Interactions Between Potentially Toxic Nanoparticles (Cu, CuO, ZnO, and TiO2) and the Cyanobacterium Arthrospira platensis: Biological Adaptations to Xenobiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. The Cyanobacterial Strain Arthrospira platensis, Mineral Media, and Experimental Conditions
2.2. Nanoparticles
2.3. Quantitative Biomass Determination
2.4. Biochemical Analysis
2.4.1. Sample Preparation for Biochemical Analysis
2.4.2. Protein Determination
2.4.3. Phycobiliprotein Determination
2.4.4. Chlorophyll and Total Carotenoid Determination
2.4.5. Carbohydrate Determination
2.4.6. Lipid Determination
2.4.7. Malondialdehyde (MDA) Determination
2.5. Statistical Analysis
3. Results
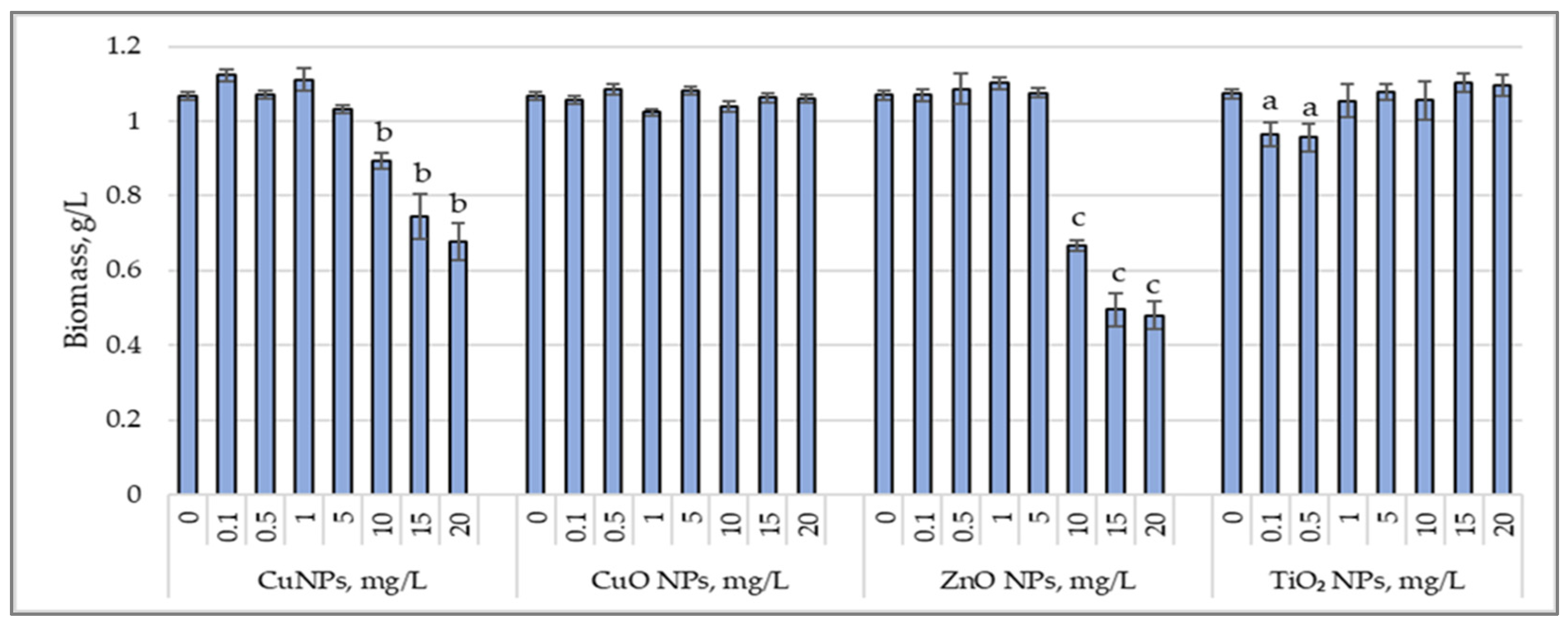
3.1. Biomass Accumulation
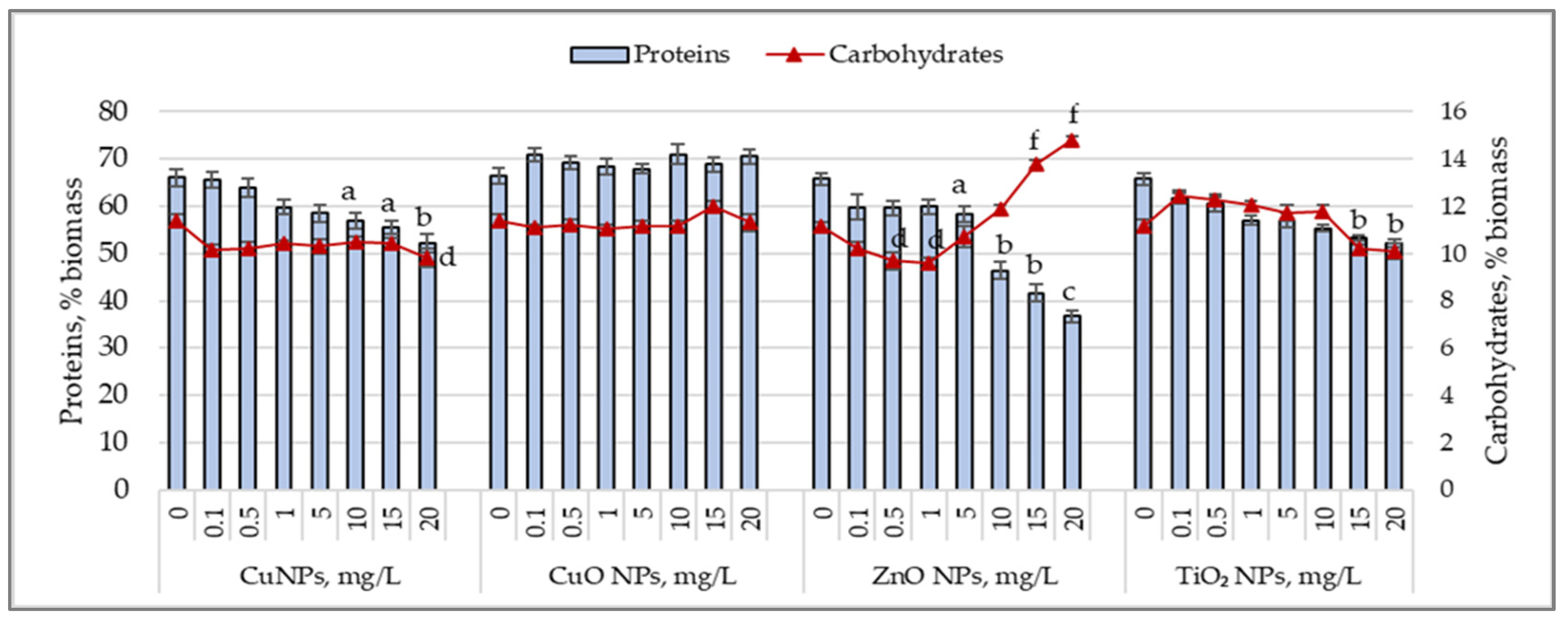
3.2. Protein and Carbohydrate Accumulation
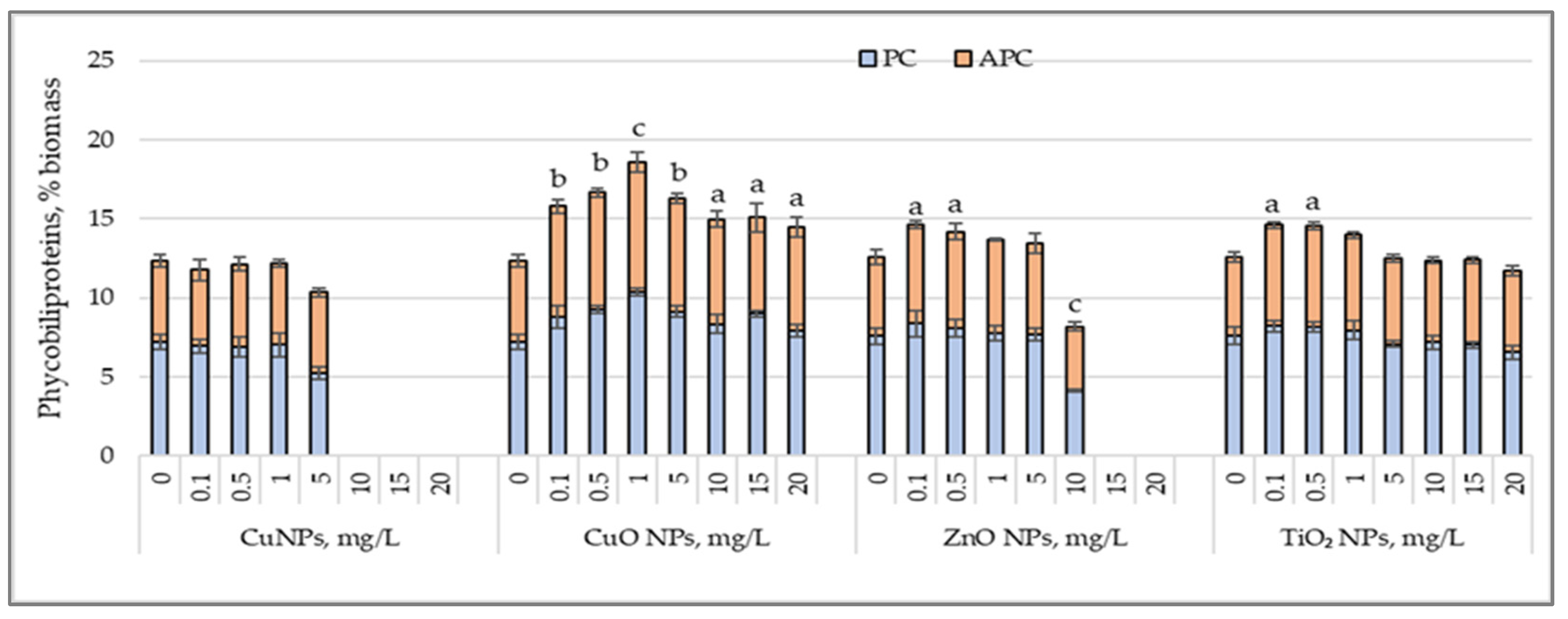
3.3. Phycobiliprotein Accumulation
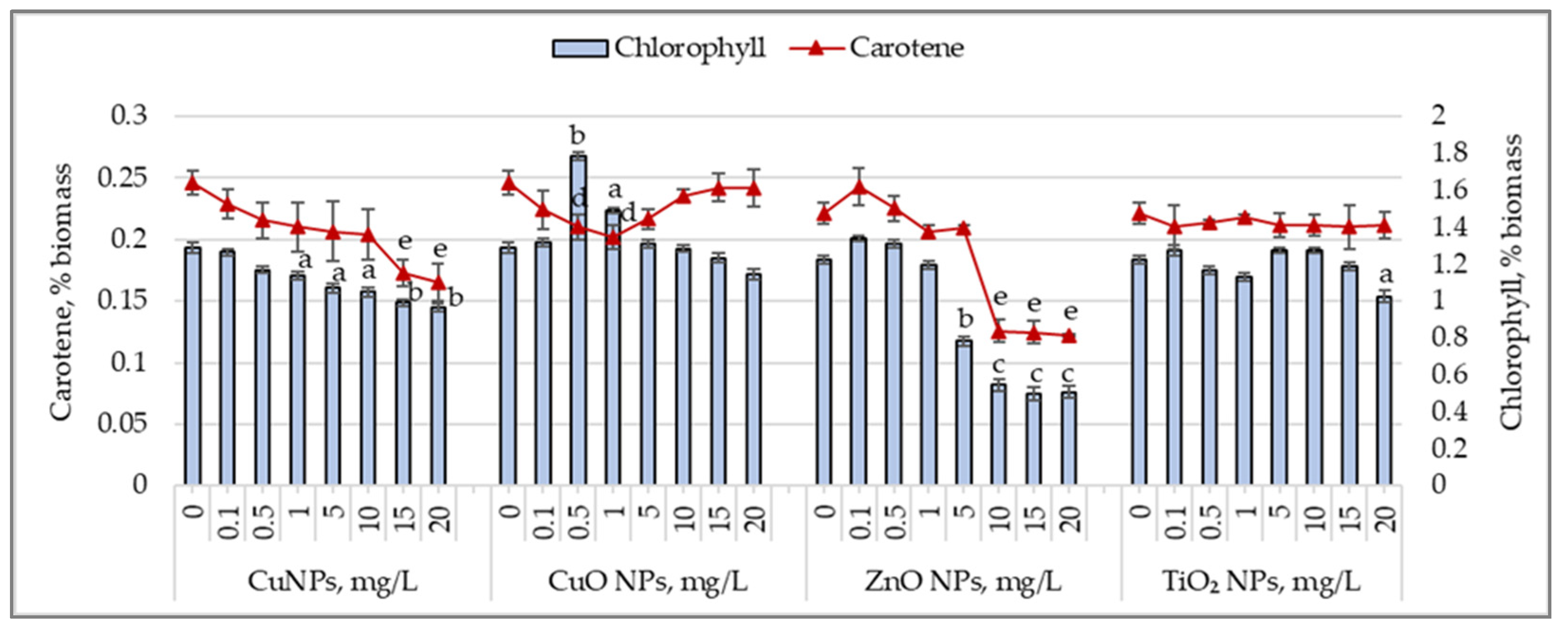
3.4. Chlorophyll and Carotenoid Accumulation
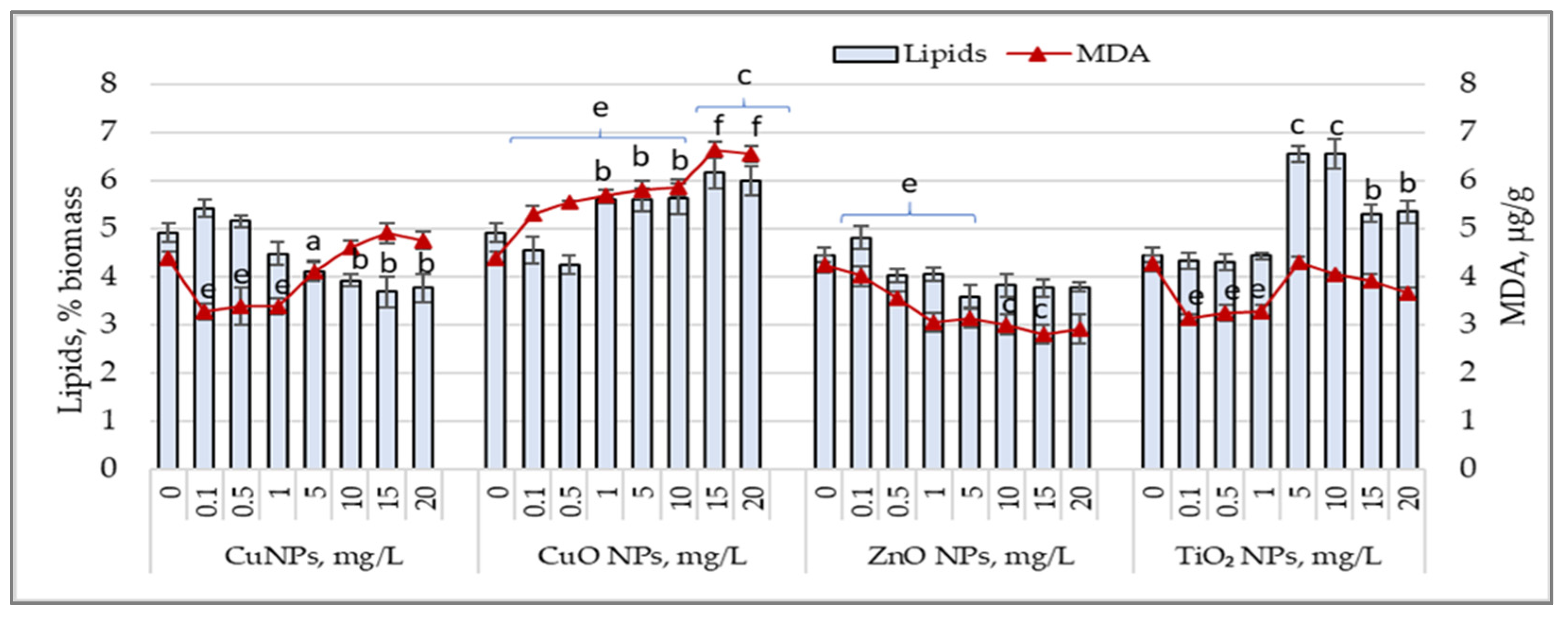
3.5. Lipid Content and Malondialdehyde (MDA) Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, X.; Gao, X.; Liu, C.; Liang, W.; Xue, H.; Li, Z.; Jin, H. Application of Nanomaterials in the Production of Biomolecules in Microalgae: A Review. Mar. Drugs 2023, 21, 594. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Estrada, L.; Domínguez-Espíndola, R.B.; Sebastian, P.J. The Influence of Fe2O3 Nanoparticles on Chlorella spp. Growth and Biochemicals Accumulation. Waste Biomass Valor 2024, 15, 3281–3295. [Google Scholar] [CrossRef]
