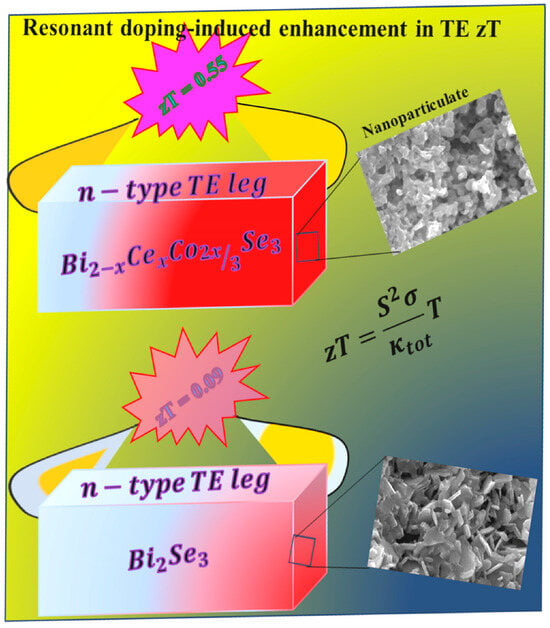
Eco-Friendly Cerium–Cobalt Counter-Doped Bi2Se3 Nanoparticulate Semiconductor: Synergistic Doping Effect for Enhanced Thermoelectric Generation
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Bi2−xCexSe3 Nanostructures (x = 0, 0.01 0.05, 0.15)
