Effect of Nitrogen Dopant Agents in the Performance of Graphene-Based Cathodes for Li-S Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide (GO)
2.2. Synthesis of Nitrogen-Functionalised rGO via Ethylenediamine (en-rGO)
2.3. Synthesis of Nitrogen-Functionalised rGO via NH3 (NH3-rGO)
2.4. Synthesis of Reduced Graphene Oxide (rGO)
2.5. Synthesis of the Graphene-Based Materials and Sulphur Composites
2.6. Cathode Electrode Preparation and Cell Assembly
2.7. Characterisation and Electrochemical Measurements
3. Results and Discussion
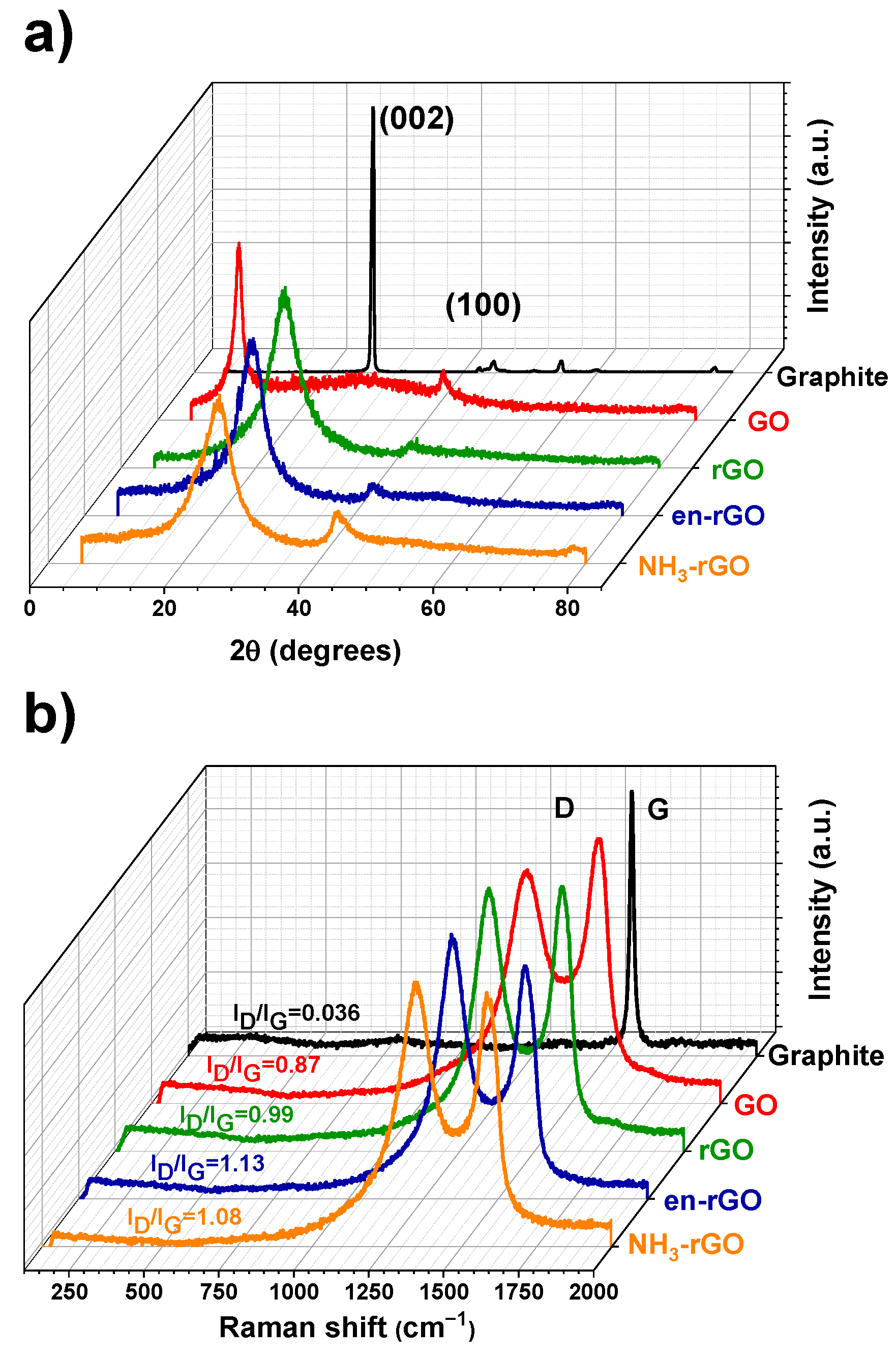
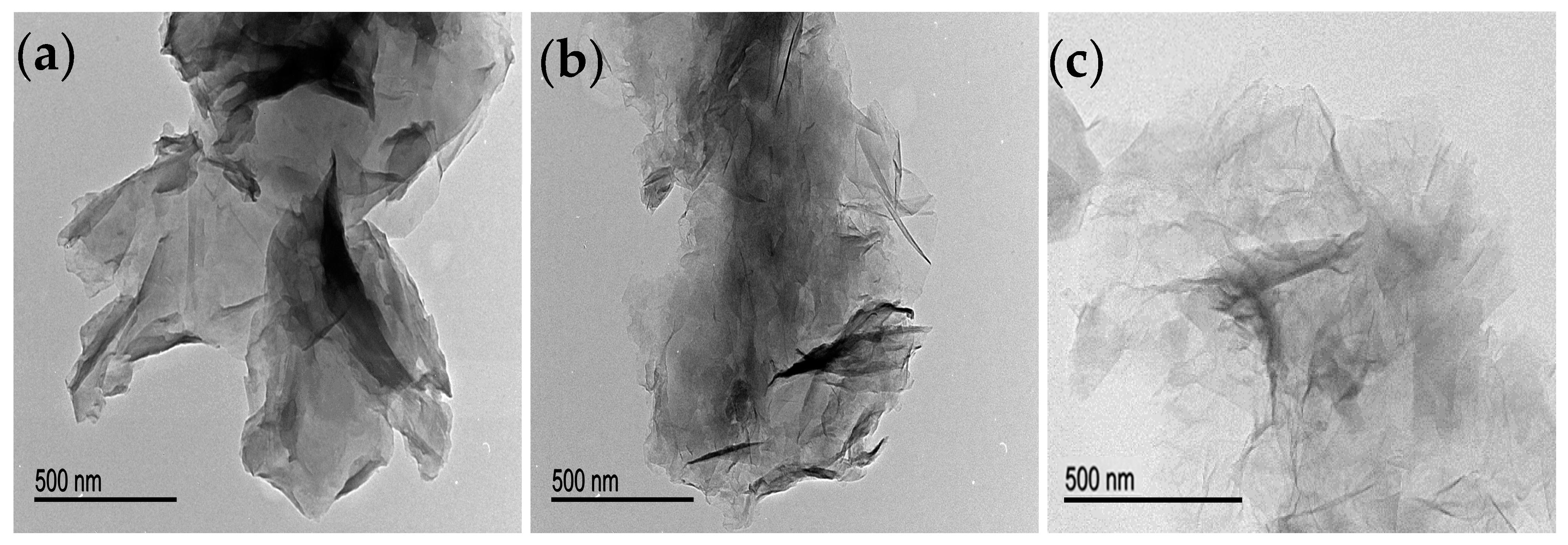
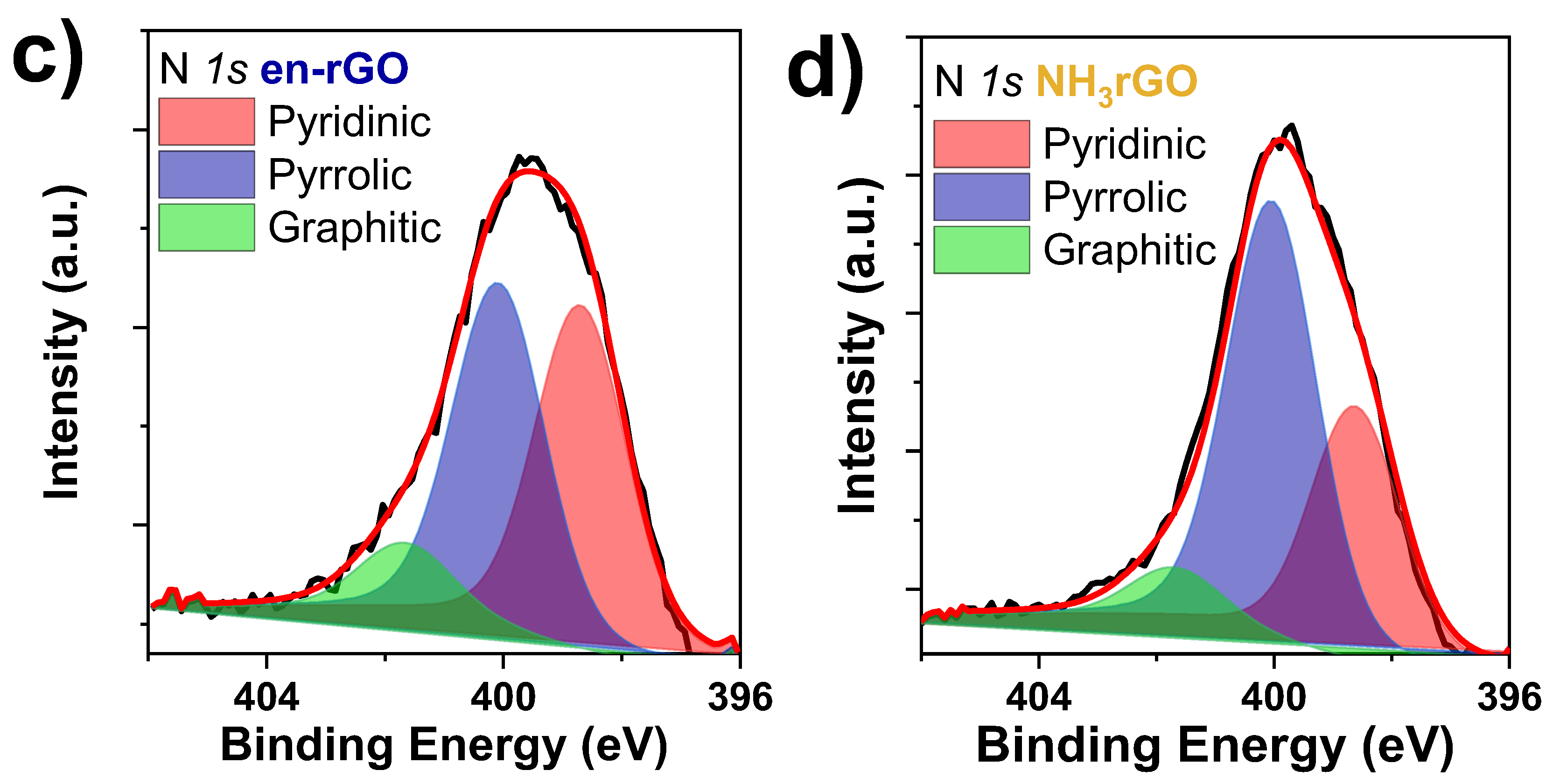
3.1. Characterisation of Graphene-Based Samples
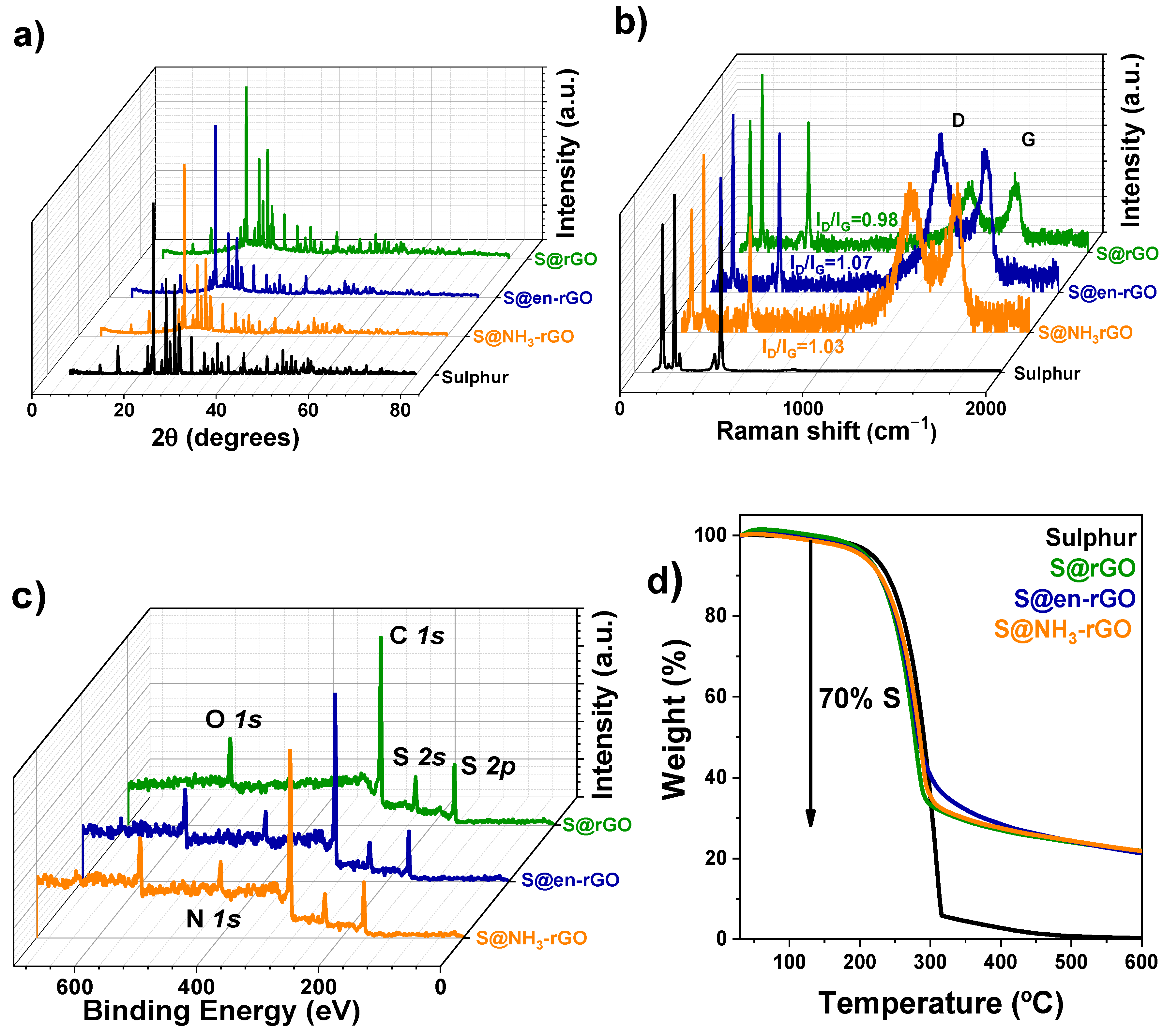
3.2. Characterisation of Sulphur Composites
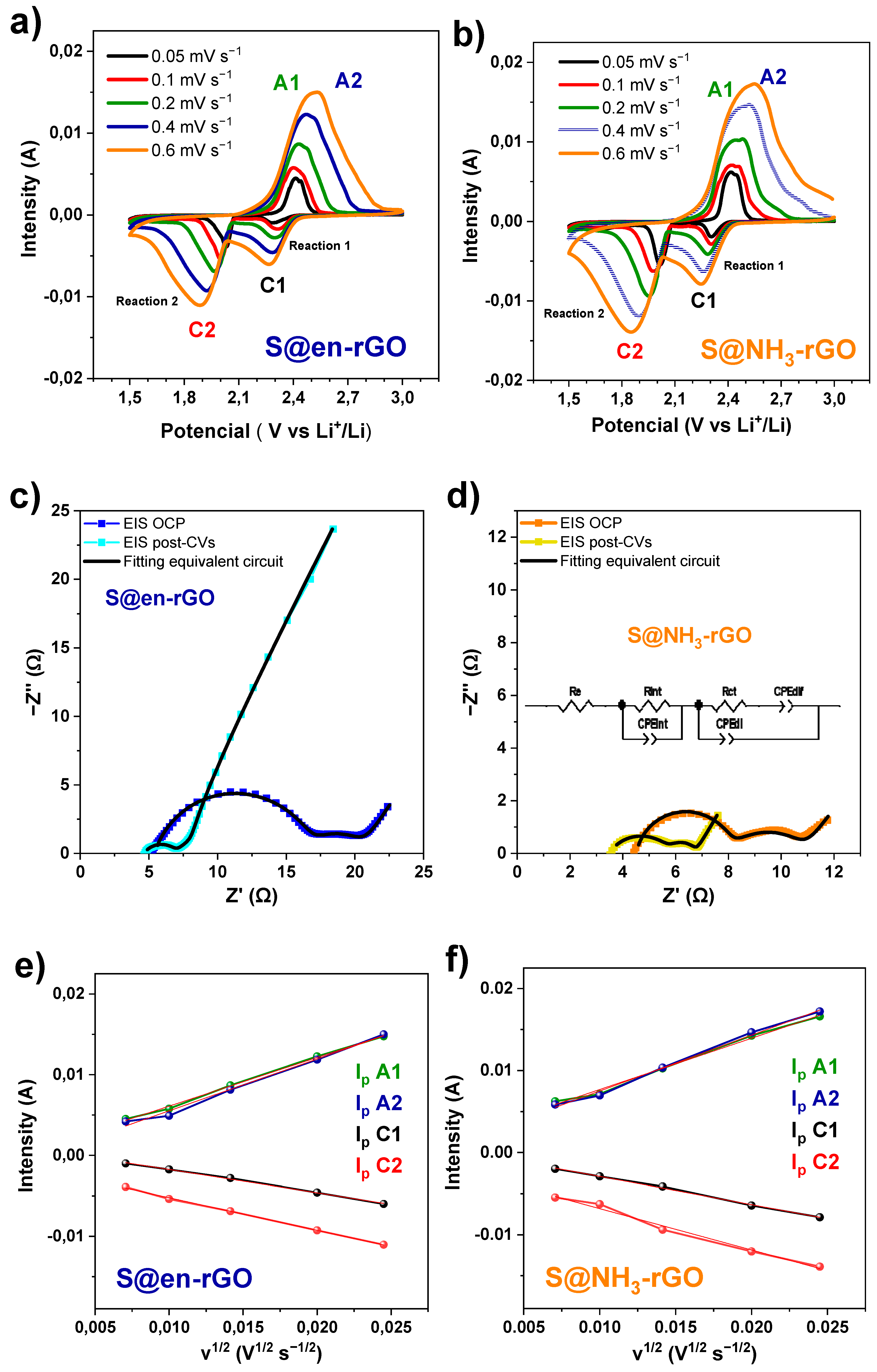
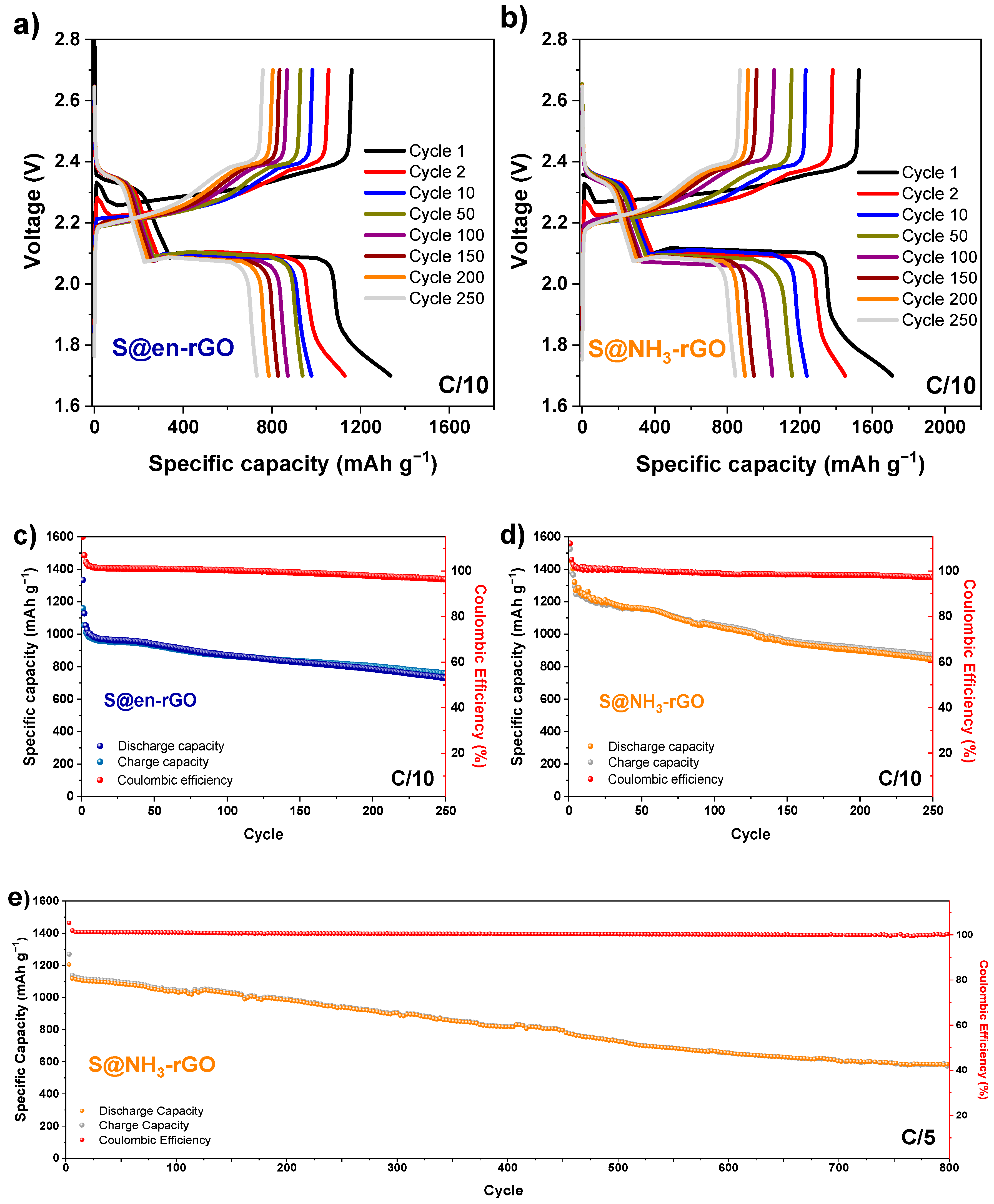
3.3. Electrochemical Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Rosado, P.; Roser, M. Fossil fuels. In Our World in Data; University of Oxford: Oxford, UK, 2023. [Google Scholar]
- Ould Amrouche, S.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of Energy Storage in Renewable Energy Systems. Int. J. Hydrog. Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- Benveniste, G.; Rallo, H.; Canals Casals, L.; Merino, A.; Amante, B. Comparison of the State of Lithium-Sulphur and Lithium-Ion Batteries Applied to Electromobility. J. Environ. Manag. 2018, 226, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S.; Wild, M. A Review on Electric Vehicle Battery Modelling: From Lithium-Ion toward Lithium–Sulphur. Renew. Sustain. Energy Rev. 2016, 56, 1008–1021. [Google Scholar] [CrossRef]
- Xu, J.; Ma, J.; Fan, Q.; Guo, S.; Dou, S. Recent Progress in the Design of Advanced Cathode Materials and Battery Models for High-Performance Lithium-X (X = O2, S, Se, Te, I2, Br2) Batteries. Adv. Mater. 2017, 29, 1606454. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Wu, F. From a Historic Review to Horizons beyond: Lithium–Sulphur Batteries Run on the Wheels. Chem. Commun. 