CoFeBP Micro Flowers (MFs) for Highly Efficient Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts
Abstract
1. Introduction
2. Electrode Fabrication and Structural Analysis
2.1. Precursor and Reaction Parameter Optimizations for CoFeBP MF
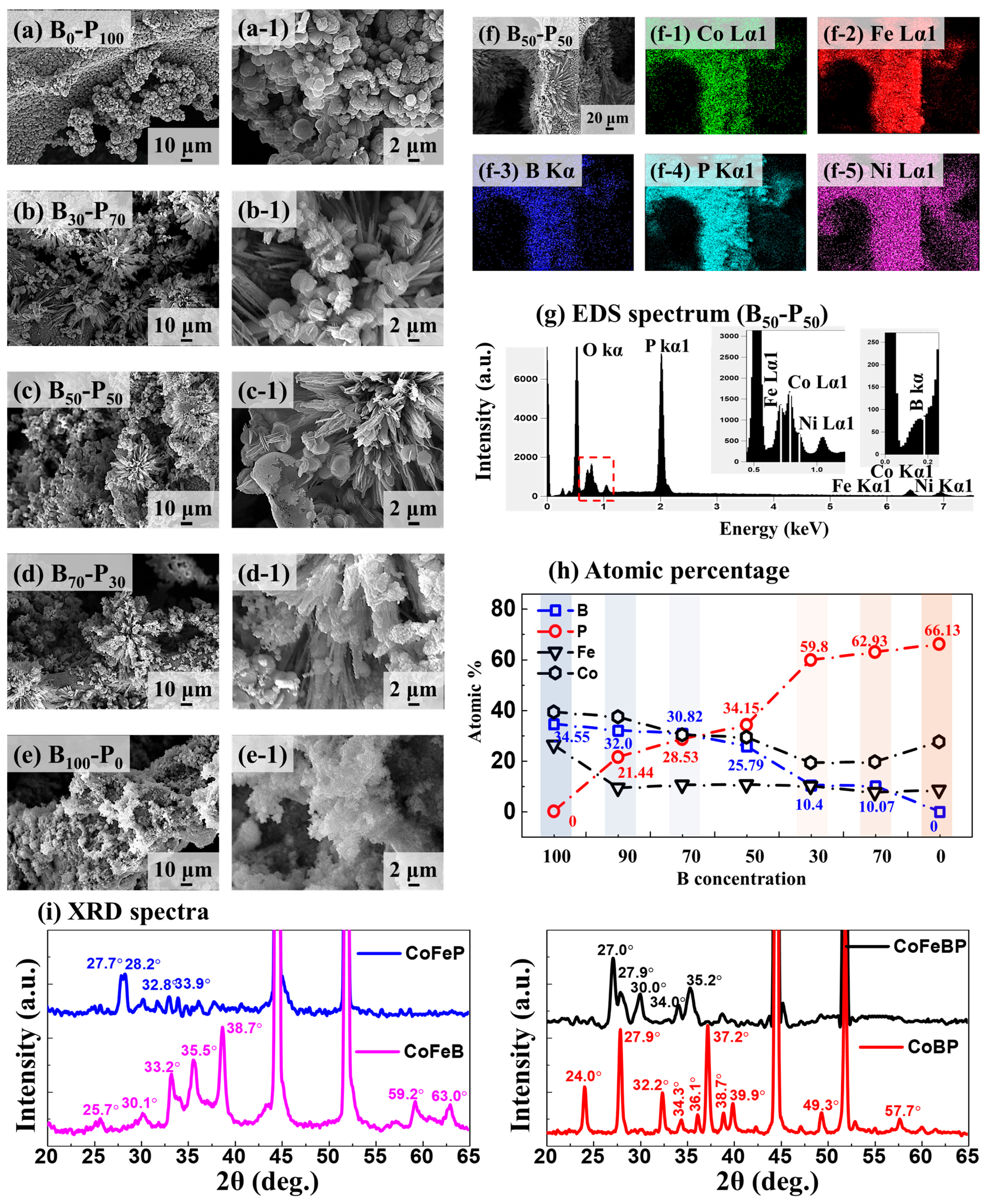
2.2. B-P Concentration Optimization for CoFeBP MF
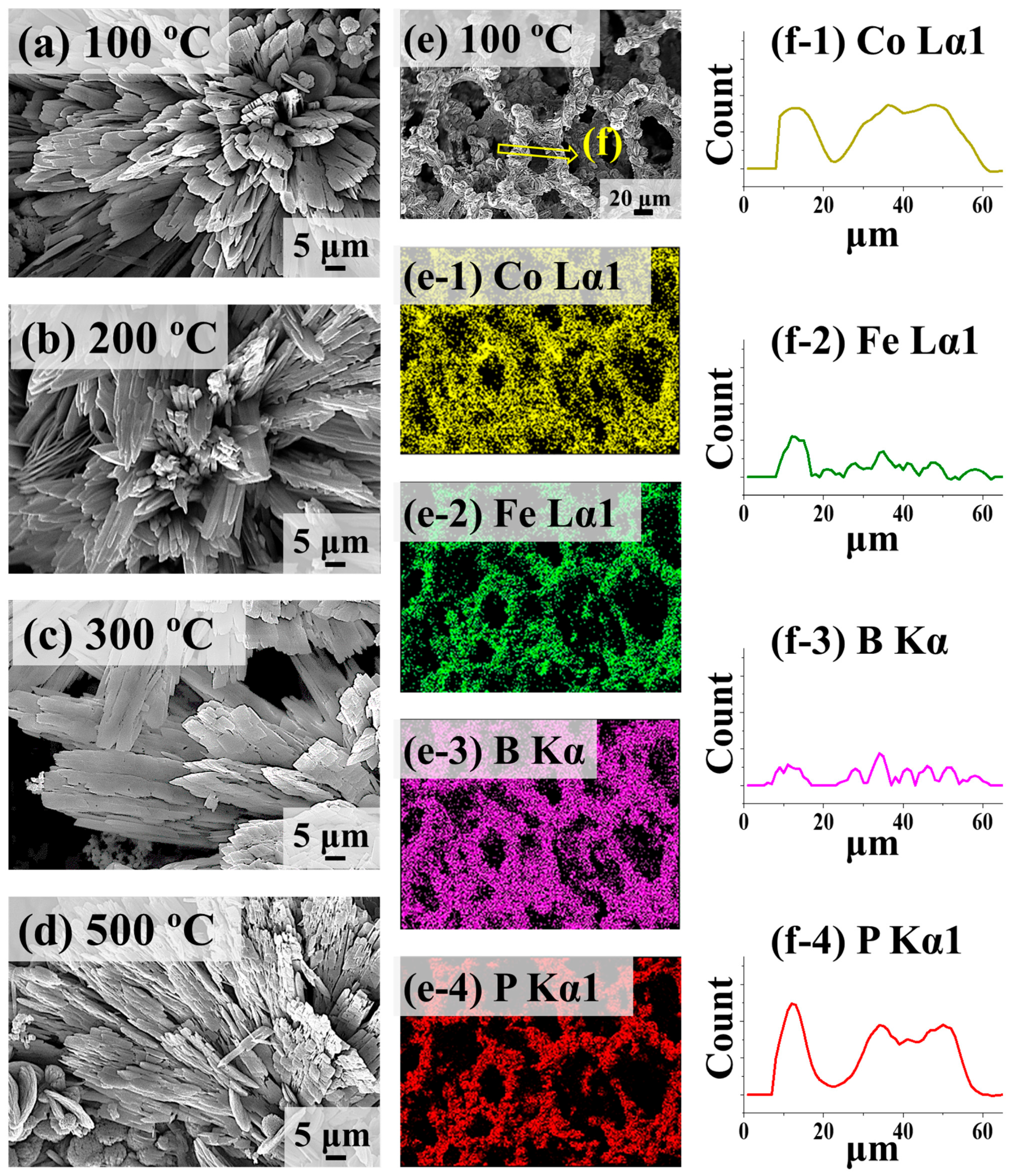
2.3. Structural Analysis of CoFeBP MF along with Post-Annealing Optimization
3. Electrochemical Analysis
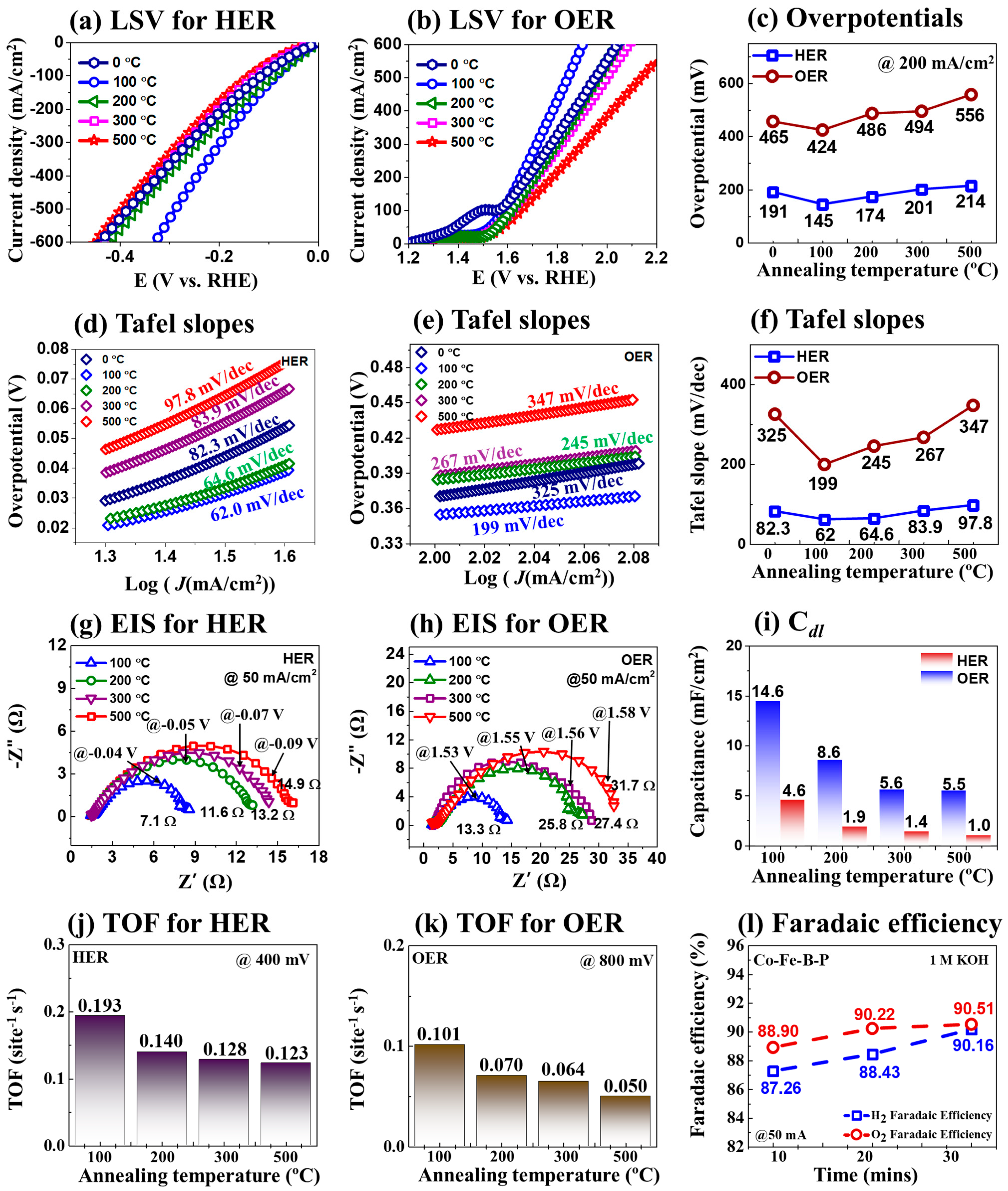
3.1. The 3-E Electrochemical Properties of CoFeBP MFs
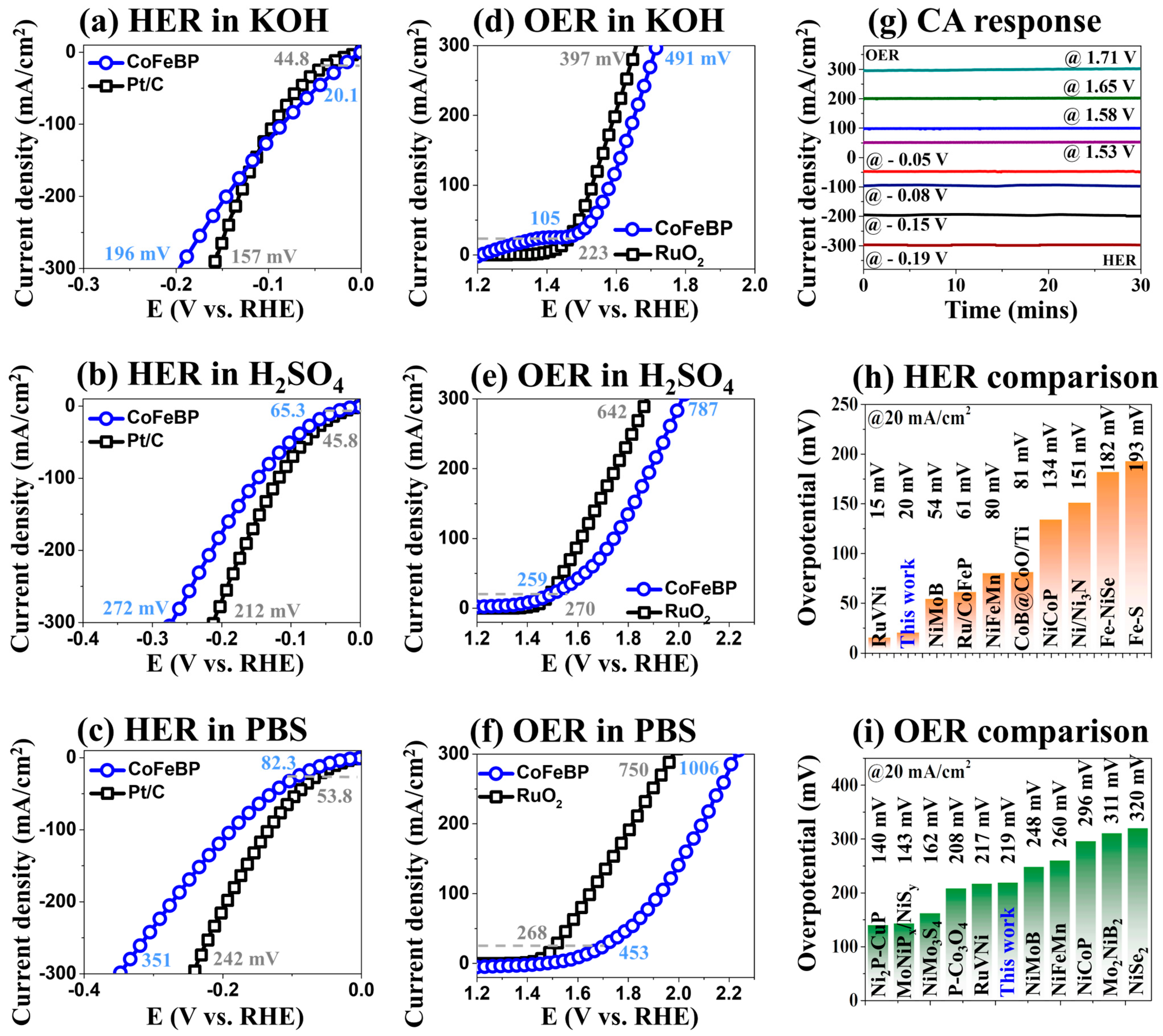
3.2. The 3-E LSV Activity of CoFeBP MFs in Different pH Media
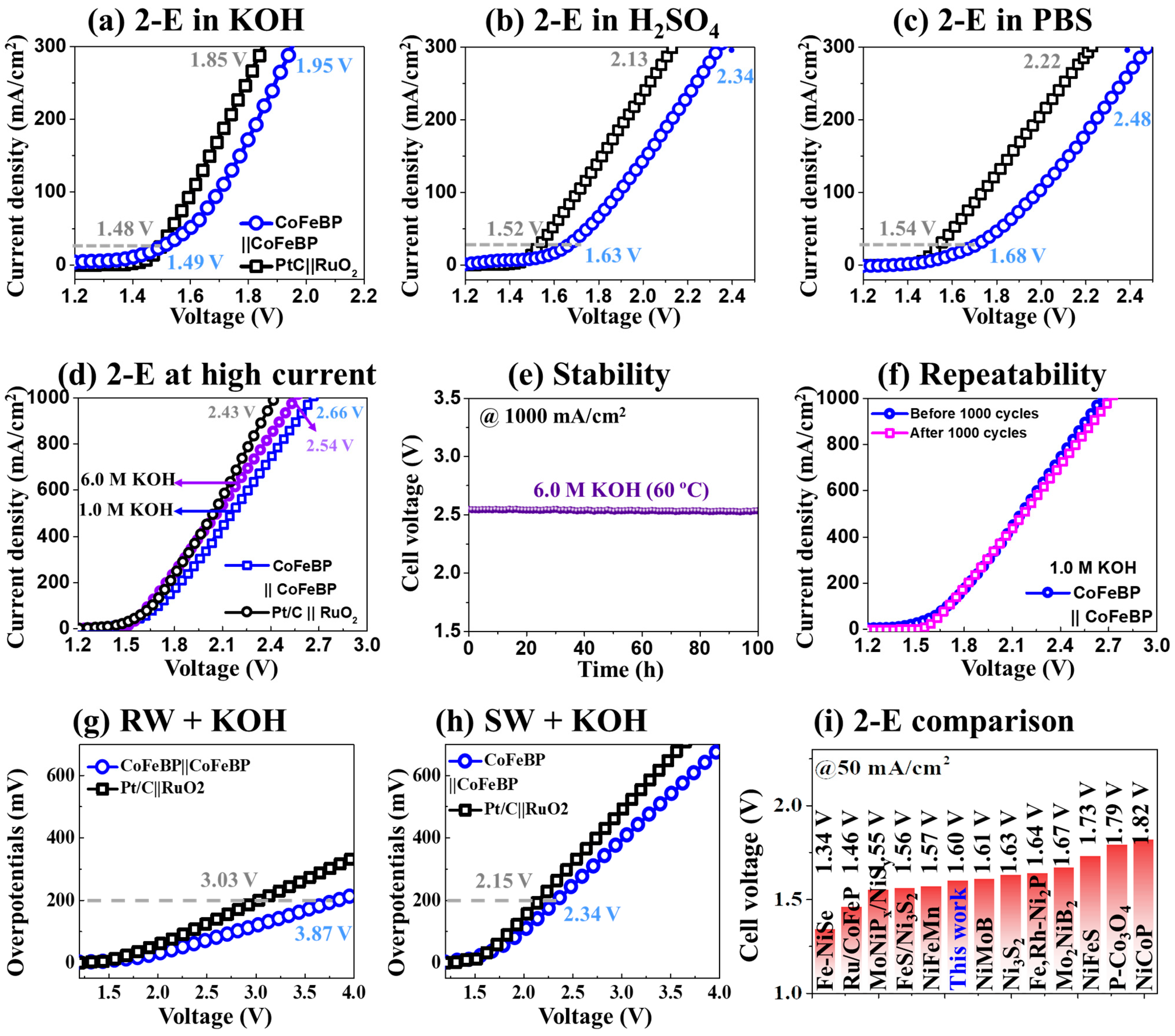
3.3. The 2-E Activity of CoFeBP MFs
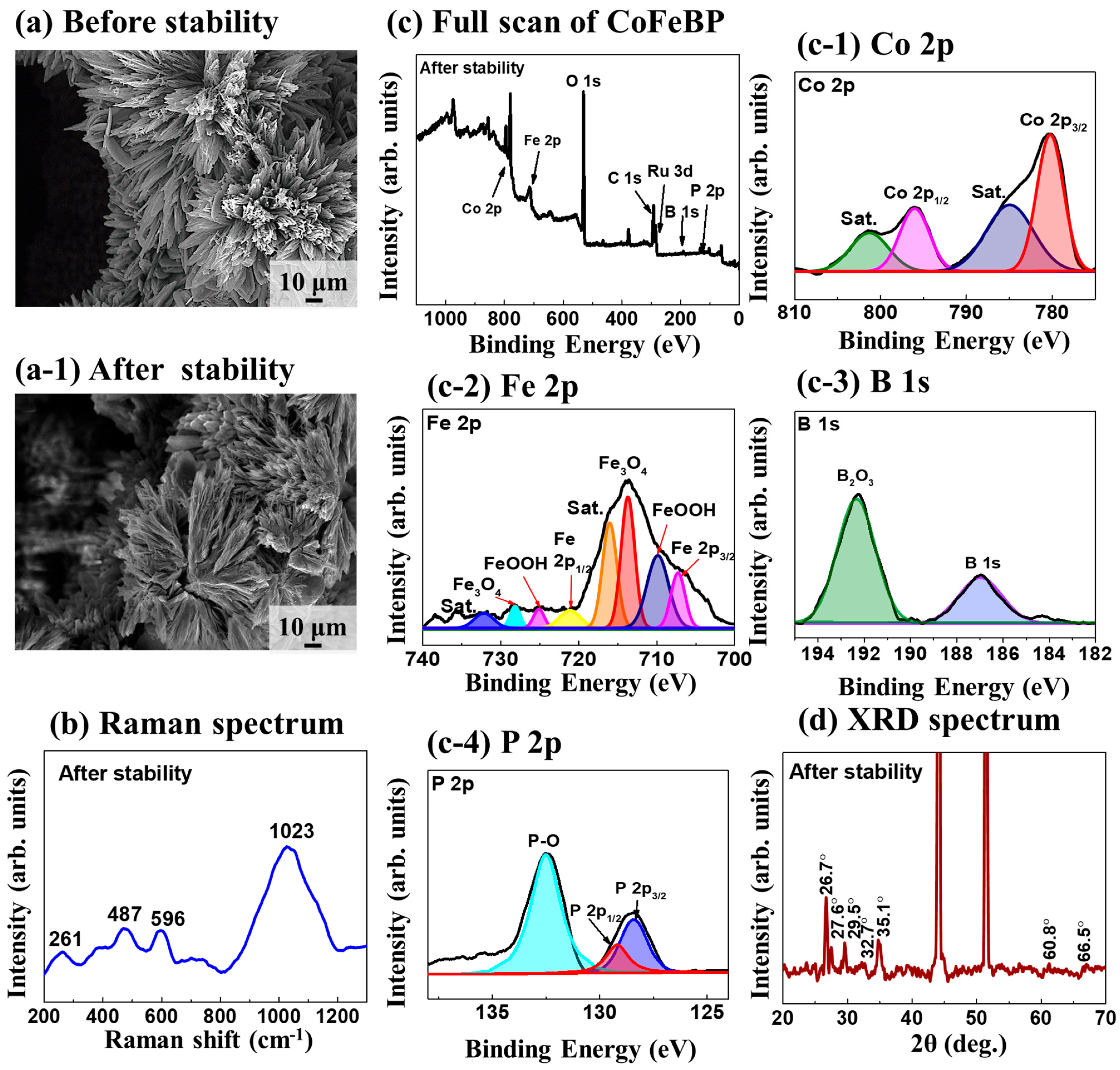
3.4. Characterization after Stability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Zhang, Z.; Chhowalla, M.; Liu, B. Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting. Adv. Mater. 2022, 34, 2108133. [Google Scholar] [CrossRef]
- Saad, A.; Gao, Y.; Owusu, K.A.; Liu, W.; Wu, Y.; Ramiere, A.; Guo, H.; Tsiakaras, P.; Cai, X. Ternary Mo2NiB2 as a Superior Bifunctional Electrocatalyst for Overall Water Splitting. Small 2022, 18, 2104303. [Google Scholar] [CrossRef] [PubMed]
- Riyajuddin, S.; Azmi, K.; Pahuja, M.; Kumar, S.; Maruyama, T.; Bera, C.; Ghosh, K. Super-Hydrophilic Hierarchical Ni-Foam-Graphene-Carbon Nanotubes-Ni2P–CuP2 Nano-Architecture as Efficient Electrocatalyst for Overall Water Splitting. ACS Nano 2021, 15, 5586–5599. [Google Scholar] [CrossRef]
- Ifkovits, Z.P.; Evans, J.M.; Meier, M.C.; Papadantonakis, K.M.; Lewis, N.S. Decoupled Electrochemical Water-Splitting Systems: A Review and Perspective. Energy Environ. Sci. 2021, 14, 4740–4759. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen Society: From Present to Future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Yue, Q.; Sun, J.; Chen, S.; Zhou, Y.; Li, H.; Chen, Y.; Zhang, R.; Wei, G.; Kang, Y. Hierarchical Mesoporous MXene-NiCoP Electrocatalyst for Water-Splitting. ACS Appl. Mater. Interfaces 2020, 12, 18570–18577. [Google Scholar] [CrossRef]
- Debata, S.; Patra, S.; Banerjee, S.; Madhuri, R.; Sharma, P.K. Controlled Hydrothermal Synthesis of Graphene Supported NiCo2O4 Coral-like Nanostructures: An Efficient Electrocatalyst for Overall Water Splitting. Appl. Surf. Sci. 2018, 449, 203–212. [Google Scholar] [CrossRef]
- Kumaravel, S.; Karthick, K.; Sam Sankar, S.; Karmakar, A.; Madhu, R.; Bera, K.; Kundu, S. Recent Progresses in Engineering of Ni and Co Based Phosphides for Effective Electrocatalytic Water Splitting. ChemElectroChem 2021, 8, 4638–4685. [Google Scholar] [CrossRef]
- Habib, M.A.; Mandavkar, R.; Lin, S.; Burse, S.; Khalid, T.; Joni, M.H.; Jeong, J.-H.; Lee, J. Ni-BP Micro Spheres for Superior Water Splitting OER Electrocatalyst Satisfying Industrial Operational Requirement. Chem. Eng. J. 2023, 462, 142177. [Google Scholar]
- Battiato, S.; Bruno, L.; Pellegrino, A.L.; Terrasi, A.; Mirabella, S. Optimized Electroless Deposition of NiCoP Electrocalysts for Enhanced Water Splitting. Catal. Today 2023, 423, 113929. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced Electrocatalysts with Unusual Active Sites for Electrochemical Water Splitting. InfoMat 2024, 6, e12494. [Google Scholar] [CrossRef]
- Loni, E.; Shokuhfar, A.; Siadati, M.H. Cobalt-Based Electrocatalysts for Water Splitting: An Overview. Catal. Surv. Asia 2021, 25, 114–147. [Google Scholar] [CrossRef]
- Hu, E.; Feng, Y.; Nai, J.; Zhao, D.; Hu, Y.; Lou, X.W. Construction of Hierarchical Ni-Co-P Hollow Nanobricks with Oriented Nanosheets for Efficient Overall Water Splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Burse, S.; Kulkarni, R.; Mandavkar, R.; Habib, M.A.; Lin, S.; Chung, Y.-U.; Jeong, J.-H.; Lee, J. Vanadium-Doped FeBP Microsphere Croissant for Significantly Enhanced Bi-Functional HER and OER Electrocatalyst. Nanomaterials 2022, 12, 3283. [Google Scholar] [CrossRef] [PubMed]
- Barati Darband, G.; Aliofkhazraei, M.; Rouhaghdam, A.S. Facile Electrodeposition of Ternary Ni-Fe-Co Alloy Nanostructure as a Binder Free, Cost-Effective and Durable Electrocatalyst for High-Performance Overall Water Splitting. J. Colloid Interface Sci. 2019, 547, 407–420. [Google Scholar] [CrossRef]
