Enhanced Separation of Palladium from Nuclear Wastewater by the Sulfur-Rich Functionalized Covalent Organic Framework
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of TAPB-BMTTPA-COF
2.3. The Adsorption Performance of TAPB-BMTTPA-COF Towards Pd(II)
2.4. Characterizations
2.5. Density Functional Theory Calculation
3. Results and Discussion
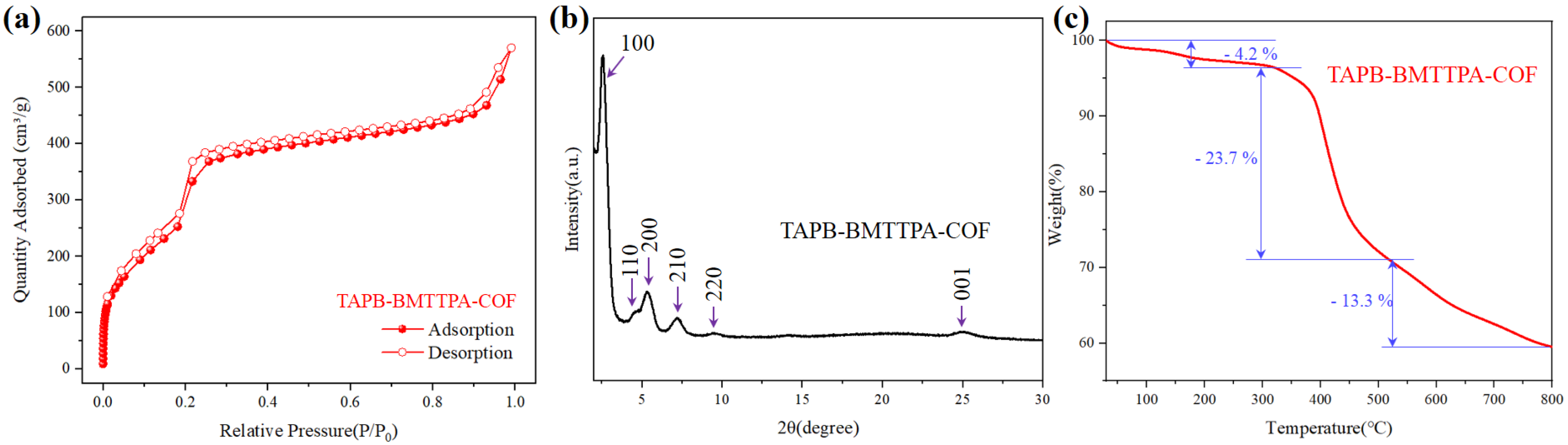
3.1. Characterizations of TAPB-BMTTPA-COF
3.2. TAPB-BMTTPA-COF Sorption Performance Towards Pd(II)
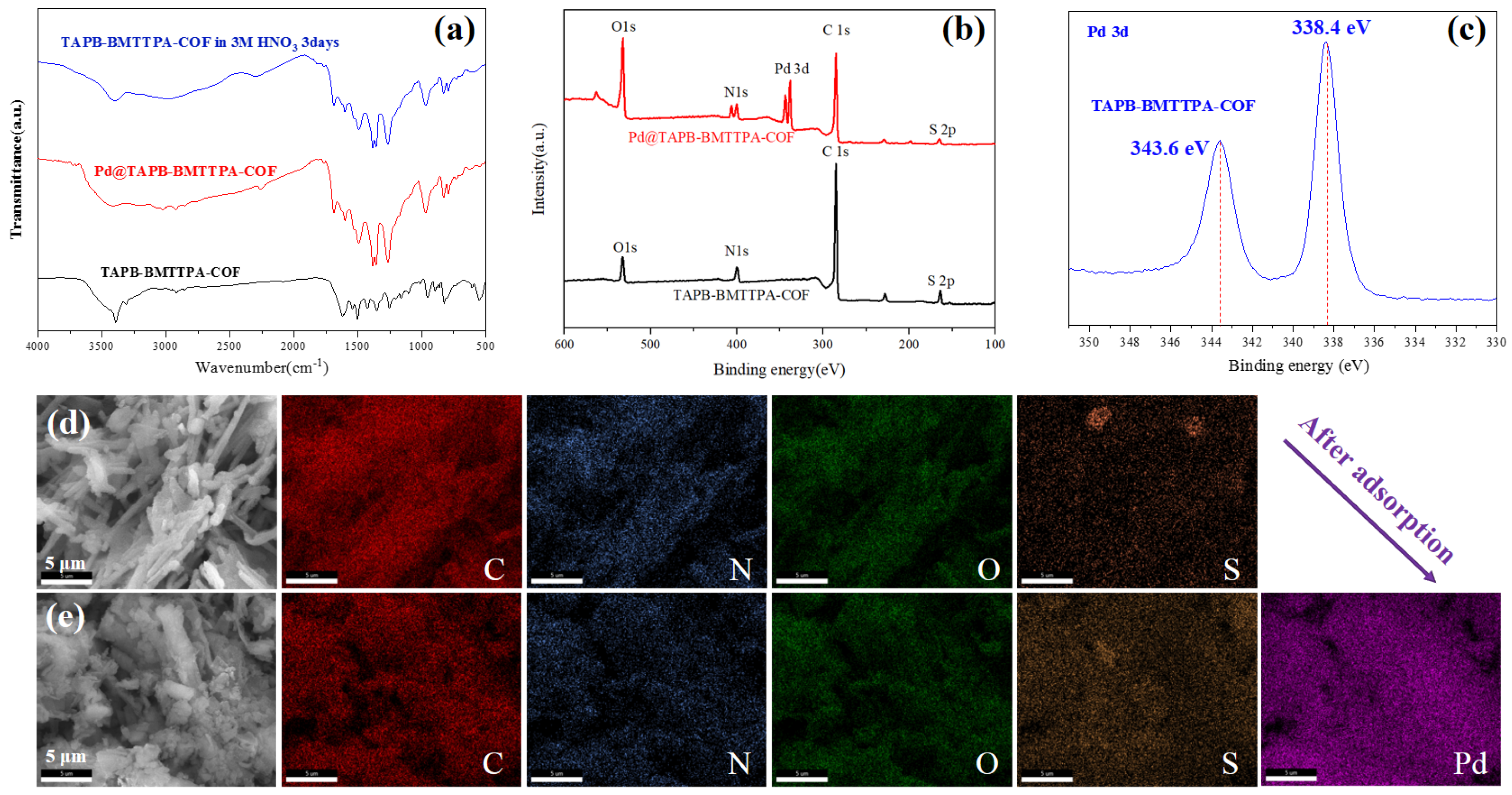
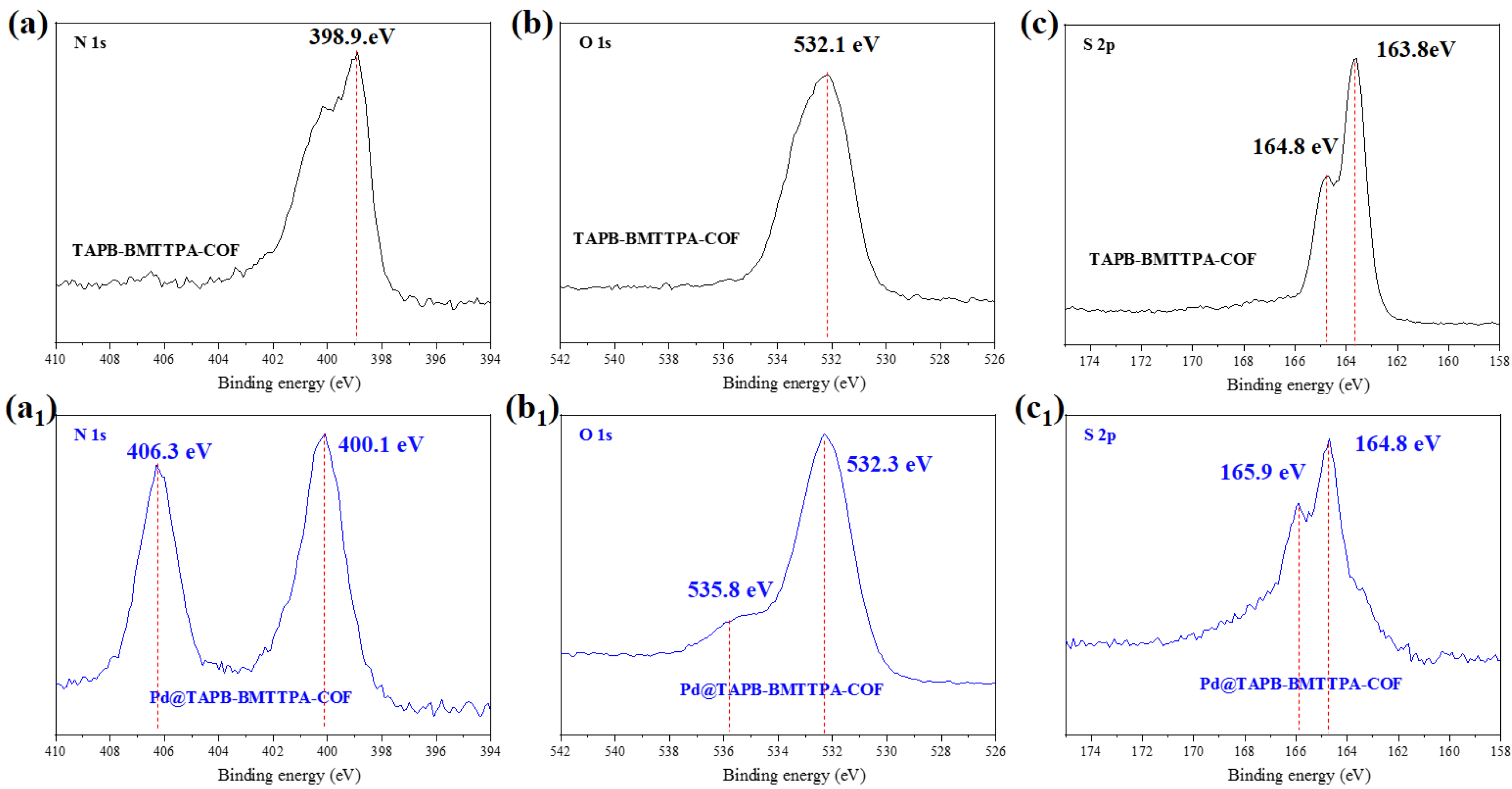
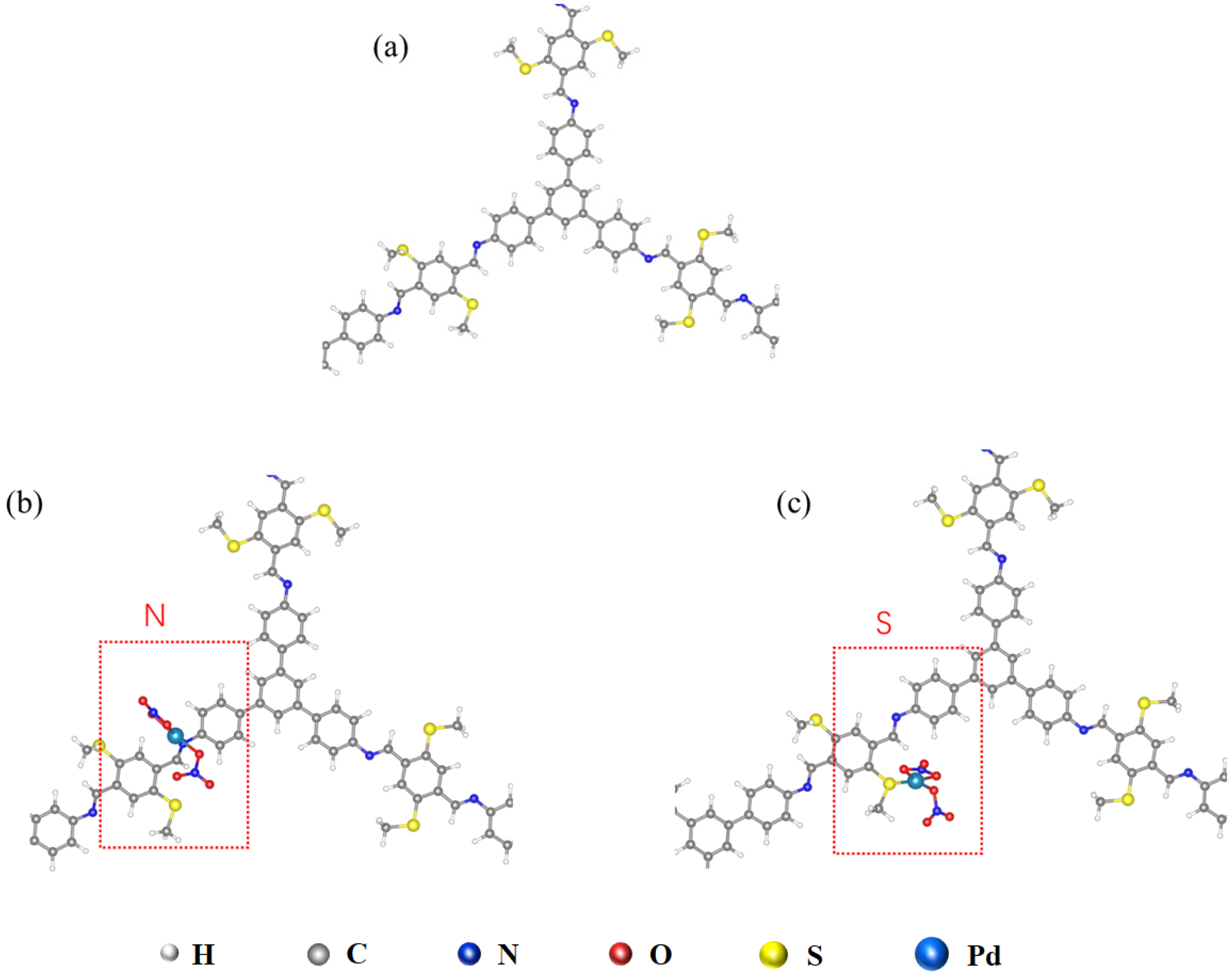
3.3. The Sorption Mechanism of TAPB-BMTTPA-COF
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rao, C.R.M.; Reddi, G.S. Platinum group metals (PGM); occurrence, use and recent trends in their determination. TrAC Trends Anal. Chem. 2000, 19, 565–586. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Antler, M. The Development and Application of Palladium Contact Materials. Platin. Met. Rev. 1987, 31, 13–19. [Google Scholar] [CrossRef]
- Uehling, M.R.; King, R.P.; Krska, S.W.; Cernak, T.; Buchwald, S.L. Pharmaceutical diversification via palladium oxidative addition complexes. Science 2019, 363, 405–408. [Google Scholar] [CrossRef]
- Tang, J.; Zuo, Y.; Tang, Y.; Xiong, J. The preparation of corrosion resistant palladium films on 316L stainless steel by brush plating. Surf. Coat. Technol. 2010, 204, 1637–1645. [Google Scholar] [CrossRef]
- Ortega-Feliu, I.; Gómez-Tubío, B.; Scrivano, S.; Ager, F.J.; de la Bandera, M.L.; Respaldiza, M.A. Palladium analysis in gold items from Punic jewellery (Cádiz, Spain). Radiat. Phys. Chem. 2020, 167, 108239. [Google Scholar] [CrossRef]