2015, 51, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.-W.; Cheng, H.-M.; Li, F. More Reliable Lithium-Sulfur Batteries: Status, Solutions and Prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Chen, S.; Zhu, J.; Ma, R.; Li, S.; Podila, R.; Rao, A.M.; Yang, G.; Wang, C.; Liu, Q.; et al. Simultaneous Suppression of the Dendrite Formation and Shuttle Effect in a Lithium–Sulfur Battery by Bilateral Solid Electrolyte Interface. Adv. Sci. 2018, 5, 1700934. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xiong, X.; Zhu, Y.; Chen, Y.; Fu, L.; Zhang, Y.; Wu, Y. Modifications of Separators for Li–S Batteries with Improved Electrochemical Performance. Russ. J. Electrochem. 2020, 56, 365–377. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, M.; Feng, J.; Mai, L.; Liu, G.; Guo, J. The Synergetic Interaction between LiNO3 and Lithium Polysulfides for Suppressing Shuttle Effect of Lithium-Sulfur Batteries. Energy Storage Mater. 2018, 11, 24–29. [Google Scholar] [CrossRef]
- Tomer, V.K.; Malik, R.; Tjong, J.; Sain, M. State and Future Implementation Perspectives of Porous Carbon-Based Hybridized Matrices for Lithium Sulfur Battery. Coord. Chem. Rev. 2023, 481, 215055. [Google Scholar] [CrossRef]
- Wang, M.; Xia, X.; Zhong, Y.; Wu, J.; Xu, R.; Yao, Z.; Wang, D.; Tang, W.; Wang, X.; Tu, J. Porous Carbon Hosts for Lithium–Sulfur Batteries. Chem. Eur. J. 2019, 25, 3710–3725. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H.-P. Graphene—How a Laboratory Curiosity Suddenly Became Extremely Interesting. Angew. Chem. Int. Ed. 2010, 49, 9332–9335. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Cheng, S.; Zhang, H.; Yang, W.; Yi, Z.; Zeng, Q.; Tang, B.; Ahmad, S.; Sun, T. Polarization Independent Tunable Bandwidth Absorber Based on Single-Layer Graphene. Diam. Relat. Mater. 2024, 142, 110793. [Google Scholar] [CrossRef]
- Jiang, B.; Hou, Y.; Wu, J.; Ma, Y.; Gan, X.; Zhao, J. In-Fiber Photoelectric Device Based on Graphene-Coated Tilted Fiber Grating. Opto-Electron. Sci. 2023, 2, 230012-1. [Google Scholar] [CrossRef]
