chem. 2017, 19, 4711–4736. [Google Scholar] [CrossRef]
- Ayoman, E.; Hosseini, S.G. Synthesis of CuO nanopowders by high-energy ball-milling method and investigation of their catalytic activity on thermal decomposition of ammonium perchlorate particles. J. Therm. Anal. Calorim. 2015, 123, 1213–1224. [Google Scholar] [CrossRef]
- Chen, T.; Hu, Y.-W.; Zhang, C.; Gao, A.-J. Recent progress on transition metal oxides and carbon-supported transition metal oxides as catalysts for thermal decomposition of ammonium perchlorate. Def. Technol. 2021, 17, 1471–1485. [Google Scholar] [CrossRef]
- Corcoran, A.; Mercati, S.; Nie, H.; Milani, M.; Montorsi, L.; Dreizin, E.L. Combustion of fine aluminum and magnesium powders in water. Combust. Flame 2013, 160, 2242–2250. [Google Scholar] [CrossRef]
- Miller, K.K.; Gottfried, J.L.; Walck, S.D.; Pantoya, M.L.; Wu, C.-C. Plasma surface treatment of aluminum nanoparticles for energetic material applications. Combust. Flame 2019, 206, 211–213. [Google Scholar] [CrossRef]
- Ao, W.; Liu, P.; Liu, H.; Wu, S.; Tao, B.; Huang, X.; Li, L.K.B. Tuning the agglomeration and combustion characteristics of aluminized propellants via a new functionalized fluoropolymer. Chem. Eng. J. 2020, 382, 122987. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, Z.; Liang, T.; Yang, R.; Li, J.; Luo, P. Establishing the interface layer on the aluminum surface through the self-assembly of tannic acid (TA): Improving the ignition and combustion properties of aluminium. Chem. Eng. J. 2021, 420, 130523. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, F.; Li, H.; Yuan, Z.; Zhang, M.; Yang, Y.; Pei, Q.; Wang, Y.; Chen, X.; Qin, Z. Improving ignition and combustion performance of Al@Ni in CMDB propellants: Effect of nickel coating. Chem. Eng. J. 2023, 456, 141010. [Google Scholar] [CrossRef]
- He, W.; Li, Z.-H.; Chen, S.; Yang, G.; Yang, Z.; Liu, P.-J.; Yan, Q.-L. Energetic metastable n-Al@PVDF/EMOF composite nanofibers with improved combustion performances. Chem. Eng. J. 2020, 383, 123146. [Google Scholar] [CrossRef]
- Rao, D.C.K.; Yadav, N.; Joshi, P.C. Cu–Co–O nano-catalysts as a burn rate modifier for composite solid propellants. Defence Technol. 2016, 12, 297–304. [Google Scholar] [CrossRef]
- Yadav, N.; Srivastava, P.K.; Varma, M. Recent advances in catalytic combustion of AP-based composite solid propellants. Defence Technol. 2021, 17, 1013–1031. [Google Scholar] [CrossRef]
- Zhao, N.; Ma, H.; Yao, E.; Yu, Z.; An, T.; Zhao, F.; Yu, X. Influence of tailored CuO and Al/CuO nanothermites on the thermocatalytic degradation of nitrocellulose and combustion performance of AP/HTPB composite propellant. Cellulose 2021, 28, 8671–8691. [Google Scholar] [CrossRef]
- Pang, W.; De Luca, L.T.; Fan, X.; Maggi, F.; Xu, H.; Xie, W.; Shi, X. Effects of different nano-sized metal oxide catalysts on the properties of composite solid propellants. Combust. Sci. Technol. 2016, 188, 315–328. [Google Scholar] [CrossRef]
