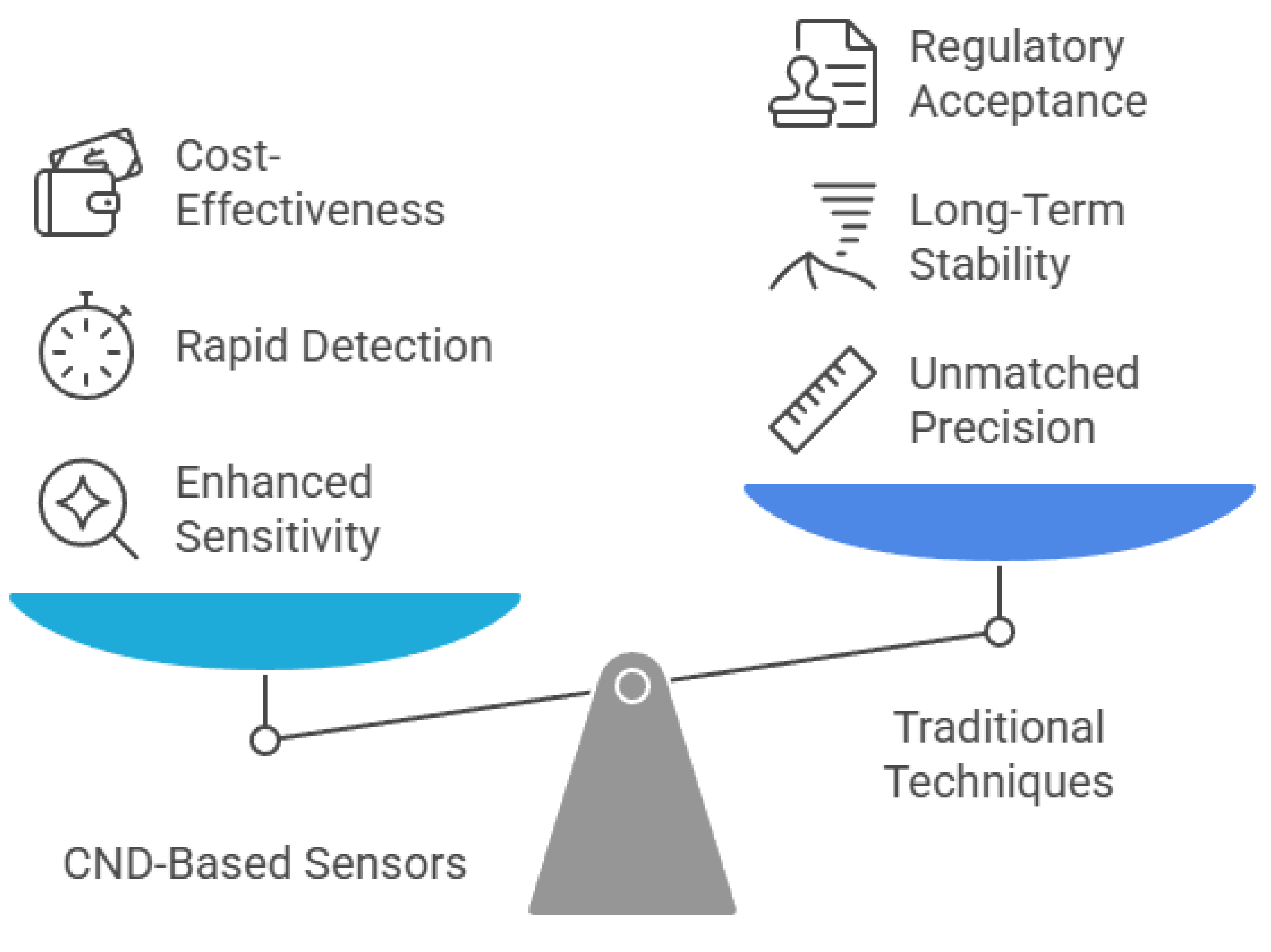
Carbon Nanodots-Based Sensors: A Promising Tool for Detecting and Monitoring Toxic Compounds
Abstract
1. Introduction
2. Fundamentals of Carbon Nanodots (CNDs) in Sensing
| Method | Advantages | Limitations | References |
|---|---|---|---|
| Arc discharge |
|
| [26] |
| Laser ablation |
|
| [26,27] |
| Hydrothermal |
|
| [28] |
| Microwave assisted |
|
| [29] |
| Pyrolysis |
|
| [30] |
3. Carbon Nanodot-Based Sensors: Design and Mechanisms
3.1. Sensor Design and Synthesis
3.2. Fluorescence-Based Detection Mechanisms
Fluorescence Quenching Mechanisms
- Static quenching: This involves the formation of non-fluorescent ground-state complexes between CNDs and analytes, often leading to altered absorption spectra. This type of quenching is sensitive to temperature changes [38]. For instance, ref. [39] used static quenching in the detection of chlortetracycline with nitrogen-doped CNDs.
- Dynamic quenching: This happens when excited-state CNDs collide with quencher molecules, transferring energy or electrons. It affects the fluorescence lifetime but not the absorption spectrum. Increasing the temperature enhances this quenching type [40,41]. Researchers applied dynamic quenching to detect malachite green in food matrices.
- Förster resonance energy transfer (FRET): In FRET-based systems, energy transfers from an excited donor (CNDs) to a nearby acceptor within ~10 nm. This leads to decreased donor fluorescence and enhanced acceptor emission. The presence of an analyte can reverse quenching by displacing the quencher and restoring CND fluorescence [42,43]. Ref. [44] demonstrated this mechanism for detecting Aflatoxin B1. Additionally, bicolor fluorescent molecular sensors offer promising capabilities for detecting cations through various mechanisms, including intramolecular charge transfer, excimer/exciplex formation, and FRET [45] to sense ultralow hazardous elemental traces [46].
- Inner filter effect (IFE): An IFE occurs when excitation or emission light is absorbed by another species in the system. This requires overlap between the absorber’s absorption spectrum and the CND’s excitation/emission wavelengths [47]. Refs. [48,49] used IFE to detect tinidazole in milk using N-doped CNDs.
- Photoinduced electron transfer (PET): PET involves electron transfer between CNDs and an analyte after photoexcitation, influencing the fluorescence output [50]. This often accompanies or overlaps with other quenching mechanisms.
3.3. Electrochemical Detection
3.4. Colorimetric Detection
- Aggregation-induced changes: Target analytes cause CND aggregation, altering their optical properties.
- Enzyme-mimicking activity: CNDs can mimic peroxidase activity, catalyzing oxidation reactions that produce colorimetric signals (e.g., in H2O2 or pesticide detection).
3.5. Comparison with Other Carbon Nanomaterials
- GQDs: Although they provide high sensitivity, their synthesis is more complex and less scalable [51].
- CNTs: These are known for their high conductivity and strength, so they are better suited for electrochemical sensing but raise environmental and health concerns due to their fibrous morphology [52].
- CNFs: CNFs have a high aspect ratio and provide good mechanical properties, similar to CNTs, making them useful for various sensor applications, particularly in electrochemical devices. However, unlike CNDs, CNFs generally lack significant photoluminescence, which limits their use in optical sensors. CNFs also require energy-intensive synthesis methods, which can be a disadvantage in terms of cost effectiveness and scalability [53].