- Zeng, C.; Lu, H.; Mao, D.; Du, Y.; Hua, H.; Zhao, W.; Zhao, J. Graphene-Empowered Dynamic Metasurfaces and Metadevices. Opto-Electron. Adv. 2022, 5, 200098. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and Graphene Oxide as Nanomaterials for Medicine and Biology Application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Hussain, S.Z.; Ismail, A.F.; Khan, I.U.; Inamuddin. Graphene and Its Derivatives: Synthesis, Modifications, and Applications in Wastewater Treatment. Environ. Chem. Lett. 2018, 16, 1301–1323. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y. (Eds.) Advances in Rechargeable Lithium–Sulfur Batteries; Springer International Publishing: Cham, Switzerland, 2022; Volume 59, ISBN 978-3-030-90898-0. [Google Scholar]
- Walle, M.D.; Zhang, Z.; Zhang, M.; You, X.; Li, Y.; Liu, Y.-N. Hierarchical 3D Nitrogen and Phosphorous Codoped Graphene/Carbon Nanotubes–Sulfur Composite with Synergistic Effect for High Performance of Lithium–Sulfur Batteries. J. Mater. Sci. 2018, 53, 2685–2696. [Google Scholar] [CrossRef]
- Rojaee, R.; Shahbazian-Yassar, R. Two-Dimensional Materials to Address the Lithium Battery Challenges. ACS Nano 2020, 14, 2628–2658. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, S.; Hou, Y. Graphene-Based Sulfur Composites for Energy Storage and Conversion in Li-S Batteries. Chin. J. Chem. 2016, 34, 13–31. [Google Scholar] [CrossRef]
- Tian, Y. Facile Hydrothermal Synthesis of Sulphur/Boron-Doped Reduced Graphene Oxide Composite Cathodes for High- Performance Li/S Batteries. Int. J. Electrochem. Sci. 2018, 13, 3441–3451. [Google Scholar] [CrossRef]
- Zhang, S.S. Heteroatom-Doped Carbons: Synthesis, Chemistry and Application in Lithium/Sulphur Batteries. Inorg. Chem. Front. 2015, 2, 1059–1069. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, W.; Zhao, W.; Li, G.; Hou, Y.; Liu, M.; Zhou, L.; Ye, F.; Li, H.; Wei, Z.; et al. High-Rate, Ultralong Cycle-Life Lithium/Sulfur Batteries Enabled by Nitrogen-Doped Graphene. Nano Lett. 2014, 14, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, Z.; Gordin, M.L.; Wang, D. Advanced Sulfur Cathode Enabled by Highly Crumpled Nitrogen-Doped Graphene Sheets for High-Energy-Density Lithium–Sulfur Batteries. Nano Lett. 2016, 16, 864–870. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, Y.-S.; Yang, X.-X.; Ren, M.-X.; Wang, Y.-Q.; Lei, B.-Y.; Zhao, D.-L. Sulfur Encapsulated in Nitrogen-Doped Graphene Aerogel as a Cathode Material for High Performance Lithium-Sulfur Batteries. Int. J. Hydrogen Energy 2021, 46, 7642–7652. [Google Scholar] [CrossRef]
- Kang, J.-H.; Chen, J.-S. Using Ethylenediamine to Prepare Three Dimensional Nitrogen-Doped Graphene Aerogel/Sulfur Composite for Lithium-sulfur Batteries. Diam. Relat. Mater. 2018, 88, 222–229. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Yin, L.; Koratkar, N.; Li, F.; Cheng, H.-M. Stabilizing Sulfur Cathodes Using Nitrogen-Doped Graphene as a Chemical Immobilizer for LiS Batteries. Carbon 2016, 108, 120–126. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Huang, C.; Zhou, Y.; Fan, B.; Li, Y.; Hu, A.; Tang, Q.; Huang, K. Redox-Active p-Phenylenediamine Functionalized Reduced Graphene Oxide Film through Covalently Grafting for Ultrahigh Areal Capacitance Zn-Ion Hybrid Supercapacitor. J. Power Sources 2021, 488, 229426. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Qu, Y.; Lai, Y.; Li, J. Nitrogen-Doped Graphene/Sulfur Composite as Cathode Material for High Capacity Lithium–Sulfur Batteries. J. Power Sources 2014, 256, 361–368. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, L.; Xu, M.; Li, Y.; Song, X.; Yin, L. Difference between Ammonia and Urea on Nitrogen Doping of Graphene Quantum Dots. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125703. [Google Scholar] [CrossRef]
- Kim, I.T.; Shin, M.W. Synthesis of Nitrogen-Doped Graphene via Simple Microwave-Hydrothermal Process. Mater. Lett. 2013, 108, 33–36. [Google Scholar] [CrossRef]
- Bonilla, Á.; Benítez, A.; Gómez-Cámer, J.L.; Caballero, Á. Impact of Composite Preparation Method on the Electrochemical Performance of Lithium–Sulfur Batteries. J. Alloys Compd. 2023, 968, 171810. [Google Scholar] [CrossRef]
