Oxygen Defects Containing TiN Films for the Hydrogen Evolution Reaction: A Robust Thin-Film Electrocatalyst with Outstanding Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
2.2. DFT Calculations
3. Results
3.1. Morphology Structure and Chemistry
3.2. HER Investigations
4. Discussion
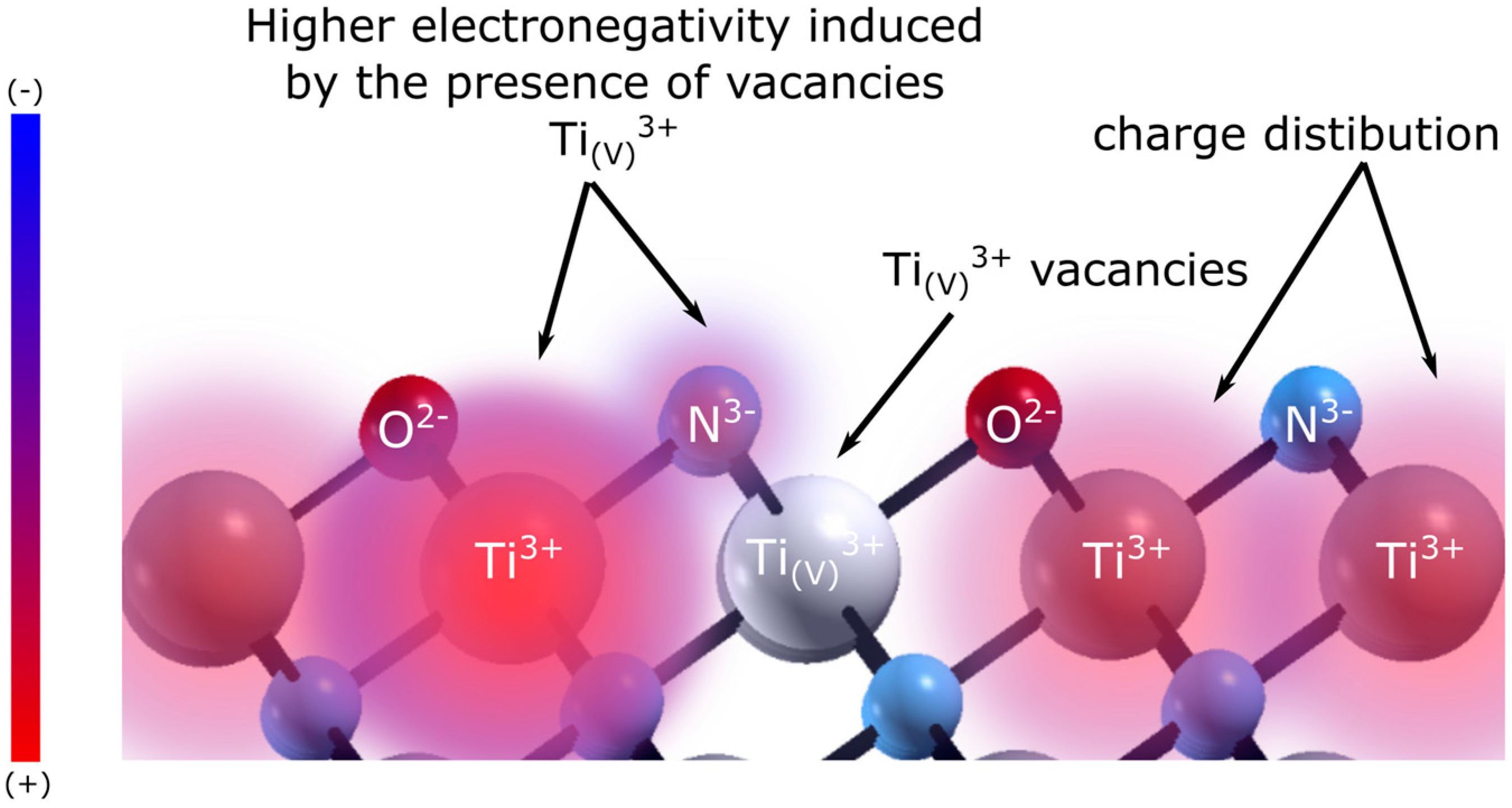
4.1. HER of Oxygen Defects Containing TiN
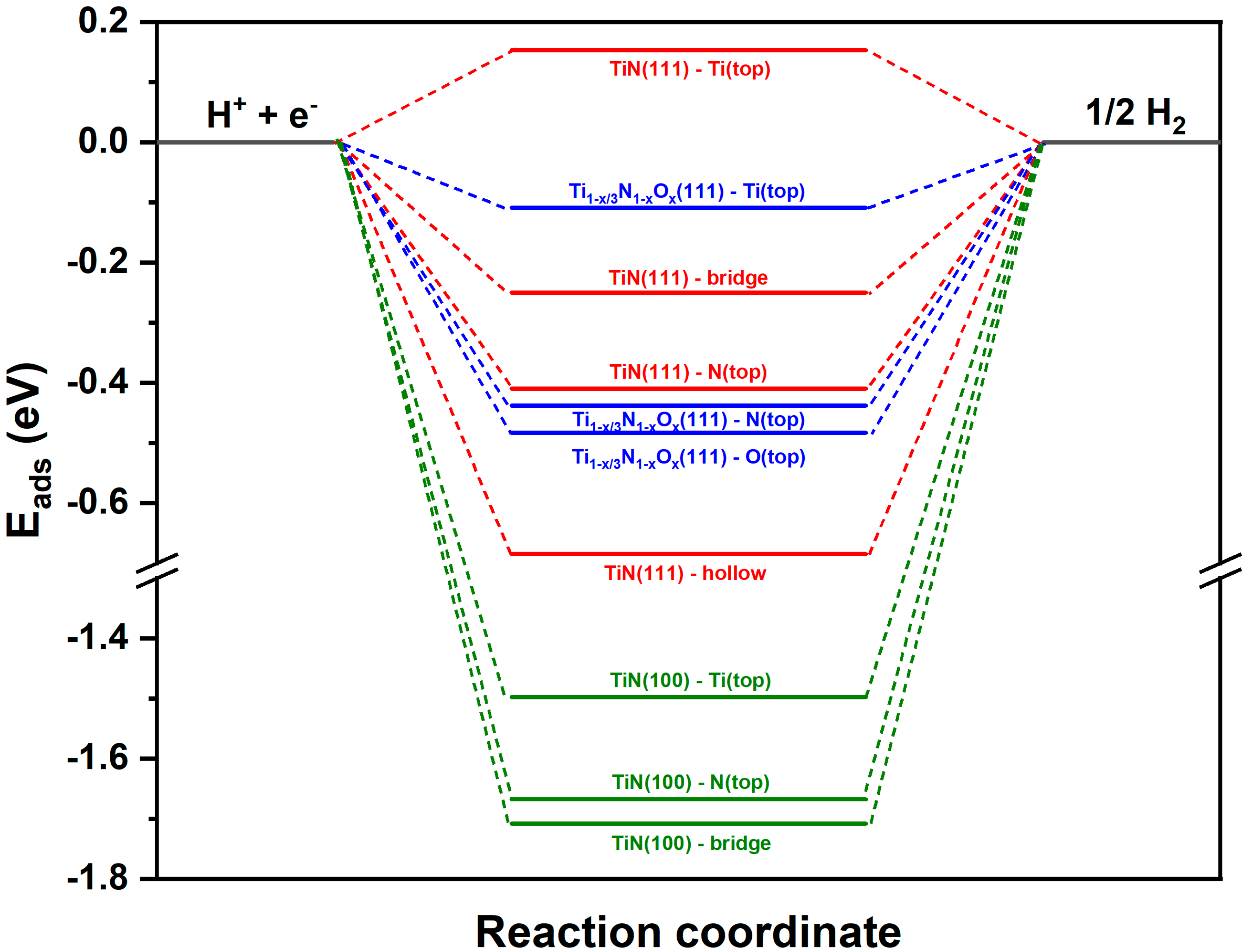
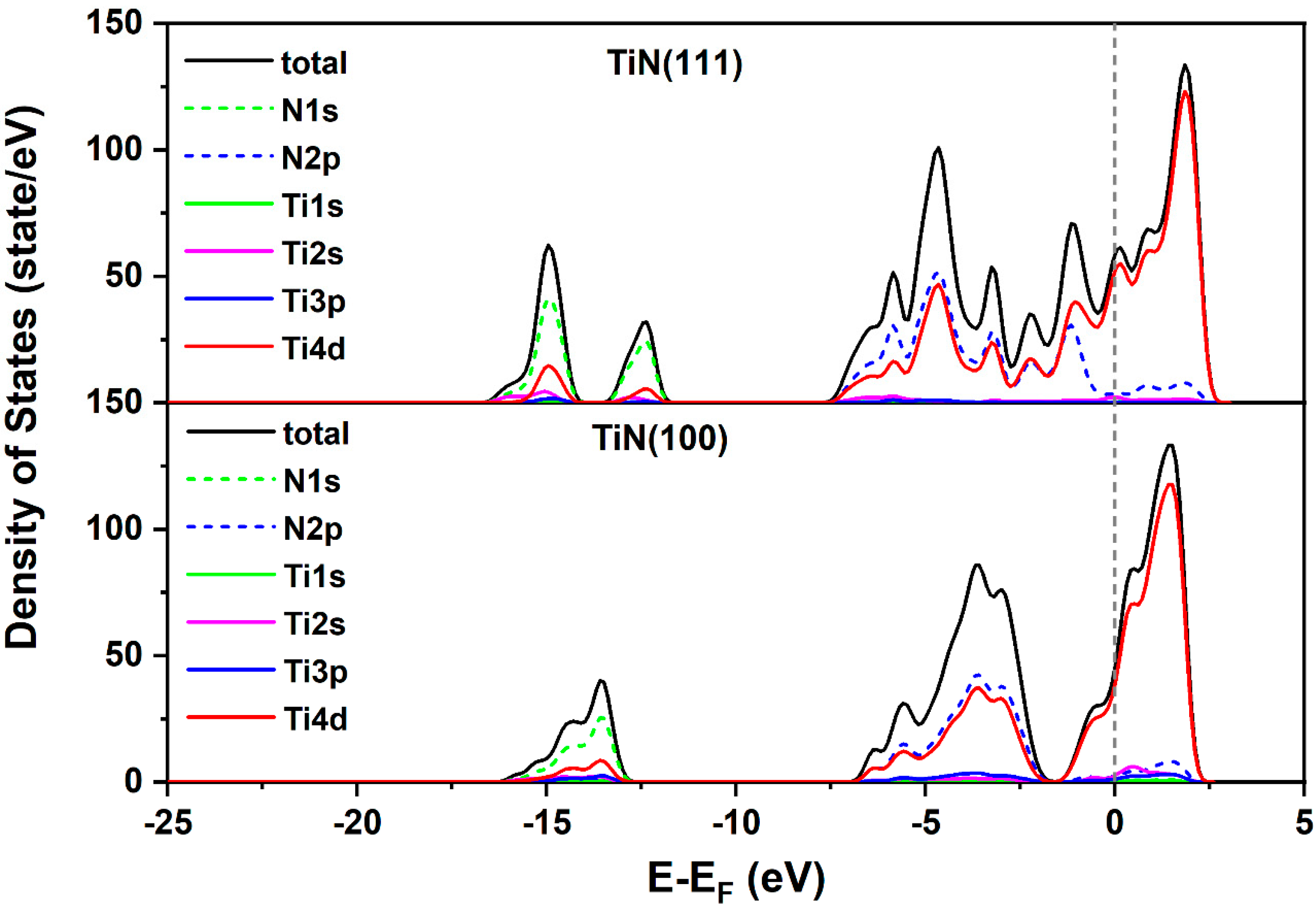
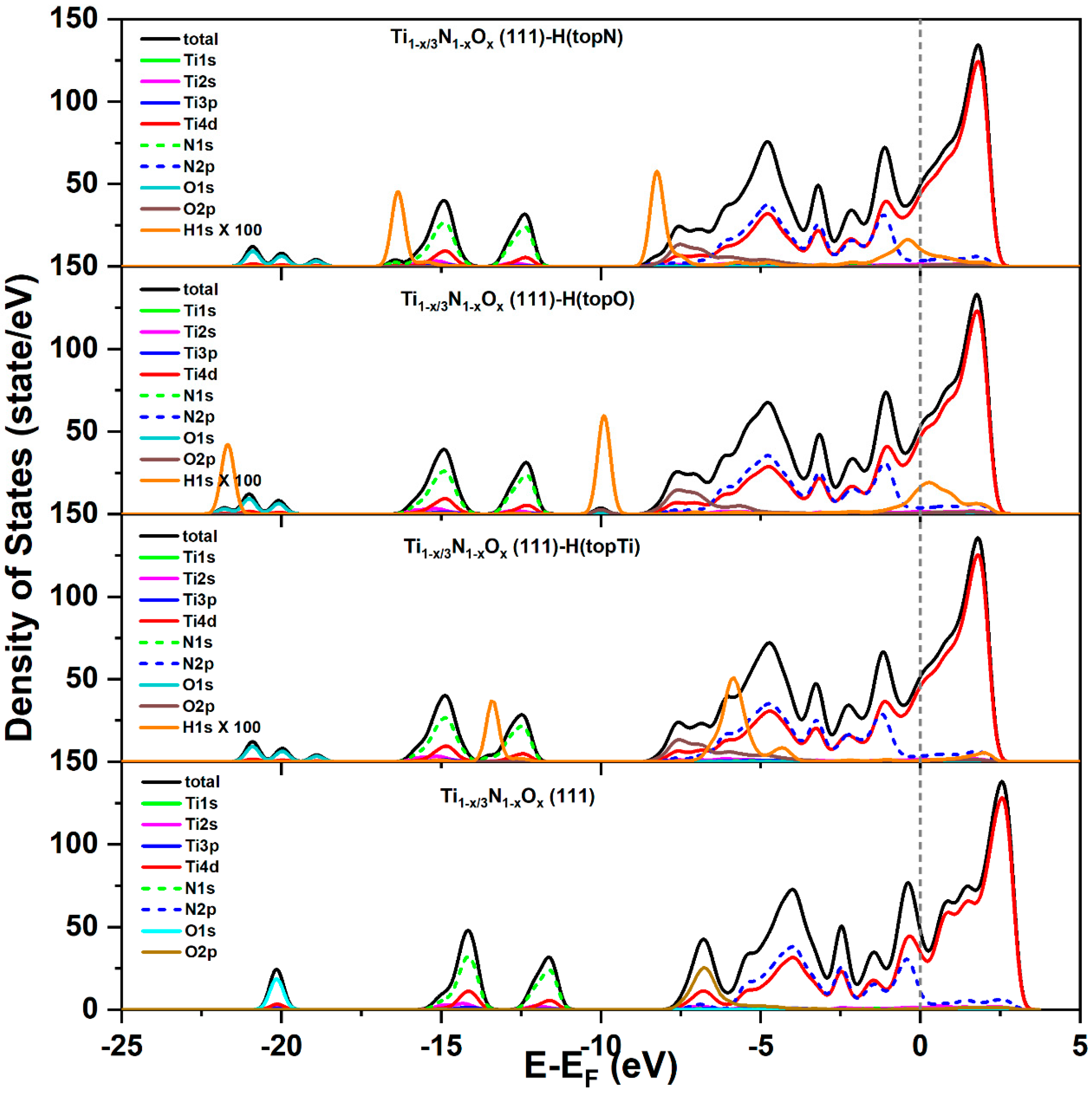
4.2. DFT Calculations
4.3. Pd-Top-Layer Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hohmeyer, O.H.; Bohm, S. Trends toward 100% renewable electricity supply in Germany and Europe: A paradigm shift in energy policies. WIREs Energy Environ. 2015, 4, 74–97. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Innovation in hydrogen production. Int. J. Hydrog. Energy 2017, 42, 14843–14864. [Google Scholar] [CrossRef]
- Fastmarkets. Available online: https://www.metalbulletin.com/lithium-prices-update (accessed on 1 June 2020).
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced electrocatalysts with unusual active sites for electrochemical water splitting. InfoMat 2023, 6, e12494. [Google Scholar] [CrossRef]