4. Toxic Compounds in Food, Agriculture, and the Environment
4.1. Heavy Metal Contaminants
4.2. Organic Pollutants
4.3. Emerging Contaminants
5. Applications of CND-Based Sensors for Detecting Toxic Compounds
5.1. Applications of CND-Based Sensors for Detecting Heavy Metals
5.2. Applications of CND-Based Sensors for Detecting Organic Pollutants
5.3. Applications of CND-Based Sensors for Detecting Emerging Contaminants
| Method of Synthesis | Precursors Used | Characterization Techniques Used | References |
|---|---|---|---|
| Hydrothermal | Gallic acid and DMF | FT-IR, DLS, HR-TEM, XRD, XPS, and FS | [160] |
| Tea bag waste | UV–Vis, PSA, Zeta potential, HR-TEM, AFM, FT-IR, DSC, and FS | [161] | |
| o-phenylenediamine, dipicolinic acid | HR-TEM, DLS, FT-IR, XPS, and FS | [162] | |
| o-phenylenediamine | FT-IR, DLS, TEM, XRD, SEM, and XPS | [163] | |
| Salvadora persica powder and m-phenylenediamine | TEM, Zeta potential, UV-Vis, FT-IR, XPS, TRPL, and FS | [164] | |
| Citric acid and polyethyleneimine | HR-TEM, EDX, FT-IR, UV-Vis, and FS | [158] | |
| Citric acid and ethylenediamine | TEM, XPS, FT-IR, UV-Vis, and FS | [157] | |
| Ethylene glycol and sodium hydroxide | UV-Vis, TEM, FT-IR, and FS | [165] | |
| Citric acid, arginine, and ethane diamine | TEM, FT-IR, Zeta potential, XPS, UV-Vis, and FS | [166] | |
| Microwave assisted | Citric acid, urea, and trisodium citrate | FT-IR, UV-Vis, XRD, FS, HR-TEM, and RS | [167] |
| Cerium nitrate, dopamine hydrochloride, and citric acid | FT-IR, XPS, XRD, TEM, RS, UV-Vis, EPR, and Zeta potential | [168] | |
| Solvothermal | Neutral red, sulfuric acid and glutathione | HR-TEM, SEM, FT-IR, XPS, PXRD, and DSC | [40] |
| Gamma irradiation | Sucrose and ammonia | Zeta potential, FT-IR, UV-Vis, XRD, XPS, TEM, and FS | [146] |
| Pyrolysis, sol–gel, and electrodeposition | Titanium oxide and citric acid | FT-IR, EM, Zeta analysis, UV–Vis, and XRD | [169] |
| Oil bath | Sucrose and urea | UV-Vis, FS, TEM, FT-IR, XRD, and XPS | [170] |
| Analyte Detected | LOD | Calibration Range | References | |
|---|---|---|---|---|
| Metal ions | As5+, Fe2+, Hg2+, and Fe3+ | As5+—31.50 μM, Fe2+—122.4 μM, Hg2+—96.40 μM, and Fe3+—161.9 μM | As5+—0.09–0.19 mM (R2 = 0.9969), Fe2+—0.01–0.8 mM (R2 = 0.9966), Hg2+—0.04–0.9 mM (R2 = 0.9962), and Fe3+—0.01–0.9 mM (R2 = 0.9967) | [146] |
| Pb2+ | 0.715 μM | 30–130 μM (R2 = 0.9902) | [160] | |
| Fe3+ and Ag+ | Fe3+—0.250 μM; Ag1+—0.140 μM | Fe3+—1–100 μM (R2 = 0.9952); Ag1+—1–200 μM (R2 = 0.9985) | [167] | |
| Hg2+ | 0.147 µg L−1 | 0.625–90 µg L−1 (R2 = 0.9960) | [168] | |
| Polymer | Melamine | 30 nM | 0–20 μM (R2 = 0.9940) | [165] |
| Melamine | 0.67 μM | 2.0 to 290 μM (R2 = 0.9981) | [166] | |
| Acrylamide | 0.354 μg L−1 | 0.5–10 μg L−1 (R2 = 0.9991) | [161] | |
| Acrylamide | 0.670 nM | 10–200 nM (R2 = 0.9876) | [169] | |
| L-asparagine | 0.31 μM | 1.0–50.0 μM (R2 = 0.9984) | [170] | |
| Food Additives | Erythrosine | 1.210 nM | 4–20 µM (R2 = 0.9970) | [164] |
| Malachite green | 1.200 nM | 0.014–300 µM (R2 = 0.9964) | [40] | |
| Mycotoxin | Aflatoxin M1 | 0.07 μg L−1 | 0.2–0.8 μg L−1 (R2 = 0.9552) | [158] |
| Gamma irradiation | Aflatoxin M1 | 0.0186 μg kg−1 | 0.003–0.81 μg kg−1 (R2 = 0.9940) | [157] |
| Insecticide | Imidacloprid | 1.870 μg kg−1 | 0.037–0.2 mg kg−1 (R2 = 0.9700) | [163] |
| Allergen | Histamine | 6.96 µM | 25–1000 µM (R2 = 0.9978) | [162] |
| Source of Carbon Dots | Studied Pesticides | References |
|---|---|---|
| Green-fluorescent C-dots from vegetables/fruits | Pesticide parathion methyl can be detected through the reliable and sensitive technique in real food samples | [171] |
| Porphyrinic Zr metal–organic framework (PCN-224@CDs) | Organo-phosphorus pesticides via carbon dots supported Zr-based metal organic framework | [172] |
| Producing carbon quantum dots from tea residue | Using Al2(SO4)3/CQDs composite in photocatalytic degradation of pesticide Fipronil (C12H4Cl2F6N4OS) | [173] |
| Using carbon dots from Boerhavia diffusa leaves | Nanocomposite of CDs/cobalt ferrite and boehmite for photo-degradation via sensing of pesticide methyl parathion | [174] |
| Producing carbon dots using gallic acid | Detecting the “organophosphate pesticide” chlorpyrifos in wastewater as fluorescent probe | [175] |
| Producing carbon dots using Grewia asiatica fruit via microwave | Detecting the organo-phosphorus pesticide of quinalphos by forming red emissive carbon dots in vegetable, water, and soil samples | [176] |
| Bio-producing CeO2@C-dots from agrowaste (chestnut peels) | Active catalyst for degradation of Rhodamine B dye and detecting 4-Nitrophenol in aqueous solutions | [177] |
| Nitrogen-doped carbon dots using hydrothermal method | Detecting imidacloprid as an effective neonicotinoid insecticide in real foods | [178] |
| Boron-nitrogen doped carbon dots (BN-C-dots) using hydrothermal protocol | Rapid detection of insecticide acephate using BN-C-dots in vegetables and water | [179] |
| Producing TiO2/ZnO-CQDs from spent coffee using hydrothermal method | Detecting carbaryl (C12H11NO2) in carbamate pesticides via photocatalytic degradation in water for environmental remediation approach | [180] |
| Producing graphene carbon dots in corn stalk pith as green sorbent | Using the studied green sorbent in detecting triazole fungicide residues in different types of rice samples | [181] |
| Nitrogen- and phosphorus-doped carbon quantum dots (NP-CQDs) via hydrothermal method | Using fluorescent NP-CQDs in detecting pesticides of chlorpyrifos in miscellaneous beans and their residues in different kinds of foods | [41] |
| Nitrogen-doped carbon dots (N-CDs) | Using N-CD-based fluorescent sensor for detecting glyphosate in organo-phosphorus pesticides | [182] |
| Source of Carbon Dots | Studied Antibiotics | Ref. |
|---|---|---|
| Producing N- and S-doped blue-fluorescent carbon dots via a one-step solvothermal protocol | Detecting chloramphenicol in environmental and food safety contexts as portable fluorescence-based sensor for several analytical purposes | [183] |
| Producing N-doped C-quantum dots via microwave-assisted hydrothermal protocol | Detecting the antibiotic meropenem using a sensor of N-CQDs-AuNPs in pharmaceuticals and plasma | [184] |
| Producing N-doped carbon quantum dots using hydrothermal protocol | N-CQDs are more effective than antibiotic levofloxacin in treating bacterial keratitis caused by multidrug-resistant Staphylococcus aureus, combating its resistance | [185] |
| Producing Curcumin-derived C-dots via hydrothermal protocol | Cur-CDs exhibit significant antibacterial effects against strains such as Escherichia coli, and Staphylococcus aureus comparing with antibiotic chloramphenicol | [186] |
| Producing fluorescent C-quantum dots from disposable water bottles | Using Polyethylene Terephthalate plastic (PET) fluorescent C-quantum dots for removing antibiotic ciprofloxacin | [187] |
| Producing N and S co-doped C-quantum dots using hydrothermal protocol | On-site detection of antibiotics (i.e., moxifloxacin, gatifloxacin, and ofloxacin) in milk using N, S co-doped CQNs in combination with a smartphone | [188] |
| Producing N-doped C-quantum dots from the peels of Citrus limetta | Detecting β-Lactam antibiotics (ampicillin) in milk and water depending on the N-CQDs by forming a greenish-blue fluorescent color | [189] |
| Producing highly water-soluble Curcumin carbon dots using the hydrothermal protocol | Studied Cur-CDs exhibited a higher antimicrobial efficacy against Escherichia coli and Staphylococcus aureus and combating drug-resistant bacterial infections | [190] |
| Producing N-doped green-fluorescent C-dots using the hydrothermal protocol | A cost-effective, reliable approach, using high-fluorescence CDs for detecting the antibiotic chlortetracycline in real samples | [191] |
| Producing stable red-fluorescent nitrogen-doped carbon dots via solvothermal method | Studying N-CDs as a fluorescent probe to detect ceftazidime antibiotic in real samples | [192] |
| Producing CDs via hydrothermal method using different quaternary ammonium salts | Detecting tetracycline antibiotics in real milk using CDs as a cost-effective approach for providing insights into food safety testing methodologies | [193] |
| Producing magnetic molecular nanomaterials coupled with CDs via hydrothermal method | Detecting doxycycline antibiotics using CDs in food matrices through fluorescence quenching mechanism and inner filter effect (IFE) | [194] |
6. Sensitivity, Selectivity, and Performance of CND-Based Sensors
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chatterjee, R. Economic Damages from Nutrient Pollution Create a “Toxic Debt”. Environ. Sci. Technol. 2009, 43, 6–7. [Google Scholar] [CrossRef]