- Luna-Lama, F.; Caballero, A.; Morales, J. Synergistic Effect between PPy:PSS Copolymers and Biomass-Derived Activated Carbons: A Simple Strategy for Designing Sustainable High-Performance Li–S Batteries. Sustain. Energy Fuels 2022, 6, 1568–1586. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Benítez, A.; Caballero, A.; Morales, J.; Hassoun, J.; Rodríguez-Castellón, E.; Canales-Vázquez, J. Physical Activation of Graphene: An Effective, Simple and Clean Procedure for Obtaining Microporous Graphene for High-Performance Li/S Batteries. Nano Res. 2019, 12, 759–766. [Google Scholar] [CrossRef]
- Das, A.; Chakraborty, B.; Sood, A.K. Raman Spectroscopy of Graphene on Different Substrates and Influence of Defects. Bull. Mater. Sci. 2008, 31, 579–584. [Google Scholar] [CrossRef]
- Benítez, A.; Di Lecce, D.; Caballero, Á.; Morales, J.; Rodríguez-Castellón, E.; Hassoun, J. Lithium Sulfur Battery Exploiting Material Design and Electrolyte Chemistry: 3D Graphene Framework and Diglyme Solution. J. Power Sources 2018, 397, 102–112. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M.; Aravindh, S.A.; Dabare, R.; Gao, X.; Jiang, Z.-T.; Wong, K.W. Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models. Nanomaterials 2022, 12, 1847. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical Porous Carbon from Mango Seed Husk for Electro-Chemical Energy Storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Schneider, P. Adsorption Isotherms of Microporous-Mesoporous Solids Revisited. Appl. Catal. Gen. 1995, 129, 157–165. [Google Scholar] [CrossRef]
- Qian, M.; Duan, M.; Gong, Z. Nitrogen-Doped Self-Shrinking Porous 3D Graphene Capacitor Deionization Electrode. Int. J. Energy Res. 2019, 43, 7583–7593. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental Review: Chemical Reduction of Graphene Oxide (GO) to Reduced Graphene Oxide (rGO) by Aqueous Chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef]