- Abdelghafar, F.; XuXiaomin, X.; Ping, X.; Ping, J. single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Conway, B.E.; Tilak, B.V. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. [Google Scholar] [CrossRef]
- Bockris, J.O.; Conway, B.E. Modern Aspects of Electrochemistry; Butterworths Scientific Publications: London, UK, 1954. [Google Scholar]
- Eley, D.D.; Pines, H.; Weisz, P.B. Advance in Catalysis; Academic Press Inc.: Bronx, NY, USA, 1992. [Google Scholar]
- Bhardwaj, M.; Balasubramaniam, R. Uncoupled non-linear equations method for determining kinetic parameters in case of hydrogen evolution reaction following Volmer–Heyrovsky–Tafel mechanism and Volmer–Heyrovsky mechanism. Int. J. Hydrog. Energy 2008, 33, 2178–2188. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Eftikhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Stratton, S.M.; Zhang, S.; Montemore, M.M. Addressing complexity in catalyst design: From volcanos and scaling to more sophisticated design strategies. Surf. Sci. Rep. 2023, 78, 100597. [Google Scholar] [CrossRef]
- Laghrissi, A.; Es-Souni, M. Au-Nanorods Supporting Pd and Pt Nanocatalysts for the Hydrogen Evolution Reaction: Pd Is Revealed to Be a Better Catalyst than Pt. Nanomaterials 2023, 13, 2007. [Google Scholar] [CrossRef]