- Macêdo, R.L.; Haubrock, P.J.; Klippel, G.; Fernandez, R.D.; Leroy, B.; Angulo, E.; Carneiro, L.; Musseau, C.L.; Rocha, O.; Cuthbert, R.N. The Economic Costs of Invasive Aquatic Plants: A Global Perspective on Ecology and Management Gaps. Sci. Total Environ. 2024, 908, 168217. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Molina, J.; Cases, F.; Moretto, L.M. Graphene-Based Materials for the Electrochemical Determination of Hazardous Ions. Anal. Chim. Acta 2016, 946, 9–39. [Google Scholar] [CrossRef]
- Álvarez-Ruiz, R.; Picó, Y. Analysis of Emerging and Related Pollutants in Aquatic Biota. Trends Environ. Anal. Chem. 2020, 25, e00082. [Google Scholar] [CrossRef]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-Based Optical Chemical Sensors for the Detection of Heavy Metals in Water: Recent Advances and Challenges. TrAC Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, A. Drinking Water Contamination and Treatment Techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of Natural Organic Matter in Drinking Water Treatment by Coagulation: A Comprehensive Review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of Nanotechnology in Water and Wastewater Treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Wang, L.; Kim, J.; Cui, T. Self-Assembled Graphene and Copper Nanoparticles Composite Sensor for Nitrate Determination. Microsyst. Technol. 2018, 24, 3623–3630. [Google Scholar] [CrossRef]
- Sankaranarayanan, A.; Amaresan, N.; Sharma, A.; Khalifa, A.Y.Z. Mycotoxins Associated Food Safety Concerns of Agricultural Crops, Prevention and Control. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 357–374. ISBN 978-0-12-821394-0. [Google Scholar]
- Molla, A.; Youk, J.H. Recent Progress on Electroanalytical Sensing of Small Molecules and Biomolecules Using Carbon Dots: A Review. J. Ind. Eng. Chem. 2023, 127, 62–81. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon Nanodots: Synthesis, Properties and Applications. J. Mater. Chem. 2012, 22, 24230. [Google Scholar] [CrossRef]
- Dhamodharan, D.; Byun, H.-S.; Varsha Shree, M.; Veeman, D.; Natrayan, L.; Stalin, B. Carbon Nanodots: Synthesis, Mechanisms for Bio-Electrical Applications. J. Ind. Eng. Chem. 2022, 110, 68–83. [Google Scholar] [CrossRef]
- Etefa, H.F.; Tessema, A.A.; Dejene, F.B. Carbon Dots for Future Prospects: Synthesis, Characterizations and Recent Applications: A Review (2019–2023). C 2024, 10, 60. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care—An Updated Review (2018–2021). Nanomaterials 2021, 11, 2525. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon Dots: Synthesis, Properties and Biomedical Applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef]
- Prokisch, J.; Törős, G.; Nguyen, D.H.H.; Neji, C.; Ferroudj, A.; Sári, D.; Muthu, A.; Brevik, E.C.; El-Ramady, H. Nano-Food Farming: Toward Sustainable Applications of Proteins, Mushrooms, Nano-Nutrients, and Nanofibers. Agronomy 2024, 14, 606. [Google Scholar] [CrossRef]
- Bai, J.; Cheng, Y.; He, F.; Liu, Q.; Qiang, S.; Zhang, L.; Yang, J.; Wang, C.; Xu, Y.; Zhang, W. Environment-Sensitive Carbon Dots Derived from Naphthalenediol for Solvent Polarity Indicator and Anti-Counterfeiting. ChemistrySelect 2022, 7, e202200588. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Keçili, R.; Hussain, C.G.; Hussain, C.M. Fluorescent Nanosensors Based on Green Carbon Dots (CDs) and Molecularly Imprinted Polymers (MIPs) for Environmental Pollutants: Emerging Trends and Future Prospects. Trends Environ. Anal. Chem. 2023, 40, e00213. [Google Scholar] [CrossRef]
- Das, R.; Shahnavaz, Z.; Ali, M.E.; Islam, M.M.; Abd Hamid, S.B. Can We Optimize Arc Discharge and Laser Ablation for Well-Controlled Carbon Nanotube Synthesis? Nanoscale Res. Lett. 2016, 11, 510. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A Review. KONA 2017, 34, 80–90. [Google Scholar] [CrossRef]
- Shen, S.; Fu, J.; Wang, H. A Facile, Effective Synthesis of Excellent Fluorescent Carbon Dots with Optical Properties. ChemistrySelect 2019, 4, 12762–12767. [Google Scholar] [CrossRef]
- Pawelski, D.; Plonska-Brzezinska, M.E. Microwave-Assisted Synthesis as a Promising Tool for the Preparation of Materials Containing Defective Carbon Nanostructures: Implications on Properties and Applications. Materials 2023, 16, 6549. [Google Scholar] [CrossRef]
- Stan, C.S.; Albu, C.; Coroaba, A.; Popa, M.; Sutiman, D. One Step Synthesis of Fluorescent Carbon Dots through Pyrolysis of N-Hydroxysuccinimide. J. Mater. Chem. C 2015, 3, 789–795. [Google Scholar] [CrossRef]
- Barhoum, A.; Alhashemi, Y.; Ahmed, Y.M.; Rizk, M.S.; Bechelany, M.; Abdel-Haleem, F.M. Innovations in Ion-Selective Optodes: A Comprehensive Exploration of Modern Designs and Nanomaterial Integration. Front. Bioeng. Biotechnol. 2024, 12, 1397587. [Google Scholar] [CrossRef]
- Chen, H.; Luo, K.; Xie, C.; Zhou, L. Nanotechnology of Carbon Dots with Their Hybrids for Biomedical Applications: A Review. Chem. Eng. J. 2024, 496, 153915. [Google Scholar] [CrossRef]
- Banger, A.; Gautam, S.; Jadoun, S.; Jangid, N.K.; Srivastava, A.; Pulidindi, I.N.; Dwivedi, J.; Srivastava, M. Synthetic Methods and Applications of Carbon Nanodots. Catalysts 2023, 13, 858. [Google Scholar] [CrossRef]
- Kayani, K.F.; Ghafoor, D.; Mohammed, S.J.; Shatery, O.B. Carbon Dots: Synthesis, Sensing Mechanisms, and Potential Applications as Promising Materials for Glucose Sensors. Nanoscale Adv. 2024, 7, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Detection of Fluoride Ion by Carbon Dots-Based Fluorescent Probes. J. Mol. Struct. 2024, 1319, 139465. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Carbon Dot-Based Fluorescent Detection of Biothiols. Biosensors 2023, 13, 335. [Google Scholar] [CrossRef]
- Abdel-Karim, R. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Materials, Applications, Challenges, and Future Scope. Biomed. Mater. Devices 2024, 2, 759–777. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The Quenching of the Fluorescence of Carbon Dots: A Review on Mechanisms and Applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Long, X.; Wu, S. Green Synthesis of Fluorescent Carbon Dots from Waste Chicken Feathers for Chlortetracycline Sensing. J. Mol. Struct. 2025, 1319, 139444. [Google Scholar] [CrossRef]
- Ali, R.; Elfadil, H.; Sirag, N.; Albalawi, A.S.; Albalawi, A.; Alharbi, S.; Al-anzi, A.; Alatawi, S.; Alhuaiti, Y.; Alsubaie, F.T.; et al. A Novel Red Emissive Glutathione-Capped Carbon Dots Embedded within Molecularly-Imprinted Polymers for Adsorption and Fluorescent Sensing of Malachite Green in Food Samples. Microchem. J. 2025, 212, 113376. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Zhang, L.; Yu, R. Rapid Detection of Chlorpyrifos in Miscellaneous Beans Based on Nitrogen and Phosphorus Doped Carbon Quantum Dots Fluorescence Probe. J. Food Compos. Anal. 2025, 137, 106884. [Google Scholar] [CrossRef]
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. Quantum Dots as a Platform for Nanoparticle Drug Delivery Vehicle Design. Adv. Drug Deliv. Rev. 2013, 65, 703–718. [Google Scholar] [CrossRef]
- Annamalai, K.; Ravichandran, R.; Annamalai, A.; Jeevarathinam, A.; Suresh, R.; Elumalai, S. Synthesis of Blue-Sparkling N, S-Doped Carbon Dots for Effective Detection of Nitro Explosive and Fe3+ Ion and Anti-Counterfeiting Studies. Mater. Res. Bull. 2025, 181, 113068. [Google Scholar] [CrossRef]
- Duong, P.V.; Thi, L.A.; Hung, P.Q.; Toan, L.D.; Chuyen, P.T.; Hieu, D.M.; Minh, P.H.; Binh, N.T.; Cuong, T.M.; Hoa, N.M. Probing Förster Resonance Energy Transfer in Carbon Quantum Dots for High-Sensitivity Aflatoxin B1 Detection. J. Fluoresc. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Auria-Luna, F.; Foss, F.W.; Molina-Canteras, J.; Velazco-Cabral, I.; Marauri, A.; Larumbe, A.; Aparicio, B.; Vázquez, J.L.; Alberro, N.; Arrastia, I.; et al. Supramolecular Chemistry in Solution and Solid–Gas Interfaces: Synthesis and Photophysical Properties of Monocolor and Bicolor Fluorescent Sensors for Barium Tagging in Neutrinoless Double Beta Decay. RSC Appl. Interfaces 2025, 2, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, X.; Liu, C.; Zhang, R.; Gu, X.; Guan, G.; Jiang, C.; Zhang, L.; Du, S.; Liu, B.; et al. Color-Multiplexing-Based Fluorescent Test Paper: Dosage-Sensitive Visualization of Arsenic(III) with Discernable Scale as Low as 5 Ppb. Anal. Chem. 2016, 88, 6105–6109. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.-L.; Wang, J.-H. Inner Filter Effect-Based Fluorescent Sensing Systems: A Review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Ilie-Mihai, R.-M.; Gheorghe, D.-C.; Stefan-van Staden, R.-I. Carbon Nanodots in Nanobiomedicines and Electrochemical Sensing Devices. In Handbook of Material Engineering in Nanobiomedicine and Diagnostics; Azad, U.P., Chandra, P., Eds.; Springer Nature: Singapore, 2025; pp. 363–380. ISBN 978-981-97-7445-6. [Google Scholar]
