Modulation of the Effect of Cisplatin on Nicotine-Stimulated A549 Lung Cancer Cells Using Analog of Marine Sponge Toxin Loaded in Gelatin Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Gelatin NPs
2.3. Characterization of Gelatin NPs
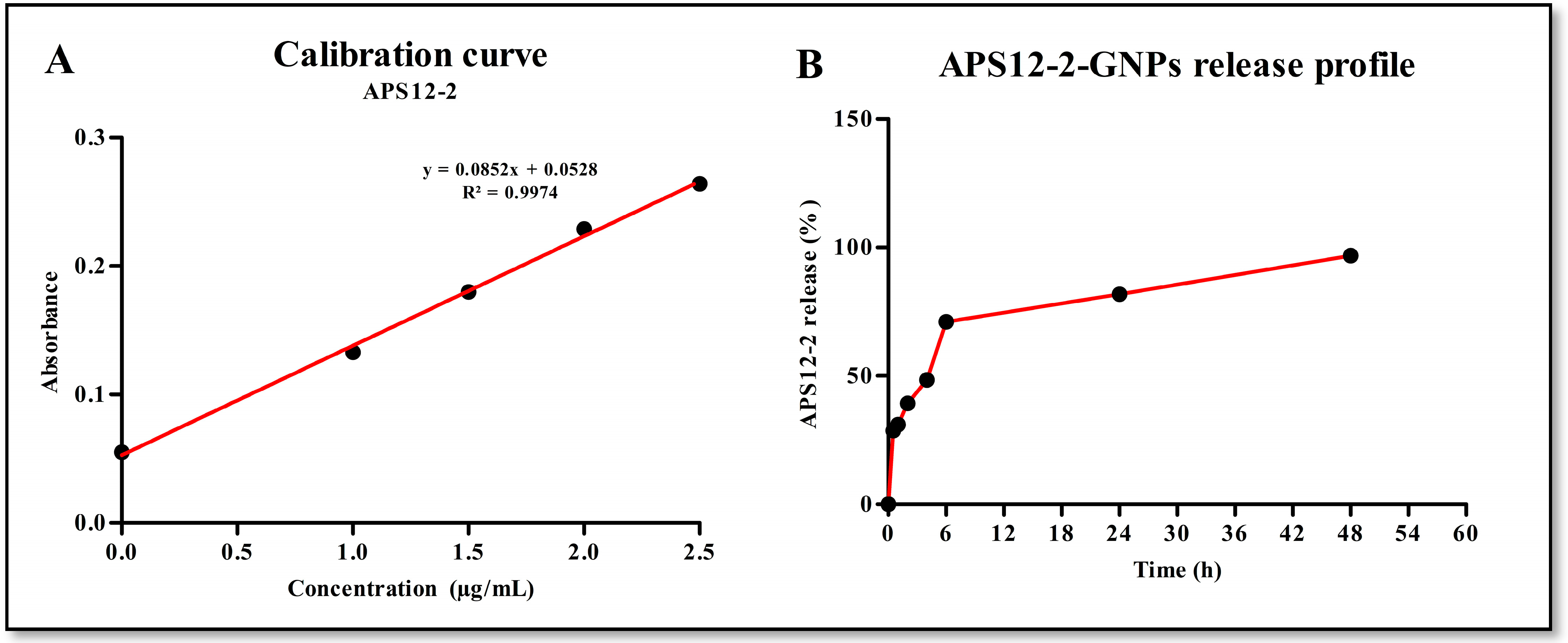
2.4. APS12-2 Loading and Profile Release
2.5. Cell Culture
2.6. Cytotoxicity Measurements
2.7. ApoTox-GloTM Triplex Assay
2.8. Intracellular ROS Measurements
2.9. Lipid Droplet Measurements
2.10. Statistical Analysis
3. Results
3.1. Nanoparticle Characteristics
3.2. Profile Release of APS12-2-GNPs
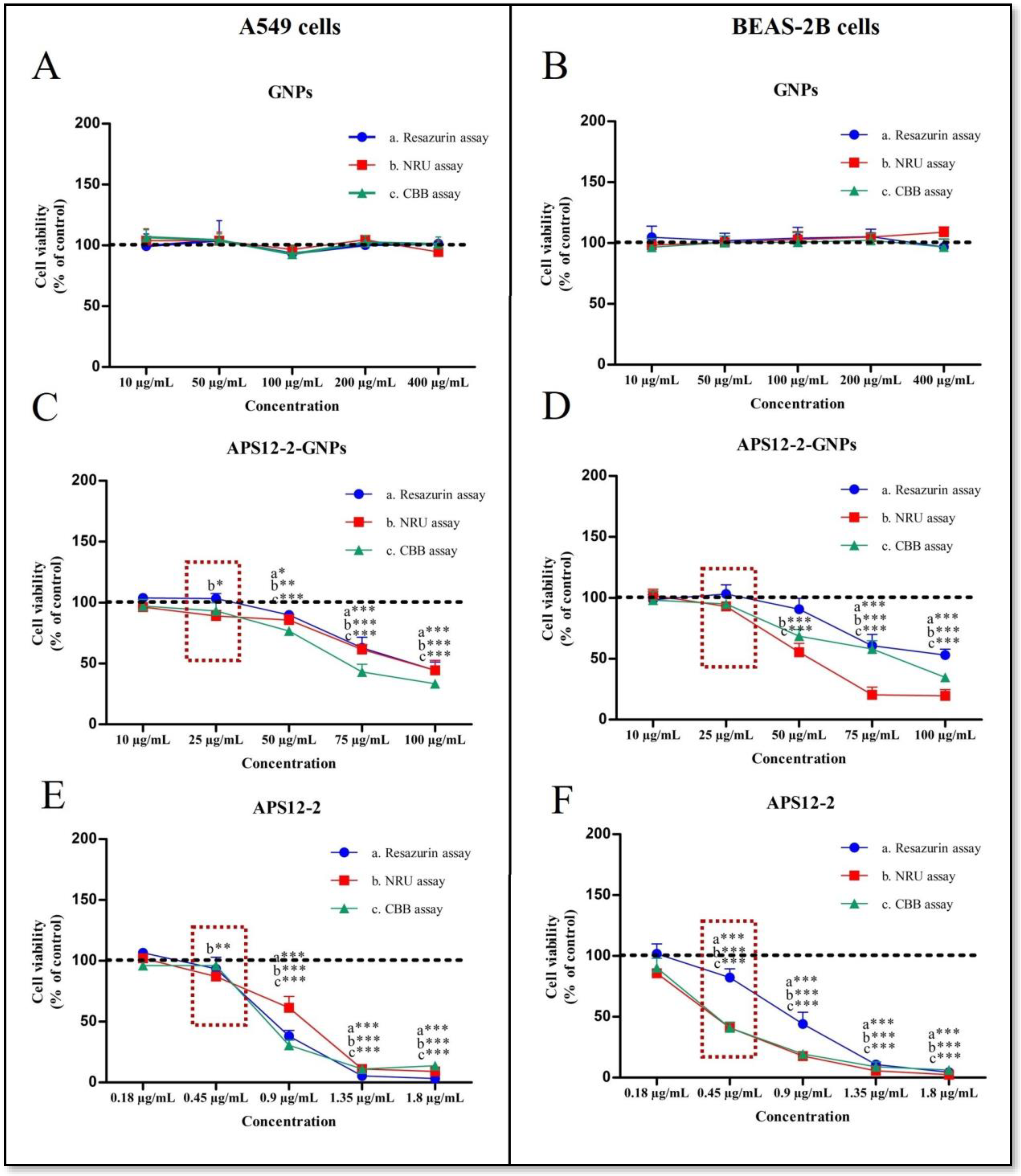
3.3. Cytotoxicity of APS12-2, GNPs, and APS12-2-GNPs
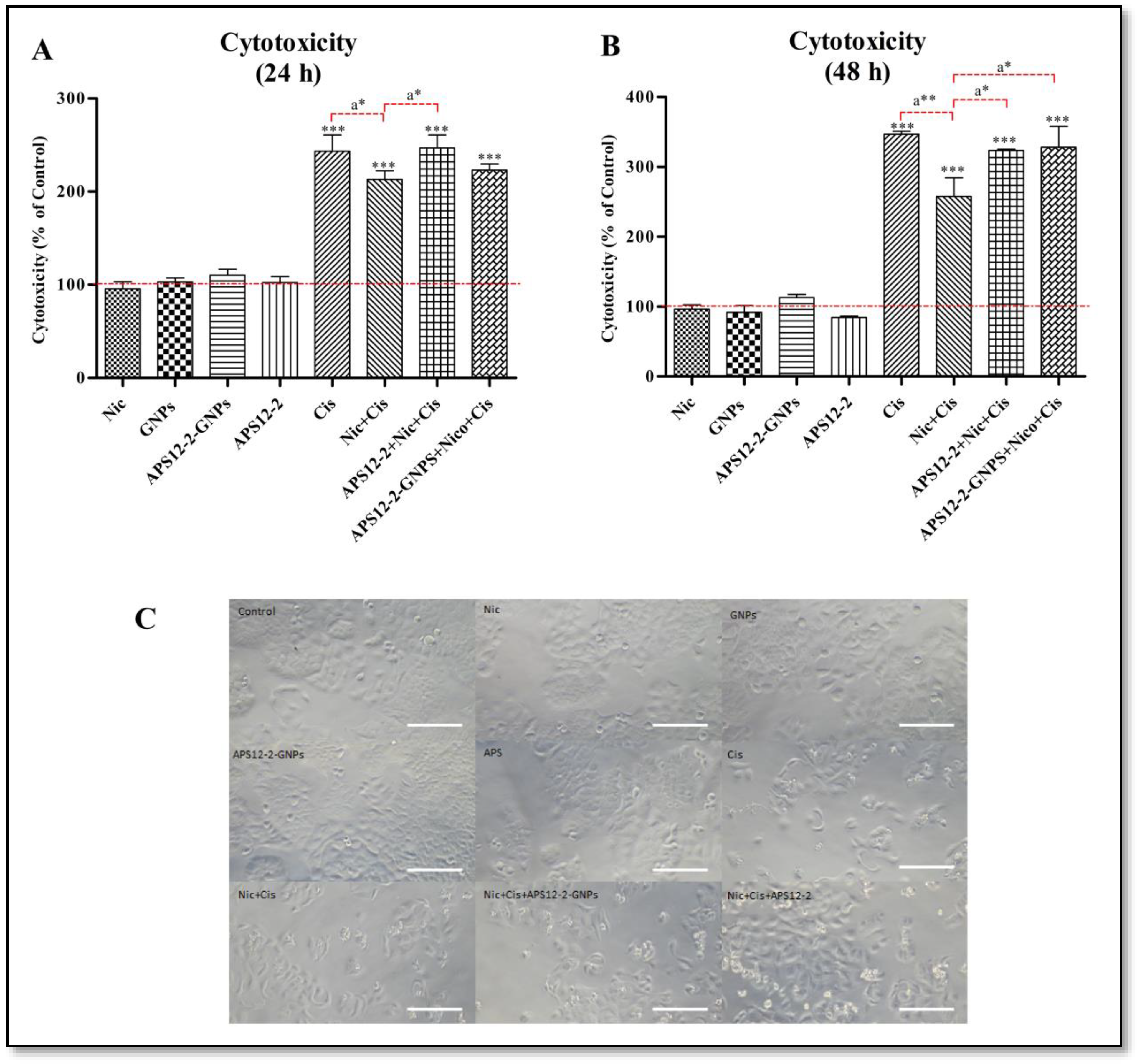
3.4. Modulation of Cisplatin-Induced Cytotoxicity in A549 Cells by Nicotine, APS12-2, or APS12-2-GNPs
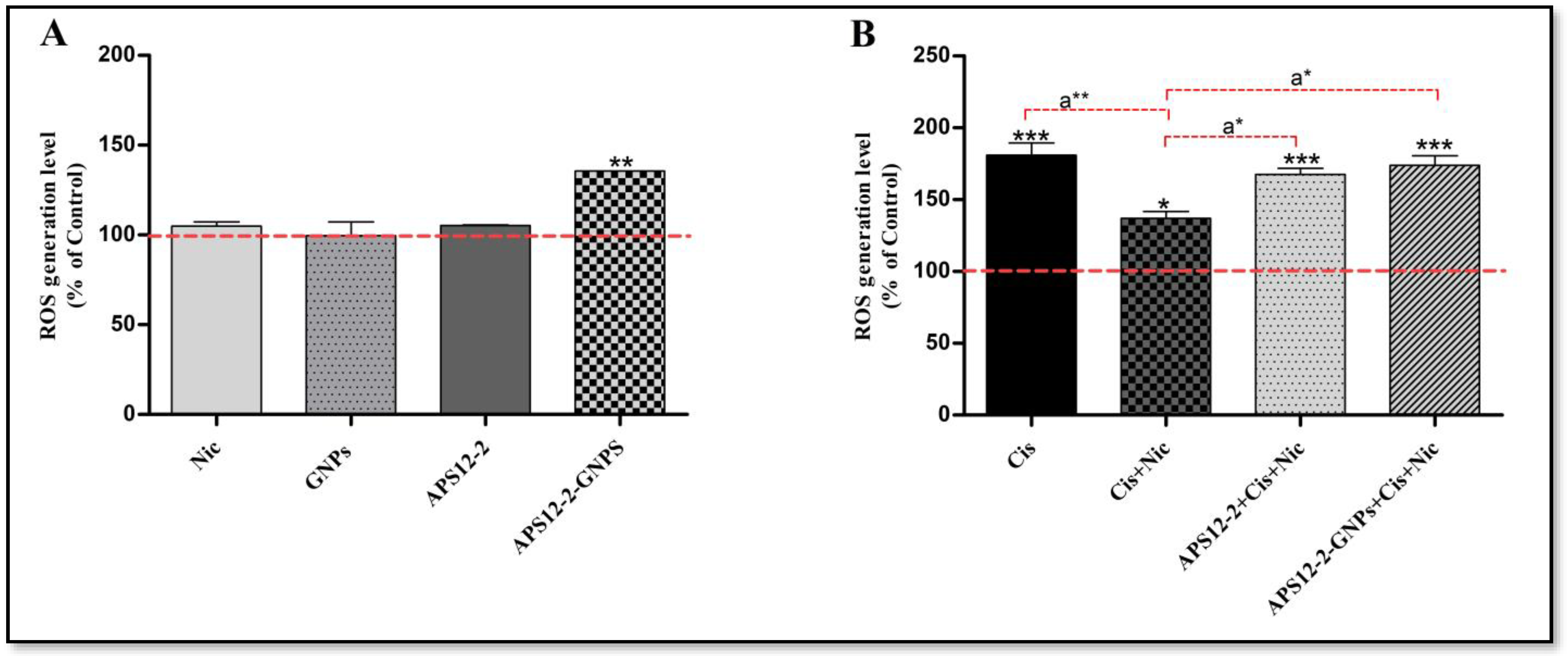
3.5. Intracellular ROS Measurement
3.6. Intracellular Amount of Lipid Droplets
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Woodman, C.; Vundu, G.; George, A.; Wilson, C.M. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. Semin. Cancer Biol. 2020, 69, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Kunda, N.K. Antimicrobial peptides as novel therapeutics for non-small cell lung cancer. Drug Discov. Today 2020, 25, 238–247. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Araghi, M.; Mannani, R.; Maleki, A.H.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef]
- Zhang, C.; Leighl, N.B.; Wu, Y.-L.; Zhong, W.-Z. Emerging therapies for non-small cell lung cancer. J. Hematol. Oncol. 2019, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Zovko, A.; Viktorsson, K.; Lewensohn, R.; Kološa, K.; Filipič, M.; Xing, H.; Kem, W.R.; Paleari, L.; Turk, T. APS8, a Polymeric Alkylpyridinium Salt Blocks α7 nAChR and Induces Apoptosis in Non-Small Cell Lung Carcinoma. Mar. Drugs 2013, 11, 2574–2594. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat. Rev. Cancer 2009, 9, 195–205. [Google Scholar] [CrossRef]