- Zhang, N.; Shao, Q.; Xiao, X.; Huang, X. Advanced Catalysts Derived from Composition-Segregated Platinum–Nickel Nanostructures: New Opportunities and Challenges. Adv. Funct. Mater. 2019, 29, 1808161. [Google Scholar] [CrossRef]
- Hu, D.; Fan, W.; Liu, Z.; Li, L. Three-Dimensionally Hierarchical Pt/C Nanocomposite with Ultra-High Dispersion of Pt Nanoparticles as a Highly Efficient Catalyst for Chemoselective Cinnamaldehyde Hydrogenation. ChemCatChem 2018, 10, 779–788. [Google Scholar] [CrossRef]
- Sanyal, U.; Song, Y.; Singh, N.; Fulton, J.L.; Herranz, J.; Jentys, A.; Gutiérrez, O.Y.; Lercher, J.A. Structure Sensitivity in Hydrogenation Reactions on Pt/C in Aqueous-phase. ChemCatChem 2019, 11, 575–582. [Google Scholar] [CrossRef]
- Ostovari Moghaddam, A.; Trofimov, E.A. Toward expanding the realm of high entropy materials to platinum group metals: A review. J. Alloys Compd. 2021, 851, 156838. [Google Scholar] [CrossRef]
- McKone, J.R.; Warren, E.L.; Bierman, M.J.; Boettcher, S.W.; Brunschwig, B.S.; Lewis, N.S.; Gray, H.B. Evaluation of Pt, Ni, and Ni–Mo electrocatalysts for hydrogen evolution on crystalline Si electrodes. Energy Environ. Sci. 2011, 4, 3573–3583. [Google Scholar] [CrossRef]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 Nanosheets as Catalysts for Hydrogen Evolution Reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef]