2.2. Characterization of Bi2−xCexSe3 Nanostructures
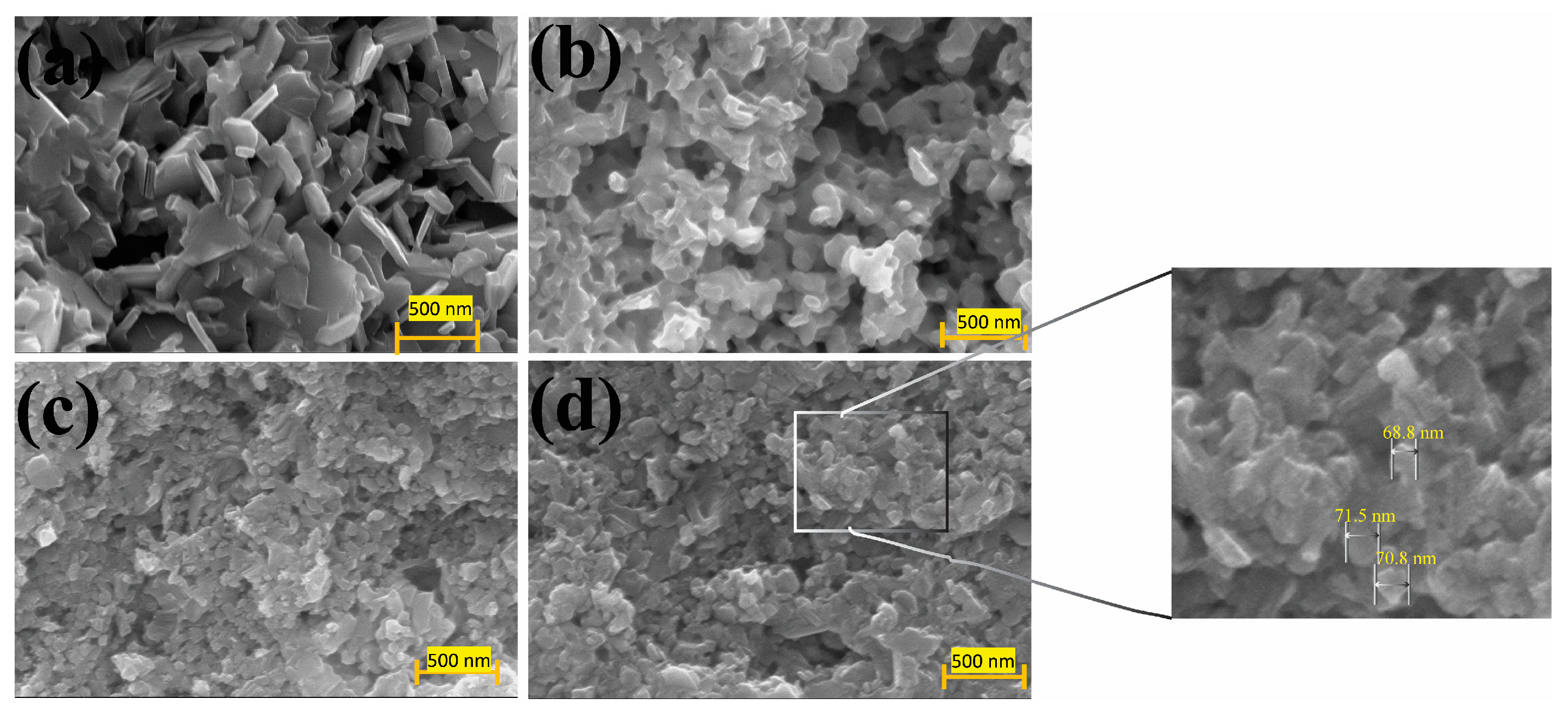
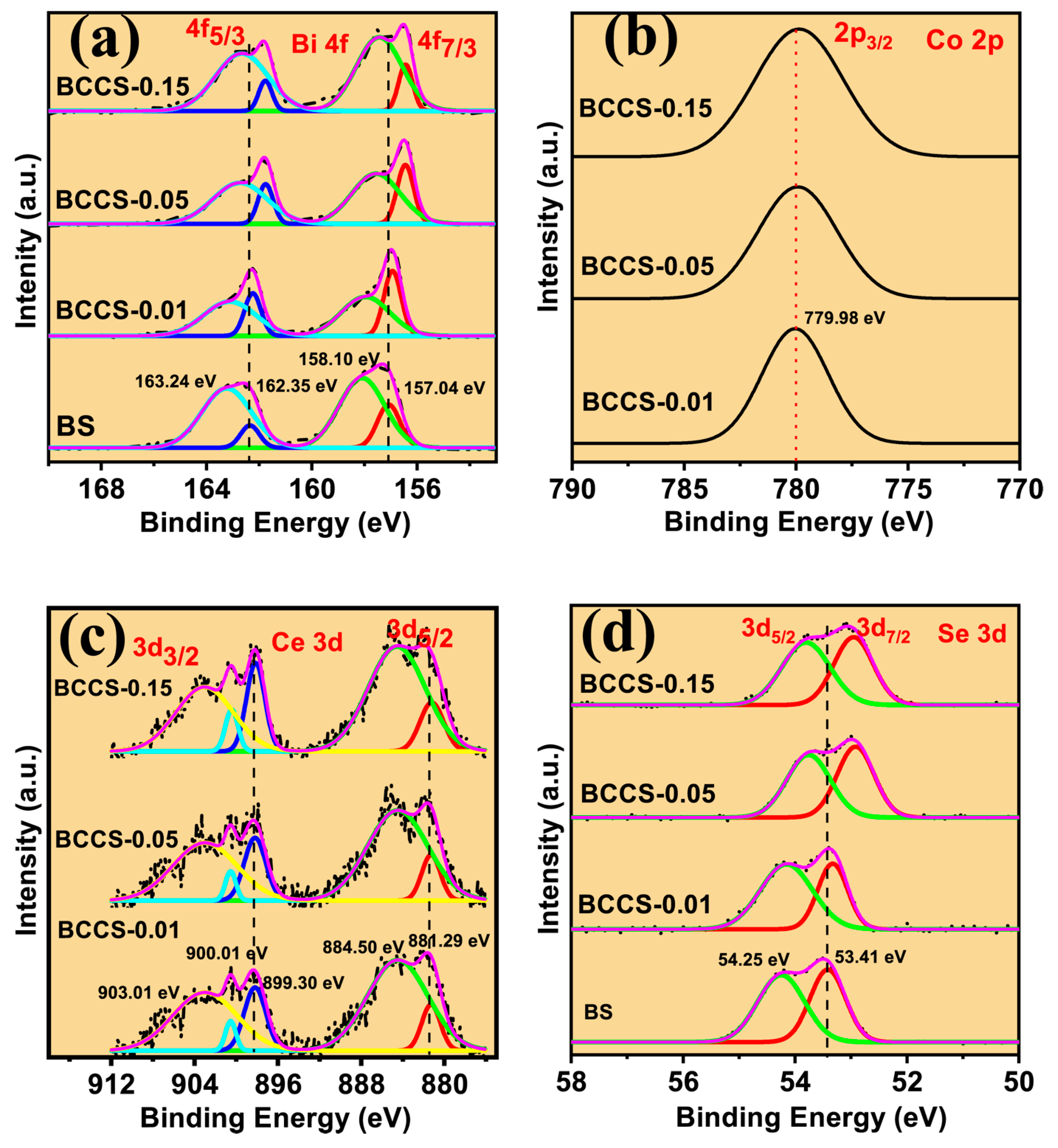
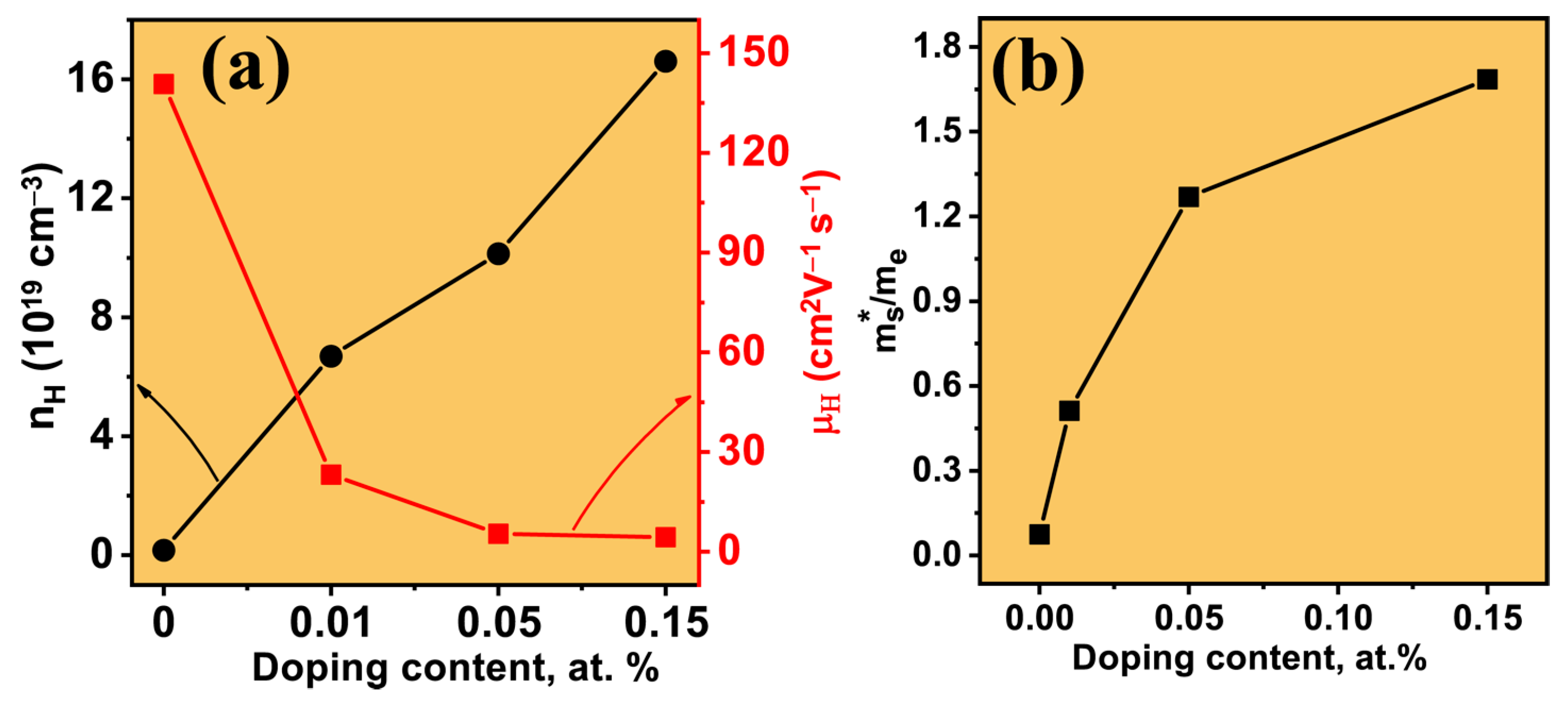
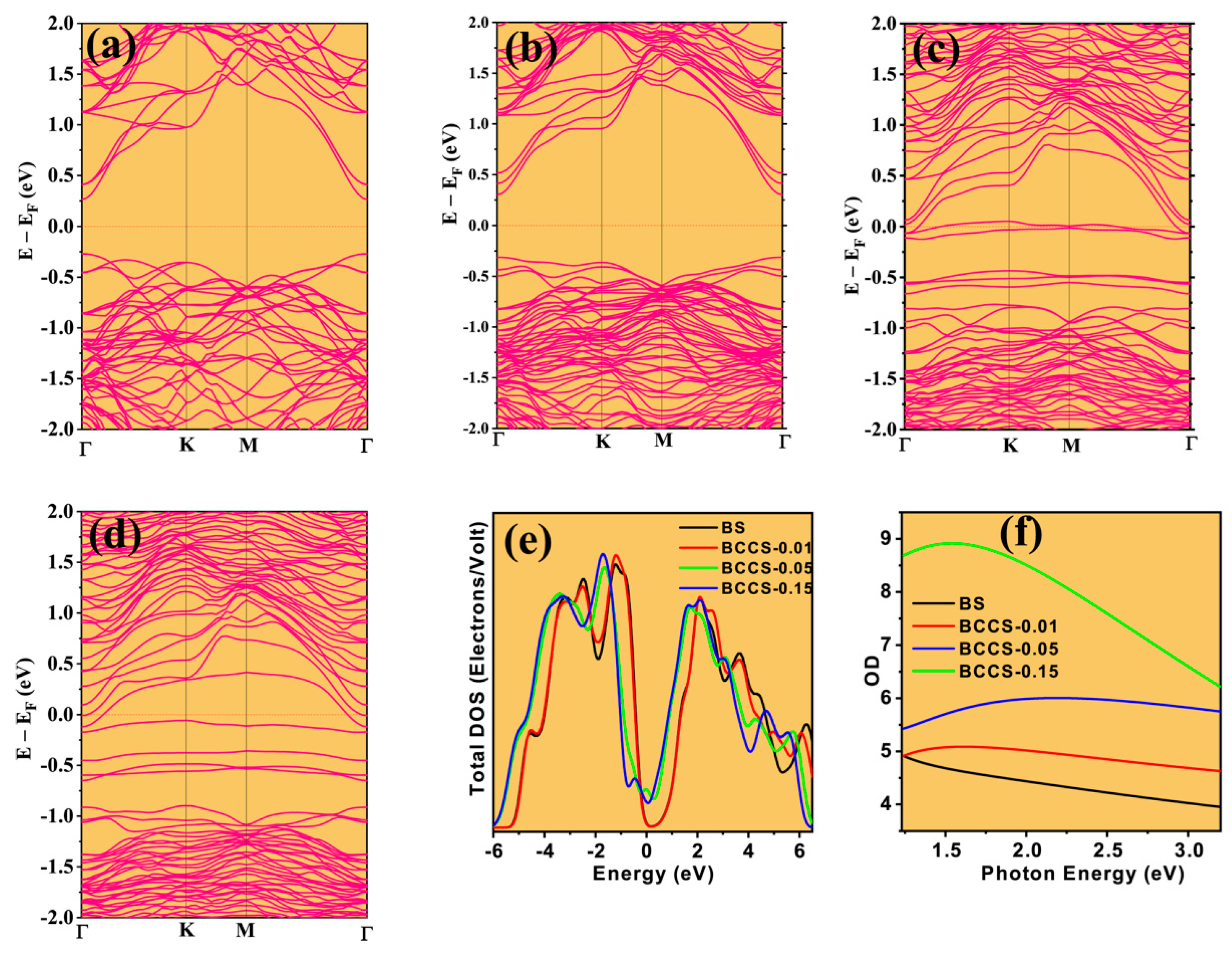
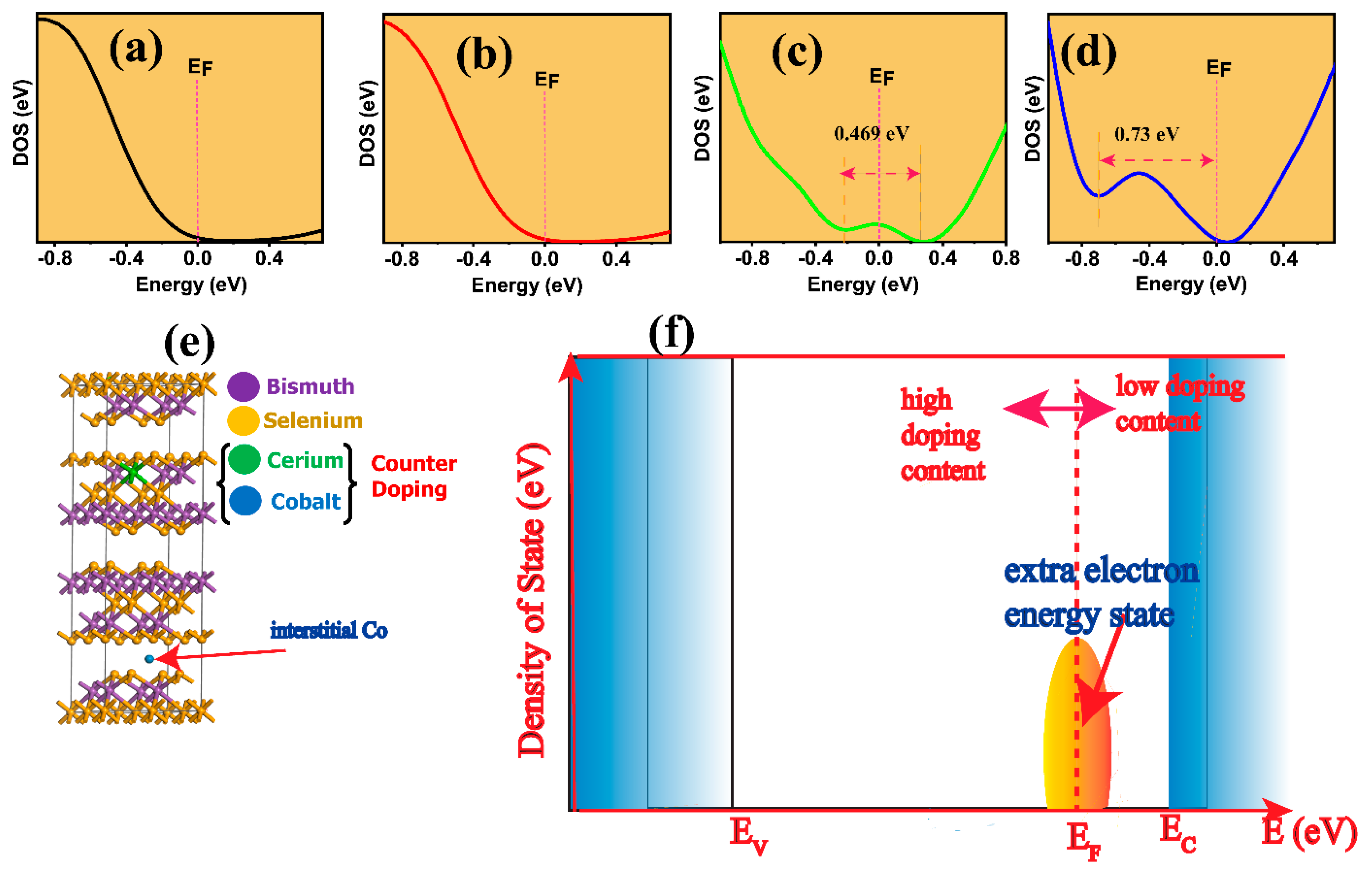
3. Results and Discussion
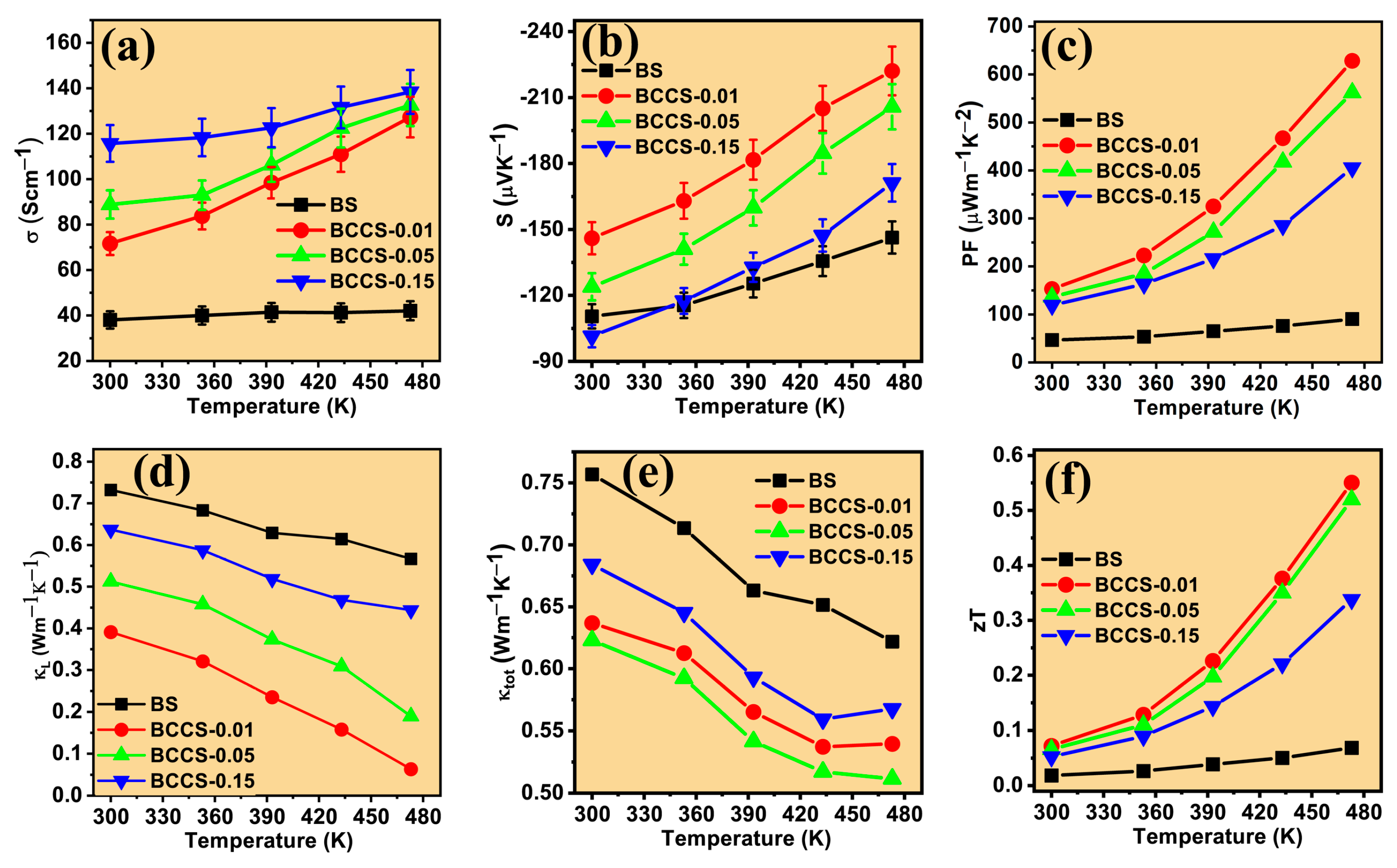