- Zegeye, T.A.; Tsai, M.C.; Cheng, J.H.; Lin, M.H.; Chen, H.M.; Rick, J.; Su, W.N.; Kuo, C.F.J.; Hwang, B.J. Controllable Embedding of Sulfur in High Surface Area Nitrogen Doped Three Dimensional Reduced Graphene Oxide by Solution Drop Impregnation Method for High Performance Lithium-Sulfur Batteries. J. Power Sources 2017, 353, 298–311. [Google Scholar] [CrossRef]
- Liu, P.; Li, G.; Chang, W.-T.; Wu, M.-Y.; Li, Y.-X.; Wang, J. Highly Dispersed Pd Nanoparticles Supported on Nitrogen-Doped Graphene with Enhanced Hydrogenation Activity. RSC Adv. 2015, 5, 72785–72792. [Google Scholar] [CrossRef]
- Farivar, F.; Lay Yap, P.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. C 2021, 7, 41. [Google Scholar] [CrossRef]
- Chen, J.-J.; Yuan, R.-M.; Feng, J.-M.; Zhang, Q.; Huang, J.-X.; Fu, G.; Zheng, M.-S.; Ren, B.; Dong, Q.-F. Conductive Lewis Base Matrix to Recover the Missing Link of Li2S8 during the Sulfur Redox Cycle in Li–S Battery. Chem. Mater. 2015, 27, 2048–2055. [Google Scholar] [CrossRef]
- Zu, C.; Manthiram, A. Hydroxylated Graphene–Sulfur Nanocomposites for High-Rate Lithium–Sulfur Batteries. Adv. Energy Mater. 2013, 3, 1008–1012. [Google Scholar] [CrossRef]
- Zhu, W.; Paolella, A.; Kim, C.-S.; Liu, D.; Feng, Z.; Gagnon, C.; Trottier, J.; Vijh, A.; Guerfi, A.; Mauger, A.; et al. Investigation of the Reaction Mechanism of Lithium Sulfur Batteries in Different Electrolyte Systems by in Situ Raman Spectroscopy and in Situ X-Ray Diffraction. Sustain. Energy Fuels 2017, 1, 737–747. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Z.; Lai, Y.; Liu, J.; Li, J.; Liu, Y. Electrochemical Impedance Spectroscopy Study of a Lithium/Sulfur Battery: Modeling and Analysis of Capacity Fading. J. Electrochem. Soc. 2013, 160, A553. [Google Scholar] [CrossRef]
- Carbone, L.; Coneglian, T.; Gobet, M.; Munoz, S.; Devany, M.; Greenbaum, S.; Hassoun, J. A Simple Approach for Making a Viable, Safe, and High-Performances Lithium-Sulfur Battery. J. Power Sources 2018, 377, 26–35. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Breitkopf, C. Determination of Diffusion Coefficients Using Impedance Spectroscopy Data. J. Electrochem. Soc. 2018, 165, E826–E831. [Google Scholar] [CrossRef]