- Mitra, A.; Sen, I.S. Anthrobiogeochemical platinum, palladium and rhodium cycles of earth: Emerging environmental contamination. Geochim. Cosmochim. Acta 2017, 216, 417–432. [Google Scholar] [CrossRef]
- Sekiba, T.; Kimura, S.; Yamamoto, H.; Okada, A. Development of automotive palladium three-way catalysts. Catal. Today 1994, 22, 113–126. [Google Scholar] [CrossRef]
- Michałek, T.; Hessel, V.; Wojnicki, M. Production, Recycling and Economy of Palladium: A Critical Review. Materials 2024, 17, 45. [Google Scholar] [CrossRef]
- Mudd, G.M.; Jowitt, S.M.; Werner, T.T. Global platinum group element resources, reserves and mining—A critical assessment. Sci. Total Environ. 2018, 622–623, 614–625. [Google Scholar] [CrossRef]
- Islam, A.; Roy, S.; Teo, S.H.; Khandaker, S.; Taufiq-Yap, Y.H.; Aziz, A.A.; Monir, M.U.; Rashid, U.; Vo, D.V.N.; Ibrahim, M.L.; et al. Functional novel ligand based palladium(II) separation and recovery from e-waste using solvent-ligand approach. Colloids Surf. A 2022, 632, 127767. [Google Scholar] [CrossRef]
- Paiva, A.P.; Ortet, O.; Carvalho, G.I.; Nogueira, C.A. Recovery of palladium from a spent industrial catalyst through leaching and solvent extraction. Hydrometallurgy 2017, 171, 394–401. [Google Scholar] [CrossRef]
- Kolarik, Z.; Renard, E.V. Recovery of value fission platinoids from spent nuclear fuel. Platin. Met. Rev. 2003, 47, 74–87. [Google Scholar] [CrossRef]
- Pokhitonov, Y.A.; Tananaev, I.G. Prospects for the use of Palladium from NPP spent nuclear fuel and ways to design the technology of its recovery at a radiochemical enterprise. Radiochemistry 2022, 64, 270–279. [Google Scholar] [CrossRef]
