Sustainable Valorization of Rice Straw into Biochar and Carbon Dots Using a Novel One-Pot Approach for Dual Applications in Detection and Removal of Lead Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Reagents
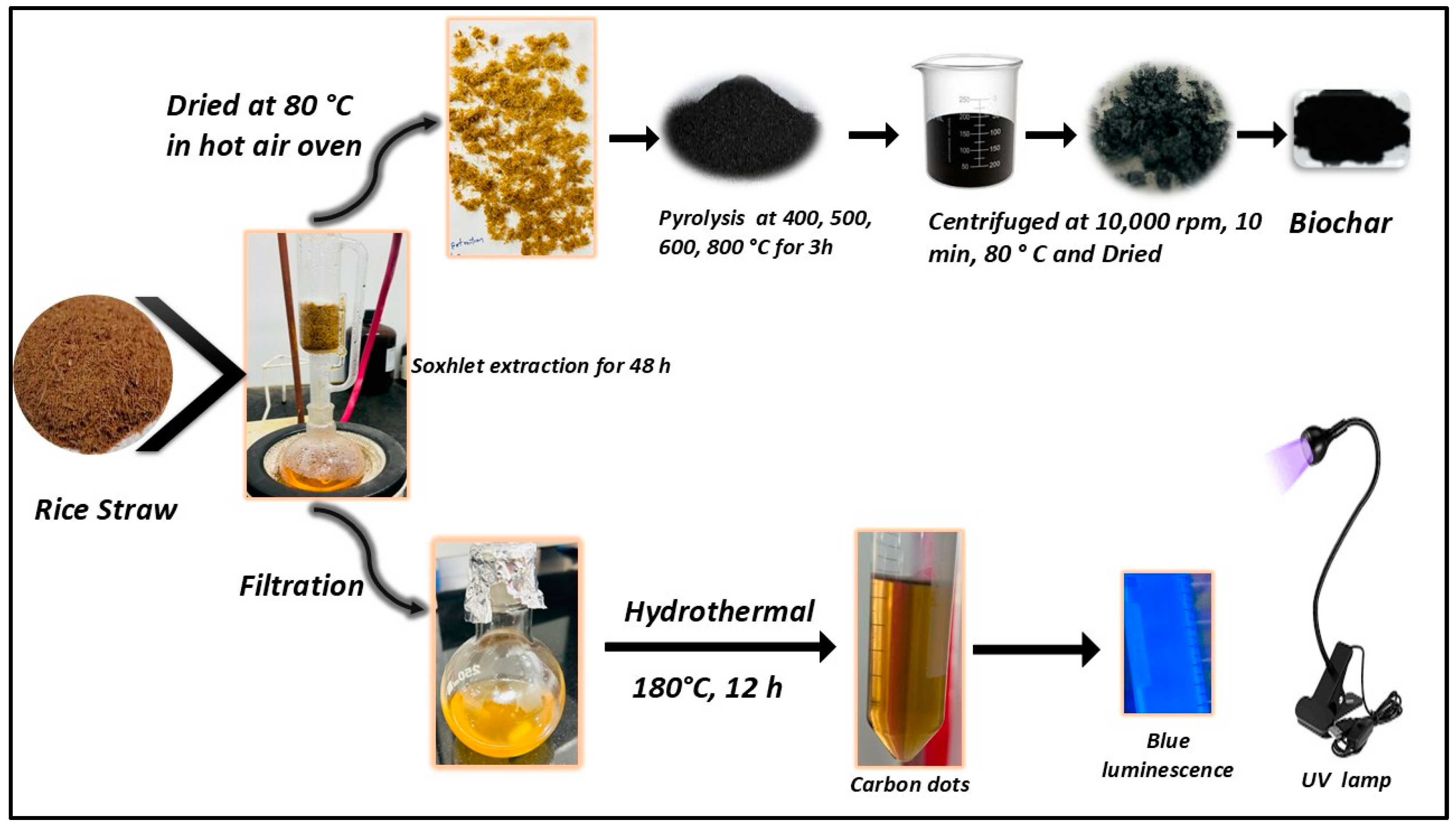
2.2. Synthesis of R-CDs
2.3. Preparation of R-BC
2.4. Characterization Techniques
2.5. Photophysical Studies
2.6. Lead Ion (Pb2+) Adsorption Study
3. Results
3.1. Characterization of R-BC
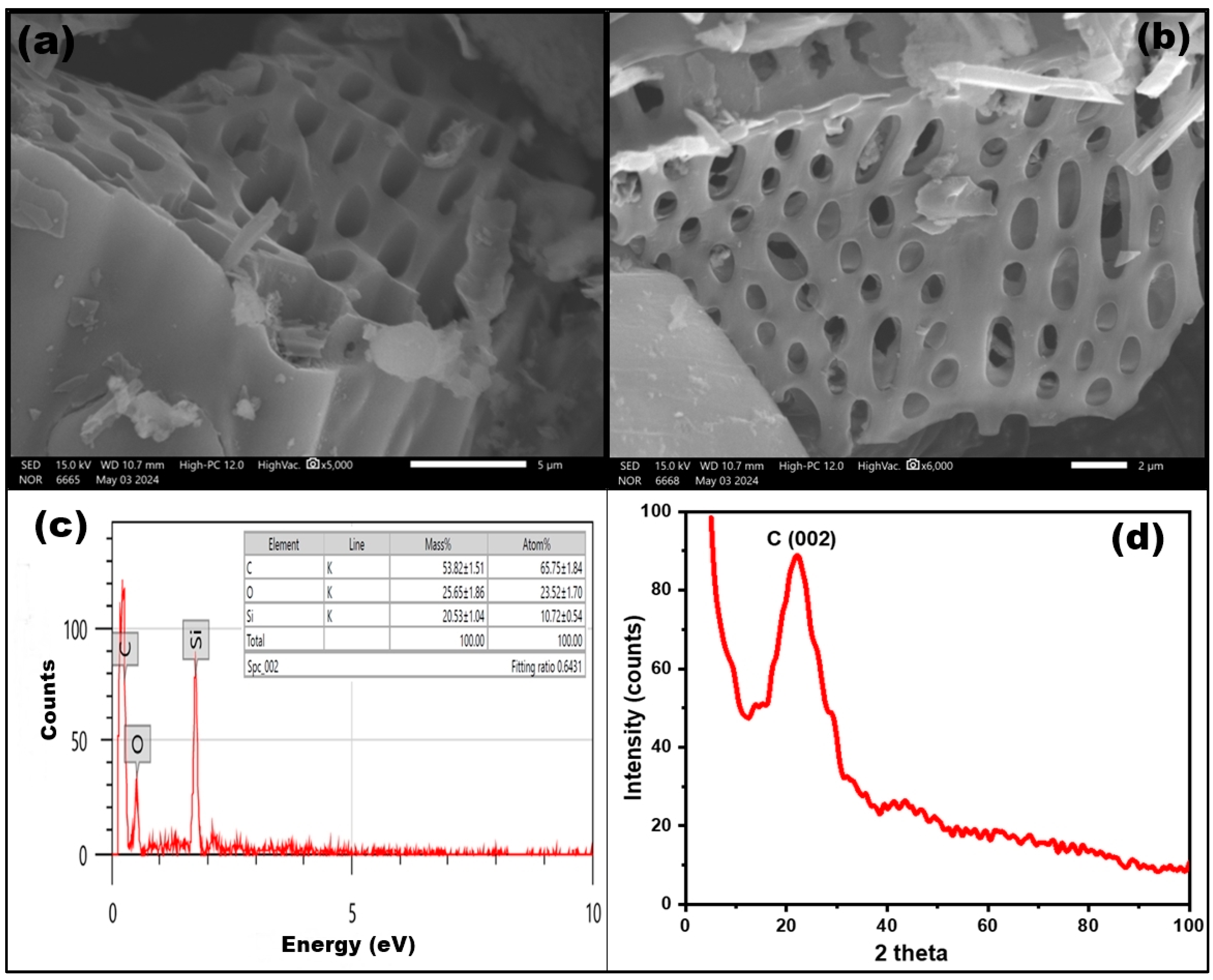
3.1.1. Scanning Electron Microscopy Analysis
3.1.2. XRD Analysis
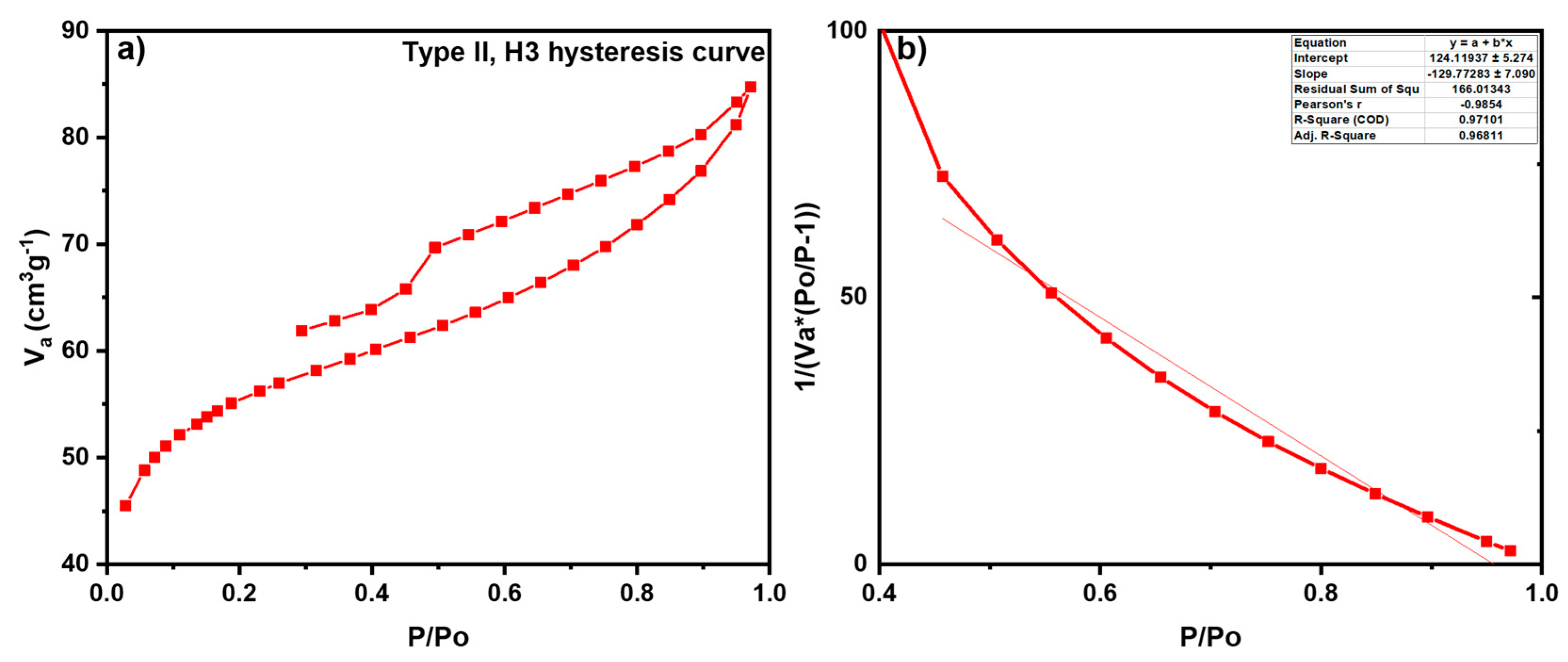
3.1.3. Brunauer–Emmett–Teller Analysis
3.2. Characterization of R-CDs
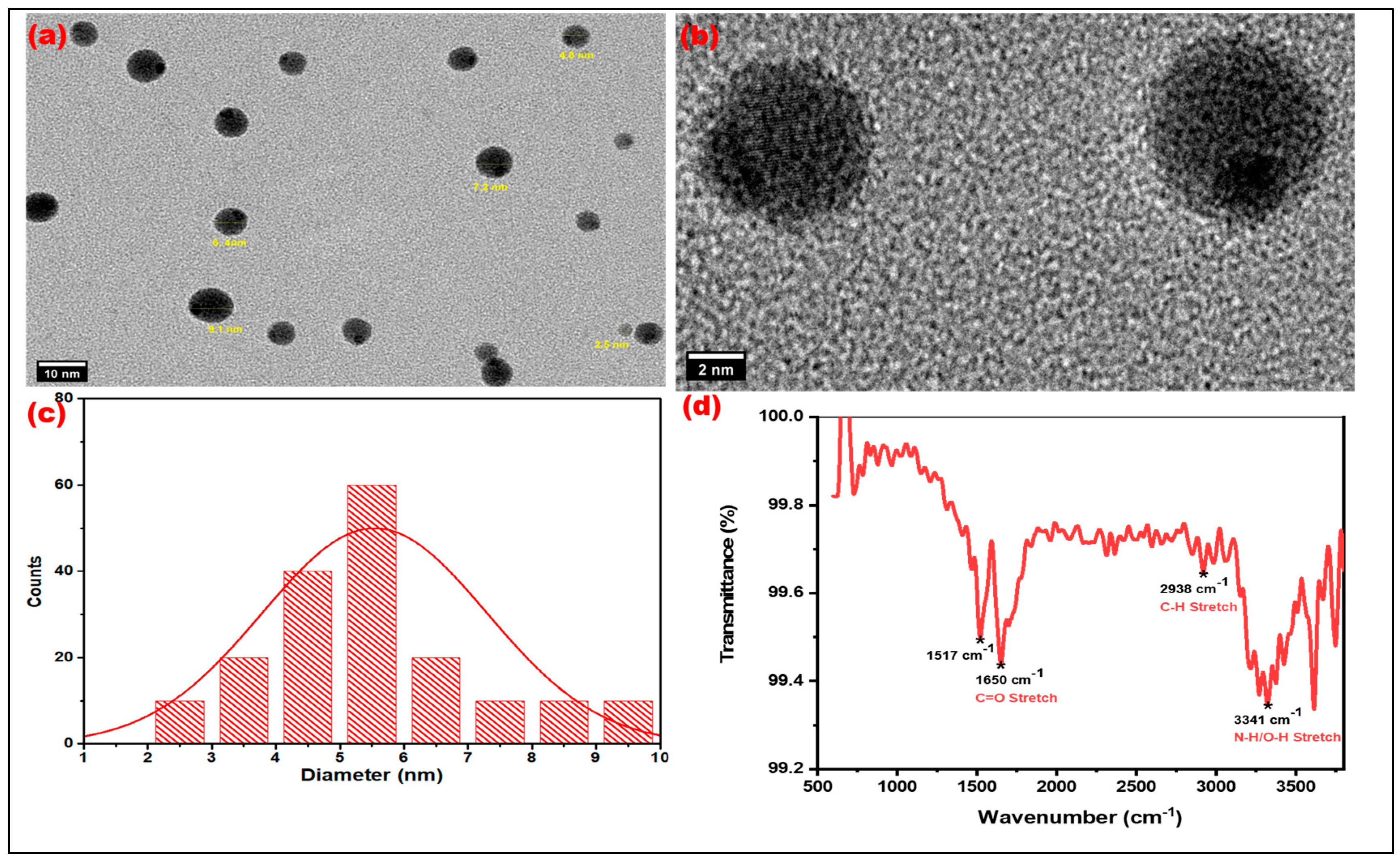
3.2.1. HRTEM Analysis
3.2.2. FTIR Spectroscopic Studies
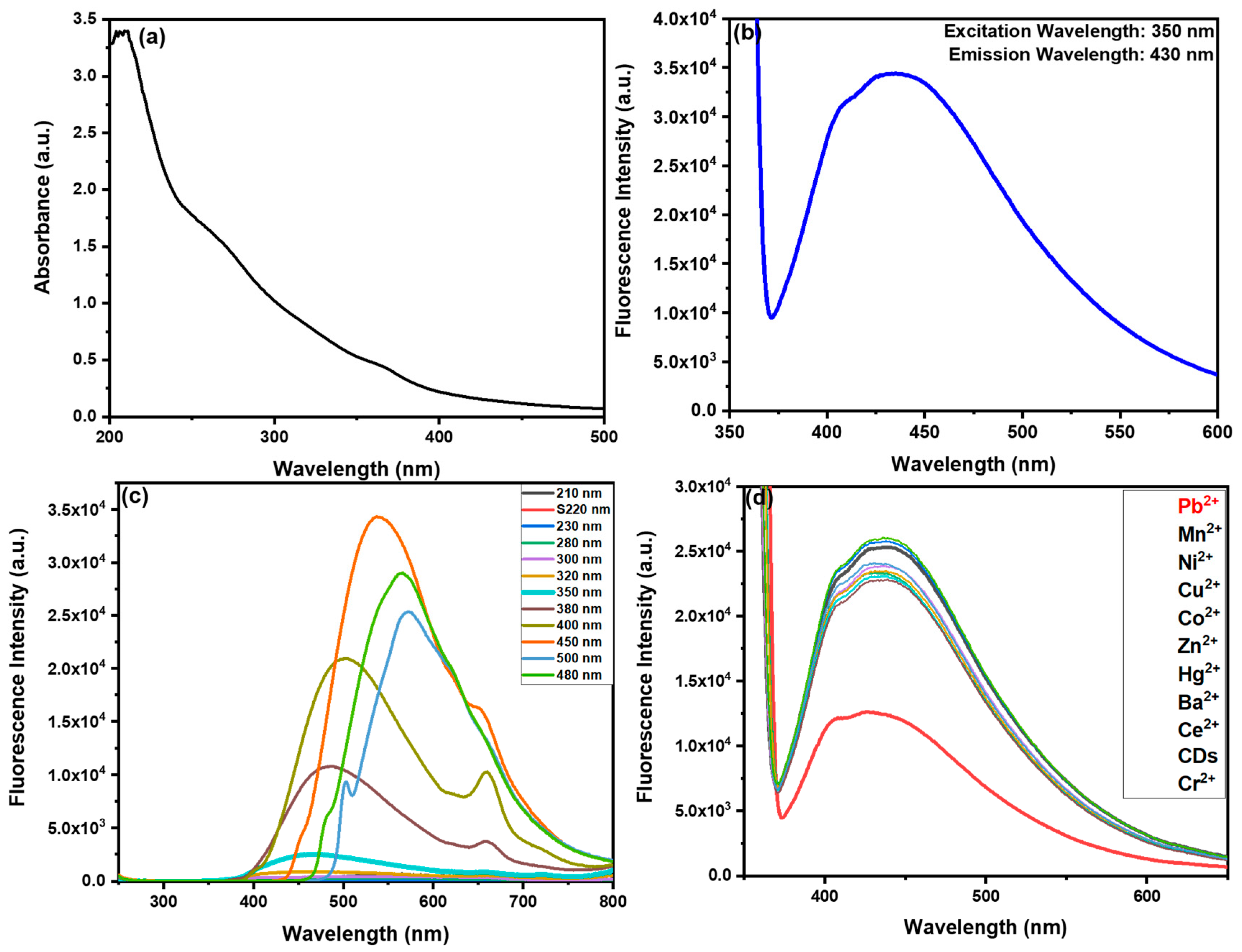
3.2.3. Optical Characterization of R-CDs
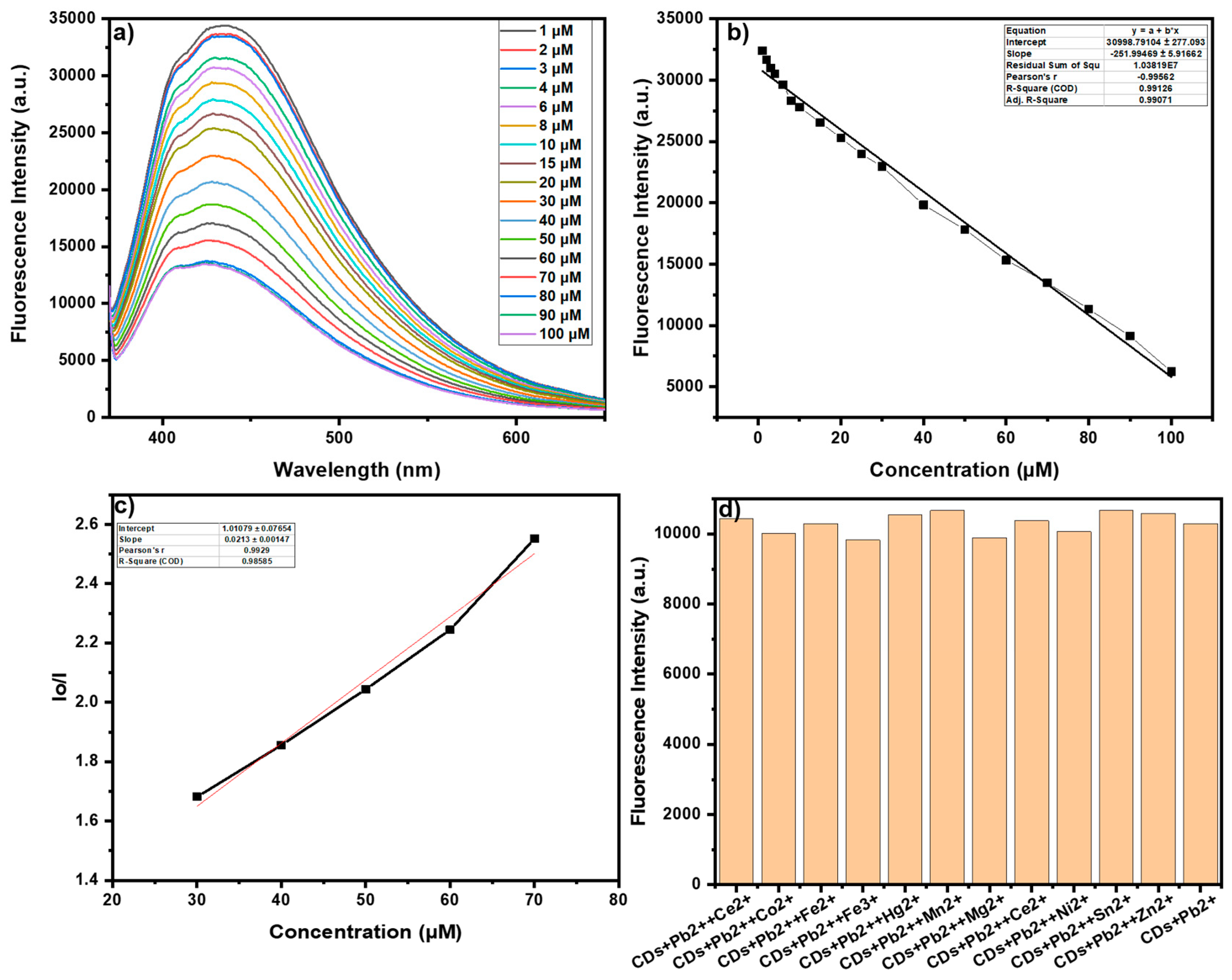
3.3. Sensing Study of Pb2+
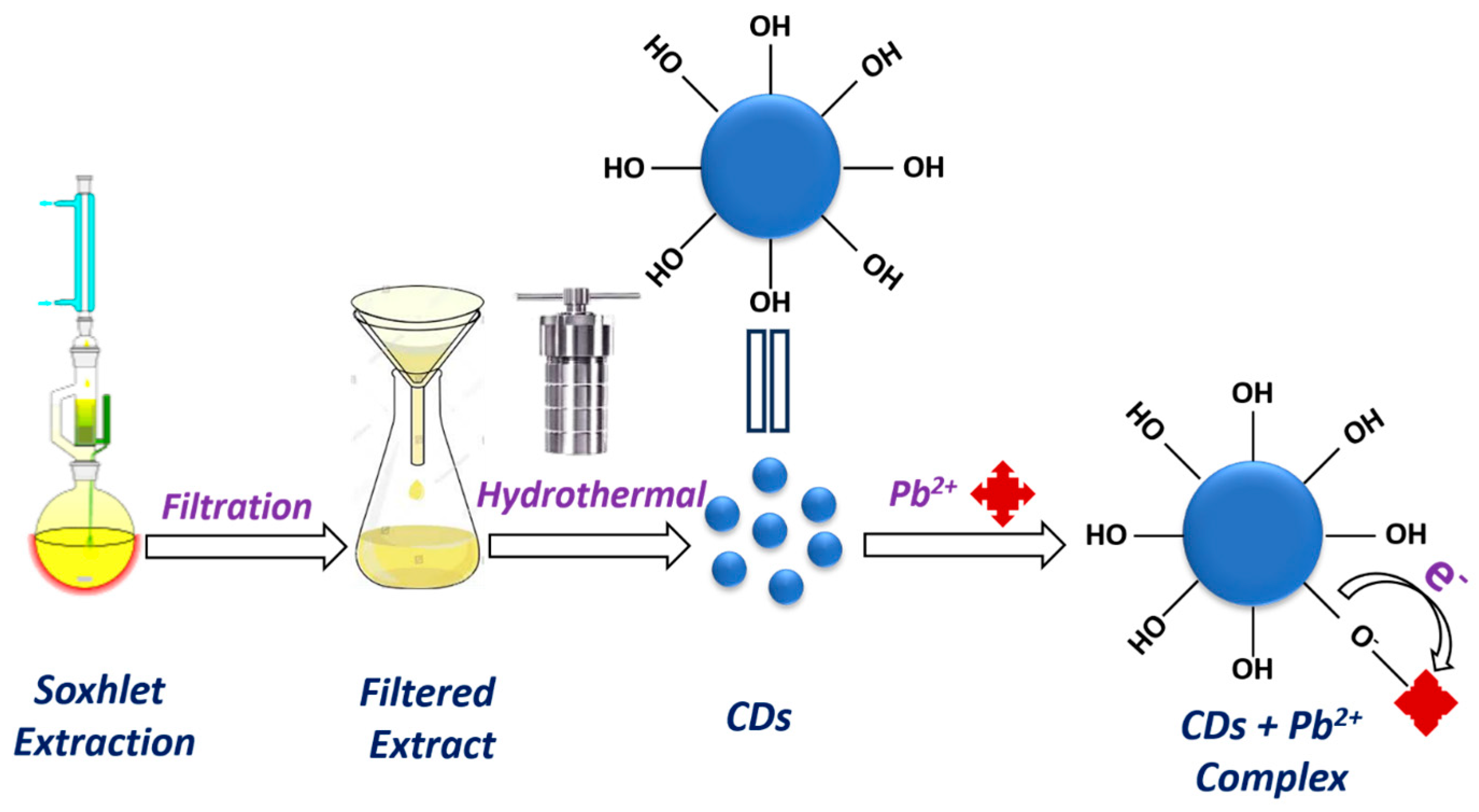
Mechanism Illustrated for Pb2+ Recognition Using R-CDs
| Techniques | Detection Range (ng/mL) | Detection Limit (ng/mL) | References |
|---|---|---|---|
| Pb2+-driven DNA molecular device | 0.02–1 µM | 20 nM | [45] |
| AGRO100 | 0–1000 nM | 1.0 nM | [46] |
| G-quadruplex DNAzyme | 0–1000 nM | 0.4 nM | [46] |
| Blue and Red CDs | Not Given | 2.89 nM | [47] |
| Fluorescent starch-based hydrogel | 5–160 μg/L | 0.06 μg/L | [48] |
| Moringa oleifera gum derived CDs | 0–100 ppb | 11.62 nM | [49] |
| Rice straw derived CDs | 1–100 µM | 0.11 µM | This Work |
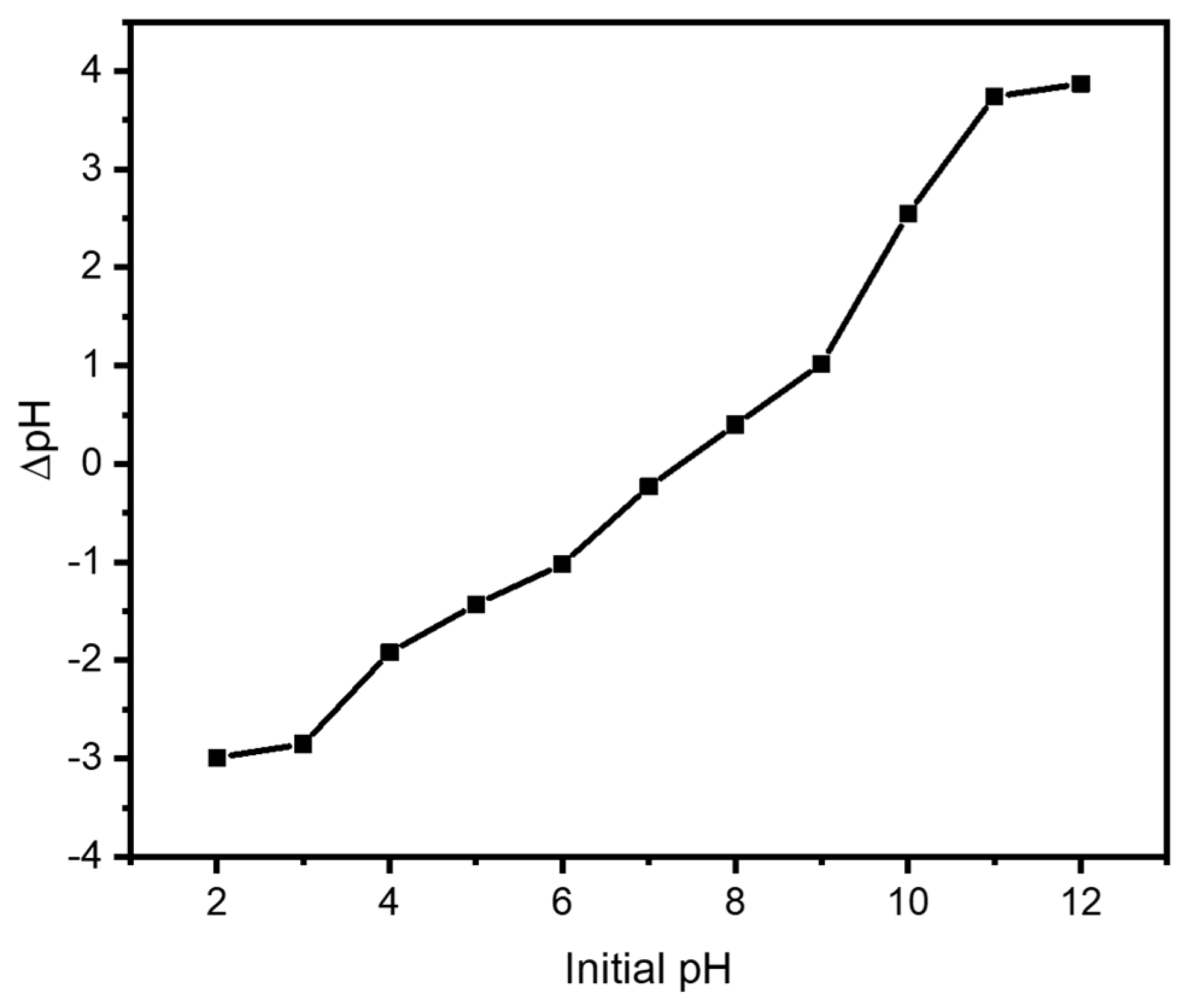
3.4. Point of Zero Charge (pHzpc)
3.5. Lead Removal Studies
3.6. Kinetics Studies
3.6.1. PFOM
3.6.2. PSOM
3.6.3. Intraparticle Diffusion Model
3.6.4. Elovich Model
4. Real Sample Analysis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gahrouei, A.E.; Rezapour, A.; Pirooz, M.; Pourebrahimi, S. From Classic to Cutting-Edge Solutions: A Comprehensive Review of Materials and Methods for Heavy Metal Removal from Water Bodies. Desalination Water Treat. 2024, 319, 100446. [Google Scholar] [CrossRef]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon Dots for Heavy-Metal Sensing, PH-Sensitive Cargo Delivery, and Antibacterial Applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Lee, A.; Chin, J.; Park, O.K.; Chung, H.; Kim, J.W.; Yoon, S.-Y.; Park, K. A Novel Near-Infrared Fluorescence Chemosensor for Copper Ion Detection Using Click Ligation and Energy Transfer. Chem. Commun. 