- Laghrissi, A.; Es-Souni, M. A TiN@Au-NR Plasmonic Structure with Tunable Surface Plasmon Resonance Depending on TiN to Au Thickness Ratio. Plasmonics 2021, 16, 49–57. [Google Scholar] [CrossRef]
- Santecchia, E.; Hamouda, A.M.S.; Musharavati, F.; Zalnezhad, E.; Cabibbo, M.; Spigarelli, S. Wear resistance investigation of titanium nitride-based coatings. Ceram. Int. 2015, 41, 10349–10379. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Liu, X.; Li, D.-S. Development of the applications of titanium nitride in fuel cells. Mater. Today Chem. 2019, 11, 42–59. [Google Scholar] [CrossRef]
- van Hove, R.P.; Sierevelt, I.N.; van Royen, B.J.; Nolte, P.A. Titanium-Nitride Coating of Orthopaedic Implants: A Review of the Literature. BioMed Res. Int. 2015, 2015, 485975. [Google Scholar] [CrossRef]
- Ahangarani, S.; Sabour Rouhaghadam, A.; Azadi, M. A Review on Titanium Nitride and Titanium Carbide Single and Multilayer Coatings Deposited by Plasma Assisted Chemical Vapor Deposition. Int. J. Eng. 2016, 29, 677–687. [Google Scholar]
- Liu, L.M.; Wang, S.Q.; Ye, H.Q. First-principles study of the effect of hydrogen on the metal–ceramic interface. J. Phys. Condens. Matter 2005, 17, 5335–5348. [Google Scholar] [CrossRef]
