Functionalization of Ordered Mesoporous Silica (MCM-48) with Task-Specific Ionic Liquid for Enhanced Carbon Capture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AAIL@MCM-48 Composite
2.3. Characterization
2.4. Adsorption Isotherms
3. Results and Discussion
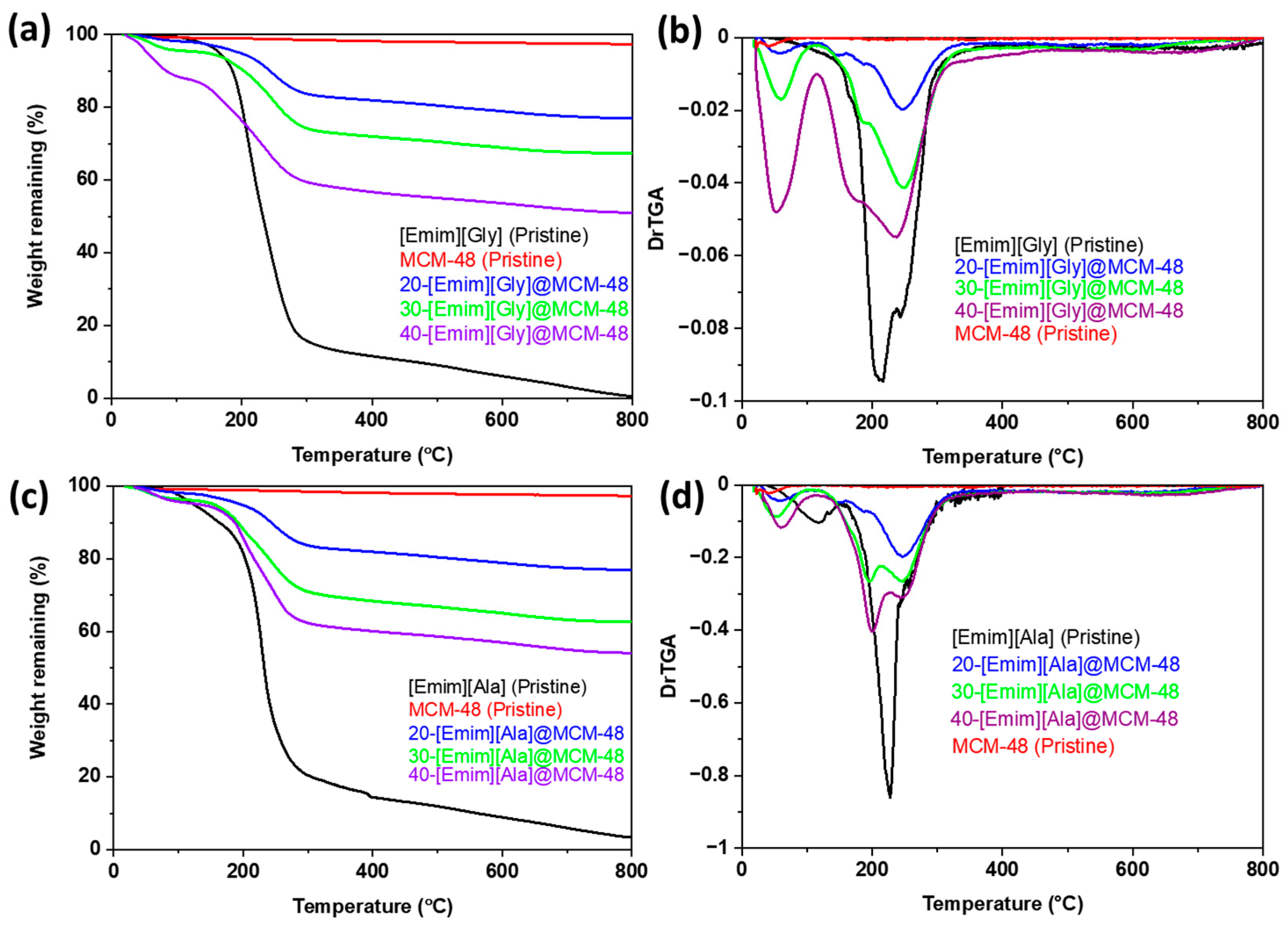
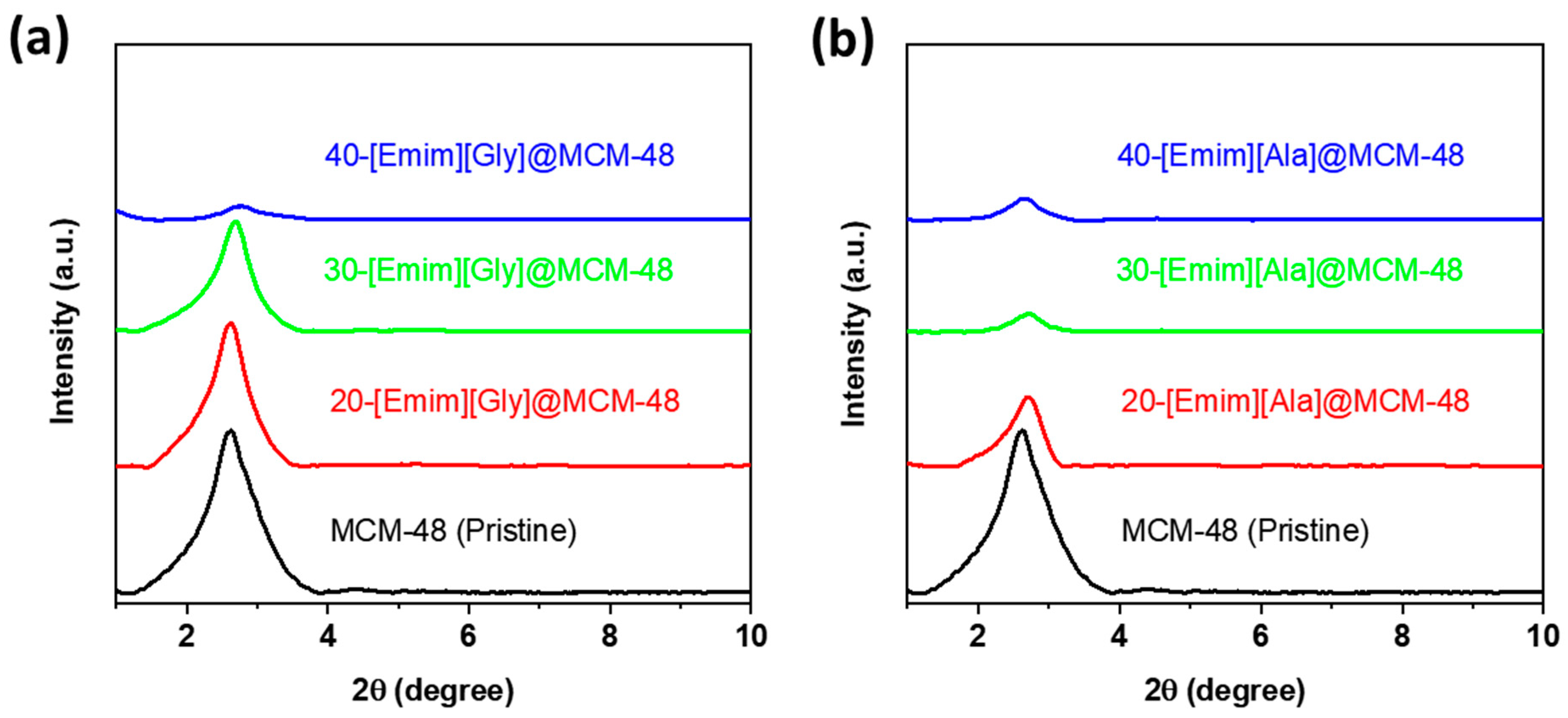
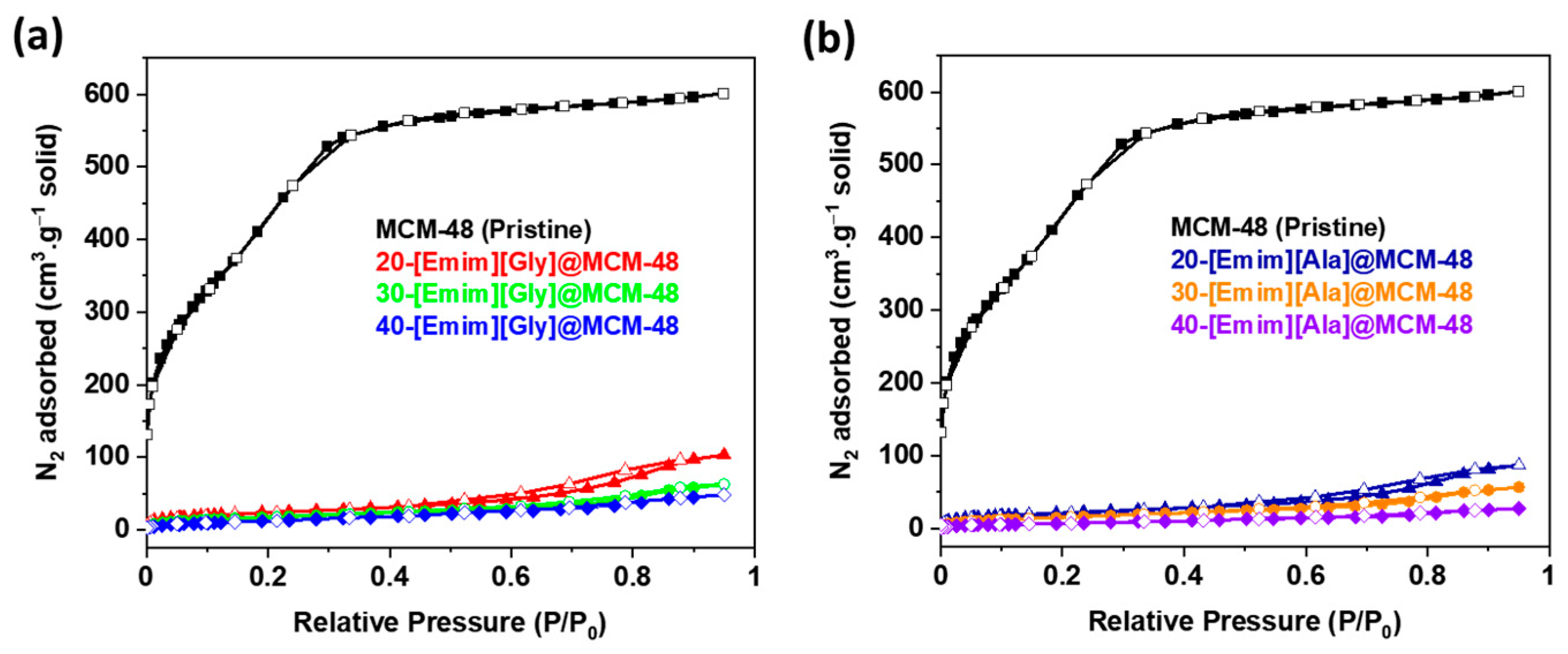
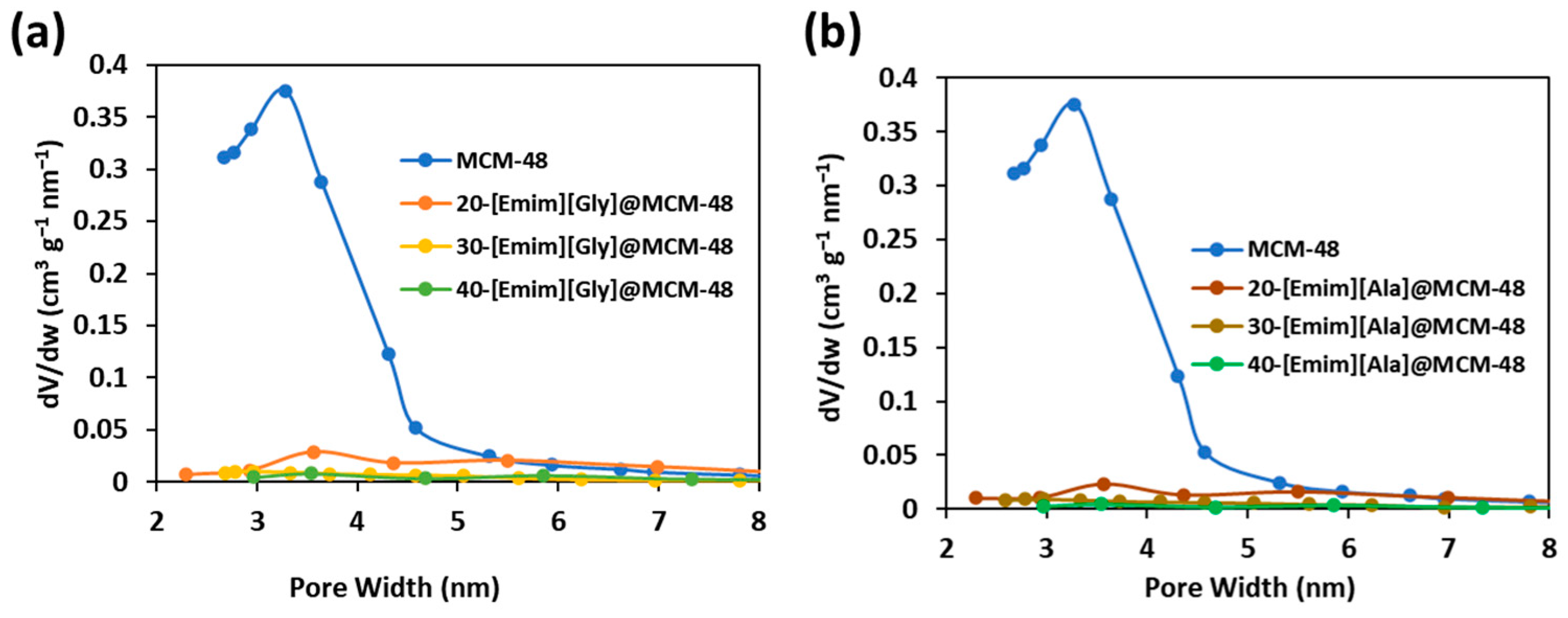
3.1. Characterizations of the AAIL-Impregnated Sorbents
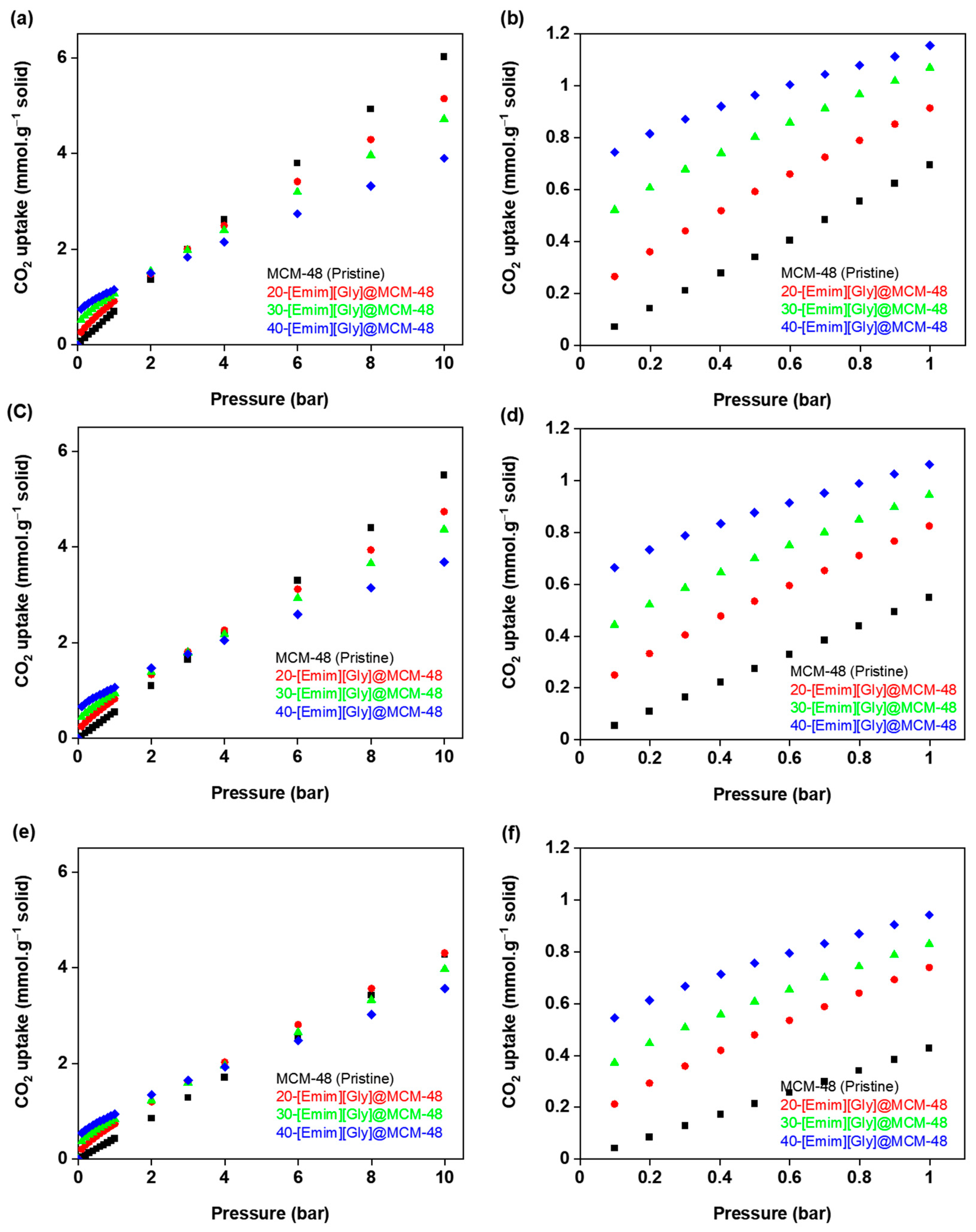
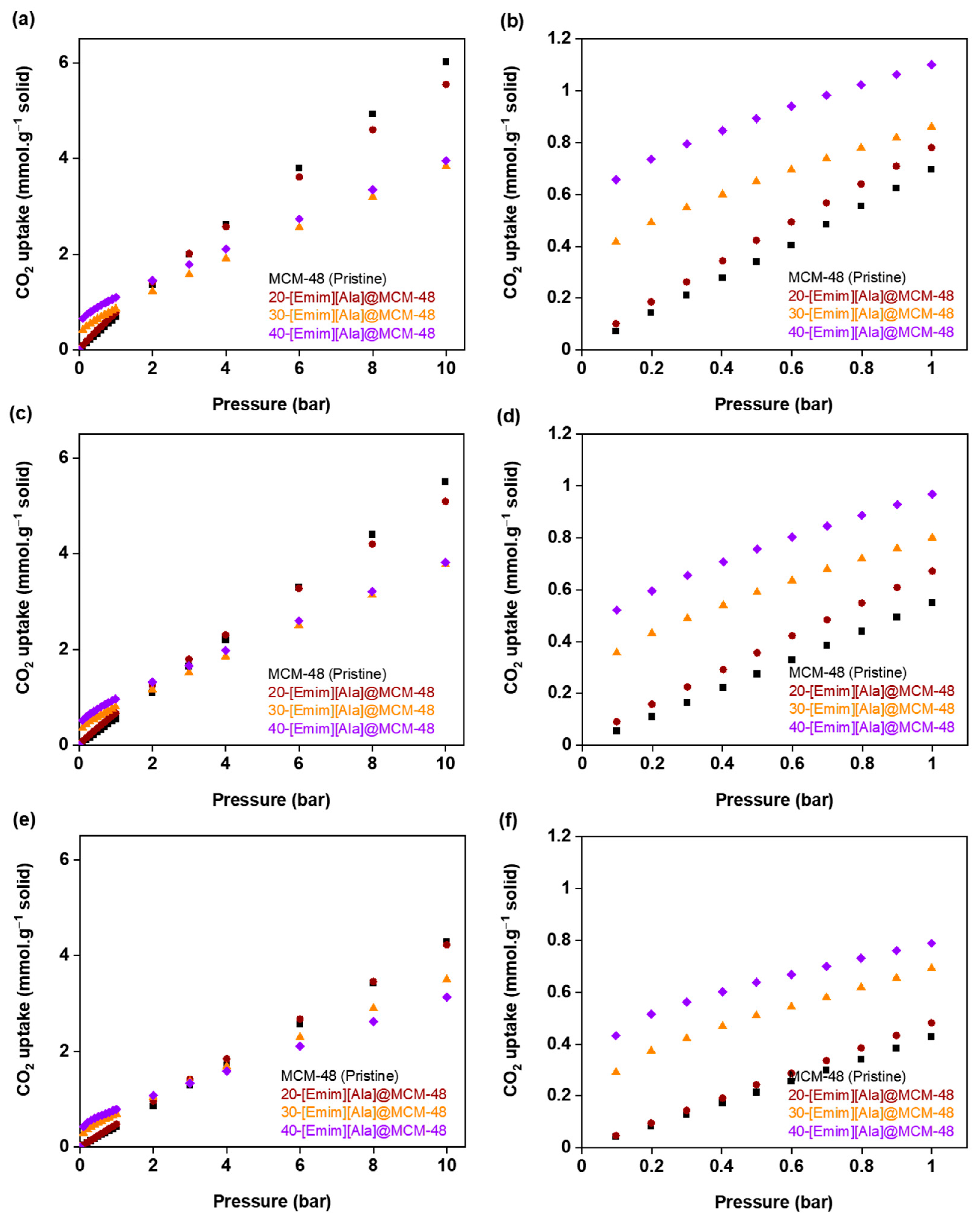
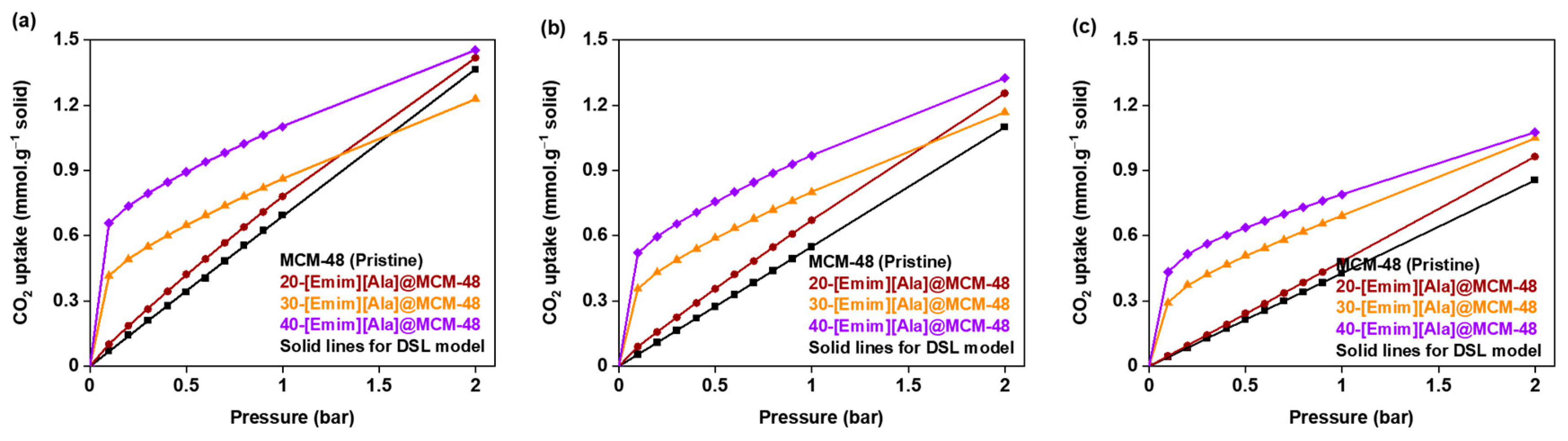
3.2. CO2 Adsorption Isotherms
| Composites | CO2 Uptake (mmol g−1) | Experimental Conditions | Ref. |
|---|---|---|---|
| APS-MCM-48 | 0.8 | 1.0 atm/298 K | [3] |
| PEI-MCM-48 | 0.4 | 1.0 atm/298 K | [3] |
| PyrPS-MCM-48 | 0.3 | 1.0 atm/298 K | [3] |
| p-APS-MCM-48 | 0.1 | 1.0 atm/298 K | [3] |
| 35PEHA-15DEA-MCM-48 | 0.51 | 1.0 atm/298 K | [34] |
| 50PEHA-MCM-48 | 0.26 | 1.0 atm/298 K | [34] |
| PEI-MCM-41 | 1.09 | 101 kPa/298 K | [35] |
| [P666][2-Op]-MCM-41 | 1.17 | 101 kPa/298 K | [36] |
| [P4444][imidazole]-MCM-41 | 0.91 | 101 kPa/293 K | [37] |
| EDA-PVC-SBA-15 | 0.5 | 50 kPa/298 K | [38] |
| 40 wt.%-[Emim][Gly]-MCM-48 | 0.82 | 20 kPa/303 K | Present Study |
| 40 wt.%-[Emim][Ala]-MCM-48 | 0.74 | 20 kPa/303 K | Present Study |
| 40 wt.%-[Emim][Gly]-MCM-48 | 1.15 | 100 kPa/303 K | Present Study |
| 40 wt.%-[Emim][Ala]-MCM-48 | 1.1 | 100 kPa/303 K | Present Study |