2013, 49, 5969–5971. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Li, B.; Wu, N.; Gao, G.; You, J.; Lan, J. Cyclen-Functionalized Perylenebisimides as Sensitive and Selective Fluorescent Sensors for Pb 2+ in Aqueous Solution. Chem. Commun. 2011, 47, 6668–6670. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, J.H.; Lu, Y. Highly Sensitive “Turn-on” Fluorescent Sensor for Hg2+ in Aqueous Solution Based on Structure-Switching DNA. Chem. Commun. 2008, 45, 6005–6007. [Google Scholar] [CrossRef] [PubMed]
- Barba-Bon, A.; Costero, A.M.; Gil, S.; Parra, M.; Soto, J.; Martínez-Máñez, R.; Sancenón, F. A New Selective Fluorogenic Probe for Trivalent Cations. Chem. Commun. 2012, 48, 3000–3002. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Patel, N.G.; Zhang, G.; Zhou, D.; Pang, Y. A Single Molecular Probe for Multi-Analyte (Cr3+, Al3+ and Fe3+) Detection in Aqueous Medium and Its Biological Application. Chem. Commun. 2014, 50, 12258–12261. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Qin, M.; Xu, R.; Gao, X.; Shen, Y.; Xu, Q.; Cao, Y.; Wang, W. A Genetically Encoded Copper (I) Sensor Based on Engineered Structural Distortion of EGFP. Chem. Commun. 2012, 48, 3890–3892. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.S.; Bakr, A.-S.A.; El Naggar, A.M.A.; Sultan, E.-S.A. Water Decontamination via the Removal of Pb (II) Using a New Generation of Highly Energetic Surface Nano-Material: Co+2Mo+6 LDH. J. Colloid Interface Sci. 2016, 461, 261–272. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Lead Toxicity, a Review of the Literature. Part I: Exposure, Evaluation, and Treatment. Altern. Med. Rev. 2006, 11, 2. [Google Scholar] [PubMed]
- Lan, T.; Furuya, K.; Lu, Y. A Highly Selective Lead Sensor Based on a Classic Lead DNAzyme. Chem. Commun. 2010, 46, 3896–3898. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Kiani, M.; Karami, C.; Taher, M.A. Colorimetric Sensor of Detection of Cr (III) and Fe (II) Ions in Aqueous Solutions Using Gold Nanoparticles Modified with Methylene Blue. Optik 2018, 158, 813–825. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.I.; Akhtar, K.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Biosynthesis of Silver Nanoparticles: A Colorimetric Optical Sensor for Detection of Hexavalent Chromium and Ammonia in Aqueous Solution. Phys. E Low-Dimens. Syst. Nanostructures 2018, 103, 367–376. [Google Scholar] [CrossRef]