- Soler-Piña, F.J.; Morales, J.; Caballero, Á. Synergy between Highly Dispersed Ni Nanocrystals and Graphitized Carbon Derived from a Single Source as a Strategy for High Performance Lithium-Sulfur Batteries. J. Colloid Interface Sci. 2023, 640, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xiang, K.; Zhu, Y.; Xiao, L.; Liao, H.; Chen, X.; Chen, H. Nb2O5-Decorated Nitrogen-Doped Carbon Nanotube Microspheres for Highly Efficient Sulfur Confinement in Lithium–Sulfur Batteries. Ind. Eng. Chem. Res. 2019, 58, 8724–8733. [Google Scholar] [CrossRef]
- Xiang, K.; Wen, X.; Hu, J.; Wang, S.; Chen, H. Rational Fabrication of Nitrogen and Sulfur Codoped Carbon Nanotubes/MoS2 for High-Performance Lithium–Sulfur Batteries. ChemSusChem 2019, 12, 3602–3614. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.-N.; Li, Y.-H.; Xu, D.-F.; Zhou, W.; Xiang, K.-X.; Chen, H. Three-Dimensional Hierarchically Porous Nitrogen-Doped Carbon from Water Hyacinth as Selenium Host for High-Performance Lithium–Selenium Batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Yin, L.-C.; Liang, J.; Zhou, G.-M.; Li, F.; Saito, R.; Cheng, H.-M. Understanding the Interactions between Lithium Polysulfides and N-Doped Graphene Using Density Functional Theory Calculations. Nano Energy 2016, 25, 203–210. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, H.; Ning, S.; Lin, J.; Wen, J.; Kang, X. Polypyrrole/Graphene Composite Interlayer: High Redox Kinetics of Polysulfides and Electrochemical Performance of Lithium–Sulfur Batteries Enabled by Unique Pyrrolic Nitrogen Sites. ChemElectroChem 2021, 8, 1798–1806. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Y.; Chen, Y.; Zhang, J.; Jiang, H.; Chen, X.; Zhang, Z.; Zeng, Y.; Sa, B.; Wei, Q.; et al. Reducing Polarization of Lithium-Sulfur Batteries via ZnS/Reduced Graphene Oxide Accelerated Lithium Polysulfide Conversion. Mater. Today Energy 2020, 18, 100519. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, Y.; Manthiram, A. Dual-Confined Flexible Sulfur Cathodes Encapsulated in Nitrogen-Doped Double-Shelled Hollow Carbon Spheres and Wrapped with Graphene for Li–S Batteries. Adv. Energy Mater. 2015, 5, 1402263. [Google Scholar] [CrossRef]