- Liang, S.X.T.; Wong, L.S.; Antony, A.C.T.; Dhanapal, A.; Djearamane, S. Toxicity of Metals and Metallic Nanoparticles on Nutritional Properties of Microalgae. Water Air Soil Pollut. 2020, 231, 52. [Google Scholar] [CrossRef]
- Wang, F.; Liu, T.; Guan, W.; Xu, L.; Huo, S.; Ma, A.; Zhuang, G.; Terry, N. Development of a strategy for enhancing the biomass growth and lipid accumulation of Chlorella sp. UJ-3 using magnetic Fe3O4 nanoparticles. Nanomaterials 2021, 11, 2802. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.K.; Lee, B.; Choi, G.G.; Moon, M.; Park, M.S.; Lim, J.; Yang, J.-W. Enhancing lipid productivity of Chlorella vulgaris using oxidative stress by TiO2 nanoparticles. Korean J. Chem. Eng. 2014, 31, 861–867. [Google Scholar] [CrossRef]
- Vargas-Estrada, L.; Torres-Arellano, S.; Longoria, A.; Arias, D.M.; Okoye, P.U.; Sebastian, P.J. Role of nanoparticles on microalgal cultivation: A review. Fuel 2020, 280, 118598. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Fais, G.; Casula, M.; Borselli, M.; Giannaccare, G.; Locci, A.M.; Lai, N.; Orrù, R.; Cao, G.; Concas, A. Nanoparticles from Microalgae and Their Biomedical Applications. Mar. Drugs 2023, 21, 352. [Google Scholar] [CrossRef]
- Sibi, G.; Ananda Kumar, D.; Gopal, T.; Harinath, K.; Banupriya, S.; Chaitra, S. Metal Nanoparticle Triggered Growth and Lipid Production in Chlorella vulgaris. Int. J. Sci. Res. Environ. Sci. Toxicol. 2017, 2, 1–8. [Google Scholar]