- Qian, Y.; Chen, G.; Ma, C.; Li, L.; Yang, T.; Zhu, C.; Gao, H.; Hu, A.; Guo, X.; Yang, W.; et al. N-Doped Carbon Nanodots as Temperature Sensors and Fluorescent Probes for the Detection of Tinidazole in Milk. J. Fluoresc. 2025, 1–11. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon Dots: Synthesis, Formation Mechanism, Fluorescence Origin and Sensing Applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Mangaiyarkkarasi, J. Harnessing Carbon Nanomaterials for Next-Generation Environmental Sensing. In Environmental Applications of Carbon-Based Materials; IGI Global: Hershey, PA, USA, 2024; pp. 144–166. [Google Scholar]
- Paramasivam, G.; Palem, V.V.; Meenakshy, S.; Suresh, L.K.; Gangopadhyay, M.; Antherjanam, S.; Sundramoorthy, A.K. Advances on Carbon Nanomaterials and Their Applications in Medical Diagnosis and Drug Delivery. Colloids Surf. B Biointerfaces 2024, 241, 114032. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkov, M.N.; Stolyarova, D.Y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Diankin, I.D.; et al. Guiding Graphene Derivatization for Covalent Immobilization of Aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]
- Maurya, N.; Mishra, S.; Gupta, M.K. Carbon Dots and Their Environmental Applications. In Green Solutions for Degradation of Pollutants; Bentham Science Publishers: Sharjah, United Arab Emirates, 2024; pp. 96–114. [Google Scholar]
- Shaik, R.; Ghosh, B.; Barman, H.C.; Rout, A.; Padhy, P.K. Green Nanotech: A Review of Carbon-Based Nanomaterials for Tackling Environmental Pollution Challenges. Nat. Environ. Pollut. Technol. 2024, 23, 1783–1794. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Z.; Guo, X. Editorial: Recent Research Advances on Heavy Metals, Microplastics, Persistent Organic Pollutants, and Solid Waste in Aquatic and Terrestrial Ecosystems. Front. Environ. Sci. 2024, 12, 1391614. [Google Scholar] [CrossRef]
- Luo, Q. Impacts of Heavy Metal Pollution on Agricultural Production and Response Strategies. Sci. Technol. Eng. Chem. Environ. Prot. 2024, 1. [Google Scholar] [CrossRef]
- Sarma, H.H.; Rajkumar, A.; Baro, A.; Das, B.C.; Talukdar, N. Impact of Heavy Metal Contamination on Soil and Crop Ecosystem with Advanced Techniques to Mitigate Them. JABB 2024, 27, 53–63. [Google Scholar] [CrossRef]
- Ullah, H.; Uddin, J.; Ijaz, M.; Haziq, M.; Muhsinah, A.B.; Ullah, I.; Jamal, M. Metabolic Dynamics and Health Risk Assessment of Heavy Metal Accumulation in Urban–Rural Interface Vegetable Systems. Environ. Monit. Assess. 2025, 197, 567. [Google Scholar] [CrossRef]
- Ma, J.; Hung, H.; Macdonald, R.W. The Influence of Global Climate Change on the Environmental Fate of Persistent Organic Pollutants: A Review with Emphasis on the Northern Hemisphere and the Arctic as a Receptor. Glob. Planet. Chang. 2016, 146, 89–108. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and Removal of Heavy Metal Ions: A Review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Anderson, A.; Anbarasu, A.; Pasupuleti, R.R.; Manigandan, S.; Praveenkumar, T.R.; Aravind Kumar, J. Treatment of Heavy Metals Containing Wastewater Using Biodegradable Adsorbents: A Review of Mechanism and Future Trends. Chemosphere 2022, 295, 133724. [Google Scholar] [CrossRef]
- Saidon, N.B.; Szabó, R.; Budai, P.; Lehel, J. Trophic Transfer and Biomagnification Potential of Environmental Contaminants (Heavy Metals) in Aquatic Ecosystems. Environ. Pollut. 2024, 340, 122815. [Google Scholar] [CrossRef]
- Nde, S.C.; Felicite, O.M.; Aruwajoye, G.S.; Palamuleni, L.G. A Meta-Analysis and Experimental Survey of Heavy Metals Pollution in Agricultural Soils. J. Trace Elem. Miner. 2024, 9, 100180. [Google Scholar] [CrossRef]
- Xiong, R.; He, X.; Gao, N.; Li, Q.; Qiu, Z.; Hou, Y.; Shen, W. Soil pH Amendment Alters the Abundance, Diversity, and Composition of Microbial Communities in Two Contrasting Agricultural Soils. Microbiol. Spectr. 2024, 12, e04165-23. [Google Scholar] [CrossRef] [PubMed]
- Moghimi Dehkordi, M.; Pournuroz Nodeh, Z.; Soleimani Dehkordi, K.; Salmanvandi, H.; Rasouli Khorjestan, R.; Ghaffarzadeh, M. Soil, Air, and Water Pollution from Mining and Industrial Activities: Sources of Pollution, Environmental Impacts, and Prevention and Control Methods. Results Eng. 2024, 23, 102729. [Google Scholar] [CrossRef]
- Shirsat, M.D.; Hianik, T. Electrochemical Detection of Heavy Metal Ions Based on Nanocomposite Materials. J. Compos. Sci. 2023, 7, 473. [Google Scholar] [CrossRef]
- Yoo, D.; Park, Y.; Cheon, B.; Park, M.-H. Carbon Dots as an Effective Fluorescent Sensing Platform for Metal Ion Detection. Nanoscale Res. Lett. 2019, 14, 272. [Google Scholar] [CrossRef]
- Devi, N.L. Persistent Organic Pollutants (POPs): Environmental Risks, Toxicological Effects, and Bioremediation for Environmental Safety and Challenges for Future Research. In Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Saxena, G., Bharagava, R.N., Eds.; Springer: Singapore, 2020; pp. 53–76. ISBN 978-981-13-1891-7. [Google Scholar]
- Akhtar, A.B.T.; Naseem, S.; Yasar, A.; Naseem, Z. Persistent Organic Pollutants (POPs): Sources, Types, Impacts, and Their Remediation. In Environmental Pollution and Remediation; Prasad, R., Ed.; Springer: Singapore, 2021; pp. 213–246. ISBN 978-981-15-5499-5. [Google Scholar]
- Ahmed, H.; Sharif, A.; Bakht, S.; Javed, F.; Hassan, W. Persistent Organic Pollutants and Neurological Disorders: From Exposure to Preventive Interventions. In Environmental Contaminants and Neurological Disorders; Akash, M.S.H., Rehman, K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 231–247. ISBN 978-3-030-66376-6. [Google Scholar]
- Said, T.O.; El Zokm, G.M. Toxicology and Ecological Risk with Emphasis on Scenario-Describing Mechanisms. In Persistent Organic Pollutants in Aquatic Systems: Classification, Toxicity, Remediation and Future; Said, T.O., El Zokm, G.M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 55–78. ISBN 978-3-031-53341-9. [Google Scholar]
- Devi, P.I.; Manjula, M.; Bhavani, R.V. Agrochemicals, Environment, and Human Health. Annu. Rev. Environ. Resour. 2022, 47, 399–421. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A Review on Effective Removal of Emerging Contaminants from Aquatic Systems: Current Trends and Scope for Further Research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Francis, B.; Aravindakumar, C.T.; Brewer, P.B.; Simon, S. Plant Nutrient Stress Adaptation: A Prospect for Fertilizer Limited Agriculture. Environ. Exp. Bot. 2023, 213, 105431. [Google Scholar] [CrossRef]
- Kumar, A.; Karn, S.K.; Hung, Y.-T. Chemicals Used in Agriculture: Hazards and Associated Toxicity Issues: Hazards and Toxicity of Agrochemicals. In Waste Treatment in the Biotechnology, Agricultural and Food Industries; Springer: Berlin/Heidelberg, Germany, 2023; Volume 2, pp. 185–198. [Google Scholar]
- Najam, L.; Alam, T. Occurrence, Distribution, and Fate of Emerging Persistent Organic Pollutants (POPs) in the Environment. In Emerging Contaminants and Plants: Interactions, Adaptations and Remediation Technologies; Aftab, T., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 135–161. ISBN 978-3-031-22269-6. [Google Scholar]
- Gupta, S. Organic Micropollutants in the Environment: Ecotoxicity Potential and Bioremediation Approaches. In Organic Micropollutants in Aquatic and Terrestrial Environments; Bhadouria, R., Tripathi, S., Singh, P., Singh, R., Singh, H.P., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 249–263. ISBN 978-3-031-48977-8. [Google Scholar]
- Jorfi, S.; Atashi, Z.; Akhbarizadeh, R.; Khorasgani, Z.N.; Ahmadi, M. Distribution and Health Risk Assessment of Organochlorine Pesticides in Agricultural Soils of the Aghili Plain, Southwest Iran. Environ. Earth Sci. 2019, 78, 603. [Google Scholar] [CrossRef]
- Sharma, S.; Pathania, S.; Bhagta, S.; Kaushal, N.; Bhardwaj, S.; Bhatia, R.K.; Walia, A. Microbial Remediation of Polluted Environment by Using Recombinant E. coli: A Review. Biotechnol. Environ. 2024, 1, 8. [Google Scholar] [CrossRef]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide Pollution in Agricultural Soils and Sustainable Remediation Methods: A Review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Belz, R.G.; Duke, S.O. Herbicides and Plant Hormesis. Pest Manag. Sci. 2014, 70, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Lahariya, V.; Vikas; Singh, A.K.; Das, A.; Yadav, A.; Gupta, G. Fluorometric Sensing and Nanomolar Level Detection of Heavy Metal Ions Using Nitrogen Doped Carbon Dots. Emergent Mater. 2024, 8, 1–15. [Google Scholar]
- Aloo, B.N. Pollution of Ground and Surface Waters with Agrochemicals. In Handbook of Water Pollution; Wiley: Hoboken, NJ, USA, 2024; pp. 65–96. [Google Scholar]