- Siodmiak, M.; Govind, N.; Andzelm, J.; Tanpipat, N.; Frenking, G.; Korkin, A. Theoretical Study of Hydrogen Adsorption and Diffusion on TiN(100) Surface. Phys. Status Solidi B 2001, 226, 29–36. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baseggio, O.; Bonfà, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; de Gironcoli, S.; Delugas, P.; Ruffino, F.F.; et al. Quantum ESPRESSO toward the exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Roy, M.; Sarkar, K.; Som, J.; Pfeifer, M.A.; Craciun, V.; Schall, J.D.; Aravamudhan, S.; Wise, F.W.; Kumar, D. Modulation of Structural, Electronic, and Optical Properties of Titanium Nitride Thin Films by Regulated In Situ Oxidation. ACS Appl. Mater. Interfaces 2023, 15, 4733–4742. [Google Scholar] [CrossRef]
- Ahmed, M.; Xinxin, G. A review of metal oxynitrides for photocatalysis. Inorg. Chem. Front. 2016, 3, 578–590. [Google Scholar] [CrossRef]
- Graciani, J.; Hamad, S.; Sanz, J.F. Changing the physical and chemical properties of titanium oxynitrides TiN1−xOx by changing the composition. Phys. Rev. B 2009, 80, 184112. [Google Scholar] [CrossRef]
- Venkataraj, S.; Severin, D.; Mohamed, S.H.; Ngaruiya, J.; Kappertz, O.; Wuttig, M. Towards understanding the superior properties of transition metal oxynitrides prepared by reactive DC magnetron sputtering. Thin Solid Film. 2006, 502, 228–234. [Google Scholar] [CrossRef]