3.3. Selectivity for CO2/N2
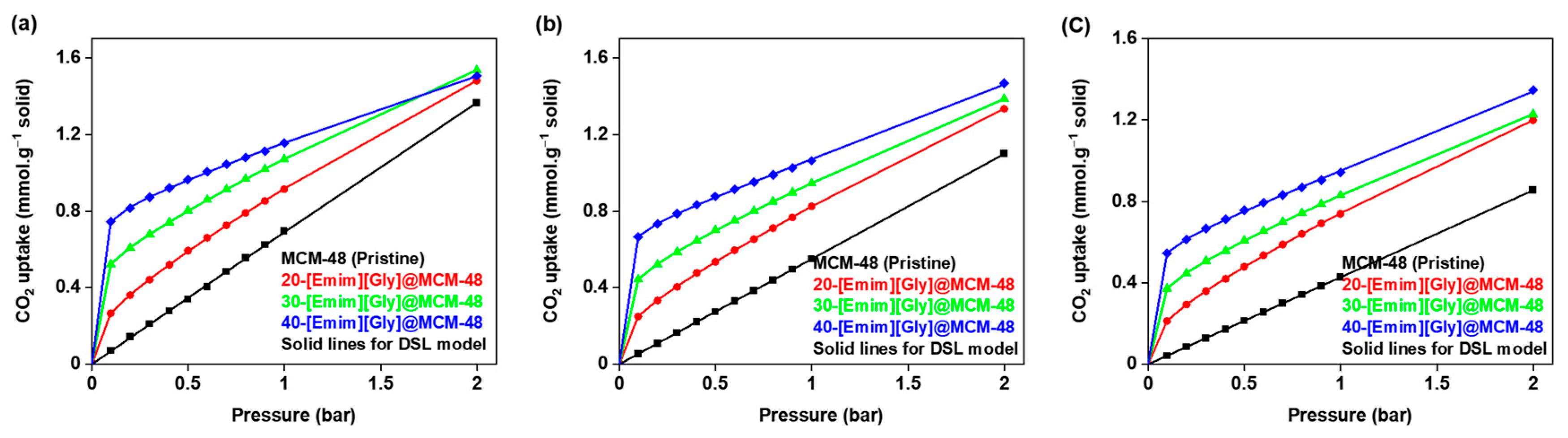
3.4. Equilibrium Isotherm Modeling
3.5. Isosteric Heat of Adsorption (Qst)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA. Net Zero Roadmap: A Global Pathway to Keep the 1.5 °C Goal in Reach; IEA: Paris, France, 2023. [Google Scholar]
- IPCC. Mitigation Pathways Compatible with 1.5 °C in the Context of Sustainable Development; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Kim, S.; Ida, J.; Guliants, V.V.; Lin, J.Y.S. Tailoring Pore Properties of MCM-48 Silica for Selective Adsorption of CO2. J. Phys. Chem. B 2005, 109, 6287–6293. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Ünveren, E.E.; Monkul, B.Ö.; Sarıoğlan, Ş.; Karademir, N.; Alper, E. Solid Amine Sorbents for CO2 Capture by Chemical Adsorption: A Review. Petroleum 2017, 3, 37–50. [Google Scholar] [CrossRef]
- Yue, M.B.; Chun, Y.; Cao, Y.; Dong, X.; Zhu, J.H. CO2 Capture by As-Prepared SBA-15 with an Occluded Organic Template. Adv. Funct. Mater. 2006, 16, 1717–1722. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T.; Chinn, D.; Munson, C.L. Amine-Grafted MCM-48 and Silica Xerogel as Superior Sorbents for Acidic Gas Removal from Natural Gas. Ind. Eng. Chem. Res. 2003, 42, 2427–2433. [Google Scholar] [CrossRef]
- Wei, J.; Liao, L.; Xiao, Y.; Zhang, P.; Shi, Y. Capture of Carbon Dioxide by Amine-Impregnated as-Synthesized MCM-41. J. Environ. Sci. 2010, 22, 1558–1563. [Google Scholar] [CrossRef]