- Kurva, R.; Gupta, G.; Dhabbe, K.I.; Jawale, L.S.; Kulkarni, P.S.; Maurya, M. Evaluation of 4-(Dimethylsilyl) butyl ferrocene grafted HTPB as a burning rate modifier in composite propellant formulation using bicurative system. Propellants Explos. Pyrotech. 2017, 42, 401–409. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Wang, R.; Xu, P.; Zhang, X.; Gao, B.; Guo, C.; Yang, G. Bio-inspired Cu-alginate to smartly enhance safety performance and the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2019, 470, 269–275. [Google Scholar] [CrossRef]
- Guo, M.F.; Li, J.; Li, K.; Zhu, G.; Hu, B.Z.; Liu, Y.; Ji, J.B. Carbon nanotube reinforced ablative material for thermal protection system with superior resistance to high-temperature dense particle erosion. Aerosp. Sci. Technol. 2020, 106, 106234. [Google Scholar] [CrossRef]
- Lan, Y.F.; Li, X.Y.; Luo, Y.J. Research progress on application of graphene in energetic materials. Chin. J. Explos. Propellants 2015, 38, 1–7. (In Chinese) [Google Scholar] [CrossRef]
- Mao, J.J.; Zhang, W.; Lu, H.M. Static and dynamic analyses of graphene-reinforced aluminium-based composite plate in thermal environment. Aerosp. Sci. Technol. 2020, 107, 106354. [Google Scholar] [CrossRef]
- Zhang, J.; Hooper, J.P.; Zhang, J.; Shreeve, J.M. Well-balanced energetic cocrystals of H5IO6/HIO3 achieved by a small acid-base gap. Chem. Eng. J. 2021, 405, 126623. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Zhang, J.; Gong, F.; Huang, B.; Zhang, Q.; Yan, Q.-L.; Yang, Z. Regulating safety and energy release of energetic materials by manipulation of molybdenum disulfide phase. Chem. Eng. J. 2021, 411, 128603. [Google Scholar] [CrossRef]
- Kumar, H.; Tengli, P.N.; Mishra, V.K.; Tripathi, P.; Bhushan, A.; Mishra, P.K. The effect of reduced graphene oxide on the catalytic activity of Cu–Cr–O–TiO2 to enhance the thermal decomposition rate of ammonium perchlorate: An efficient fuel oxidizer for solid rocket motors and missiles. RSC Adv. 2017, 7, 36594–36604. [Google Scholar] [CrossRef]
- Dey, A.; Nangare, V.; More, P.V.; Khan, M.A.S.; Khanna, P.K.; Sikder, A.K.; Chattopadhyay, S. A graphene titanium dioxide nanocomposite (GTNC): One pot green synthesis and its application in a solid rocket propellant. RSC Adv. 2015, 5, 63777–63785. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, J.Q.; Luo, Y.J. Preparation and thermal decomposition behaviour of ammonium perchlorate/graphene aerogel nanocomposites. Chin. J. Explos. Propellants 2012, 35, 76–80. (In Chinese) [Google Scholar] [CrossRef]
- Yang, D.; Bai, C.; Zhu, F.; Liu, C.; Tu, C.Z.; Li, G.; Luo, Y.; Zhang, T. Highly efficient thermal decomposition of AP through intimately encapsulating in 3DOM CoFe2O4 spinel. Chem. Eng. J. 2024, 488, 150748. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Nie, J.-X.; Jiao, G.-L.; Xu, X.; Guo, X.-Y.; Yan, S.; Jiao, Q.-J. Effect of the microporous structure of ammonium perchlorate on thermal behaviour and combustion characteristics. Defence Technol. 2022, 18, 1156–1166. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, X.; Zhu, J.; Li, Y.; Wang, X.; Hu, X. Catalytic activity of nanometer-sized CuO/Fe2O3 on thermal decomposition of AP and combustion of AP-based propellant. Combust. Sci. Technol. 2010, 183, 154–162. [Google Scholar] [CrossRef]