- Mezhov, E.A.; Kuchumov, V.A.; Druzhenkov, V.V. Study of extraction of palladium from nitric acid solutions with nitrogen-containing compounds, as applied to recovery of fission palladium from spent nuclear fuel of nuclear power plants: 1. Extraction and backwashing conditions. Radiochemistry 2002, 44, 135–140. [Google Scholar] [CrossRef]
- Hartmann, T.; Pentinghaus, H.J. The ternary system palladium–rhodium–tellurium: A Study to understand phase formation in the vitrification process of high-level waste concentrates (HLWC). Nucl. Mater. 2012, 422, 124–130. [Google Scholar] [CrossRef]
- Ruhela, R.; Singh, A.K.; Tomar, B.S.; Hubli, R.C. Separation of palladium from high level liquid waste—A review. RSC Adv. 2014, 4, 24344–24350. [Google Scholar] [CrossRef]
- Uruga, K.; Sawada, K.; Enokida, Y.; Yamamoto, I. Vitrification of high-level radioactive waste considering the behavior of platinum group metals. Prog. Nucl. Energy 2008, 50, 514–517. [Google Scholar] [CrossRef]
- Huang, H.; Huang, C.; Wu, Y.; Ding, S.; Liu, N.; Su, D.; Lv, T. Extraction of palladium (II) from nitric acid solutions with diglycolthioamide. Hydrometallurgy 2015, 156, 6–11. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, X.; Song, L.; Li, F.; Li, Q.; He, L.; Shen, Z.; Luo, F.; Ding, S. Highly efficient and selective adsorption of palladium (II) from simulated nuclear waste solution using Amberlite XAD-7 resin impregnated with a phenanthroline-derived diamide. Hydrometallurgy 2023, 221, 106127. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baron, P.; Nilsson, M. Advanced Separation Techniques for Nuclear Fuel Reprocessing and Radioactive Waste Treatment; Elsevier: Amsterdam, The Netherlands, 2011; pp. 141–175. [Google Scholar]