- Sang, F.; Li, X.; Zhang, Z.; Liu, J.; Chen, G. Recyclable Colorimetric Sensor of Cr3+ and Pb2+ Ions Simultaneously Using a Zwitterionic Amino Acid Modified Gold Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 109–116. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, L.; Xiong, X.; Zhang, Q.; Feng, J.; Deng, S.; Zhou, L. A New Impedimetric Sensor Based on Anionic Intercalator for Detection of Lead Ions with Low Cost and High Sensitivity. J. Electroanal. Chem. 2018, 827, 175–180. [Google Scholar] [CrossRef]
- Bhatt, R.; Bhatt, R.; Padmaja, P. DTPA Capped Gold and Silver Nanofluids-Facile Synthesis and Their Application as Chromium Sensors. Sens. Actuators B Chem. 2018, 258, 602–611. [Google Scholar] [CrossRef]
- Sekar, A.; Yadav, R.; Basavaraj, N. Fluorescence Quenching Mechanism and the Application of Green Carbon Nanodots in the Detection of Heavy Metal Ions: A Review. New J. Chem. 2021, 45, 2326–2360. [Google Scholar] [CrossRef]
- Bhattu, M.; Verma, M.; Wani, A.A.; Bharatam, P.V.; Sareen, S.; Kathuria, D. Tuning of (E)-(4-Fluorophenyl)-1, 1-Diamino-2, 3-Diazabuta-1, 3-Diene Nanostructures for the Selective Detection of Imidacloprid. Environ. Res. 2023, 216, 114494. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jin, X.; Lu, X.-Q.; Chen, Z. Adsorption of Pb (II), Cd (II), Ni (II) and Cu (II) onto Natural Kaolinite Clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Helal, A.; Nguyen, H.L.; Al-Ahmed, A.; Cordova, K.E.; Yamani, Z.H. An Ultrasensitive and Selective Metal–Organic Framework Chemosensor for Palladium Detection in Water. Inorg. Chem. 2019, 58, 1738–1741. [Google Scholar] [CrossRef]
- Chen, M.; Kutsanedzie, F.Y.H.; Cheng, W.; Li, H.; Chen, Q. Ratiometric Fluorescence Detection of Cd2+ and Pb2+ by Inner Filter-Based Upconversion Nanoparticle-Dithizone Nanosystem. Microchem. J. 2019, 144, 296–302. [Google Scholar] [CrossRef]
- Pankajakshan, A.; Kuznetsov, D.; Mandal, S. Ultrasensitive Detection of Hg (II) Ions in Aqueous Medium Using Zinc-Based Metal–Organic Framework. Inorg. Chem. 2019, 58, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Bhattu, M.; Verma, M.; Acevedo, R.; Duc, N.D.; Singh, J. Biogenic Silver Based Nanostructures: Synthesis, Mechanistic Approach and Biological Applications. Environ. Res. 2023, 231, 116045. [Google Scholar] [CrossRef]