- Meena, A.; Thangavel, P.; Jeong, D.S.; Singh, A.N.; Jana, A.; Im, H.; Nguyen, D.A.; Kim, K.S. Crystalline-Amorphous Interface of Mesoporous Ni2P@FePOxHy for Oxygen Evolution at High Current Density in Alkaline-Anion-Exchange-Membrane Water-Electrolyzer. Appl. Catal. B Environ. 2022, 306, 121127. [Google Scholar] [CrossRef]
- Mohili, R.; Hemanth, N.R.; Jin, H.; Lee, K.; Chaudhari, N. Emerging High Entropy Metal Sulphides and Phosphides for Electrochemical Water Splitting. J. Mater. Chem. A 2023, 11, 10463–10472. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Ge, R.; Ren, X.; Ren, J.; Yang, D.; Zhang, L.; Sun, X. Phosphorus-Doped Co3O4 Nanowire Array: A Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Catal. 2018, 8, 2236–2241. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Ge, L.; Peng, C.; Zhu, L.; Zou, W.; Chen, J.; Wu, Q.; Zhang, Y.; Huang, H.; et al. Accelerating Hydrogen Evolution in Ru-Doped FeCoP Nanoarrays with Lattice Distortion toward Highly Efficient Overall Water Splitting. Catal. Sci. Technol. 2020, 10, 8314–8324. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Y.; He, H.; Zhang, P. Novel Electrocatalyst of Nickel Sulfide Boron Coating for Hydrogen Evolution Reaction in Alkaline Solution. Appl. Surf. Sci. 2019, 480, 689–696. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.-L.; Huang, Y.-C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-Doped Nitrogen-Deficient Carbon Nitride-Based Z-Scheme Heterostructures for Photocatalytic Overall Water Splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Habib, M.A.; Mandavkar, R.; Burse, S.; Lin, S.; Kulkarni, R.; Patil, C.S.; Jeong, J.-H.; Lee, J. Design of Boron-Based Ternary W3CoB3 Electrocatalyst for the Improved HER and OER Performances. Mater. Today Energy 2022, 26, 101021. [Google Scholar] [CrossRef]
- Nyarige, J.S.; Krüger, T.P.J.; Diale, M. Influence of Precursor Concentration and Deposition Temperature on the Photoactivity of Hematite Electrodes for Water Splitting. Mater. Today Commun. 2020, 25, 101459. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Zhang, W.; Guo, S. Electronic-Structure Tuning of Water-Splitting Nanocatalysts. Trends Chem. 2019, 1, 259–271. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, Y.; Wang, G.; Yan, K.; Zhao, J. Effect of NH4F Concentration and Controlled-Charge Consumption on the Photocatalytic Hydrogen Generation of TiO2 Nanotube Arrays. Electrochim. Acta 2015, 155, 312–320. [Google Scholar] [CrossRef]
- Patil, S.J.; Chodankar, N.R.; Hwang, S.-K.; Rama Raju, G.S.; Huh, Y.-S.; Han, Y.-K. Fluorine Engineered Self-Supported Ultrathin 2D Nickel Hydroxide Nanosheets as Highly Robust and Stable Bifunctional Electrocatalysts for Oxygen Evolution and Urea Oxidation Reactions. Small 2022, 18, 2103326. [Google Scholar] [CrossRef]
- Oliveira, F.G.S.; Santos, L.P.M.; da Silva, R.B.; Correa, M.A.; Bohn, F.; Correia, A.N.; Vieira, L.; Vasconcelos, I.F.; de Lima-Neto, P. FexNi(1−x) Coatings Electrodeposited from Choline Chloride-Urea Mixture: Magnetic and Electrocatalytic Properties for Water Electrolysis. Mater. Chem. Phys. 2022, 279, 125738. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Mu, Z.; Wang, Y.; Ali, U.; Jing, S.; Xing, S. Urea-Assisted Enhanced Electrocatalytic Activity of MoS2-Ni3S2for Overall Water Splitting. Inorg. Chem. Front. 2020, 7, 3588–3597. [Google Scholar] [CrossRef]
- Xu, T.; Yang, L.; Li, J.; Usoltseva, N.; An, V.; Jin, X.; Zhang, C.; Zhang, X.; Liu, B. NH4F-Induced Morphology Control of CoP Nanostructures to Enhance the Hydrogen Evolution Reaction. Inorg. Chem. 2021, 60, 10781–10790. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y. Designing Transition-Metal-Boride-Based Electrocatalysts for Applications in Electrochemical Water Splitting. Nanoscale 2020, 12, 9327–9351. [Google Scholar] [CrossRef]
- Chen, L.; Ren, J.-T.; Yuan, Z.-Y. Design Strategies of Phosphorus-Containing Catalysts for Photocatalytic, Photoelectrochemical and Electrocatalytic Water Splitting. Green Chem. 2022, 24, 713–747. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, W.; Zuo, P.; Kang, L.; Yin, G. A Novel Spherical Boron Phosphide as a High-Efficiency Overall Water Splitting Catalyst: A Density Functional Theory Study. Catal. Lett. 2020, 150, 544–554. [Google Scholar] [CrossRef]