- Chen, H.; Liang, Z.; Yang, X.; Zhang, Z.; Zhang, Z. Experimental Investigation of CO2 Capture Capacity: Exploring Mesoporous Silica SBA-15 Material Impregnated with Monoethanolamine and Diethanolamine. Energy Fuels 2016, 30, 9554–9562. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Guo, Q. Development of Hybrid Amine-Functionalized MCM-41 Sorbents for CO2 capture. Chem. Eng. J. 2015, 260, 573–581. [Google Scholar] [CrossRef]
- Santos, S.C.G.; Pedrosa, A.M.G.; Souza, M.J.B.; Cecilia, J.A.; Rodríguez-Castellón, E. Carbon Dioxide Adsorption on Micro-Mesoporous Composite Materials of ZSM-12/MCM-48 Type: The Role of the Contents of Zeolite and Functionalized Amine. Mater. Res. Bull. 2015, 70, 663–672. [Google Scholar] [CrossRef]
- Kinik, F.P.; Altintas, C.; Balci, V.; Koyuturk, B.; Uzun, A.; Keskin, S. [BMIM][PF6] Incorporation Doubles CO2 Selectivity of ZIF-8: Elucidation of Interactions and Their Consequences on Performance. ACS Appl. Mater. Interfaces 2016, 8, 30992–31005. [Google Scholar] [CrossRef]
- Koyuturk, B.; Altintas, C.; Kinik, F.P.; Keskin, S.; Uzun, A. Improving Gas Separation Performance of ZIF-8 by [BMIM][BF4] Incorporation: Interactions and Their Consequences on Performance. J. Phys. Chem. C 2017, 121, 10370–10381. [Google Scholar] [CrossRef]