- Amin, M.A.; Fadlallah, S.A.; Alosaimi, G.S. In situ aqueous synthesis of silver nanoparticles supported on titanium as active electrocatalyst for the hydrogen evolution reaction. Int. J. Hydrog. Energy 2014, 39, 19519–19540. [Google Scholar] [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H.A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef]
- Han, Y.; Xin Yue, X.; Jin, Y.; Huang, X.; Shen, P.K. Hydrogen evolution reaction in acidic media on single-crystalline titanium nitride nanowires as an efficient non-noble metal electrocatalyst. J. Mater. Chem. A 2016, 4, 3673–3677. [Google Scholar] [CrossRef]
- El-Khodary, S.A.; Cui, Y.; Bu, Y.; Lian, J. Electric double-layer capacitors. Electrochem. Capacit. 2023, 1, 1–60. [Google Scholar]
- Kumar, R.; Nayak, S.; Garbrecht, M.; Bhatia, V.; Pillai, A.I.K.; Gupta, M.; Shivaprasad, S.M.; Saha, B. Clustering of oxygen point defects in transition metal nitrides. J. Appl. Phys. 2021, 129, 055305. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, C.; Wu, Y.; Yu, H.; Zhang, S.; Du, A.; Ostrikov, K.K.; Zheng, J.; Li, X. Direct conversion of metal organic frameworks into ultrafine phosphide nanocomposites in multicomponent plasma for wide pH hydrogen evolution. J. Mater. Chem. A 2020, 8, 10402. [Google Scholar] [CrossRef]