- Wang, H.; Zou, H.; Liu, Y.; Liu, Z.; Sun, W.; Lin, K.A.; Li, T.; Luo, S. Ni2P Nanocrystals Embedded Ni-MOF Nanosheets Supported on Nickel Foam as Bifunctional Electrocatalyst for Urea Electrolysis. Sci. Rep. 2021, 11, 21414. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, F.; Faid, A.Y.; Sunde, S.; Pollet, B.G. Sonoactivated Polycrystalline Ni Electrodes for Alkaline Oxygen Evolution Reaction. Ultrason. Sonochem. 2022, 86, 106013. [Google Scholar] [CrossRef]
- Lin, S.; Habib, M.A.; Mandavkar, R.; Kulkarni, R.; Burse, S.; Chung, Y.-U.; Liu, C.; Wang, Z.; Lin, S.; Jeong, J.-H.; et al. Higher Water-Splitting Performance of Boron-Based Porous CoMnB Electrocatalyst over the Benchmarks at High Current in 1 m KOH and Real Sea Water. Adv. Sustain. Syst. 2022, 6, 2200213. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, R.; Dong, C.; Liu, H.; Yang, J.; Du, X. Polycrystalline CoO–Co9S8 Heterostructure Nanoneedle Arrays as Bifunctional Catalysts for Efficient Overall Water Splitting. ChemElectroChem 2022, 9, e202101566. [Google Scholar] [CrossRef]
- Guo, C.; Shi, Y.; Lu, S.; Yu, Y.; Zhang, B. Amorphous Nanomaterials in Electrocatalytic Water Splitting. Chin. J. Catal. 2021, 42, 1287–1296. [Google Scholar] [CrossRef]
- Xu, Y.; Tu, W.; Zhang, B.; Yin, S.; Huang, Y.; Kraft, M.; Xu, R. Nickel Nanoparticles Encapsulated in Few-Layer Nitrogen-Doped Graphene Derived from Metal–Organic Frameworks as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mater. 2017, 29, 1605957. [Google Scholar] [CrossRef]
- Makimizu, Y.; Yoo, J.; Poornajar, M.; Nguyen, N.T.; Ahn, H.-J.; Hwang, I.; Kment, S.; Schmuki, P. Effects of Low Oxygen Annealing on the Photoelectrochemical Water Splitting Properties of α-Fe2O3. J. Mater. Chem. A 2020, 8, 1315–1325. [Google Scholar] [CrossRef]
- Mandavkar, R.; Habib, M.A.; Lin, S.; Kulkarni, R.; Burse, S.; Jeong, J.-H.; Lee, J. Electron Enriched Ternary NiMoB Electrocatalyst for Improved Overall Water Splitting: Better Performance as Compared to the Pt/C || RuO2 at High Current Density. Appl. Mater. Today 2022, 29, 101579. [Google Scholar] [CrossRef]
- Diaz-Morales, O.; Ferrus-Suspedra, D.; Koper, M.T.M. The Importance of Nickel Oxyhydroxide Deprotonation on Its Activity towards Electrochemical Water Oxidation. Chem. Sci. 2016, 7, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, B.; Wu, Y.; Ruan, Q.; Liu, L.; Su, J.; Tang, Y.; Liu, R.; Chu, P.K. Experimental and Theoretical Investigation of Reconstruction and Active Phases on Honeycombed Ni3N-Co3N/C in Water Splitting. Appl. Catal. B Environ. 2021, 297, 120461. [Google Scholar] [CrossRef]
- Guo, M.; Meng, H.; Jin, J.; Mi, J. Amine-Assisted Synthesis of the Ni3Fe Alloy Encapsulated in Nitrogen-Doped Carbon for High-Performance Water Splitting. J. Mater. Chem. A 2023, 11, 6452–6464. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Waltham, MA, USA, 1992; Volume 40, p. 221. [Google Scholar]
- Guo, T.; Chen, L.; Li, Y.; Shen, K. Controllable Synthesis of Ultrathin Defect-Rich LDH Nanoarrays Coupled with MOF-Derived Co-NC Microarrays for Efficient Overall Water Splitting. Small 2022, 18, 2107739. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Li, C.-F.; Zhao, J.-W.; Xie, L.-J.; Wu, J.-Q.; Li, G.-R. Fe Doping and Oxygen Vacancy Modulated Fe-Ni5P4/NiFeOH Nanosheets as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. Appl. Catal. B Environ. 2021, 291, 119987. [Google Scholar] [CrossRef]
- Teng, W.; Huo, M.; Sun, Z.; Yang, W.; Zheng, X.; Ding, C.; Zhang, S. FeCoNi Sulfides Derived from in Situ Sulfurization of Precursor Oxides as Oxygen Evolution Reaction Catalyst. Front. Chem. 2020, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Yan, P.; Sun, B.; Elshekh, H.; Yan, B. An Excellent Soft Magnetic Fe/Fe3O4-FeSiAl Composite with High Permeability and Low Core Loss. Results Phys. 2019, 14, 102498. [Google Scholar] [CrossRef]
- Li, X.; Xiao, L.; Zhou, L.; Xu, Q.; Weng, J.; Xu, J.; Liu, B. Adaptive Bifunctional Electrocatalyst of Amorphous CoFe Oxide @ 2D Black Phosphorus for Overall Water Splitting. Angew. Chem. 2020, 132, 21292–21299. [Google Scholar] [CrossRef]
- Hao, W.; Yao, D.; Xu, Q.; Wang, R.; Zhang, C.; Guo, Y.; Sun, R.; Huang, M.; Chen, Z. Highly Efficient Overall-Water Splitting Enabled via Grafting Boron-Inserted Fe-Ni Solid Solution Nanosheets onto Unconventional Skeleton. Appl. Catal. B Environ. 2021, 292, 120188. [Google Scholar] [CrossRef]