- Marjanović, B.; Benković, M.; Jurina, T.; Sokač Cvetnić, T.; Valinger, D.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Bioactive Compounds from Spirulina spp.—Nutritional Value, Extraction, and Application in Food Industry. Separations 2024, 11, 257. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Maciel, G.M.; Fernandes, I.A.; Pedro, A.C.; Rubio, F.T.V.; Branco, I.G.; Haminiuk, C.W.I. Functional properties of bioactive compounds from Spirulina spp.: Current status and future trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Li, J.; Zhong, H.; Xie, J.; Zhang, P.; Lu, Q.; Li, J.; Xu, P.; Chen, P.; Leng, L.; et al. Anti-oxidation properties and therapeutic potentials of Spirulina. Algal Res. 2021, 55, 102240. [Google Scholar] [CrossRef]
- Wan, D.; Wu, Q.; Kuča, K. Chapter 57—Spirulina. In Nutraceuticals. Efficacy, Safety, and Toxicity, 2nd ed.; Gupta, R.C., Lall, R., Srivastava, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 959–974. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, J.; Meng, F. Adaptive laboratory evolution of microalgae: A review of the regulation of growth, stress resistance, metabolic processes, and biodegradation of pollutants. Front. Microbiol. 2021, 12, 737248. [Google Scholar] [CrossRef] [PubMed]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Djur, S.; Yushin, N.; Grozdov, D. Assessment of Metal Accumulation by Arthrospira platensis and Its Adaptation to Iterative Action of Nickel Mono- and Polymetallic Synthetic Effluents. Microorganisms 2022, 10, 1041. [Google Scholar] [CrossRef]
- Diaconu, M.; Soreanu, G.; Balan, C.D.; Buciscanu, I.I.; Maier, V.; Cretescu, I. Study of Spirulina platensis (Arthrospira) Development under the Heavy Metals Influence, as a Potential Promoter of Wastewater Remediation. Water 2023, 15, 3962. [Google Scholar] [CrossRef]