- Kulak, H.; Polat, H.M.; Kavak, S.; Keskin, S.; Uzun, A. Improving CO2 Separation Performance of MIL-53(Al) by Incorporating 1-n-Butyl-3-Methylimidazolium Methyl Sulfate. Energy Technol. 2019, 7, 1900157. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room Temperature Ionic Liquids from 20 Natural Amino Acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Man, Z.B.; Bustam, M.A.; Mutalib, M.I.A.; Wilfred, C.D.; Rafiq, S. Synthesis and Thermophysical Properties of Low Viscosity Amino Acid-Based Ionic Liquids. J. Chem. Eng. Data 2011, 56, 3157–3162. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. CO2 Absorption Studies in Amino Acid-Anion Based Ionic Liquids. Chem. Eng. J. 2015, 273, 268–276. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, P. Investigation of the Absorption Performance and Viscosity for CO2 Capture Process Using [Bmim][Gly] Promoted MDEA (N-Methyldiethanolamine) Aqueous Solution. Energy 2015, 87, 165–172. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, P.; Wang, L.M. Absorption Performance of CO2 in High Concentrated [Bmim][Lys]-MDEA Aqueous Solution. Energy 2016, 113, 1–8. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, X.F.; Fu, D. CO2 Removal in Tray Tower by Using AAILs Activated MDEA Aqueous Solution. Energy 2018, 161, 1122–1132. [Google Scholar] [CrossRef]
- Wang, X.; Akhmedov, N.G.; Duan, Y.; Luebke, D.; Li, B. Immobilization of Amino Acid Ionic Liquids into Nanoporous Microspheres as Robust Sorbents for CO2 Capture. J. Mater. Chem. A 2013, 1, 2978–2982. [Google Scholar] [CrossRef]