- Wei, Y.; Zou, P.; Yue, Y.; Wang, M.; Fu, W.; Si, S.; Wei, L.; Zhao, X.; Hu, G.; Xin, H.L. One-Pot Synthesis of B/P-Codoped Co-Mo Dual-Nanowafer Electrocatalysts for Overall Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 20024–20033. [Google Scholar] [CrossRef] [PubMed]
- Anantharaj, S.; Noda, S.; Driess, M.; Menezes, P.W. The Pitfalls of Using Potentiodynamic Polarization Curves for Tafel Analysis in Electrocatalytic Water Splitting. ACS Energy Lett. 2021, 6, 1607–1611. [Google Scholar] [CrossRef]
- Peng, Y.; Mak, C.H.; Kai, J.-J.; Du, M.; Ji, L.; Yuan, M.; Zou, X.; Shen, H.-H.; Santoso, S.P.; Colmenares, J.C.; et al. Recent Progress on Post-Synthetic Treatments of Photoelectrodes for Photoelectrochemical Water Splitting. J. Mater. Chem. A 2021, 9, 26628–26649. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.; Gu, Y.; Xu, H.; Wang, C.; Shao, Z.; Guo, Y. Tuning Synergy between Nickel and Iron in Ruddlesden–Popper Perovskites through Controllable Crystal Dimensionalities towards Enhanced Oxygen-evolving Activity and Stability. Carbon Energy 2024, e465. [Google Scholar] [CrossRef]
- Al-Naggar, A.H.; Shinde, N.M.; Kim, J.-S.; Mane, R.S. Water Splitting Performance of Metal and Non-Metal-Doped Transition Metal Oxide Electrocatalysts. Coord. Chem. Rev. 2023, 474, 214864. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, S.; Ma, Y.; Qiu, Y.; An, Y.; Cheng, L. Interfacial Engineering of Cobalt Nitrides and Mesoporous Nitrogen-Doped Carbon: Toward Efficient Overall Water-Splitting Activity with Enhanced Charge-Transfer Efficiency. ACS Energy Lett. 2020, 5, 692–700. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent Progress Made in the Mechanism Comprehension and Design of Electrocatalysts for Alkaline Water Splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Shalom, M.; Ressnig, D.; Yang, X.; Clavel, G.; Fellinger, T.P.; Antonietti, M. Nickel Nitride as an Efficient Electrocatalyst for Water Splitting. J. Mater. Chem. A 2015, 3, 8171–8177. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, H.; Li, T.; Liang, J.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Wu, Q.; Sun, X. Iron-Based Phosphides as Electrocatalysts for the Hydrogen Evolution Reaction: Recent Advances and Future Prospects. J. Mater. Chem. A 2020, 8, 19729–19745. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, M.K.; Miotello, A.; Patel, N. Metal Boride-Based Catalysts for Electrochemical Water-Splitting: A Review. Adv. Funct. Mater. 2020, 30, 1906481. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kunwar, S.; Pandit, S.; Lee, J. CoP2Nanoparticles Deposited on Nanometer-Thick Pt-Coated Fluorine-Doped Tin Oxide Substrates as Electrocatalysts for Simultaneous Hydrogen Evolution and Oxygen Evolution. ACS Appl. Nano Mater. 2020, 3, 6507–6515. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S. Do the Evaluation Parameters Reflect Intrinsic Activity of Electrocatalysts in Electrochemical Water Splitting? ACS Energy Lett. 2019, 4, 1260–1264. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen Production from Water Electrolysis: Role of Catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, Z.; Jiao, L. Development Strategies in Transition Metal Borides for Electrochemical Water Splitting. Energy Environ. Mater. 2022, 5, 470–485. [Google Scholar] [CrossRef]
- Bahadur, A.; Hussain, W.; Iqbal, S.; Ullah, F.; Shoaib, M.; Liu, G.; Feng, K. A Morphology Controlled Surface Sulfurized CoMn2O4 Microspike Electrocatalyst for Water Splitting with Excellent OER Rate for Binder-Free Electrocatalytic Oxygen Evolution. J. Mater. Chem. A 2021, 9, 12255–12264. [Google Scholar] [CrossRef]
- Friebel, D.; Bajdich, M.; Yeo, B.S.; Louie, M.W.; Miller, D.J.; Sanchez Casalongue, H.; Mbuga, F.; Weng, T.-C.; Nordlund, D.; Sokaras, D.; et al. On the Chemical State of Co Oxide Electrocatalysts during Alkaline Water Splitting. Phys. Chem. Chem. Phys. 2013, 15, 17460–17467. [Google Scholar] [CrossRef] [PubMed]
- Behl, W.K.; Toni, J.E. Anodic Oxidation of Cobalt in Potassium Hydroxide Electrolytes. J. Electroanal. Chem. Interfacial Electrochem. 1971, 31, 63–75. [Google Scholar] [CrossRef]
- Shit, S.; Bolar, S.; Murmu, N.C.; Kuila, T. An Account of the Strategies to Enhance the Water Splitting Efficiency of Noble-Metal-Free Electrocatalysts. J. Energy Chem. 2021, 59, 160–190. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Xu, X.; Zhang, C. Advances in Noble Metal (Ru, Rh, and Ir) Doping for Boosting Water Splitting Electrocatalysis. J. Mater. Chem. A 2021, 9, 13459–13470. [Google Scholar] [CrossRef]