- Grando, S.A. Connections of nicotine to cancer. Nat. Rev. Cancer 2014, 14, 419–429. [Google Scholar] [CrossRef]
- Mucchietto, V.; Fasoli, F.; Pucci, S.; Moretti, M.; Benfante, R.; Mclntosh, M.; Clementi, F.; Gotti, C. A9- and A7-Containing Receptors Mediate the pro-Proliferative Effects of Nicotine in the A549 Adenocarcinoma Cell Line. Br. J. Pharmacol. 2018, 175, 1957–1972. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tsai, K.-Y.; Su, Y.-F.; Chien, C.-Y.; Chen, Y.-C.; Wu, Y.-C.; Liu, S.-Y.; Shieh, Y.S. α7-Nicotine acetylcholine receptor mediated nicotine induced cell survival and cisplatin resistance in oral cancer. Arch. Oral Biol. 2020, 111, 104653. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M. Regulatory Role of the A7nAChR in Cancer. Curr. Drug Targets 2012, 13, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Wang, Z.-W.; Lu, T.-L.; Gomez, C.B.; Fang, H.-W.; Wei, Y.; Tseng, C.-L. The Synergistic Anticancer Effect of Dual Drug- (Cisplatin/Epigallocatechin Gallate) Loaded Gelatin Nanoparticles for Lung Cancer Treatment. J. Nanomater. 2020, 2020, 9181549. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Kim, H.-K.; Shim, W.; Anwar, M.A.; Kwon, J.-W.; Kwon, H.-K.; Kim, H.J.; Jeong, H.; Kim, H.M.; Hwang, D.; et al. Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS ONE 2015, 10, e0135083. [Google Scholar] [CrossRef] [PubMed]