- Bhattu, M.; Singh, J. Recent Advances in Nanomaterials Based Sustainable Approaches for Mitigation of Emerging Organic Pollutants. Chemosphere 2023, 321, 138072. [Google Scholar] [CrossRef] [PubMed]
- Soni, H.; Bhattu, M.; Priya, S.D.; Kaur, M.; Verma, M.; Singh, J. Recent Advances in Waste-Derived Carbon Dots and Their Nanocomposites for Environmental Remediation and Biological Applications. Environ. Res. 2024, 251, 118560. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, H.; Ling, L.; Li, G.; Cheng, R.; Lu, X.; Xie, A.-Q.; Li, Q.; Wang, C.-F.; Chen, S. Green Synthesis of Carbon Dots toward Anti-Counterfeiting. ACS Sustain. Chem. Eng. 2019, 8, 1566–1572. [Google Scholar] [CrossRef]
- Monje, D.S.; Chacon, K.M.; Galindo, I.C.; Castaño, C.; Ballesteros-Rueda, L.M.; Valencia, G.C.; Gonzalez, M.C.; Mercado, D.F. Carbon Dots from Agroindustrial Residues: A Critical Comparison of the Effect of Physicochemical Properties on Their Performance as Photocatalyst and Emulsion Stabilizer. Mater. Today Chem. 2021, 20, 100445. [Google Scholar] [CrossRef]
- Manjubaashini, N.; Bargavi, P.; Balakumar, S. Carbon Quantum Dots Derived from Agro Waste Biomass for Pioneering Bioanalysis and in Vivo Bioimaging. J. Photochem. Photobiol. A Chem. 2024, 454, 115702. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, R.; Zhou, J. Shrimp-Shell-Derived Carbon Dots for Quantitative Detection by Fluorometry and Colorimetry: A New Analytic Chemistry Experiment for University Education. J. Chem. Educ. 2024, 101, 2784–2789. [Google Scholar] [CrossRef]
- Kumar, M.; Chinnathambi, S.; Bakhori, N.; Abu, N.; Etezadi, F.; Thangavel, V.; Packwood, D.; Sivaniah, E.; Pandian, G.N. Biomass-Derived Carbon Dots as Fluorescent Quantum Probes to Visualize and Modulate Inflammation. Sci. Rep. 2024, 14, 12665. [Google Scholar] [CrossRef]
- Singh, J.; Bhattu, M.; Verma, M. Rice Straw Derived Mesoporous Biochar for the Removal of Coomassie Brilliant Blue Dye. Top. Catal. 2024, 1–10. [Google Scholar] [CrossRef]
- Zhao, B.; Tan, Z. Fluorescent Carbon Dots: Fantastic Electroluminescent Materials for Light-emitting Diodes. Adv. Sci. 2021, 8, 2001977. [Google Scholar] [CrossRef]