- Hausmann, J.N.; Menezes, P.W. Why Should Transition Metal Chalcogenides Be Investigated as Water Splitting Precatalysts Even Though They Transform into (Oxyhydr)Oxides? Curr. Opin. Electrochem. 2022, 34, 100991. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Han, C.; Lu, Q.; Xing, Z.; Yang, X. Atomic and Electronic Modulation of Self-Supported Nickel-Vanadium Layered Double Hydroxide to Accelerate Water Splitting Kinetics. Nat. Commun. 2019, 10, 3899. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Li, C.; Pham, B.T.; Zhang, D. Electrodeposition of Ni–Fe–Mn Ternary Nanosheets as Affordable and Efficient Electrocatalyst for Both Hydrogen and Oxygen Evolution Reactions. Int. J. Hydrogen Energy 2020, 45, 24670–24683. [Google Scholar] [CrossRef]
- Lu, W.; Liu, T.; Xie, L.; Tang, C.; Liu, D.; Hao, S.; Qu, F.; Du, G.; Ma, Y.; Asiri, A.M.; et al. In Situ Derived Co—B Nanoarray: A High-Efficiency and Durable 3D Bifunctional Electrocatalyst for Overall Alkaline Water Splitting. Small 2017, 13, 1700805. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, J.; Chen, Y.; Wei, M.; Liu, X.; Li, X.; Wu, Q.; Feng, B.; Zhang, Y.; Yang, L. Regulation of the Morphology and Electrochemical Properties of Ni0.85Se via Fe Doping for Overall Water Splitting and Supercapacitors. CrystEngComm 2022, 24, 1704–1718. [Google Scholar] [CrossRef]
- Shit, S.; Bolar, S.; Murmu, N.C.; Kuila, T. Binder-Free Growth of Nickel-Doped Iron Sulfide on Nickel Foam via Electrochemical Deposition for Electrocatalytic Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 18015–18026. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Yang, G.; Song, W.; Zhong, W.; Wang, X.; Wang, M.; Sun, T.; Tang, Y. Heterogeneous Bimetallic Mo-NiPx/NiSy as a Highly Efficient Electrocatalyst for Robust Overall Water Splitting. Adv. Funct. Mater. 2021, 31, 2101532. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y.; Huang, S.; Von Lim, Y.; Wang, M.; Xu, T.; Zang, J.; Li, X.; Yang, H.Y. Defect-Engineered 3D Hierarchical NiMo3S4 Nanoflowers as Bifunctional Electrocatalyst for Overall Water Splitting. J. Colloid Interface Sci. 2022, 607, 1876–1887. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Perovskite for Electrocatalytic Oxygen Evolution at Elevated Temperatures. ChemSusChem 2024, e202301534. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Wei, W.; Zhang, M.; Jiang, Z.; Yan, Z.; Xie, J. Chrysanthemum-like FeS/Ni3S2 Heterostructure Nanoarray as a Robust Bifunctional Electrocatalyst for Overall Water Splitting. J. Colloid Interface Sci. 2022, 608, 536–548. [Google Scholar] [CrossRef]
- Ren, G.; Hao, Q.; Mao, J.; Liang, L.; Liu, H.; Liu, C.; Zhang, J. Ultrafast Fabrication of Nickel Sulfide Film on Ni Foam for Efficient Overall Water Splitting. Nanoscale 2018, 10, 17347–17353. [Google Scholar] [CrossRef]
- Chen, M.-T.; Duan, J.-J.; Feng, J.-J.; Mei, L.-P.; Jiao, Y.; Zhang, L.; Wang, A.-J. Iron, Rhodium-Codoped Ni2P Nanosheets Arrays Supported on Nickel Foam as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. J. Colloid Interface Sci. 2022, 605, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Yuan, M.; Hao, H.; San, X.; Lv, Z.; Xu, L.; Wei, B. Operando Capturing of Surface Self-Reconstruction of Ni3S2/FeNi2S4 Hybrid Nanosheet Array for Overall Water Splitting. Chem. Eng. J. 2022, 427, 131944. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, M.; Yuan, J.; Luo, J.; Zhang, J.; Lu, Z.; Chen, D.; Fu, X.; Wang, L.; Liu, C. Oxygen Vacancies and Interface Engineering on Amorphous/Crystalline CrOx-Ni3N Heterostructures toward High-Durability and Kinetically Accelerated Water Splitting. Small 2022, 18, 2106554. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Amorphous Catalysts and Electrochemical Water Splitting: An Untold Story of Harmony. Small 2020, 16, 1905779. [Google Scholar] [CrossRef]