| Samples | SBET (m2 g−1) | VT (cm3 g−1) |
|---|---|---|
| GO | 160 | 0.16 |
| rGO | 128 | 0.28 |
| en-rGO | 136 | 0.17 |
| NH3-rGO | 214 | 0.27 |
| N Content (wt.%) | S Content (wt.%) | S Loading (mg cm−2) | C-Rate | Cycle Number | Ci (mAh g−1) | Cf (mAh g−1) | Capacity Fading (% Cycle−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 3.9 | 60 | 0.8 | 0.2C | 150 | 1200 | 750 | 0.25 | [27] |
| 6.53 | 80 | ~1.5 | 0.46C | 300 | 1150 | 850 | 0.09 | [28] |
| 9.23 | 75 | ND | 0.1C | 50 | 1200 | 880 | 0.53 | [29] |
| ND | 77 | 1.2 | 0.1C | 75 | 1250 | 1050 | 0.21 | [30] |
| 7.1 | 70 | 2 | 0.2C | 100 | 1200 | 850 | 0.29 | [31] |
| 9.21 | 70 | 2.5 | 0.1C | 250 | 1000 | 740 | 0.11 | This work |
| 9.41 | 70 | 2.5 | 0.2C | 800 | 1200 | 590 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licari, A.; Benítez, A.; Gómez-Cámer, J.L.; Trócoli, R.; Caballero, Á. Effect of Nitrogen Dopant Agents in the Performance of Graphene-Based Cathodes for Li-S Batteries. Nanomaterials 2024, 14, 489. https://doi.org/10.3390/nano14060489
Licari A, Benítez A, Gómez-Cámer JL, Trócoli R, Caballero Á. Effect of Nitrogen Dopant Agents in the Performance of Graphene-Based Cathodes for Li-S Batteries. Nanomaterials. 2024; 14(6):489. https://doi.org/10.3390/nano14060489
Chicago/Turabian StyleLicari, Adrián, Almudena Benítez, Juan Luis Gómez-Cámer, Rafael Trócoli, and Álvaro Caballero. 2024. "Effect of Nitrogen Dopant Agents in the Performance of Graphene-Based Cathodes for Li-S Batteries" Nanomaterials 14, no. 6: 489. https://doi.org/10.3390/nano14060489
APA StyleLicari, A., Benítez, A., Gómez-Cámer, J. L., Trócoli, R., & Caballero, Á. (2024). Effect of Nitrogen Dopant Agents in the Performance of Graphene-Based Cathodes for Li-S Batteries. Nanomaterials, 14(6), 489. https://doi.org/10.3390/nano14060489