- Goralczyk, K. A Review of the Impact of Selected Anthropogenic Chemicals from the Group of Endocrine Disruptors on Human Health. Toxics 2021, 9, 146. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A Review on Metabolism, Toxicity, Occurrence in Food, Occupational Exposure, and Detoxification Methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Bansal, A.; Sharma, M.; Pandey, A.; Shankar, J. Aflatoxins: Occurrence, Biosynthesis Pathway, Management, and Impact on Health. In Fungal Resources for Sustainable Economy: Current Status and Future Perspectives; Singh, I., Rajpal, V.R., Navi, S.S., Eds.; Springer Nature: Singapore, 2023; pp. 565–594. ISBN 978-981-19-9103-5. [Google Scholar]
- Gbashi, S.; Njobeh, P.B.; Madala, N.E.; De Boevre, M.; Kagot, V.; De Saeger, S. Parallel Validation of a Green-Solvent Extraction Method and Quantitative Estimation of Multi-Mycotoxins in Staple Cereals Using LC-MS/MS. Sci. Rep. 2020, 10, 10334. [Google Scholar] [CrossRef]
- Okechukwu, V.O.; Adelusi, O.A.; Kappo, A.P.; Njobeh, P.B.; Mamo, M.A. Aflatoxins: Occurrence, Biosynthesis, Mechanism of Action and Effects, Conventional/Emerging Detection Techniques. Food Chem. 2024, 436, 137775. [Google Scholar] [CrossRef]
- Miklós, G.; Angeli, C.; Ambrus, Á.; Nagy, A.; Kardos, V.; Zentai, A.; Kerekes, K.; Farkas, Z.; Jóźwiak, Á.; Bartók, T. Detection of Aflatoxins in Different Matrices and Food-Chain Positions. Front. Microbiol. 2020, 11, 1916. [Google Scholar] [CrossRef]
- IARC. Aflatoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks on Humans; IARC: Lyon, France, 2012. [Google Scholar]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M.; et al. Mycotoxin Exposure and Human Cancer Risk: A Systematic Review of Epidemiological Studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef]
- Jaćević, V.; Dumanović, J.; Alomar, S.Y.; Resanović, R.; Milovanović, Z.; Nepovimova, E.; Wu, Q.; Franca, T.C.C.; Wu, W.; Kuča, K. Research Update on Aflatoxins Toxicity, Metabolism, Distribution, and Detection: A Concise Overview. Toxicology 2023, 492, 153549. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; et al. Risk Assessment of Aflatoxins in Food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- Omer, A.K.; Mohammed, R.R.; Ameen, P.S.M.; Abas, Z.A.; Ekici, K. Presence of Biogenic Amines in Food and Their Public Health Implications: A Review. J. Food Prot. 2021, 84, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Özogul, Y.; Özogul, F. Biogenic Amines Formation, Toxicity, Regulations in Food; The Royal Society of Chemistry: London, UK, 2019. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Lai, S.; Song, J.; Wu, X.; Wang, D.; Pang, L.; Chai, T. Rapid Determination of Histamine Level in Seafood Using Read-out Strips Based on High-Performance Thin Layer Chromatography Modified with Self-Visualization Nanomaterials. Food Control 2021, 122, 107816. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. US Food and Drug Administration Guidelines for the Validation of Chemical Methods for the FDA Foods Program. In FdA Foods program Guidelines for Chemical Methods, Version 2012; FDA: Silver Spring, MD, USA, 2012; Volume 1, p. 35. [Google Scholar]
- Xu, X.; Liu, X.; Wang, S.; Zou, Y.; Zhang, J.; Liang, L.; Wen, C.; Li, Y.; Xu, X.; He, X.; et al. Relationship between PAH4 Formation and Thermal Reaction Products in Model Lipids and Possible Pathways of PAHs Formation. J. Hazard. Mater. 2024, 465, 133374. [Google Scholar] [CrossRef]
- Singh, L.; Agarwal, T.; Simal-Gandara, J. PAHs, Diet and Cancer Prevention: Cooking Process Driven-Strategies. Trends Food Sci. Technol. 2020, 99, 487–506. [Google Scholar] [CrossRef]
- Du, W.; Jiang, S.; Lei, Y.; Wang, J.; Cui, Z.; Xiang, P.; Chang, Z.; Duan, W.; Shen, G.; Qin, Y.; et al. Occurrence, Formation Mechanism, and Health Risk of Polycyclic Aromatic Hydrocarbons in Barbecued Food. Ecotoxicol. Environ. Saf. 2025, 293, 118046. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Polycyclic Aromatic Hydrocarbons in Food-scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 6, 724. [Google Scholar]
- Singh, S.; Sivaram, N.; Dhanjal, D.S.; Assefa, H.; Singh, J.; Ramamurthy, P.C. Navigating the Complexity of Emerging Contaminants: Sources, Impacts, and Remediation Strategies. J. Indian Inst. Sci. 2024, 104, 519–553. [Google Scholar] [CrossRef]
- Belay, W.Y.; Getachew, M.; Tegegne, B.A.; Teffera, Z.H.; Dagne, A.; Zeleke, T.K.; Abebe, R.B.; Gedif, A.A.; Fenta, A.; Yirdaw, G.; et al. Mechanism of Antibacterial Resistance, Strategies and next-Generation Antimicrobials to Contain Antimicrobial Resistance: A Review. Front. Pharmacol. 2024, 15, 1444781. [Google Scholar] [CrossRef]
- Sudarsan, J.S.; Dogra, K.; Kumar, R.; Raval, N.P.; Leifels, M.; Mukherjee, S.; Trivedi, M.H.; Jain, M.S.; Zang, J.; Barceló, D.; et al. Tricks and Tracks of Prevalence, Occurrences, Treatment Technologies, and Challenges of Mixtures of Emerging Contaminants in the Environment: With Special Emphasis on Microplastic. J. Contam. Hydrol. 2024, 265, 104389. [Google Scholar] [CrossRef]
- Cechinel, M.A.P.; Macuvele, D.L.P.; Padoin, N.; Riella, H.G.; Soares, C. Occurrence of Emerging Contaminants in the Environment Causes and Effects. In Occurrence, Distribution and Toxic Effects of Emerging Contaminantsx; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-003-33575-7. [Google Scholar]
- Eze, C.G.; Nwankwo, C.E.; Dey, S.; Sundaramurthy, S.; Okeke, E.S. Food Chain Microplastics Contamination and Impact on Human Health: A Review. Environ. Chem. Lett. 2024, 22, 1889–1927. [Google Scholar] [CrossRef]
- Kumar, P.; Chaudhary, S.; Bhalla, A. Occurrence and Fate of Emerging Contaminants with Microplastics Current Scenario, Sources and Effects. In Occurrence, Distribution and Toxic Effects of Emerging Contaminantsx; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-003-33575-7. [Google Scholar]
- Molins-Delgado, D.; Díaz-Cruz, M.S.; Barceló, D. Introduction: Personal Care Products in the Aquatic Environment. In Personal Care Products in the Aquatic Environment; Díaz-Cruz, M.S., Barceló, D., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–34. ISBN 978-3-319-18809-6. [Google Scholar]
- Naeem, M.; Gill, R.; Gill, S.S.; Singh, K.; Sofo, A.; Tuteja, N. Emerging Contaminants and Their Effect on Agricultural Crops; Frontiers Media SA: Lausanne, Switzerland, 2023; Volume 14, p. 1296252. ISBN 1664-462X. [Google Scholar]
- Bhavadharini, B.; Kavimughil, M.; Malini, B.; Vallath, A.; Prajapati, H.K.; Sunil, C.K. Recent Advances in Biosensors for Detection of Chemical Contaminants in Food—A Review. Food Anal. Methods 2022, 15, 1545–1564. [Google Scholar] [CrossRef]
- Mirza Alizadeh, A.; Mohammadi, M.; Hashempour-baltork, F.; Hosseini, H.; Shahidi, F. Process-Induced Toxicants in Food: An Overview on Structures, Formation Pathways, Sensory Properties, Safety and Health Implications. Food Prod. Process. Nutr. 2025, 7, 7. [Google Scholar] [CrossRef]
- Lee, J.; Roux, S.; Le Roux, E.; Keller, S.; Rega, B.; Bonazzi, C. Unravelling Caramelization and Maillard Reactions in Glucose and Glucose + Leucine Model Cakes: Formation and Degradation Kinetics of Precursors, α-Dicarbonyl Intermediates and Furanic Compounds during Baking. Food Chem. 2022, 376, 131917. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, G.; Liu, H.; Han, B. Development Trends in Selective Hydrogenation Upgrading of 5-Hydroxymethylfurfural Catalyzed by Heterogeneous Metal Catalysts. Top. Catal. 2024, 1–13. [Google Scholar] [CrossRef]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and Risk Assessment of 5-Hydroxymethylfurfural in Food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef]
- Czerwonka, M.; Pietrzak-Sajjad, R.; and Bobrowska-Korczak, B. Evaluation of 5-Hydroxymethylfurfural Content in Market Milk Products. Food Addit. Contam. Part. A 2020, 37, 1135–1144. [Google Scholar] [CrossRef]
- Althaiban, M.A. Investigation of Hydroxymethylfurfural Levels in Commercial Acacia Honey for Quality Control: A Systematic Review. Discov. Appl. Sci. 2024, 6, 515. [Google Scholar] [CrossRef]
- Elbashir, A.A.; Omar, M.M.A.; Ibrahim, W.A.W.; Schmitz, O.J.; Aboul-Enein, H.Y. Acrylamide Analysis in Food by Liquid Chromatographic and Gas Chromatographic Methods. Crit. Rev. Anal. Chem. 2014, 44, 107–141. [Google Scholar] [CrossRef]
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Ghasemi, J.; Hosseini-Esfahani, F.; Mohammadi, A. Investigation and Determination of Acrylamide in the Main Group of Cereal Products Using Advanced Microextraction Method Coupled with Gas Chromatography-Mass Spectrometry. J. Cereal Sci. 2019, 87, 157–164. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, D.; Rachamalla, M.; Jangra, A. Toxic Effects of Acrylamide and Their Underlying Mechanisms. In Sustainable Development Goals Towards Environmental Toxicity and Green Chemistry: Environment and Sustainability; Prakash, C., Kesari, K.K., Negi, A., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 225–248. ISBN 978-3-031-77327-3. [Google Scholar]