- Browne, M.P.; Vasconcelos, J.M.; Coelho, J.; O’Brien, M.; Rovetta, A.A.; McCarthy, E.K.; Nolan, H.; Duesberg, G.S.; Nicolosi, V.; Colavita, P.E.; et al. Improving the Performance of Porous Nickel Foam for Water Oxidation Using Hydrothermally Prepared Ni and Fe Metal Oxides. Sustain. Energy Fuels 2017, 1, 207–216. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sam Sankar, S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and Correctness in the Evaluation of Electrocatalytic Water Splitting: Revisiting Activity Parameters with a Critical Assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Feng, R.; Zhu, Q.; Chu, M.; Jia, S.; Zhai, J.; Wu, H.; Wu, P.; Han, B. Electrodeposited Cu–Pd Bimetallic Catalysts for the Selective Electroreduction of CO2 to Ethylene. Green Chem. 2020, 22, 7560–7565. [Google Scholar] [CrossRef]
- Han, A.; Chen, H.; Sun, Z.; Xu, J.; Du, P. High Catalytic Activity for Water Oxidation Based on Nanostructured Nickel Phosphide Precursors. Chem. Commun. 2015, 51, 11626–11629. [Google Scholar] [CrossRef]
- Wang, G.; Hua, C.; Chen, W.; Fan, H.; Feng, P.; Zhu, Y. Intriguing 3D Micro-Flower Structure of Co1.11Te2 Deposited on Te Nanosheets Showing an Efficient Bifunctional Electrocatalytic Property for Overall Water Splitting. Electrochim. Acta 2023, 447, 142133. [Google Scholar] [CrossRef]
- Sun, H.; Meng, J.; Jiao, L.; Cheng, F.; Chen, J. A Review of Transition-Metal Boride/Phosphide-Based Materials for Catalytic Hydrogen Generation from Hydrolysis of Boron-Hydrides. Inorg. Chem. Front. 2018, 5, 760–772. [Google Scholar] [CrossRef]
- Yang, G.; Jiao, Y.; Yan, H.; Xie, Y.; Wu, A.; Dong, X.; Guo, D.; Tian, C.; Fu, H. Interfacial Engineering of MoO2-FeP Heterojunction for Highly Efficient Hydrogen Evolution Coupled with Biomass Electrooxidation. Adv. Mater. 2020, 32, 2000455. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, X.; Zhang, D.; Wang, Y.; Wang, J.; Pi, M.; Zhou, H.; Li, J.; Chen, S. Porous Mn-Doped CoP3 Nanowires as a Janus Electrocatalyst for Efficient Overall Water Splitting in Alkaline Solution. J. Electrochem. Soc. 2018, 165, F1323. [Google Scholar] [CrossRef]
- Yu, X.; Yu, Z.-Y.; Zhang, X.-L.; Li, P.; Sun, B.; Gao, X.; Yan, K.; Liu, H.; Duan, Y.; Gao, M.-R.; et al. Highly Disordered Cobalt Oxide Nanostructure Induced by Sulfur Incorporation for Efficient Overall Water Splitting. Nano Energy 2020, 71, 104652. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Qian, J.; Li, Y.; Shen, K. Intensified Kirkendall Effect Assisted Construction of Double-Shell Hollow Cu-Doped CoP Nanoparticles Anchored by Carbon Arrays for Water Splitting. Appl. Catal. B Environ. 2023, 325, 122295. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Han, C.; Lu, R.; Yang, S.; Song, X. Electrospun Hollow Cage-like α-Fe2O3 Microspheres: Synthesis, Formation Mechanism, and Morphology-Preserved Conversion to Fe Nanostructures. CrystEngComm 2014, 16, 10618–10623. [Google Scholar] [CrossRef]
- Zhang, H.; Lee, J.S. Hybrid Microwave Annealing Synthesizes Highly Crystalline Nanostructures for (Photo)Electrocatalytic Water Splitting. Acc. Chem. Res. 2019, 52, 3132–3142. [Google Scholar] [CrossRef]
- Kim, T.; Roy, S.B.; Moon, S.; Yoo, S.; Choi, H.; Parale, V.G.; Kim, Y.; Lee, J.; Jun, S.C.; Kang, K. Highly Dispersed Pt Clusters on F-Doped Tin (IV) Oxide Aerogel Matrix: An Ultra-Robust Hybrid Catalyst for Enhanced Hydrogen Evolution. ACS Nano 2022, 16, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Gong, S.; Chen, Y.; Xie, R.; Wu, Q.; Tao, J.; Meng, F.; Zhao, P. A Novel Non-Enzymatic Electrochemical Biosensor Based on the Nanohybrid of Bimetallic PdCu Nanoparticles/Carbon Black for Highly Sensitive Detection of H2O2 Released from Living Cells. Sens. Actuators B Chem. 2019, 290, 249–257. [Google Scholar] [CrossRef]
- Sultan, S.; Ha, M.; Kim, D.Y.; Tiwari, J.N.; Myung, C.W.; Meena, A.; Shin, T.J.; Chae, K.H.; Kim, K.S. Superb Water Splitting Activity of the Electrocatalyst Fe3Co (PO4)4 Designed with Computation Aid. Nat. Commun. 2019, 10, 5195. [Google Scholar] [CrossRef]
- Yang, J.; An, Y.; Guo, K.; Ren, X.; Jiang, B. Nitrogen Doped FeCoNiS Nanoparticles on N, S-Co-Doped Vertical Graphene as Bifunctional Electrocatalyst for Water Splitting. Int. J. Hydrogen Energy 2023, 48, 4143–4157. [Google Scholar] [CrossRef]
- Surendran, S.; Jesudass, S.C.; Janani, G.; Kim, J.Y.; Lim, Y.; Park, J.; Han, M.-K.; Cho, I.S.; Sim, U. Sulphur Assisted Nitrogen-Rich CNF for Improving Electronic Interactions in Co-NiO Heterostructures toward Accelerated Overall Water Splitting. Adv. Mater. Technol. 2023, 8, 2200572. [Google Scholar] [CrossRef]