- Cepoi, L.; Zinicovscaia, I. Chapter 39—Spirulina platensis as a model object for the environment bioremediation studies. In Handbook of Algal Science, Technology and Medicine; Konur, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 629–640. [Google Scholar] [CrossRef]
- Hazeem, L. Single and Combined Toxicity Effects of Zinc Oxide Nanoparticles: Uptake and Accumulation in Marine Microalgae, Toxicity Mechanisms, and Their Fate in the Marine Environment. Water 2022, 14, 2669. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Parada, J.; de Oliveira, H.C.; Benavides-Mendoza, A.; Leiva, S.; Fernandez-Baldo, M.; Seabra, A.B. Copper nanoparticles as a potential emerging pollutant: Divergent effects in the agriculture, risk-benefit balance and integrated strategies for its use. Emerg. Contam. 2024, 10, 100352. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Tripathi, R.; Kumar, A. Advancing of Zinc Oxide Nanoparticles for Cosmetic Applications. In Handbook of Consumer Nanoproducts; Mallakpour, S., Hussain, C.M., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2022; pp. 1057–10721. [Google Scholar] [CrossRef]
- Gutiérrez-Ramírez, J.A.; Betancourt-Galindo, R.; Aguirre-Uribe, L.A.; Cerna-Chávez, E.; Sandoval-Rangel, A.; Ángel, E.C.D.; Chacón-Hernández, J.C.; García-López, J.I.; Hernández-Juárez, A. Insecticidal Effect of Zinc Oxide and Titanium Dioxide Nanoparticles against Bactericera cockerelli Sulc. (Hemiptera: Triozidae) on Tomato Solanum lycopersicum. Agronomy 2021, 11, 1460. [Google Scholar] [CrossRef]