- Renschler, M.F. The emerging role of reactive oxygen species in cancer therapy. Eur. J. Cancer 2004, 40, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Casares, C.; Ramírez-Camacho, R.; Trinidad, A.; Roldán, A.; Jorge, E.; García-Berrocal, J.R. Reactive oxygen species in apoptosis induced by cisplatin: Review of physiopathological mechanisms in animal models. Eur. Arch. Oto-Rhino-Laryngol. 2012, 269, 2455–2459. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, V.F.; Marta, G.N.; da Silva, E.M.; Gois, A.F.; de Castria, T.B.; Riera, R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2020, 2020, CD009256. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wen, W.; Hou, X.; Wu, J.; Yi, L.; Zhi, Y.; Lv, Y.; Tan, X.; Liu, L.; Wang, P.; et al. Inhibitory effect of sinomenine on lung cancer cells via negative regulation of α7 nicotinic acetylcholine receptor. J. Leukoc. Biol. 2021, 109, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Fan, W.; Gao, T.; Li, T.; Yin, Z.; Guo, H.; Wang, L.; Han, Y.; Jiang, J.-D. Analgesic Mechanism of Sinomenine against Chronic Pain. Pain Res. Manag. 2020, 2020, 1876862. [Google Scholar] [CrossRef]
- Witayateeraporn, W.; Arunrungvichian, K.; Pothongsrisit, S.; Doungchawee, J.; Vajragupta, O.; Pongrakhananon, V. α7-Nicotinic acetylcholine receptor antagonist QND7 suppresses non-small cell lung cancer cell proliferation and migration via inhibition of Akt/mTOR signaling. Biochem. Biophys. Res. Commun. 2020, 521, 977–983. [Google Scholar] [CrossRef]
- Brown, K.C.; Lau, J.K.; Dom, A.M.; Witte, T.R.; Luo, H.; Crabtree, C.M.; Shah, Y.H.; Shiflett, B.S.; Marcelo, A.J.; Proper, N.A.; et al. MG624, an α7-nAChR antagonist, inhibits angiogenesis via the Egr-1/FGF2 pathway. Angiogenesis 2011, 15, 99–114. [Google Scholar] [CrossRef]