- Cheng, Y.; Sun, X.; Liao, X.; Shi, B. Adsorptive recovery of uranium from nuclear fuel industrial wastewater by titanium loaded collagen fiber. Chin. J. Chem. Eng. 2011, 19, 592–597. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.X.; Wei, Y.Z.; Mimura, H. Development of adsorption and solidification process for decontamination of Cs-contaminated radioactive water in Fukushima through silica-based AMP hybrid adsorbent. Sep. Purif. Technol. 2017, 181, 76–84. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Ma, S. Opportunities of porous organic polymers for radionuclide sequestration. Trends Chem. 2019, 1, 292–303. [Google Scholar] [CrossRef]
- Pan, X.; Ding, C.; Zhang, Z.; Ke, H.; Cheng, G. Functional porous organic polymer with high S and N for reversible iodine capture. Microporous Mesoporous Mater. 2020, 300, 110161. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Wu, P.; Ouyang, X.; Bai, W.; Wan, Y.; Yuan, L.; Feng, W. High acidity-and radiation-resistant triazine-based POPs for recovery of Pd (II) from nuclear fission products. Chem. Eng. J. 2022, 430, 132618. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Mellah, A.; Fernandes, S.P.S.; Rodríguez, R.; Otero, J.; Paz, J.; Cruces, J.; Medina, D.D.; Djamila, H.; Espiña, B.; Salonen, L.M. Cover Feature: Adsorption of Pharmaceutical Pollutants from Water Using Covalent Organic Frameworks (Chem. Eur. J. 42/2018). Chem.—Eur. J. 2018, 24, 10558. [Google Scholar] [CrossRef]