- Song, X.; Yu, J.; Yu, X.; Zhang, F.; Zeng, J.; Wan, X.; Zhang, Y. Cracking the Code of Acrylamide and Nε-(Carboxymethyl)Lysine: Fish Oil Use and Predictive Strategies in Potato Chips during Thermal Processing. Food Chem. 2025, 473, 143034. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chai, X.; He, A.; Huang, Z.; Chen, S.; Rao, P.; Ke, L.; Xiang, L. Inhibition of Acrylamide Toxicity In Vivo by Arginine-Glucose Maillard Reaction Products. Food Chem. Toxicol. 2021, 154, 112315. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Song, Z.; Xu, X.; Yang, X.; Wei, S.; Chen, F.; Dong, X.; Zhang, X.; Zhu, Y. The Neurotoxicity of Acrylamide in Ultra-Processed Foods: Interventions of Polysaccharides through the Microbiota–Gut–Brain Axis. Food Funct. 2025, 16, 10–23. [Google Scholar] [CrossRef]
- Sebastià, A.; Fernández-Matarredona, C.; Castagnini, J.M.; Barba, F.J.; Berrada, H.; Moltó, J.C.; Pardo, O.; Esteve-Turrillas, F.A.; Ferrer, E. Acrylamide Content in Popcorn from Spanish Market: Risk Assessment. Food Chem. Toxicol. 2025, 196, 115145. [Google Scholar] [CrossRef]
- Usman, A.; Ahmad, M. From BPA to Its Analogues: Is. it a Safe Journey? Chemosphere 2016, 158, 131–142. [Google Scholar] [CrossRef]
- Kawamura, Y.; Inoue, K.; Nakazawa, H.; Yamada, T.; Maitani, T. Cause of bisphenol A migration from cans for drinks and assessment of improved cans. Shokuhin Eiseigaku Zasshi 2001, 42, 13–17. [Google Scholar] [CrossRef]
- Noonan, G.O.; Ackerman, L.K.; Begley, T.H. Concentration of Bisphenol A in Highly Consumed Canned Foods on the U.S. Market. J. Agric. Food Chem. 2011, 59, 7178–7185. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Tariq, T.; Fatima, B.; Sahar, A.; Tariq, F.; Munir, S.; Khan, S.; Nawaz Ranjha, M.M.A.; Sameen, A.; Zeng, X.-A.; et al. An Insight into Bisphenol A, Food Exposure and Its Adverse Effects on Health: A Review. Front. Nutr. 2022, 9, 1047827. [Google Scholar] [CrossRef]
- Costa, H.E.; Cairrao, E. Effect of Bisphenol A on the Neurological System: A Review Update. Arch. Toxicol. 2024, 98, 1–73. [Google Scholar] [CrossRef]
- Agarwal, A.; Gandhi, S.; Tripathi, A.D.; Gupta, A.; Iammarino, M.; Sidhu, J.K. Food Contamination from Packaging Material with Special Focus on the Bisphenol-A. Crit. Rev. Biotechnol. 2025, 45, 69–79. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Deveci, G.; Tek, N.A. N-Nitrosamines: A Potential Hazard in Processed Meat Products. J. Sci. Food Agric. 2024, 104, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, Z.; Jing, Y.; Wei, X.; Li, H.; Xie, J.; Shen, M. Formation of Advanced Glycation End-Products and N-Nitrosamines in Salami of Different Recipes and Fermented at Different Stages. Food Chem. 2025, 474, 143228. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Huang, X.; Xiao, Z.; Yang, Y.; Yu, Q.; Chen, S.; He, L.; Liu, A.; Liu, S.; et al. A Review on Mechanistic Overview on the Formation of Toxic Substances during the Traditional Fermented Food Processing. Food Rev. Int. 2023, 39, 1275–1292. [Google Scholar] [CrossRef]
- Zhou, F.; Liao, X.; Li, L.; Huang, Y.; Qi, H.; Li, Q.; Zou, J.; Zhou, Z.; Tu, F.; Wei, M.; et al. Laws and Mechanisms of Inorganic Ammonia Mediates Organic NDMA Formation during Ozonation of Amines. Sep. Purif. Technol. 2025, 354, 128841. [Google Scholar] [CrossRef]
- Paustenbach, D.J.; Brown, S.E.; Heywood, J.J.; Donnell, M.T.; Eaton, D.L. Risk Characterization of N-Nitrosodimethylamine in Pharmaceuticals. Food Chem. Toxicol. 2024, 186, 114498. [Google Scholar] [CrossRef]
- Tayel, D.I.; Farrag, N.K.; Aborhyem, S.M. Dietary Intake and Risk Assessment of Nitrosamine in Processed Meat Products among Medical Staff during Their Night Shift. Sci. Rep. 2025, 15, 1898. [Google Scholar] [CrossRef]
- Ramezani, H.; Hosseini, H.; Kamankesh, M.; Ghasemzadeh-Mohammadi, V.; Mohammadi, A. Rapid Determination of Nitrosamines in Sausage and Salami Using Microwave-Assisted Extraction and Dispersive Liquid–Liquid Microextraction Followed by Gas Chromatography–Mass Spectrometry. Eur. Food Res. Technol. 2015, 240, 441–450. [Google Scholar] [CrossRef]
- Devi, P.; Saini, S.; Kim, K.-H. The Advanced Role of Carbon Quantum Dots in Nanomedical Applications. Biosens. Bioelectron. 2019, 141, 111158. [Google Scholar] [CrossRef]
- Ji, C.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Recent Developments of Carbon Dots in Biosensing: A Review. ACS Sens. 2020, 5, 2724–2741. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gadly, T.; Gupta, A.; Ballal, A.; Ghosh, S.K.; Kumbhakar, M. Origin of Excitation Dependent Fluorescence in Carbon Nanodots. J. Phys. Chem. Lett. 2016, 7, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, J.; Duan, Y.; Liu, W.; Li, D.; Yan, Z.; Yang, K. Highly Luminescent Nitrogen-Doped Carbon Dots for Simultaneous Determination of Chlortetracycline and Sulfasalazine. Luminescence 2018, 33, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Chobpattana, V.; Sangtawesin, T.; Khaopueak, P.; Wechakorn, K. Sugar Derived-Fluorescent Carbon Quantum Dots Conjugated Glutathione for Sensing Heavy Metal Ions and Antioxidant Activity. Mater. Sci. Eng. B 2025, 313, 117956. [Google Scholar] [CrossRef]
- Arvapalli, D.M.; Sheardy, A.T.; Alapati, K.C.; Wei, J. High Quantum Yield Fluorescent Carbon Nanodots for Detection of Fe (III) Ions and Electrochemical Study of Quenching Mechanism. Talanta 2020, 209, 120538. [Google Scholar] [CrossRef]
- Wahyudi, S.; Rizoputra, I.; Panatarani, C.; Faizal, F.; Bahtiar, A. Green Synthesis of Carbon Nanodots (CNDs) Moderated by Flavonoid Extracts from Moringa Oleifera Leaves and Co-Doped Sulfur/Nitrogen (NS–CNDs–Fla) and Their Potential for Heavy Metals Sensing Application. J. Fluoresc. 2024, 1–13. [Google Scholar] [CrossRef]
- Kim, Y.; Jeon, Y.; Na, M.; Hwang, S.-J.; Yoon, Y. Recent Trends in Chemical Sensors for Detecting Toxic Materials. Sensors 2024, 24, 431. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Mujumdar, A.S.; Wang, H. Application of Carbon Dots in Food Preservation: A Critical Review for Packaging Enhancers and Food Preservatives. Crit. Rev. Food Sci. Nutr. 2023, 63, 6738–6756. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, X.; Wang, X.; Xu, B.; Zhang, F.; Wu, W. Synthesis of a Core–Shell Magnetic Covalent Organic Framework for the Enrichment and Detection of Aflatoxin in Food Using HPLC-MS/MS. Microchim. Acta 2023, 190, 488. [Google Scholar] [CrossRef]
- Alsammarraie, F.K.; Lin, M. Using Standing Gold Nanorod Arrays as Surface-Enhanced Raman Spectroscopy (SERS) Substrates for Detection of Carbaryl Residues in Fruit Juice and Milk. J. Agric. Food Chem. 2017, 65, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent Metal Nanoclusters with Aggregation-Induced Emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Buzuk, M. Chemical Sensors for Toxic Chemical Detection. Sensors 2024, 24, 6072. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-Assisted Synthesis of Carbon Dots and Their Applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Nguyen, D.H.H.; El-Ramady, H.; Prokisch, J. Food Safety Aspects of Carbon Dots: A Review. Environ. Chem. Lett. 2025, 23, 337–360. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Zhang, X.; Luo, P.; Lin, G.; Jiang, W. Highly Photoluminescent Carbon Dots-Based Immunosensors for Ultrasensitive Detection of Aflatoxin M1 Residues in Milk. Food Chem. 2021, 355, 129443. [Google Scholar] [CrossRef]
- Singh, H.; Singh, S.; Bhardwaj, S.K.; Kaur, G.; Khatri, M.; Deep, A.; Bhardwaj, N. Development of Carbon Quantum Dot-Based Lateral Flow Immunoassay for Sensitive Detection of Aflatoxin M1 in Milk. Food Chem. 2022, 393, 133374. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Törős, G.; Prokisch, J. Assessing the Efficacy of Carbon Nanodots Derived from Curcumin on Infectious Diseases. Expert Rev. Anti Infect. Ther. 2024, 22, 1107–1121. [Google Scholar] [CrossRef]
- Kaur, H.; Bhattu, M.; Chakroborty, S.; Aulakh, M.K.; Mutreja, V.; Verma, M.; Tiwari, K.; Chakraborty, C.; Darwish, I.A. Highly Green Fluorescent Carbon Dots from Gallic Acid: A Turn-On Sensor toward Pb2+ Ions. ACS Omega 2025, 10, 2354–2363. [Google Scholar] [CrossRef]
- Sharma, N.; Thakur, S.; Bains, A.; Goksen, G.; Ali, N.; Ansari, M.A.; Kopsacheili, A.; Proestos, C.; Chawla, P. Green Hydrothermal Approach for the Synthesis of Carbon Quantum Dots from Waste Tea Bags for Acrylamide Detection in Drinking Water: A Fluorescence Assay Validated by HPLC-PDA Analysis. Food Chem. X 2025, 25, 102043. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Hasan, E.; Sun, X.; Asif, M.; Aziz, A.; Lu, W.; Dong, C.; Shuang, S. Bridging Biological and Food Monitoring: A Colorimetric and Fluorescent Dual-Mode Sensor Based on N-Doped Carbon Dots for Detection of pH and Histamine. J. Hazard. Mater. 2024, 470, 134271. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Guo, W. Carbon Dots-Based Fluorescent Probe for the Detection of Imidacloprid Residue in Leafy Vegetables. Food Chem. 2024, 435, 137578. [Google Scholar] [CrossRef]