- Grandič, M.; Bajuk, B.P.; Sepčić, K.; Košorok, M.D.; Frangež, R. Effects of synthetic analogues of poly-APS on contractile response of porcine coronary arteries. Toxicol. Vitr. 2013, 27, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Houssen, W.E.; Lu, Z.; Edrada-Ebel, R.; Chatzi, C.; Tucker, S.J.; Sepčić, K.; Turk, T.; Zovko, A.; Shen, S.; Mancini, I.; et al. Chemical synthesis and biological activities of 3-alkyl pyridinium polymeric analogues of marine toxins. J. Chem. Biol. 2010, 3, 113–125. [Google Scholar] [CrossRef]
- Zovko, A.; Gabrič, M.V.; Sepčić, K.; Pohleven, F.; Jaklič, D.; Gunde-Cimerman, N.; Lu, Z.; Edrada-Ebel, R.; Houssen, W.E.; Mancini, I.; et al. Antifungal and antibacterial activity of 3-alkylpyridinium polymeric analogs of marine toxins. Int. Biodeterior. Biodegrad. 2012, 68, 71–77. [Google Scholar] [CrossRef]
- Joukhan, A.; Kononenko, V.; Bele, T.; Dolenc, M.S.; Peigneur, S.; Pinheiro-Junior, E.L.; Tytgat, J.; Turk, T.; Križaj, I.; Drobne, D. Attenuation of Nicotine Effects on A549 Lung Cancer Cells by Synthetic α7 nAChR Antagonists APS7-2 and APS8-2. Mar. Drugs 2024, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Grandič, M.; Sepčić, K.; Turk, T.; Juntes, P.; Frangež, R. In vivo toxic and lethal cardiovascular effects of a synthetic polymeric 1,3-dodecylpyridinium salt in rodents. Toxicol. Appl. Pharmacol. 2011, 255, 86–93. [Google Scholar] [CrossRef]
- Grandič, M.; Aráoz, R.; Molgó, J.; Turk, T.; Sepčić, K.; Benoit, E.; Frangež, R. The non-competitive acetylcholinesterase inhibitor APS12-2 is a potent antagonist of skeletal muscle nicotinic acetylcholine receptors. Toxicol. Appl. Pharmacol. 2012, 265, 221–228. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Asadi, A.; Gurushankar, K.; Karimi Shayan, T.; Abedi Sarvestani, F. Importance of Nano Medicine and New Drug Therapies for Cancer. Adv. Pharm. Bull. 2021, 11, 450–457. [Google Scholar] [CrossRef]