- Vats, M.; Bhardwaj, S.; Chhabra, A. Green Synthesis of Copper Oxide Nanoparticles using Cucumis sativus (Cucumber) Extracts and their Bio-Physical and Biochemical Characterization for Cosmetic and Dermatologic Applications. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 726–733. [Google Scholar] [CrossRef]
- Solomonova, E.S.; Shoman, N.Y.; Akimov, A.I.; Rylkova, O.A. Comparative assessment of stress responses of the microalgae Prorocentrum cordatum (Ostenfeld) Dodge and Dunaliella salina (Teod.) to the presence of copper oxide nanoparticles. Microbiology 2023, 92, 66–74. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, Y.; Zhao, T.; Tan, L.; Wang, J. The effects of copper ions and copper nanomaterials on the output of amino acids from marine microalgae. Environ. Sci. Pollut. Res. 2022, 29, 9780–9791. [Google Scholar] [CrossRef] [PubMed]
- Samei, M.; Sarrafzadeh, M.H.; Faramarzi, M.A. The impact of morphology and size of zinc oxide nanoparticles on its toxicity to the freshwater microalga, Raphidocelis subcapitata. Environ. Sci. Pollut. Res. 2019, 26, 2409–2420. [Google Scholar] [CrossRef] [PubMed]
- Thenarasu, A.; Chai, M.K.; Shing, W.L.; Djearamane, S.; Thirunavukkarasu, C. Effect of Titanium, Silver and Zinc Nanoparticles on Microalgae in the Aquatic Environment. J. Exp. Biol. Agric. Sci. 2022, 10, 767–772. [Google Scholar] [CrossRef]
- Déniel, M.; Couzinet-Mossion, A.; Jubeau, S.; Viau, M.; Fievet, F.; Wadouachi, A. Current methods to monitor microalgae-nanoparticle interaction and associated effects. Aquat. Toxicol. 2019, 210, 105311. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.K.; Chu, W.L.; Kok, Y.Y.; Cheong, K.W. Assessing the toxicity of copper oxide nanoparticles and copper sulfate in a tropical Chlorella. J. Appl. Phycol. 2018, 30, 3153–3165. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Siegelman, H.W.; Kycia, J.H. Algal biliproteins. In Handbook of Phycological Methods: Physiological and Biochemical Methods; Hellebust, J.A., Craigie, J.S., Eds.; Cambridge University Press: Cambridge, UK, 1978; pp. 71–79. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Park, J.Y.; Jeong, H.J.; Yoon, E.Y.; Moon, S.J. Easy and rapid quantification of lipid content of marine dinoflagellates using the sulpho-phospho-vanillin method. Algae 2016, 31, 391–401. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.-Y.; Lee, Y.-C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Saf. 2020, 201, 110781. [Google Scholar] [CrossRef]
- Barreto, D.M.; Tonietto, A.E.; Lombardi, A.T. Environmental concentrations of copper nanoparticles affect vital functions in Ankistrodesmus densus. Aquat. Toxicol. 2021, 231, 105720. [Google Scholar] [CrossRef]
- Pikula, K.; Mintcheva, N.; Kulinich, S.A.; Zakharenko, A.; Markina, Z.; Chaika, V.; Orlova, T.; Mezhuev, Y.; Kokkinakis, E.; Tsatsakis, A.; et al. Aquatic toxicity and mode of action of CdS and ZnS nanoparticles in four microalgae species. Environ. Res. 2020, 186, 109513. [Google Scholar] [CrossRef]
- Cavalletti, E.; Romano, G.; Palma Esposito, F.; Barra, L.; Chiaiese, P.; Balzano, S.; Sardo, A. Copper Effect on Microalgae: Toxicity and Bioremediation Strategies. Toxics 2022, 10, 527. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, J.; Lu, T.; Zhang, M.; Ke, M.; Fu, Z.; Pan, X.; Qian, H. A comparison of the effects of copper nanoparticles and copper sulfate on Phaeodactylum tricornutum physiology and transcription. Environ. Toxicol. Pharmacol. 2017, 56, 43–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, D.; Gao, W.; Zhang, X.; Ye, W.; Zhang, Z. The metabolic mechanisms of Cd-induced hormesis in photosynthetic microalgae, Chromochloris zofingiensis. Sci. Total Environ. 2024, 912, 168966. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, Y. Toxic effects of different particle size ZnO NPs on marine microalgae Chlorella sp. IOP Conf. Ser. Earth Environ. Sci. 2021, 770, 012022. [Google Scholar] [CrossRef]
- Aravantinou, A.F.; Andreou, F.; Manariotis, I.D. Long-term toxicity of ZnO nanoparticles to Scenedesmus rubescens cultivated in different media. Sci. Rep. 2017, 7, 13454. [Google Scholar] [CrossRef]
- Djearamane, S.; Lim, Y.M.; Wong, L.S.; Lee, P.F. Cytotoxic effects of zinc oxide nanoparticles on cyanobacterium Spirulina (Arthrospira) platensis. PeerJ 2018, 6, e4682. [Google Scholar] [CrossRef]
- Tzanakis, N.; Aravantinou, A.F.; Manariotis, I.D. Short-term toxicity of ZnO nanoparticles on microalgae at different initial nutrient concentrations. Sustainability 2023, 15, 7853. [Google Scholar] [CrossRef]
- Lau, Z.L.; Low, S.S.; Ezeigwe, E.R.; Chew, K.W.; Chai, W.S.; Bhatnagar, A.; Yap, Y.J.; Show, P.L. A review on the diverse interactions between microalgae and nanomaterials: Growth variation, photosynthetic performance and toxicity. Bioresour. Technol. 2022, 351, 127048. [Google Scholar] [CrossRef]