- Durrani, S.; Zhang, J.; Durrani, F.; Wang, Z.; Mukramin; Xu, K.-F.; Wang, H.; Khan, H.; Wu, F.-G.; Lin, F. Triple Channel Fluorescence Na-Ca-Cl-Doped Carbon Dots for Erythrosine Detection in Food Samples and Living Cells. J. Mol. Struct. 2025, 1321, 139934. [Google Scholar] [CrossRef]
- Li, N.; Liu, T.; Liu, S.G.; Lin, S.M.; Fan, Y.Z.; Luo, H.Q.; Li, N.B. Visible and Fluorescent Detection of Melamine in Raw Milk with One-Step Synthesized Silver Nanoparticles Using Carbon Dots as the Reductant and Stabilizer. Sens. Actuators B Chem. 2017, 248, 597–604. [Google Scholar] [CrossRef]
- Zhuang, Q.; Li, L.; Ding, Y.; Zeng, H.; Wu, Y. Highly Luminescent Nitrogen-Doped Carbon Dots as “Turn-On” Fluorescence Probe for Selective Detection of Melamine. ChemistrySelect 2019, 4, 84–89. [Google Scholar] [CrossRef]
- Waluyo, R.; Manopo, J.; Isnaeni; Darma, Y. Unraveling Excitation-Dependent Fluorescence of Nitrogen and Sodium Co-Doped Carbon Dots for Dual Detection of Fe3+ and Ag+. Colloids Surf. A Physicochem. Eng. Asp. 2025, 707, 135810. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Li, H.; Wang, Y.; Xiao, F.; Yang, D.; Xia, Q.; Yang, Y. SERS Detection of Hg2+ and Aflatoxin B1 through on–off Strategy of Oxidase-like Au@HgNPs/Carbon Dots. Food Chem. 2023, 424, 136443. [Google Scholar] [CrossRef]
- Aafria, S.; Sharma, M. Development of a Rapid and Ultrasensitive Acrylamide Nanosensor Based on TiO2 NPs/GQDs Nanocomposite. J. Food Compos. Anal. 2025, 140, 107188. [Google Scholar] [CrossRef]
- Alhazzani, K.; Alanazi, A.Z.; Ibrahim, H.; Mostafa, A.M.; Barker, J.; Mahmoud, A.M.; El-Wekil, M.M.; Ali, A.-M.B.H. L-Asparaginase-Mediated pH Shift and Carbon Dot Fluorescence Modulation: A Sensitive Ratiometric Method for Quantifying L-Asparagine in Diverse Potato Varieties under Variable Storage Conditions. Food Chem. 2025, 463, 141396. [Google Scholar] [CrossRef]
- Medhi, M.; Yumnam, M.; Mudoi, P.; Mishra, P. Green Florescent Carbon Dots Synthesized from Various Household Green Wastes for Detection of Parathion Methyl Pesticide. J. Lumin. 2025, 277, 120926. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Wang, M.; Shan, R.; Li, X.; Zhang, H. Novel Ratiometric Fluorescence Sensor for Organophosphorus Pesticides via Carbon Dots Supported Zirconium-Based Metal Organic Frameworks. Food Control 2025, 171, 111095. [Google Scholar] [CrossRef]
- Rahmah, A.S.; Heryanto, H.; Rinovian, A.; Yudasari, N.; Tahir, D. Structural Characterization of Carbon Quantum Dots Derived from Tea Residue and Their Photocatalytic Application in CQDs-Modified Al2(SO4)3 Nanoparticles for Sustainable Pesticide Degradation. Mater. Chem. Phys. 2025, 334, 130401. [Google Scholar] [CrossRef]
- Renu; Nidhi; Kaur, P.; Komal; Minakshi; Paulik, C.; Kaushik, A.; Singhal, S. Rational Design of Boerhavia Diffusa Derived CoFe2O4-Carbon dots@Boehmite Platform for Photocatalysis and Ultra Trace Monitoring of Hazardous Pesticide and UO22+ Ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 325, 125111. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Jain, V.; Renuka Jyothi, S.; Bhanot, D.; Kumari, B.; Verma, M.; Mutreja, V.; Bhattu, M. Dual-Emission Carbon Dots from Gallic Acid for Selective Turn off Fluorescent Detection of Chlorpyrifos in Wastewater. J. Mol. Struct. 2025, 1327, 141147. [Google Scholar] [CrossRef]
- Vadia, F.Y.; Jha, S.; Mehta, V.N.; Park, T.J.; Malek, N.I.; Kailasa, S.K. Development of Sustainable Fluorescence Approach with Red Emissive Carbon Dots Derived from Grewia Asiatica Fruit for the Detection of Quinalphos. J. Photochem. Photobiol. A Chem. 2025, 458, 115948. [Google Scholar] [CrossRef]
- Kumar, V.; Kaushal, I.; Sharma, A.K.; Sharma, S.; Kumar, A.; Bhukal, S.; Rani, J.; Saharan, P. Biowaste-Derived Multifunctional CeO2@carbon Dot Nanospheres for Efficient Sono-Catalytic Degradation of Rhodamine B Dye and Electrochemical Sensing of 4-Nitrophenol. Appl. Surf. Sci. 2025, 688, 162351. [Google Scholar] [CrossRef]
- Pan, M.; Gao, M.; Cui, J.; Gao, R.; Li, H.; Sun, J.; Chen, W.; Wang, S. Fluorescent Molecularly Imprinted Hydrogel Sensing Strip Based on Nitrogen-Doped Carbon Dots and Inverse Opal Photonic Crystals Applying for Effective Detection for Imidacloprid in Fruits and Vegetables. Food Chem. 2025, 477, 143497. [Google Scholar] [CrossRef]
- Mallik, A.; Hazra, M.; Adak, M.K.; Nag, R.; Pandey, A.; Sahoo, G.P. Fluorescent Probe Based on Boron-nitrogen Co-Doped Carbon Dots for the Rapid Detection of Acephate Residue in Vegetables and Water. Diam. Relat. Mater. 2025, 153, 112107. [Google Scholar] [CrossRef]
- Muangmora, R.; Rojviroon, O.; Kemacheevakul, P.; Chuangchote, S.; Rajendran, R.; Phouheuanghong, P.; Arumugam, P.; Paramasivam, S.; Rojviroon, T. Spent Coffee Ground-Derived Carbon Quantum Dot Composite with Metal Oxides for Photocatalytic Degradation of Carbaryl in Water and Antibacterial Application. J. Water Process Eng. 2025, 70, 107145. [Google Scholar] [CrossRef]
- Silaram, W.; Boonlerd, R.; Rattanaphonsaen, P.; Khiaophong, W.; Kachangoon, R.; Teshima, N.; Pakkethati, K.; Mukdasai, S.; Ponhong, K.; Vichapong, J. Functional Graphene Carbon Dots in Corn Stalk Pith as an Efficient Sorbent for Preconcentration and Trace Determination of Triazole Residues in Rice Samples. Microchem. J. 2025, 211, 113087. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, S.; Tao, C.; Yan, J.; Jiang, Y.; Lu, Y. Nitrogen-Doped Carbon Dots as Efficient Turn-on Fluorescent Probe for Assay of Organophosphorus Pesticides. ChemPhysMater 2024, 3, 462–469. [Google Scholar] [CrossRef]
- Liu, H.; Wu, S. Blue Fluorescent Carbon Dots Doped with Nitrogen and Sulfur as a Dual-Functional Fluorescent Probe for the Detection of Hg2+ and Chloramphenicol. J. Mol. Struct. 2025, 1329, 141459. [Google Scholar] [CrossRef]
- Tran, N.B.; Nguyen, Q.K.; Ngoc Dang, T.M.; Tran, T.D.; Nguyen, T.M.; Thuong Nguyen, T.K.; Vu, D.T.; Pham, B.; Huong Nguyen, T.A.; Mai Pham, T.N. Nitrogen Doped Carbon Quantum Dots (N-CQDs) Synthesized by a Microwave Assisted Hydrothermal Method in Combination with Gold Nanoparticles (AuNPs) as Sensitive Dual Sensor for Antibiotic Meropenem Detection. Microchem. J. 2025, 210, 112977. [Google Scholar] [CrossRef]
- Xia, W.; Wu, Z.; Hou, B.; Cheng, Z.; Bi, D.; Chen, L.; Chen, W.; Yuan, H.; Koole, L.H.; Qi, L. Inactivation of Antibiotic Resistant Bacteria by Nitrogen-Doped Carbon Quantum Dots through Spontaneous Generation of Intracellular and Extracellular Reactive Oxygen Species. Mater. Today Bio 2025, 30, 101428. [Google Scholar] [CrossRef]
- Miao, H.; Wang, P.; Wu, J.; Li, X.; Du, Y.; Yan, H.; You, Q.; Dong, W.; Li, L. Highly Efficient and Broad-Spectrum Antibacterial Carbon Dots Combat Antibiotic Resistance. Talanta 2025, 281, 126926. [Google Scholar] [CrossRef]
- Ebere Enyoh, C.; Wang, Q. Box–Behnken Design and Machine Learning Optimization of PET Fluorescent Carbon Quantum Dots for Removing Fluoxetine and Ciprofloxacin with Molecular Dynamics and Docking Studies as Potential Antidepressant and Antibiotic. Sep. Purif. Technol. 2025, 362, 131975. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Zhang, Y.; Li, H.; Jiang, S.; Han, J.; Gong, W.; Li, D.; Yao, Z. A Ratiometric Fluorescent Probe for the Determination of Quinolone Antibiotics in Milk Based on N and S Co-Doped Carbon Quantum Dots. Food Control 2025, 167, 110812. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Hemalatha, M.; Venkatasubbu, D.G.; Abimanyu, S.; Aravind, M.; Rathinsabapathi, P. Label-Free Detection of Ampicillin in Milk and Water Using Fluorescence Carbon Dots Synthesised from Citrus Limetta Peel. Diam. Relat. Mater. 2025, 152, 111892. [Google Scholar] [CrossRef]
- Du, J.; Liu, S.; Hou, J.; Wu, X.; Si, H.; Guo, X.; Zhuo, S. Curcumin-Derived Water-Soluble Carbon Dots without Detectable Resistance: Dual Potentials for Antimicrobial Activity and Infected Wound Healing. Diam. Relat. Mater. 2025, 153, 112075. [Google Scholar] [CrossRef]
- Hu, J.; Long, X.; Wu, S. High-Performance Nitrogen-Doped Green Fluorescent Carbon Dots for Applications in Rapid Detection of Chlortetracycline and Fluorescent Film. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 329, 125564. [Google Scholar] [CrossRef]
- Hu, J.; Ma, Y.; Wu, S. Synthesis and Application of Optically Stable Red Fluorescent Carbon Dots for Sensitive and Selective Detection of Ceftazidime. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 327, 125341. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Qi, H.; Yi, T.; Jing, T.; Li, J.; Gao, Y.; Zeng, Q.; Zhao, H. Quaternary Ammonium Salt-Derived Carbon Dots for Antibacterial Efficacy and Tetracycline Sensing. J. Mol. Struct. 2025, 1322, 140620. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, H.; Liu, J.; Jamil, A.; Hou, Y.; Li, Q.; Zhao, M.; Zhao, C.; Li, W.; Hong, B. Deep Eutectic Solvent-Based Magnetic Molecular Nanomaterials Coupled with Fluorescent Carbon Dots as a New Strategy to Highly Enrich and Sensitively Detect Doxycycline in Food Matrices. Food Chem. X 2025, 25, 102202. [Google Scholar] [CrossRef] [PubMed]