- Exner, K.S. On the optimum binding energy for the hydrogen evolution reaction: How do experiments contribute? Electrochem. Sci. Adv. 2022, 2, e2100101. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhou, X.; Nie, H.; Xu, X.; Zheng, X.; Hou, J.; Duan, H.; Huang, S.; Yang, Z. Designing Pd/O co-doped MoSx for boosting the hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 15599. [Google Scholar] [CrossRef]
- Bennett, R.A.; Stone, P.; Bowker, M. Pd nanoparticle enhanced re-oxidation of non-stoichiometric TiO2: STM imaging of spillover and a new form of SMSI. Catal. Lett. 1999, 59, 99–105. [Google Scholar] [CrossRef]
- Chenakin, S.P.; Melaet, G.; Szukiewicz, R.; Kruse, N. XPS study of the surface chemical state of a Pd/(SiO2 + TiO2) catalyst after methane oxidation and SO2 treatment. J. Catal. 2014, 312, 1–11. [Google Scholar] [CrossRef]


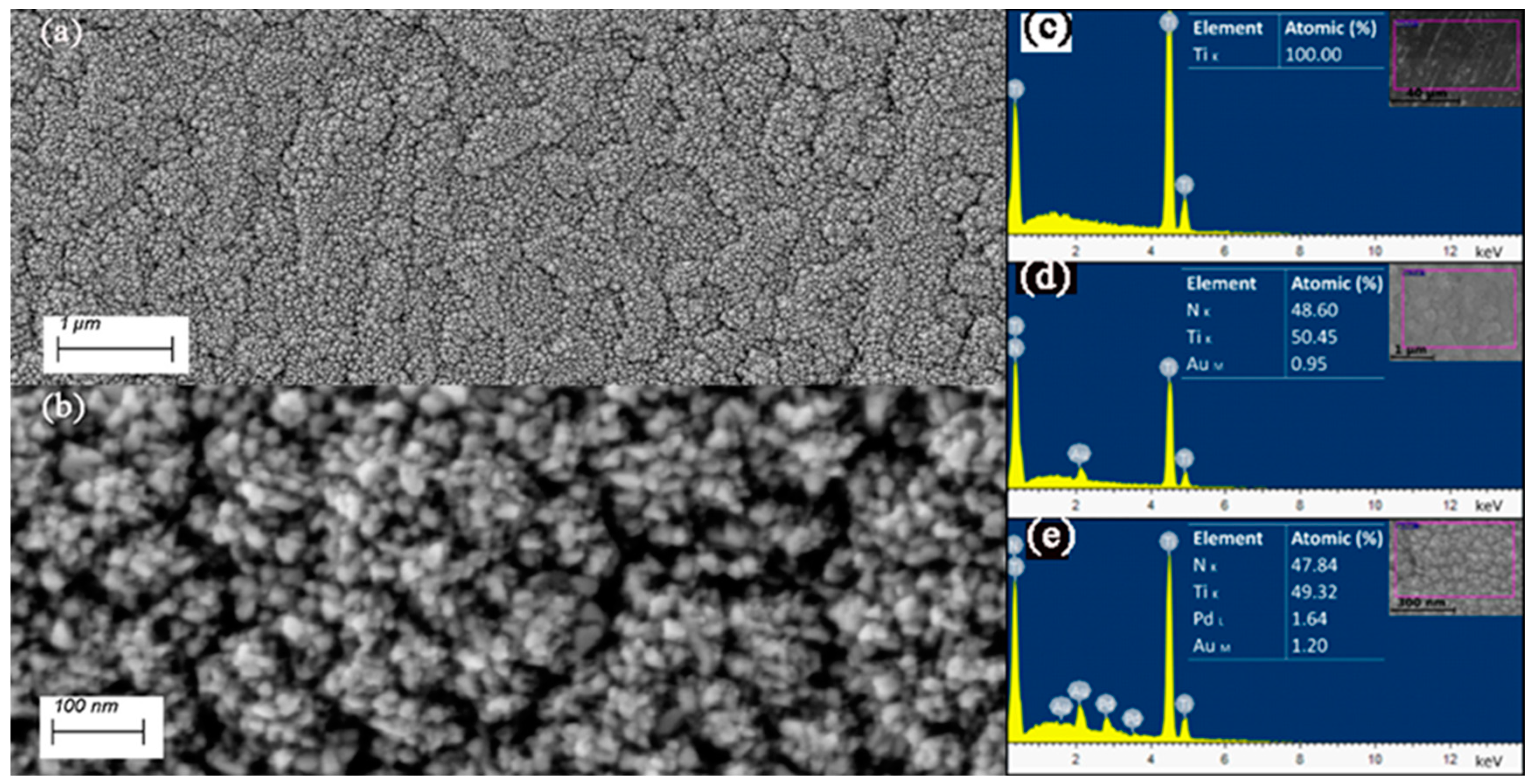
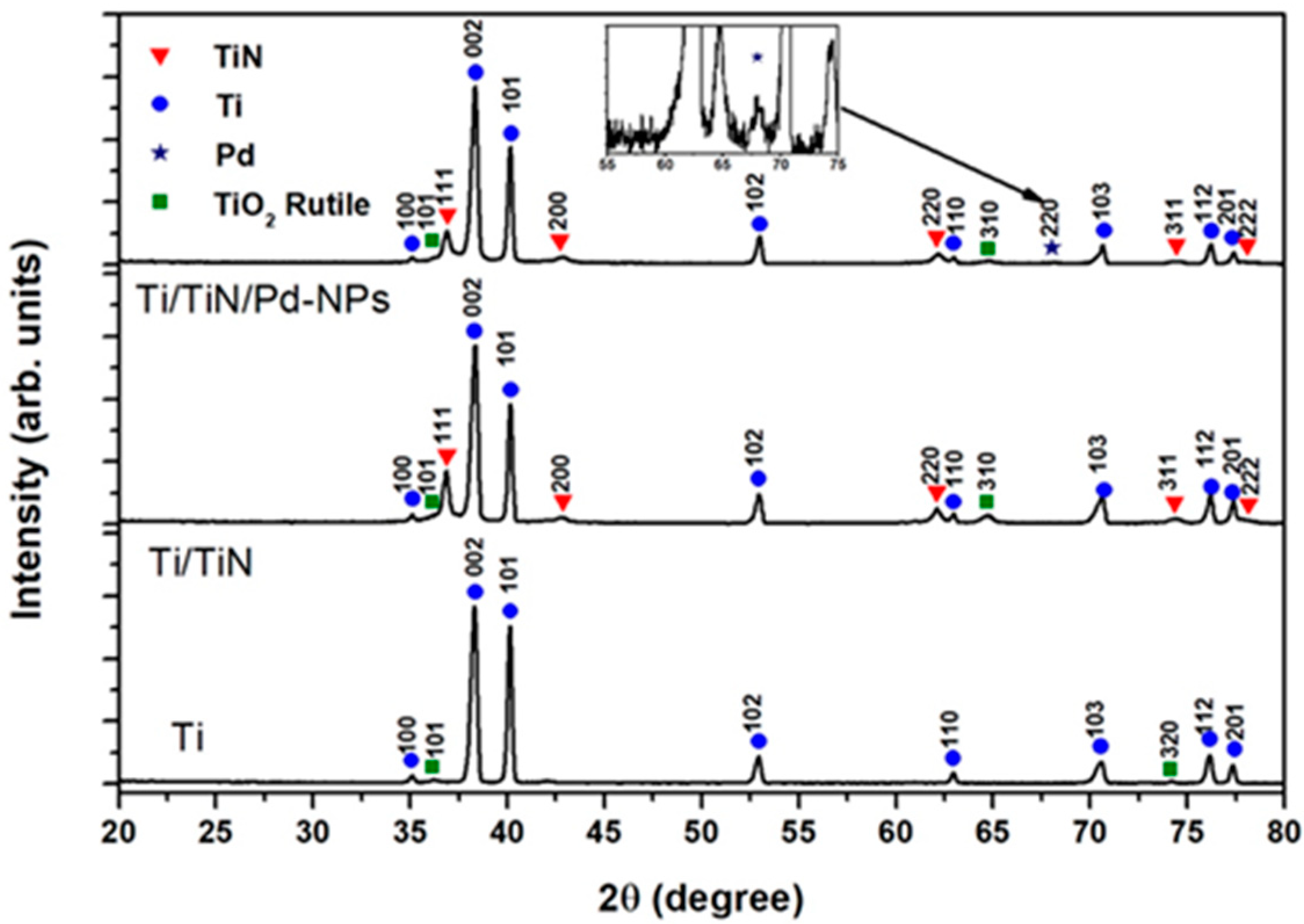
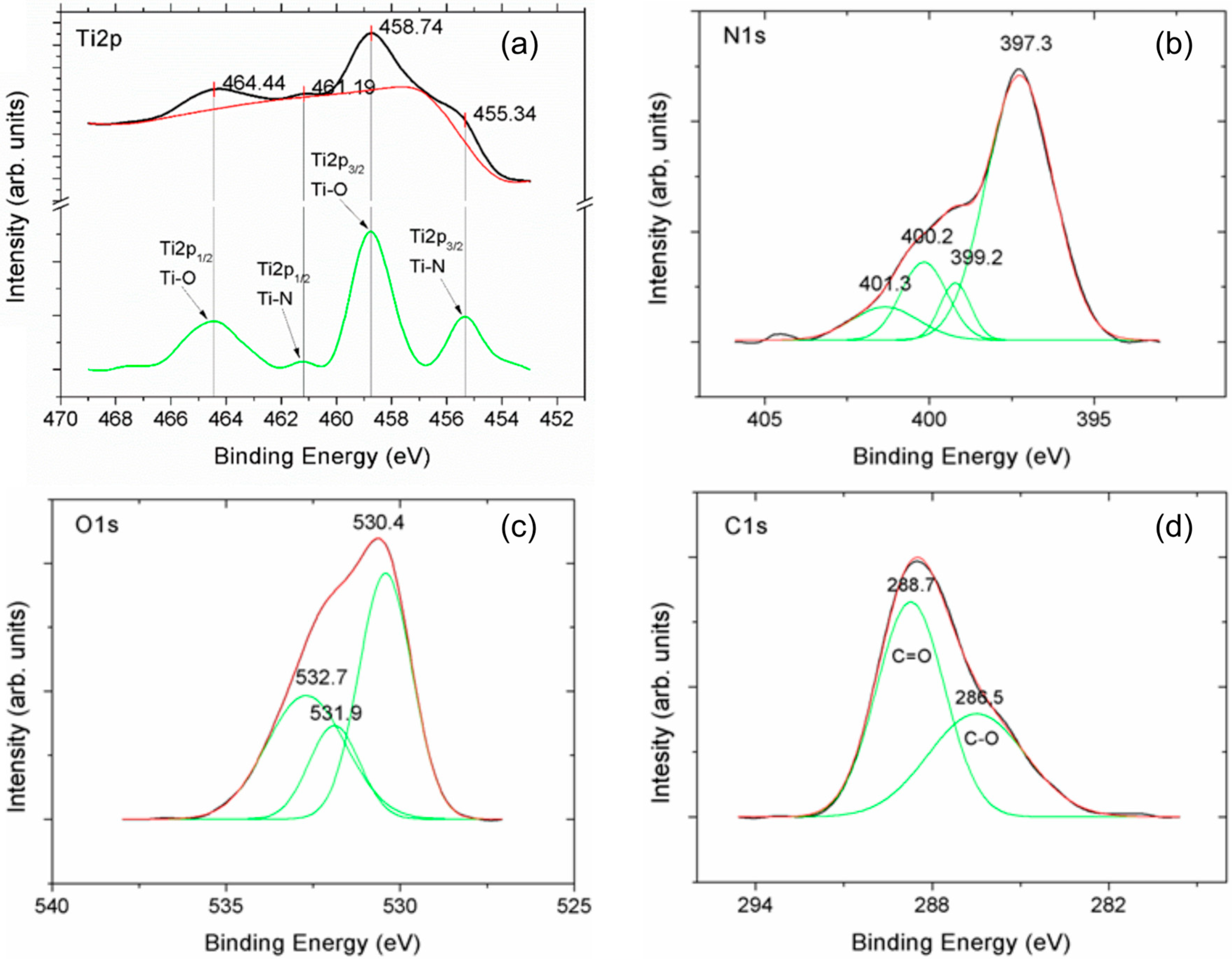

| Peak | Binding Energy (eV) | Assignment |
|---|---|---|
| N1s | 397.3 | TiN |
| 399.2 | Ti-O-N | |
| 400.2; 401.3 | N-Ox; adsorbed N2 | |
| 530.4 | TiO2 | |
| O1s | 531.9 | Defective oxide TiOx |
| 532.7 | Hydroxyl |
| Overvoltage (V) | Rs (Ohm) | Rct (Ohm) | ||