- Wolfram, J.; Ferrari, M. Clinical cancer nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Jiang, X.; Du, Z.; Zhang, X.; Zaman, F.; Song, Z.; Guan, Y.; Yu, T.; Huang, Y. Gelatin-based anticancer drug delivery nanosystems: A mini review. Front. Bioeng. Biotechnol. 2023, 11, 1158749. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Schneider, M. Improvement of Nanoprecipitation Technique for Preparation of Gelatin Nanoparticles and Potential Macromolecular Drug Loading. Macromol. Biosci. 2013, 13, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Liwata, H.N.; Liyongo, C.I.; Mayaliwa, J.C.M.; Ilunga, E.M.; Mumba, C.M.; Mbombo, C.M.; Muzele, T.K. Phytochemical Screening and Antibacterial Activity of Phytomedecine Mathesia, a Drug Use against Buruli Ulcer in Republic Democratic of the Congo (DRC). Eur. J. Pharm. Med. Res. 2020, 7, 52–56. [Google Scholar]
- Wardini, T.H.; Afifa, I.N.; Esyanti, R.R.; Astutiningsih, N.T.; Pujisiswanto, H. The potential of invasive species Praxelis clematidea extract as a bioherbicide for Asystasia gangetica. Biodiversitas J. Biol. Divers. 2023, 24, 4738–4746. [Google Scholar] [CrossRef]
- Kononenko, V.; Drobne, D. In Vitro Cytotoxicity Evaluation of the Magnéli Phase Titanium Suboxides (TixO2x−1) on A549 Human Lung Cells. Int. J. Mol. Sci. 2019, 20, 196. [Google Scholar] [CrossRef]
- Carrasco-esteban, E.; Domínguez-rullán, J.A.; Barrionuevo-castillo, P.; Pelari-mici, L.; López-campos, F. Current Role of Nanoparticles in the Treatment of Lung Cancer. J. Clin. Transl. Res. 2021, 7, 140–155. [Google Scholar] [CrossRef]
- Yazdi, M.E.T.; Qayoomian, M.; Beigoli, S.; Boskabady, M.H. Recent advances in nanoparticle applications in respiratory disorders: A review. Front. Pharmacol. 2023, 14, 1059343. [Google Scholar] [CrossRef]