- Limosani, F.; Bauer, E.M.; Cecchetti, D.; Biagioni, S.; Orlando, V.; Pizzoferrato, R.; Prosposito, P.; Carbone, M. Top-down n-Doped Carbon Quantum Dots for Multiple Purposes: Heavy Metal Detection and Intracellular Fluorescence. Nanomaterials 2021, 11, 2249. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, H.; Csapó, E.; Wojnicki, M. Carbon Quantum Dots: The Role of Surface Functional Groups and Proposed Mechanisms for Metal Ion Sensing. Inorganics 2023, 11, 262. [Google Scholar] [CrossRef]
- Ren, J.; Weber, F.; Weigert, F.; Wang, Y.; Choudhury, S.; Xiao, J.; Lauermann, I.; Resch-Genger, U.; Bande, A.; Petit, T. Influence of Surface Chemistry on Optical, Chemical and Electronic Properties of Blue Luminescent Carbon Dots. Nanoscale 2019, 11, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- van Dam, B.; Nie, H.; Ju, B.; Marino, E.; Paulusse, J.M.J.; Schall, P.; Li, M.; Dohnalová, K. Excitation-Dependent Photoluminescence from Single-Carbon Dots. Small 2017, 13, 1702098. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, N.; Titirici, M.M.; Sapelkin, A. Investigating the Effect of Reaction Time on Carbon Dot Formation, Structure, and Optical Properties. ACS Omega 2019, 4, 21658–21665. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Rashid, S.; Mahdi, M.A.; Ibrahim, N.A.; Pudza, M.Y. Facile Synthesis of Nitrogen-Doped Carbon Dots from Lignocellulosic Waste. Nanomaterials 2019, 9, 1500. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Krysmann, M.J.; Kelarakis, A.; Koutnik, P.; Anzenbacher, P.; Roland, P.J.; Ellingson, R.; Sun, L. Understanding the Photoluminescence Mechanism of Carbon Dots. MRS Adv. 2017, 2, 2927–2934. [Google Scholar] [CrossRef]
- Yang, M.; Li, H.; Liu, J.; Kong, W.; Zhao, S.; Li, C.; Huang, H.; Liu, Y.; Kang, Z. Convenient and Sensitive Detection of Norfloxacin with Fluorescent Carbon Dots. J. Mater. Chem. B 2014, 2, 7964–7970. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Karak, N. A Green and Facile Approach for the Synthesis of Water Soluble Fluorescent Carbon Dots from Banana Juice. Rsc Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, M.; Liu, Y.; Feng, X.; Yin, X.; He, X.; Zhang, Y. Nitrogen-doped Carbon Dots: A Facile and General Preparation Method, Photoluminescence Investigation, and Imaging Applications. Chem. –A Eur. J. 2013, 19, 2276–2283. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-Pot Electrochemical Synthesis of Functionalized Fluorescent Carbon Dots and Their Selective Sensing for Mercury Ion. Anal. Chim. Acta 2015, 866, 69–74. [Google Scholar] [CrossRef]