- Miao, L.; Sui, L.; Shen, X.; Yang, D.; Huang, H.; Kuang, Y. Realizing High Performance Bifunctional Energy Storage Devices and Electrocatalytic Water Splitting Catalysts through Regulated Interface Engineering of ZnCo2O4@Co3O4 Nanosheets. CrystEngComm 2023, 25, 4812–4821. [Google Scholar] [CrossRef]
- Tsai, F.-T.; Deng, Y.-T.; Pao, C.-W.; Chen, J.-L.; Lee, J.-F.; Lai, K.-T.; Liaw, W.-F. The HER/OER Mechanistic Study of an FeCoNi-Based Electrocatalyst for Alkaline Water Splitting. J. Mater. Chem. A 2020, 8, 9939–9950. [Google Scholar] [CrossRef]
- Du, Y.; Li, Z.; Liu, H.; Qiao, S.; Chen, Y.; Zhu, Z.; Tang, Y.; Liu, C. Scalable Oxygen-Assisted-Fe2+ Etching Approach towards Amorphous/Crystalline Structure Fe-Ni2P Nanoarray for Efficient Water Splitting. J. Alloy. Compd. 2023, 936, 168073. [Google Scholar] [CrossRef]
- Yang, N.; Tian, S.; Feng, Y.; Hu, Z.; Liu, H.; Tian, X.; Xu, L.; Hu, C.; Yang, J. Introducing High-Valence Iridium Single Atoms into Bimetal Phosphides toward High-Efficiency Oxygen Evolution and Overall Water Splitting. Small 2023, 19, 2207253. [Google Scholar] [CrossRef]
- Dai, Z.; Du, X.; Zhang, X. The Synthesis of Ni-Co-Fe-Se@NiCo-LDH Nanoarrays on Ni Foam as Efficient Overall Water Splitting Electrocatalyst. J. Alloy. Compd. 2023, 946, 169451. [Google Scholar] [CrossRef]
- Chen, M.; Kitiphatpiboon, N.; Feng, C.; Zhao, Q.; Abudula, A.; Ma, Y.; Yan, K.; Guan, G. Tuning Octahedron Sites in MnFe2O4 Spinel by Boron Doping for Highly Efficient Seawater Splitting. Appl. Catal. B Environ. 2023, 330, 122577. [Google Scholar] [CrossRef]
- Guo, F.; Li, W.; Liu, Y.; Chen, Q.; Zhong, Q. Heterogeneous Fe-Doped NiCoP–MoO3 Efficient Electrocatalysts for Overall Water Splitting. Langmuir 2023, 39, 1042–1050. [Google Scholar] [CrossRef]
- Lei, B.; Xu, D.; Wei, B.; Xie, T.; Xiao, C.; Jin, W.; Xu, L. In Situ Synthesis of α-Fe2O3/Fe3O4 Heterojunction Photoanode via Fast Flame Annealing for Enhanced Charge Separation and Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 4785–4795. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Zhou, B.; Xiao, W.; Wang, J.; Wang, X.; Xu, G.; Li, B.; Li, Z.; Wu, Z.; et al. Corrosive Engineering Assisted in Situ Construction of an Fe–Ni-Based Compound for Industrial Overall Water-Splitting under Large-Current Density in Alkaline Freshwater and Seawater Media. J. Mater. Chem. A 2023, 11, 1886–1893. [Google Scholar] [CrossRef]
- Xiao, Y.; Shen, Y.; Su, D.; Zhang, S.; Yang, J.; Yan, D.; Fang, S.; Wang, X. Engineering Cu1.96S/Co9S8 with Sulfur Vacancy and Heterostructure as an Efficient Bifunctional Electrocatalyst for Water Splitting. J. Mater. Sci. Technol. 2023, 154, 1–8. [Google Scholar] [CrossRef]
- He, B.; Wang, X.-C.; Xia, L.-X.; Guo, Y.-Q.; Tang, Y.-W.; Zhao, Y.; Hao, Q.-L.; Yu, T.; Liu, H.-K.; Su, Z. Metal-Organic Framework-Derived Fe-Doped Co1.11Te2 Embedded in Nitrogen-Doped Carbon Nanotube for Water Splitting. ChemSusChem 2020, 13, 5239–5247. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, A.-L.; Xiao, W.; Chao, D.; Zhang, X.; Tiep, N.H.; Chen, S.; Kang, J.; Wang, X.; Ding, J.; et al. In Situ Grown Epitaxial Heterojunction Exhibits High-Performance Electrocatalytic Water Splitting. Adv. Mater. 2018, 30, 1705516. [Google Scholar] [CrossRef]
- Lv, J.; Liu, P.; Yang, F.; Xing, L.; Wang, D.; Chen, X.; Gao, H.; Huang, X.; Lu, Y.; Wang, G. 3D Hydrangea Macrophylla-like Nickel–Vanadium Metal–Organic Frameworks Formed by Self-Assembly of Ultrathin 2D Nanosheets for Overall Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 48495–48510. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ouyang, T.; Zou, Y.; Li, N.; Liu, Z.-Q. Ultrathin NiCo2Px Nanosheets Strongly Coupled with CNTs as Efficient and Robust Electrocatalysts for Overall Water Splitting. J. Mater. Chem. A 2018, 6, 7420–7427. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Wang, C.; Gao, B.; Li, J.; Zhu, M.; Wu, H.; Zhang, C.; Qin, Y. Photothermal–Magnetic Synergistic Effects in an Electrocatalyst for Efficient Water Splitting under Optical–Magnetic Fields. Adv. Mater. 2023, 35, 2303741. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-B.; Li, X.; Wei, W.-B.; Wu, X.-T.; Zhu, Q.-L. Nano-Engineering of Ru-Based Hierarchical Porous Nanoreactors for Highly Efficient PH-Universal Overall Water Splitting. Appl. Catal. B Environ. 2021, 294, 120230. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J.; Gao, Y.; Zhang, J.; Huang, C.; Shi, Q.; Mu, S.; Xiao, Q.; Huo, S.; Xia, Z.; et al. D-Orbital Manipulated Ru Nanoclusters for High-Efficiency Overall Water Splitting at Industrial-Level Current Densities. Adv. Funct. Mater. 2023, 34, 2307917. [Google Scholar] [CrossRef]