- Das, S.; Feng, J.; Wang, W. Covalent organic frameworks in separation. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 131–153. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Thomas, A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021, 50, 6871–6913. [Google Scholar] [CrossRef]
- Lohse, M.S.; Bein, T. Covalent organic frameworks: Structures, synthesis, and applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Zheng, H.; Zhang, B.; Zuo, Q.; Fan, K. Functionalized dual modification of covalent organic framework for efficient and rapid trace heavy metals removal from drinking water. Chemosphere 2022, 290, 133215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, T.; Ma, R.; Wu, K.; Li, H.; Feng, B.; Li, C.; Shen, Y.J. Facile synthesis of sulfonated covalent organic framework for the adsorption of heavy metal ions. Taiwan Inst. Chem. E 2020, 112, 122–129. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.; Xu, H.; Jiang, D.J. Stable covalent organic frameworks for exceptional mercury removal from aqueous solutions. Am. Chem. Soc. 2017, 139, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nishimura, T.; Mori, T.; Miyazaki, E.; Osaka, I.; Takimiya, K. Largely π-extended thienoacenes with internal thieno [3, 2-b] thiophene substructures: Synthesis, characterization, and organic field-effect transistor applications. Org. Lett. 2012, 14, 4914–4917. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H.J. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. Chem. Phys. 2010, 132, 154104. [Google Scholar]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, G.; Chen, L. 2D conjugated covalent organic frameworks: Defined synthesis and tailor-made functions. Acc. Chem. Res. 2022, 55, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.G. X-ray diffraction and the Bragg equation. J. Chem. Educ. 1997, 74, 129. [Google Scholar] [CrossRef]
- Song, S.; Shi, Y.; Liu, N.; Liu, F. Theoretical screening and experimental synthesis of ultrahigh-iodine capture covalent organic frameworks. ACS Appl. Mater. Interfaces 2021, 13, 10513–10523. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Luo, C.; Wang, W.; Wang, H.; Liu, Y.; Li, J.; Yan, T. Enhanced Separation of Palladium from Nuclear Wastewater by the Sulfur-Rich Functionalized Covalent Organic Framework. Nanomaterials 2025, 15, 714. https://doi.org/10.3390/nano15100714
Wang J, Luo C, Wang W, Wang H, Liu Y, Li J, Yan T. Enhanced Separation of Palladium from Nuclear Wastewater by the Sulfur-Rich Functionalized Covalent Organic Framework. Nanomaterials. 2025; 15(10):714. https://doi.org/10.3390/nano15100714
Chicago/Turabian StyleWang, Junli, Chen Luo, Wentao Wang, Hui Wang, Yao Liu, Jianwei Li, and Taihong Yan. 2025. "Enhanced Separation of Palladium from Nuclear Wastewater by the Sulfur-Rich Functionalized Covalent Organic Framework" Nanomaterials 15, no. 10: 714. https://doi.org/10.3390/nano15100714
APA StyleWang, J., Luo, C., Wang, W., Wang, H., Liu, Y., Li, J., & Yan, T. (2025). Enhanced Separation of Palladium from Nuclear Wastewater by the Sulfur-Rich Functionalized Covalent Organic Framework. Nanomaterials, 15(10), 714. https://doi.org/10.3390/nano15100714