|---|---|---|---|---|
| Ti/TiN | Ti/TiNO-PdNPs | Ti/TiN | Ti/TiNO-PdNPs | |
| −0.4 | 3.36 | 3.45 | 1.121 | 0.52 |
| −0.3 | 3.48 | 3.32 | 1.845 | 0.76 |
| −0.2 | 3.51 | 3.33 | 5.477 | 1.16 |
| −0.1 | 3.52 | 3.35 | 38.97 | 2.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laghrissi, A.; Es-Souni, M. Oxygen Defects Containing TiN Films for the Hydrogen Evolution Reaction: A Robust Thin-Film Electrocatalyst with Outstanding Performance. Nanomaterials 2024, 14, 770. https://doi.org/10.3390/nano14090770
Laghrissi A, Es-Souni M. Oxygen Defects Containing TiN Films for the Hydrogen Evolution Reaction: A Robust Thin-Film Electrocatalyst with Outstanding Performance. Nanomaterials. 2024; 14(9):770. https://doi.org/10.3390/nano14090770
Chicago/Turabian StyleLaghrissi, Ayoub, and Mohammed Es-Souni. 2024. "Oxygen Defects Containing TiN Films for the Hydrogen Evolution Reaction: A Robust Thin-Film Electrocatalyst with Outstanding Performance" Nanomaterials 14, no. 9: 770. https://doi.org/10.3390/nano14090770
APA StyleLaghrissi, A., & Es-Souni, M. (2024). Oxygen Defects Containing TiN Films for the Hydrogen Evolution Reaction: A Robust Thin-Film Electrocatalyst with Outstanding Performance. Nanomaterials, 14(9), 770. https://doi.org/10.3390/nano14090770