- Buzid, A.; Luong, J.H.T. Electrochemical Sensing and Biosensing-Based on Carbon Nanodots. In Handbook of Nanobioelectrochemistry: Application in Devices and Biomolecular Sensing; Azad, U.P., Chandra, P., Eds.; Springer Nature: Singapore, 2023; pp. 339–362. ISBN 978-981-19-9437-1. [Google Scholar]
- Mei, Y.; He, C.; Zeng, W.; Luo, Y.; Liu, C.; Yang, M.; Kuang, Y.; Lin, X.; Huang, Q. Electrochemical Biosensors for Foodborne Pathogens Detection Based on Carbon Nanomaterials: Recent Advances and Challenges. Food Bioprocess Technol. 2022, 15, 498–513. [Google Scholar] [CrossRef]
- Gauglitz, G. Analytical Evaluation of Sensor Measurements. Anal. Bioanal. Chem. 2018, 410, 5–13. [Google Scholar] [CrossRef]
- Rajoriya, K.; Pratibha; Abhijeet; Meena, R.; Kumari, A. Synthesis of Fluorometric Carbon Nano Dots(CNDs) for Selective Sensing of Biologically Important Fe3+ and Cu2+ Metal Ions and Evaluating Their Antioxidant Capacity. J. Fluoresc. 2024, 1–10. [Google Scholar] [CrossRef]
- Zito, C.A.; Orlandi, M.O.; Volanti, D.P. Accelerated Microwave-Assisted Hydrothermal/Solvothermal Processing: Fundamentals, Morphologies, and Applications. J. Electroceram 2018, 40, 271–292. [Google Scholar] [CrossRef]
- Khan, S.; Dunphy, A.; Anike, M.S.; Belperain, S.; Patel, K.; Chiu, N.H.L.; Jia, Z. Recent Advances in Carbon Nanodots: A Promising Nanomaterial for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 6786. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface Modification and Chemical Functionalization of Carbon Dots: A Review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef]
- Qi, X.; Jin, W.; Tang, C.; Xiao, X.; Li, R.; Ma, Y.; Ma, L. pH Monitoring in High Ionic Concentration Environments: Performance Study of Graphene-Based Sensors. Anal. Sci. 2025, 41, 127–135. [Google Scholar] [CrossRef]
- Sankhla, L.; Kushwaha, H.S. Development of an Opto-Electrochemical Sensor for the Detection of Malathion Using Manganese Metal–Organic Framework (Mn-MOF). J. Mater. Sci. Mater. Eng. 2024, 19, 11. [Google Scholar] [CrossRef]
- Yoo, H.; Jo, H.; Soo Oh, S. Detection and beyond: Challenges and Advances in Aptamer-Based Biosensors. Mater. Adv. 2020, 1, 2663–2687. [Google Scholar] [CrossRef]
- Chen, M.; Li, H.; Xue, X.; Tan, F.; Ye, L. Signal Amplification in Molecular Sensing by Imprinted Polymers. Microchim. Acta 2024, 191, 574. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.G.L.; Lopes, P.A.; Pochapski, D.J.; de Oliveira, L.C.A.; Pinto, F.G.; Neto, J.L.; Tronto, J. Effect of pH, Ionic Strength, and Temperature on the Adsorption Behavior of Acid Blue 113 onto Mesoporous Carbon. Environ. Sci. Pollut. Res. 2022, 29, 77188–77198. [Google Scholar] [CrossRef] [PubMed]
- Saputra, H.A. Electrochemical Sensors: Basic Principles, Engineering, and State of the Art. Monatsh Chem. 2023, 154, 1083–1100. [Google Scholar] [CrossRef]
- He, X.; Liu, Z.; Shen, G.; He, X.; Liang, J.; Zhong, Y.; Liang, T.; He, J.; Xin, Y.; Zhang, C.; et al. Microstructured Capacitive Sensor with Broad Detection Range and Long-Term Stability for Human Activity Detection. npj Flex. Electron. 2021, 5, 17. [Google Scholar] [CrossRef]
- Yaroshenko, I.; Kirsanov, D.; Marjanovic, M.; Lieberzeit, P.A.; Korostynska, O.; Mason, A.; Frau, I.; Legin, A. Real-Time Water Quality Monitoring with Chemical Sensors. Sensors 2020, 20, 3432. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Guo, X.; Li, Y.; Li, L.; Pan, L. Recent Advances in Conductive Polymers-Based Electrochemical Sensors for Biomedical and Environmental Applications. Polymers 2024, 16, 1597. [Google Scholar] [CrossRef]
- Boonruang, S.; Naksen, P.; Anutrasakda, W.; Chansaenpak, K.; Promarak, V.; Saenmuangchin, R.; Phechkrajang, C.; Jarujamrus, P. Use of Nitrogen-Doped Amorphous Carbon Nanodots (N-CNDs) as a Fluorometric Paper-Based Sensor: A New Approach for Sensitive Determination of Lead(II) at a Trace Level in Highly Ionic Matrices. Anal. Methods 2021, 13, 3551–3560. [Google Scholar] [CrossRef]
- Vaks, J.E.; Hemyari, P.; Rullkoetter, M.; Santulli, M.J.; Schoenbrunner, N. Verification of Claimed Limit of Detection in Molecular Diagnostics. J. Appl. Lab. Med. 2016, 1, 260–270. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Verma, C. Carbon Nanodots: Recent Advances in Synthesis and Applications. Carbon Lett. 2022, 32, 1603–1629. [Google Scholar] [CrossRef]
- Hnasko, R.M. Bioconjugation of Antibodies to Horseradish Peroxidase (HRP). In ELISA: Methods and Protocols; Hnasko, R., Ed.; Springer: New York, NY, USA, 2015; pp. 43–50. ISBN 978-1-4939-2742-5. [Google Scholar]
- Nabi Duman, A.; Jalilov, A.S. Machine Learning for Carbon Dot Synthesis and Applications. Mater. Adv. 2024, 5, 7097–7112. [Google Scholar] [CrossRef]
- Singh, P.; Arpita; Kumar, S.; Kumar, P.; Kataria, N.; Bhankar, V.; Kumar, K.; Kumar, R.; Hsieh, C.-T.; Shiong Khoo, K. Assessment of Biomass-Derived Carbon Dots as Highly Sensitive and Selective Templates for the Sensing of Hazardous Ions. Nanoscale 2023, 15, 16241–16267. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A.; Niu, J. Doping Strategy, Properties and Application of Heteroatom-Doped Ordered Mesoporous Carbon. RSC Adv. 2021, 11, 5361–5383. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Ji, Y.; Schwaneberg, U. Empowering Site-Specific Bioconjugations In Vitro and In Vivo: Advances in Sortase Engineering and Sortase-Mediated Ligation. Angew. Chem. Int. Ed. 2024, 63, e202310910. [Google Scholar] [CrossRef]
- Hu, Y.; Seivert, O.; Tang, Y.; Karahan, H.E.; Bianco, A. Carbon Dot Synthesis and Purification: Trends, Challenges and Recommendations. Angew. Chem. Int. Ed. 2024, 63, e202412341. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Guo, Y.-T.; Rinawati, M.; Chang, C.-C.; Chang, L.-Y.; Yeh, M.-H. Developing Flexible Carbon Nitride Quantum Dots Decorated Polyaniline Nanocomposite Layer for a Non-Invasive Wearable Sweat Biosensor for Glucose Monitoring. ECS Meet. Abstr. 2024, MA2024-01, 1643. [Google Scholar] [CrossRef]
- Liu, C.; Lin, X.; Liao, J.; Yang, M.; Jiang, M.; Huang, Y.; Du, Z.; Chen, L.; Fan, S.; Huang, Q. Carbon Dots-Based Dopamine Sensors: Recent Advances and Challenges. Chin. Chem. Lett. 2024, 35, 109598. [Google Scholar] [CrossRef]
- Sun, F.; Dong, G.; Jiang, F.; Wang, X.; Diao, B.; Li, X.; Joo, S.W.; Zhang, L.; Kim, S.H.; Cong, C.; et al. Carbon Quantum Dot/Multiwalled Carbon Nanotube-Based Self-Powered Strain Sensors for Remote Human Motion Detection. ACS Appl. Nano Mater. 2024, 7, 27706–27716. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Decadal Journey of CNT-Based Analytical Biosensing Platforms in the Detection of Human Viruses. Nanomaterials 2022, 12, 4132. [Google Scholar] [CrossRef]
- Banerjee, S.L.; Tirrell, M.V. Carbon Nanotube-Based Chemical Sensors. In Materials for Chemical Sensors; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-003-03977-8. [Google Scholar]
- Munawar, A.; Hussain Khan, R.F.; Iqbal, M.Z.; Rehman, A.; Bajwa, S.Z.; Khan, W.S. 18−Drug-Detection Performance of Carbon Nanotubes Decorated with Metal Oxide Nanoparticles. In Metal Oxide-Carbon Hybrid Materials; Chaudhry, M.A., Hussain, R., Butt, F.K., Eds.; Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2022; pp. 475–493. ISBN 978-0-12-822694-0. [Google Scholar]
- Meskher, H.; Mustansar, H.C.; Thakur, A.K.; Sathyamurthy, R.; Lynch, I.; Singh, P.; Han, T.K.; Saidur, R. Recent Trends in Carbon Nanotube (CNT)-Based Biosensors for the Fast and Sensitive Detection of Human Viruses: A Critical Review. Nanoscale Adv. 2023, 5, 992–1010. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.H.H.; Muthu, A.; Elsakhawy, T.; Sheta, M.H.; Abdalla, N.; El-Ramady, H.; Prokisch, J. Carbon Nanodots-Based Sensors: A Promising Tool for Detecting and Monitoring Toxic Compounds. Nanomaterials 2025, 15, 725. https://doi.org/10.3390/nano15100725
Nguyen DHH, Muthu A, Elsakhawy T, Sheta MH, Abdalla N, El-Ramady H, Prokisch J. Carbon Nanodots-Based Sensors: A Promising Tool for Detecting and Monitoring Toxic Compounds. Nanomaterials. 2025; 15(10):725. https://doi.org/10.3390/nano15100725
Chicago/Turabian StyleNguyen, Duyen H. H., Arjun Muthu, Tamer Elsakhawy, Mohamed H. Sheta, Neama Abdalla, Hassan El-Ramady, and József Prokisch. 2025. "Carbon Nanodots-Based Sensors: A Promising Tool for Detecting and Monitoring Toxic Compounds" Nanomaterials 15, no. 10: 725. https://doi.org/10.3390/nano15100725
APA StyleNguyen, D. H. H., Muthu, A., Elsakhawy, T., Sheta, M. H., Abdalla, N., El-Ramady, H., & Prokisch, J. (2025). Carbon Nanodots-Based Sensors: A Promising Tool for Detecting and Monitoring Toxic Compounds. Nanomaterials, 15(10), 725. https://doi.org/10.3390/nano15100725