- Philip, F.A.; Henni, A. Enhancement of Post-Combustion CO2 Capture Capacity by Incorporation of Task-Specific Ionic Liquid into ZIF-8. Microporous Mesoporous Mater. 2021, 330, 111580. [Google Scholar] [CrossRef]
- Philip, F.A.; Henni, A. Incorporation of Amino Acid-Functionalized Ionic Liquids into Highly Porous MOF-177 to Improve the Post-Combustion CO2 Capture Capacity. Molecules 2023, 28, 7185. [Google Scholar] [CrossRef]
- Schumacher, K.; Ravikovitch, P.I.; Du Chesne, A.; Neimark, A.V.; Unger, K.K. Characterization of MCM-48 Materials. Langmuir 2000, 16, 4648–4654. [Google Scholar] [CrossRef]
- Xu, J.; Luan, Z.; He, H.; Zhou, W.; Kevan, L. A Reliable Synthesis of Cubic Mesoporous MCM-48 Molecular Sieve. Chem. Mater. 1998, 10, 3690–3698. [Google Scholar] [CrossRef]
- Thi Le, M.U.; Lee, S.Y.; Park, S.J. Preparation and Characterization of PEI-Loaded MCM-41 for CO2 Capture. Int. J. Hydrogen Energy 2014, 39, 12340–12346. [Google Scholar] [CrossRef]
- Boote, B.; Subramanian, H.; Ranjit, K.T. Rapid and Facile Synthesis of Siliceous MCM-48 Mesoporous Materials. Chem. Commun. 2007, 43, 4543–4545. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wu, L.; Li, B.G. Preparation and CO2 Sorption/Desorption of N -(3-Aminopropyl)Aminoethyl Tributylphosphonium Amino Acid Salt Ionic Liquids Supported into Porous Silica Particles. Ind. Eng. Chem. Res. 2012, 51, 7901–7909. [Google Scholar] [CrossRef]
- Gurkan, B.E.; De La Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 Absorption by Anion-Functionalized Ionic Liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef]