- Li, T.; Dong, S.; Wang, E. A Lead (II)-Driven DNA Molecular Device for Turn-on Fluorescence Detection of Lead (II) Ion with High Selectivity and Sensitivity. J. Am. Chem. Soc. 2010, 132, 13156–13157. [Google Scholar] [CrossRef]
- Li, C.-L.; Liu, K.-T.; Lin, Y.-W.; Chang, H.-T. Fluorescence Detection of Lead (II) Ions through Their Induced Catalytic Activity of DNAzymes. Anal. Chem. 2011, 83, 225–230. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Chu, S.; Liu, B.; Zhang, Q.; Zou, L.; Yu, S.; Jiang, C. Semiquantitative Visual Detection of Lead Ions with a Smartphone via a Colorimetric Paper-Based Analytical Device. Anal. Chem. 2019, 91, 9292–9299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, Z.; Qin, D.; Liu, D.; Guo, X.; Lin, H. Fluorescent Starch-Based Hydrogel with Cellulose Nanofibrils and Carbon Dots for Simultaneous Adsorption and Detection of Pb (II). Carbohydr. Polym. 2024, 323, 121427. [Google Scholar] [CrossRef]
- Mandal, A.; Karmakar, A.; Varanasi, S. “Turn ON–OFF” Detection of Lead Using Moringa Oleifera Gum-Derived Cyan-Emissive Carbon Dots Integrated with a Smartphone-Assisted Sensing Platform. Chem. Pap. 2024, 78, 2493–2507. [Google Scholar] [CrossRef]
- Okolo, B.I.; Oke, E.O.; Agu, C.M.; Adeyi, O.; Nwoso-Obieogu, K.; Akatobi, K.N. Adsorption of Lead (II) from Aqueous Solution Using Africa Elemi Seed, Mucuna Shell and Oyster Shell as Adsorbents and Optimization Using Box–Behnken Design. Appl. Water Sci. 2020, 10, 201. [Google Scholar] [CrossRef]
- Filipović, K.; Petrović, M.; Najdanović, S.; Velinov, N.; Hurt, A.; Bojić, A.; Kostić, M. Highly Efficient Nano Sorbent as a Superior Material for the Purification of Wastewater Contaminated with Anthraquinone Dye RB19. J. Water Process Eng. 2024, 67, 106118. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy Metals Removal Using Activated Carbon, Silica and Silica Activated Carbon Composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef]
- Novoseltseva, V.; Yankovych, H.; Kovalenko, O.; Václavíková, M.; Melnyk, I. Production of High-Performance Lead (II) Ions Adsorbents from Pea Peels Waste as a Sustainable Resource. Waste Manag. Res. 2021, 39, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, C.; Li, F.; Bo, X.; Liu, G.; Zhou, Q. Equilibrium and Kinetics Study on the Adsorption of Perfluorooctanoic Acid from Aqueous Solution onto Powdered Activated Carbon. J. Hazard. Mater. 2009, 169, 146–152. [Google Scholar] [CrossRef]