- Lee, E.J.; Khan, S.A.; Lim, K.-H. Gelatin Nanoparticle Preparation by Nanoprecipitation. J. Biomater. Sci. Polym. Ed. 2011, 22, 753–771. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Jazani, R.S.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Mei, D.; Zhao, L.; Chen, B.; Zhang, X.; Wang, X.; Yu, Z.; Ni, X.; Zhang, Q. α-Conotoxin ImI-modified polymeric micelles as potential nanocarriers for targeted docetaxel delivery to α7-nAChR overexpressed non-small cell lung cancer. Drug Deliv. 2018, 25, 493–503. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Liu, J.; Wu, H.; Zhang, Q.; Tang, X.-R.; Li, D.; Li, C.-S.; Liu, Y.; Cao, A.; Wang, H. Endocytosis, Distribution, and Exocytosis of Polystyrene Nanoparticles in Human Lung Cells. Nanomaterials 2023, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Chernyavsky, A.I.; Shchepotin, I.B.; Galitovkiy, V.; Grando, S.A. Mechanisms of tumor-promoting activities of nicotine in lung cancer: Synergistic effects of cell membrane and mitochondrial nicotinic acetylcholine receptors. BMC Cancer 2015, 15, 152. [Google Scholar] [CrossRef]
- Skok, M.; Gergalova, G.; Lykhmus, O.; Kalashnyk, O.; Koval, L.; Uspenska, K. Nicotinic acetylcholine receptors in mitochondria: Subunit composition, function and signaling. Neurotransmitter 2016, 3, e1290. [Google Scholar] [CrossRef]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine signaling system in progression of lung cancers. Pharmacol. Ther. 2018, 194, 222–254. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Bi, W.; Zheng, K.; Zhu, E.; Wang, S.; Xiong, Y.; Chang, J.; Jiang, J.; Liu, B.; Lu, Z.; et al. Nicotine Prevents Oxidative Stress-Induced Hippocampal Neuronal Injury through A7-NAChR/Erk1/2 Signaling Pathway. Front. Mol. Neurosci. 