- Ferreira, T.J.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Rebelo, L.P.N.; Esperança, J.M.S.S.; Esteves, I.A.A.C. Ionic Liquid-Impregnated Metal-Organic Frameworks for CO2/CH4 Separation. ACS Appl. Nano Mater. 2019, 2, 7933–7950. [Google Scholar] [CrossRef]
- Anbia, M.; Hoseini, V.; Mandegarzad, S. Synthesis and Characterization of Nanocomposite MCM-48-PEHA-DEA and Its Application as CO2 Adsorbent. Korean J. Chem. Eng. 2012, 29, 1776–1781. [Google Scholar] [CrossRef]
- Ahmed, S.; Ramli, A.; Yusup, S. Development of Polyethylenimine-Functionalized Mesoporous Si-MCM-41 for CO2 Adsorption. Fuel Process. Technol. 2017, 167, 622–630. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Y.; Hu, L.; Zhou, J.; Cen, K. CO2 Adsorption Performance of Ionic Liquid [P66614][2-Op] Loaded onto Molecular Sieve MCM-41 Compared to Pure Ionic Liquid in Biohythane/Pure CO2 Atmospheres. Energy Fuels 2016, 30, 3251–3256. [Google Scholar] [CrossRef]
- Wan, M.M.; Zhu, H.Y.; Li, Y.Y.; Ma, J.; Liu, S.; Zhu, J.H. Novel CO2-Capture Derived from the Basic Ionic Liquids Orientated on Mesoporous Materials. ACS Appl. Mater. Interfaces 2014, 6, 12947–12955. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, G.; McGlynn, J.C.; Neumann, M.S.; Aydin, H.M.; Yiu, H.H.P.; Ganin, A.Y. Aminated Poly(Vinyl Chloride) Solid State Adsorbents with Hydrophobic Function for Post-Combustion CO2 Capture. J. Mater. Chem. A 2017, 5, 11864–11872. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Mohamedali, M.; Ibrahim, H.; Henni, A. Incorporation of Acetate-Based Ionic Liquids into a Zeolitic Imidazolate Framework (ZIF-8) as Efficient Sorbents for Carbon Dioxide Capture. Chem. Eng. J. 2018, 334, 817–828. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
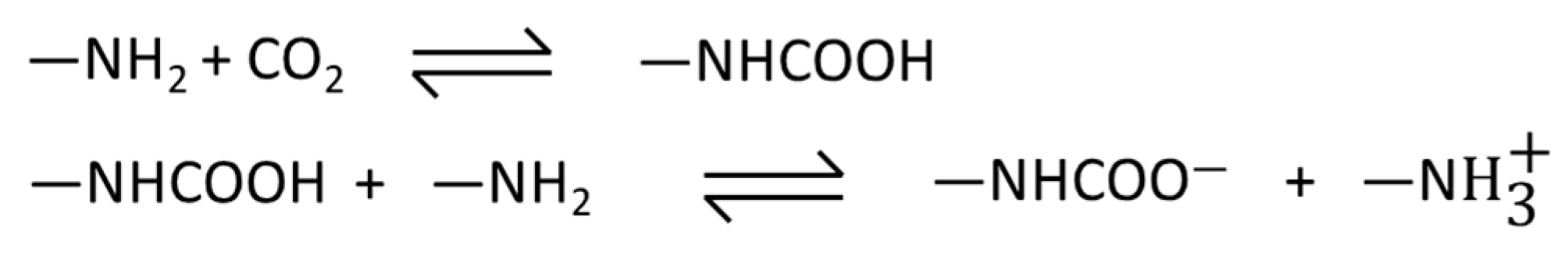


| Name | Abbreviation | Structure |
|---|---|---|
| 1-Ethyl-3-methylimidazolium | [Emim] | 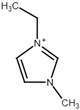 |
| Glycine | [Gly] | 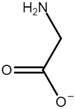 |
| Alanine | [Ala] | 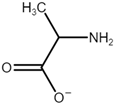 |
| Samples | SBET (m2g−1) | SLangmuir (m2g−1) | Pore Volume (cm3g−1) |
|---|---|---|---|
| MCM-48 | 1638 | 2700 | 0.93 |
| 20-[Emim][Gly]@MCM-48 | 87 | 287 | 0.16 |
| 30-[Emim][Gly]@MCM-48 | 66 | 192 | 0.10 |
| 40-[Emim][Gly]@MCM-48 | 50 | 155 | 0.07 |
| 20-[Emim][Ala]@MCM-48 | 79 | 236 | 0.13 |
| 30-[Emim][Ala]@MCM-48 | 60 | 196 | 0.09 |
| 40-[Emim][Ala]@MCM-48 | 29 | 117 | 0.04 |
| Model Parameters | 20-[Emim][Gly]@MCM-48 | 30-[Emim][Gly]@MCM-48 | 40-[Emim][Gly]@MCM-48 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | |
| NA | 7.277 | 0.239 | 6.204 | 0.520 | 0.450 | 0.390 | 0.780 | 0.691 | 0.572 |
| bA | 0.102 | 37.406 | 0.095 | 83.374 | 67.387 | 52.263 | 95.804 | 101.737 | 81.843 |
| NB | 0.248 | 7.722 | 0.209 | 5.896 | 7.090 | 7.041 | 7.017 | 731.290 | 531.196 |
| bB | 36.92 | 0.083 | 29.783 | 0.105 | 0.076 | 0.068 | 0.058 | 0.001 | 0.001 |