- Fagbayigbo, B.O.; Opeolu, B.O.; Fatoki, O.S.; Akenga, T.A.; Olatunji, O.S. Removal of PFOA and PFOS from Aqueous Solutions Using Activated Carbon Produced from Vitis Vinifera Leaf Litter. Environ. Sci. Pollut. Res. 2017, 24, 13107–13120. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Q.; Yang, M.; Wang, Y.; Liu, N.; Zhang, X. Magnetic Ion Exchange Resin for Effective Removal of Perfluorooctanoate from Water: Study of a Response Surface Methodology and Adsorption Performances. Environ. Sci. Pollut. Res. 2018, 25, 29267–29278. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Geng, D.; Mehler, W.T.; Goss, G.; Wang, T.; Yang, S.; Niu, Y.; Zheng, Y.; Zhang, Y. Removal of Perfluorooctanoic Acid (PFOA) from Aqueous Solution by Amino-Functionalized Graphene Oxide (AGO) Aerogels: Influencing Factors, Kinetics, Isotherms, and Thermodynamic Studies. Sci. Total Environ. 2021, 783, 147041. [Google Scholar] [CrossRef] [PubMed]

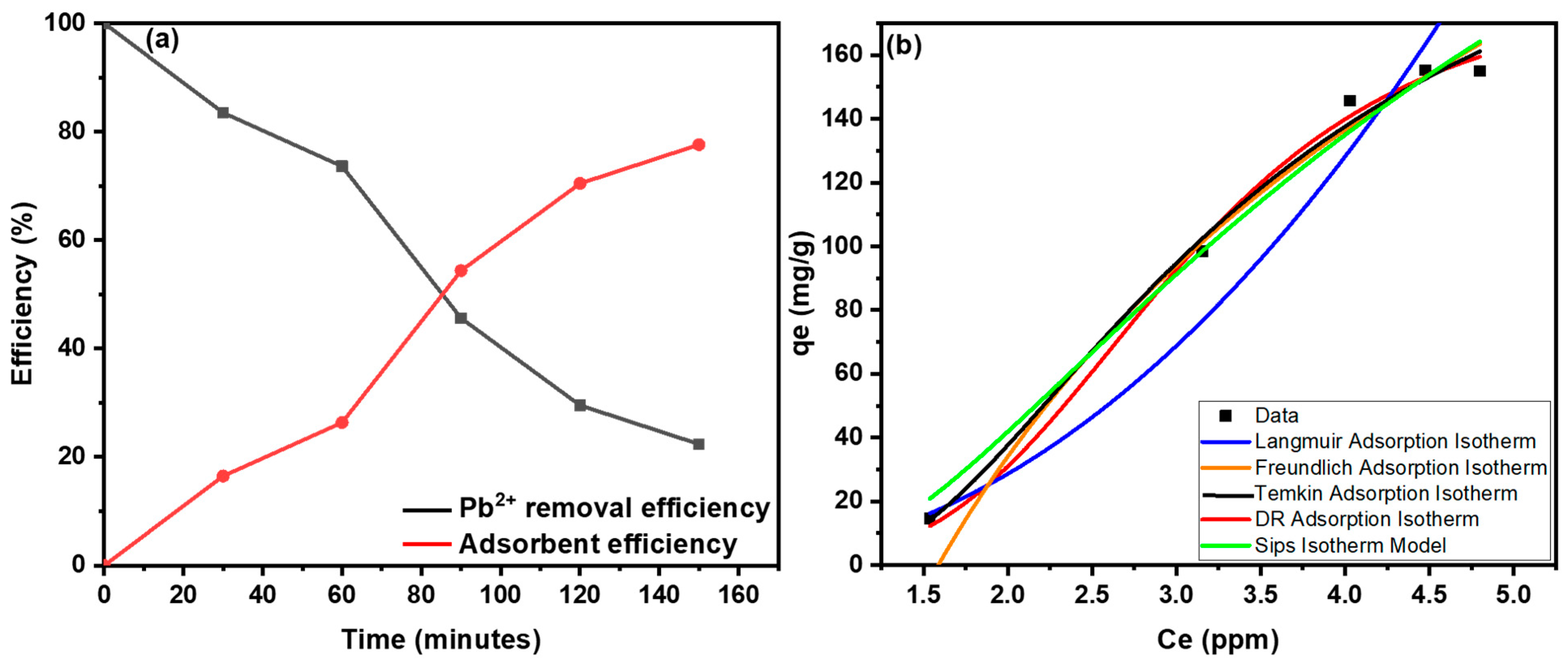

| Sr. No. | Models | Equations | Parameters |
|---|---|---|---|
| 1. | Langmuir | qe = amount of adsorbate per mass of adsorbent in equilibrium (mg/g), qm = adsorption capacity in monolayer (mg/g) KL = constant of the Langmuir model related to the binding energy. | |
| 2. | Freundlich | Kf = Freundlich capacity constant 1/n = Freundlich intensity | |
| 3. | Temkin Isotherm Model | T = reaction temperature in Kelvin (K), R = gas constant b = Temkin constant | |
| 4. | Dubinin–Radushkevich Isotherm Model | T = reaction temperature (K) R = gas constant K = D–R constant qm = maximal adsorption capacity | |
| 5. | Sips Isotherm model | K = Sips Constant |
| Sr. No. | Isotherms | Determination Constants | |||
|---|---|---|---|---|---|
| 1. | Langmuir | qm | KL | RL | R2 |
| 183 | 0.01295 | 0.794281176 | 0.9909 | ||
| 2. | Freundlich | 1/n | Kf | R2 | |
| 2.16 | 6.41 | 0.81 | |||
| 3. | Temkin | BT | AT | R2 | |
| 147.82 | 0.63 | 0.9 | |||
| 4. | Dubinin–Radushkevich | qm | KDR | R2 | |
| 241.6 | 11.31 | 0.98 | |||
| 5. | Sips model | qm | Ks | R2 | |
| 440.28 | 24.09 | 0.94 | |||
| Sr. No. | Models | Equations | Parameters |
|---|---|---|---|
| 1. | PFOM | qe (mg/g) and qt (mg/g) = quantity of Pb2+ adsorbed initially and at time t; k1 (L/min) = PFO rate constant. | |