2020, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tan, Y.; Chen, L.; Liu, Y.; Ren, Z. Reactive Oxygen Species Induces Lipid Droplet Accumulation in HepG2 Cells by Increasing Perilipin 2 Expression. Int. J. Mol. Sci. 2018, 19, 3445. [Google Scholar] [CrossRef]
- Welte, M.A.; Gould, A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1260–1272. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joukhan, A.; Kononenko, V.; Sollner Dolenc, M.; Hočevar, M.; Turk, T.; Drobne, D. Modulation of the Effect of Cisplatin on Nicotine-Stimulated A549 Lung Cancer Cells Using Analog of Marine Sponge Toxin Loaded in Gelatin Nanoparticles. Nanomaterials 2024, 14, 777. https://doi.org/10.3390/nano14090777
Joukhan A, Kononenko V, Sollner Dolenc M, Hočevar M, Turk T, Drobne D. Modulation of the Effect of Cisplatin on Nicotine-Stimulated A549 Lung Cancer Cells Using Analog of Marine Sponge Toxin Loaded in Gelatin Nanoparticles. Nanomaterials. 2024; 14(9):777. https://doi.org/10.3390/nano14090777
Chicago/Turabian StyleJoukhan, Ahmad, Veno Kononenko, Marija Sollner Dolenc, Matej Hočevar, Tom Turk, and Damjana Drobne. 2024. "Modulation of the Effect of Cisplatin on Nicotine-Stimulated A549 Lung Cancer Cells Using Analog of Marine Sponge Toxin Loaded in Gelatin Nanoparticles" Nanomaterials 14, no. 9: 777. https://doi.org/10.3390/nano14090777
APA StyleJoukhan, A., Kononenko, V., Sollner Dolenc, M., Hočevar, M., Turk, T., & Drobne, D. (2024). Modulation of the Effect of Cisplatin on Nicotine-Stimulated A549 Lung Cancer Cells Using Analog of Marine Sponge Toxin Loaded in Gelatin Nanoparticles. Nanomaterials, 14(9), 777. https://doi.org/10.3390/nano14090777