- Xia, B.; Chen, B.; Sun, X.; Qu, K.; Ma, F.; Du, M. Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: Growth inhibition, oxidative stress and internalization. Sci. Total Environ. 2015, 508, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Solomonova, E.; Shoman, N.; Akimov, A.; Rylkova, O. Impact of copper oxide nanoparticles on the physiology of different microalgal species. Reg. Stud. Mar. Sci. 2023, 66, 103128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudi, L.; Cepoi, L.; Chiriac, T.; Djur, S. Interactions Between Potentially Toxic Nanoparticles (Cu, CuO, ZnO, and TiO2) and the Cyanobacterium Arthrospira platensis: Biological Adaptations to Xenobiotics. Nanomaterials 2025, 15, 46. https://doi.org/10.3390/nano15010046
Rudi L, Cepoi L, Chiriac T, Djur S. Interactions Between Potentially Toxic Nanoparticles (Cu, CuO, ZnO, and TiO2) and the Cyanobacterium Arthrospira platensis: Biological Adaptations to Xenobiotics. Nanomaterials. 2025; 15(1):46. https://doi.org/10.3390/nano15010046
Chicago/Turabian StyleRudi, Ludmila, Liliana Cepoi, Tatiana Chiriac, and Svetlana Djur. 2025. "Interactions Between Potentially Toxic Nanoparticles (Cu, CuO, ZnO, and TiO2) and the Cyanobacterium Arthrospira platensis: Biological Adaptations to Xenobiotics" Nanomaterials 15, no. 1: 46. https://doi.org/10.3390/nano15010046
APA StyleRudi, L., Cepoi, L., Chiriac, T., & Djur, S. (2025). Interactions Between Potentially Toxic Nanoparticles (Cu, CuO, ZnO, and TiO2) and the Cyanobacterium Arthrospira platensis: Biological Adaptations to Xenobiotics. Nanomaterials, 15(1), 46. https://doi.org/10.3390/nano15010046