- Nagappan, S.; Karmakar, A.; Madhu, R.; Dhandapani, H.N.; Singha Roy, S.; Kundu, S. Tuning the Active Sites and Optimizing the D-Spacing Value in CoFe-LDH by Ex Situ Intercalation of Guest Anions: An Innovative Electrocatalyst for Overall Water Splitting Reaction. Catal. Sci. Technol. 2023, 13, 6377–6391. [Google Scholar] [CrossRef]

| Electrochemical Properties | HER | OER |
|---|---|---|
| EIS values | 7.1 Ω | 13.3 Ω |
| Tafel slopes | 62 mV/dec | 199 mV/dec |
| Cdl values | 4.6 mF/cm2 | 14.6 mF/cm2 |
| ECSA | 28.75 cm2 | 91.25 cm2 |
| TOF values (at 500 and 800 mA/cm2) | 0.193 site−1s−1 | 0.101 site−1s−1 |
| Faradic efficiency (FE) | 90.51% | 90.16% |
| Electrocatalyst | Overpotentials [mV] | Year | References | ||
|---|---|---|---|---|---|
| @20 mA/cm2 | @50 mA/cm2 | @200 mA/cm2 | |||
| RuVNi | 15 | 26 | 48 | 2019 | [72] |
| CoFeBP | 20.1 | 46 | 145 | - | This work |
| NiMoB | 54 | 97 | 210 | 2022 | [40] |
| Ru/CoFeP | 61 | 82 | - | 2020 | [19] |
| NiFeMn | 80 | 121 | - | 2020 | [73] |
| CoB@CoO/Ti | 81 | 110 | 181 | 2017 | [74] |
| NiCoP | 134 | 165 | 204 | 2018 | [13] |
| Ni/Ni3N | 151 | 232 | 420 | 2015 | [59] |
| Fe-NiSe | 182 | 265 | - | 2022 | [75] |
| Fe-S | 193 | 235 | 324 | 2019 | [76] |
| Electrocatalyst | Overpotentials [mV] | Year | References | ||
|---|---|---|---|---|---|
| @20 mA/cm2 | @50 mA/cm2 | @200 mA/cm2 | |||
| Ni2P-CuP | 140 | 190 | - | 2021 | [3] |
| MoNiPx/NiSy | 143 | 156 | 221 | 2021 | [77] |
| NiMo3S4 | 162 | 252 | 617 | 2022 | [78] |
| P-Co3O4 | 208 | 295 | 330 | 2018 | [18] |
| RuVNi | 217 | 227 | 312 | 2019 | [72] |
| CoFeBP | 219 | 303 | 426 | - | This work |
| NiMoB | 248 | 267 | 500 | 2022 | [40] |
| NiFeMn | 260 | 291 | 352 | 2020 | [73] |
| NiCoP | 296 | 328 | 370 | 2018 | [13] |
| Mo2NiB2 | 311 | 342 | - | 2021 | [2] |
| NiSe2 | 320 | 521 | - | 2022 | [75] |
| Electrolytes | @200 mA/cm2 | @1000 mA/cm2 |
|---|---|---|
| 1 M KOH | 1.84 V | 2.66 V |
| 6 M KOH | 1.76 V | 2.54 V |
| 0.5 M H2SO4 | 2.13 V | - |
| 1 M PBS | 2.25 V | - |
| River water | - | - |
| River water + 1 M KOH | 3.87 V | - |
| Seawater | - | - |
| Seawater + 1 M KOH | 2.34 V | - |
| Electrocatalyst | Electrolyte | Cell Voltage (V) | Year | References | |
|---|---|---|---|---|---|
| @50 mA/cm2 | @200 mA/cm2 | ||||
| Fe-NiSe | 1 M KOH | 1.34 V | 1.61 V | 2022 | [75] |
| Ru/CoFeP | 1 M KOH | 1.46 V | - | 2020 | [19] |
| MoNiPx/NiSy | 1 M KOH | 1.55 V | 1.88 V | 2021 | [77] |
| FeS/Ni3S2 | 1 M KOH | 1.56 V | - | 2022 | [80] |
| NiFeMn | 1 M KOH | 1.57 V | 1.66 V | 2020 | [73] |
| CoFeBP | 1 M KOH | 1.60 V | 1.84 V | - | This work |
| NiMoB | 1 M KOH | 1.61 V | 1.96 V | 2022 | [40] |
| Ni3S2 | 1 M KOH | 1.63 V | - | 2018 | [81] |
| Fe,Rh-Ni2P | 1 M KOH | 1.64 V | 1.85 V | 2022 | [82] |
| Mo2NiB2 | 1 M KOH | 1.67 V | - | 2021 | [2] |
| NiFeS | 1 M KOH | 1.73 V | - | 2022 | [83] |
| P-Co3O4 | 1 M KOH | 1.79 V | - | 2018 | [18] |
| NiCoP | 1 M KOH | 1.82 V | - | 2018 | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Habib, M.A.; Joni, M.H.; Dristy, S.A.; Mandavkar, R.; Jeong, J.-H.; Chung, Y.-U.; Lee, J. CoFeBP Micro Flowers (MFs) for Highly Efficient Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts. Nanomaterials 2024, 14, 698. https://doi.org/10.3390/nano14080698
Lin S, Habib MA, Joni MH, Dristy SA, Mandavkar R, Jeong J-H, Chung Y-U, Lee J. CoFeBP Micro Flowers (MFs) for Highly Efficient Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts. Nanomaterials. 2024; 14(8):698. https://doi.org/10.3390/nano14080698
Chicago/Turabian StyleLin, Shusen, Md Ahasan Habib, Mehedi Hasan Joni, Sumiya Akter Dristy, Rutuja Mandavkar, Jae-Hun Jeong, Young-Uk Chung, and Jihoon Lee. 2024. "CoFeBP Micro Flowers (MFs) for Highly Efficient Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts" Nanomaterials 14, no. 8: 698. https://doi.org/10.3390/nano14080698
APA StyleLin, S., Habib, M. A., Joni, M. H., Dristy, S. A., Mandavkar, R., Jeong, J.-H., Chung, Y.-U., & Lee, J. (2024). CoFeBP Micro Flowers (MFs) for Highly Efficient Hydrogen Evolution Reaction and Oxygen Evolution Reaction Electrocatalysts. Nanomaterials, 14(8), 698. https://doi.org/10.3390/nano14080698