| 2. | PSOM | qe (mg/g) = quantity of Pb2+ adsorbed at equilibrium; k2 (g/mgmin) = PSO rate constant. | |
| 3. | Intraparticle diffusion model | Kip = intraparticle diffusion apparent adsorption rate constant C = intraparticle diffusion model constant | |
| 4. | Elovich model | a = primary rate of adsorption b = desorption constant during each experiment |
| Sr. No. | Kinetics Model | Parameters | ||
|---|---|---|---|---|
| 1. | PFOM | K1 (gmg−1 min−1) | R2 | MRD (%) |
| 0.0257 | 0.93 | 7.57 | ||
| 2. | PSOM | K2 (gmg−1 min−1) | R2 | MRD (%) |
| 0.000047 | 0.98 | 2.77 | ||
| 3. | Intraparticle diffusion model | Kip | R2 | MRD (%) |
| 13.29 | 0.89 | 27.23 | ||
| 4. | Elovich model | b | R2 | MRD (%) |
| 0.012 | 0.90 | 21.53 | ||
| Real Sample | Concentration of Pb2+ (µM) | Recovery of Pb2+ (µM) | Recovery of Pb2+ (%) |
|---|---|---|---|
| River Water | 0.11 | 0.107 | 97.27 |
| 1 | 0.98 | 98 | |
| 10 | 9.8 | 98 | |
| Tap Water | 0.11 | 0.105 | 95.45 |
| 1 | 0.96 | 96 | |
| 10 | 9.7 | 97 | |
| RO Water | 0.11 | 0.107 | 97.27 |
| 1 | 0.99 | 99 | |
| 10 | 9.753 | 97.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, J.; Bhattu, M.; Verma, M.; Bechelany, M.; Brar, S.K.; Jadeja, R. Sustainable Valorization of Rice Straw into Biochar and Carbon Dots Using a Novel One-Pot Approach for Dual Applications in Detection and Removal of Lead Ions. Nanomaterials 2025, 15, 66. https://doi.org/10.3390/nano15010066
Singh J, Bhattu M, Verma M, Bechelany M, Brar SK, Jadeja R. Sustainable Valorization of Rice Straw into Biochar and Carbon Dots Using a Novel One-Pot Approach for Dual Applications in Detection and Removal of Lead Ions. Nanomaterials. 2025; 15(1):66. https://doi.org/10.3390/nano15010066
Chicago/Turabian StyleSingh, Jagpreet, Monika Bhattu, Meenakshi Verma, Mikhael Bechelany, Satinder Kaur Brar, and Rajendrasinh Jadeja. 2025. "Sustainable Valorization of Rice Straw into Biochar and Carbon Dots Using a Novel One-Pot Approach for Dual Applications in Detection and Removal of Lead Ions" Nanomaterials 15, no. 1: 66. https://doi.org/10.3390/nano15010066
APA StyleSingh, J., Bhattu, M., Verma, M., Bechelany, M., Brar, S. K., & Jadeja, R. (2025). Sustainable Valorization of Rice Straw into Biochar and Carbon Dots Using a Novel One-Pot Approach for Dual Applications in Detection and Removal of Lead Ions. Nanomaterials, 15(1), 66. https://doi.org/10.3390/nano15010066