| R2 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Model Parameters | 20-[Emim][Ala]@MCM-48 | 30-[Emim][Ala]@MCM-48 | 40-[Emim][Ala]@MCM-48 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | |
| NA | 0.026 | 0.023 | 349.867 | 0.433 | 0.374 | 0.357 | 0.679 | 0.531 | 0.525 |
| bA | 53.033 | 10,000 | 0.000 | 58.632 | 48.183 | 26.971 | 90.057 | 79.650 | 34.622 |
| NB | 8.784 | 12.152 | 579.453 | 4.647 | 4.692 | 227.845 | 3.908 | 3.753 | 94.954 |
| bB | 0.094 | 0.056 | 0.001 | 0.104 | 0.102 | 0.002 | 0.124 | 0.135 | 0.003 |
| R2 | 1.000 | 0.999 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philip, F.A.; Henni, A. Functionalization of Ordered Mesoporous Silica (MCM-48) with Task-Specific Ionic Liquid for Enhanced Carbon Capture. Nanomaterials 2024, 14, 514. https://doi.org/10.3390/nano14060514
Philip FA, Henni A. Functionalization of Ordered Mesoporous Silica (MCM-48) with Task-Specific Ionic Liquid for Enhanced Carbon Capture. Nanomaterials. 2024; 14(6):514. https://doi.org/10.3390/nano14060514
Chicago/Turabian StylePhilip, Firuz A., and Amr Henni. 2024. "Functionalization of Ordered Mesoporous Silica (MCM-48) with Task-Specific Ionic Liquid for Enhanced Carbon Capture" Nanomaterials 14, no. 6: 514. https://doi.org/10.3390/nano14060514
APA StylePhilip, F. A., & Henni, A. (2024). Functionalization of Ordered Mesoporous Silica (MCM-48) with Task-Specific Ionic Liquid for Enhanced Carbon Capture. Nanomaterials, 14(6), 514. https://doi.org/10.3390/nano14060514