- Alizadeh-Gheshlaghi, E.; Shaabani, B.; Khodayari, A.; Azizian-Kalandaragh, Y.; Rahimi, R. Investigation of the catalytic activity of nano-sized CuO, Co3O4 and CuCo2O4 powders on thermal decomposition of ammonium perchlorate. Powder Technol. 2012, 217, 330–339. [Google Scholar] [CrossRef]
- Melo, J.P.; Ríos, P.L.; Povea, P.; Morales-Verdejo, C.; Camarada, M.B. Graphene oxide quantum dots as the support for the synthesis of gold nanoparticles and their applications as new catalysts for the decomposition of composite solid propellants. ACS Omega 2018, 3, 7278–7287. [Google Scholar] [CrossRef]









| Material | Specification | Manufacturer |
|---|---|---|
| AP | Class II | Liming Chemical Co., Ltd., Luoyang, China |
| Al | 50 nm | Zhongke Detong Technology Co., Ltd.,Beijing, China |
| Graphene | Less than three layers | Kegong Metallurgical Materials Co., Ltd., Xingtai, China |
| N,N-Dimethylformamide (DMF) | ACS | J&K Scientific, Beijing, China |
| Methanol | HPLC | Beijing Tongguang Fine Chemicals Company, Beijing, China |
| Ethyl acetate | AR | Beijing Tongguang Fine Chemicals Company, Beijing, China |
| No. | AP (g) | Al (g) | Graphene (g) |
|---|---|---|---|
| G-1 | 1.6 | 0.4 | 0 |
| G-2 | 1.6 | 0.4 | 0.01 |
| G-3 | 1.6 | 0.4 | 0.02 |
| G-4 | 1.6 | 0.4 | 0.04 |
| G-5 | 1.6 | 0.4 | 0.08 |
| Sample | Pressure (kPa) | ΔP/Δt (kPa ms−1) |
|---|---|---|
| G-1 | 108.35 | 5.46 |
| G-2 | 110.62 | 11.69 |
| G-3 | 114.65 | 13.29 |
| G-4 | 85.27 | 12.10 |
| G-5 | 71.79 | 6.54 |
| Sample | TLTD (°C) | THTD (°C) | Pressure (kPa) | BAM Impact/Friction |
|---|---|---|---|---|
| G-1 | 332.77 | 411.77 | 108.35 | 2.5 J/84 N |
| G-2 | 310.35 | 408.15 | 110.62 | 5.0 J/240 N |
| G-3 | 308.00 | 410.80 | 114.65 | 6.0 J/>240 N |
| G-4 | 308.60 | 403.60 | 85.27 | 7.5 J/>240 N |
| G-5 | 307.29 | 395.09 | 71.79 | 10.0 J/>240 N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Liang, J.; Sun, X.; Li, Y.; Zhang, H.; Guo, X.; Yan, S.; Li, J.; Nie, J. Synergistic Regulation of Combustion Behavior and Safety Characteristics of Graphene Modified Core–Shell Al@AP Composites. Nanomaterials 2025, 15, 853. https://doi.org/10.3390/nano15110853
Shi J, Liang J, Sun X, Li Y, Zhang H, Guo X, Yan S, Li J, Nie J. Synergistic Regulation of Combustion Behavior and Safety Characteristics of Graphene Modified Core–Shell Al@AP Composites. Nanomaterials. 2025; 15(11):853. https://doi.org/10.3390/nano15110853
Chicago/Turabian StyleShi, Jiahui, Jiahao Liang, Xiaole Sun, Yingjun Li, Haijun Zhang, Xueyong Guo, Shi Yan, Junwei Li, and Jianxin Nie. 2025. "Synergistic Regulation of Combustion Behavior and Safety Characteristics of Graphene Modified Core–Shell Al@AP Composites" Nanomaterials 15, no. 11: 853. https://doi.org/10.3390/nano15110853
APA StyleShi, J., Liang, J., Sun, X., Li, Y., Zhang, H., Guo, X., Yan, S., Li, J., & Nie, J. (2025). Synergistic Regulation of Combustion Behavior and Safety Characteristics of Graphene Modified Core–Shell Al@AP Composites. Nanomaterials, 15(11), 853. https://doi.org/10.3390/nano15110853