Thermoelectric Properties of the Co–Ce Counter Doping in Bi2Se3 Nanomaterials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Musah, J.-D.; Yanjun, X.; Ilyas, A.M.; Novak, T.G.; Jeon, S.; Arava, C.; Novikov, S.V.; Nikulin, D.S.; Xu, W.; Liu, L.; et al. Simultaneous enhancement of Thermopower and Electrical Conductivity through Isovalent Substitution of Cerium in Bismuth Selenide Thermoelectric Materials. ACS Appl. Mater. Interfaces 2019, 11, 44026–44035. [Google Scholar] [CrossRef]
- Musah, J.; Ilyas, A.M.; Novitskii, A.; Serhiienko, I.; Egbo, K.O.; Saianand, G.; Khovaylo, V.; Kwo, S.; Man, K.; Roy, V.A.L. Effective Decoupling of Seebeck Coefficient and the Electrical Conductivity through Isovalent Substitution of Erbium in Bismuth Selenide Thermoelectric Material. J. Alloys Compd. 2020, 857, 157559. [Google Scholar]
- Devender; Gehring, P.; Gaul, A.; Hoyer, A.; Vaklinova, K.; Mehta, R.J.; Burghard, M.; Borca-Tasciuc, T.; Singh, D.J.; Kern, K.; et al. Harnessing Topological Band Effects in Bismuth Telluride Selenide for Large Enhancement in Thermoelectric Properties through Isovalent Doping. Adv. Mater. 2016, 28, 6436–6441. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wang, H.; Snyder, G.J. Band Engineering of Thermoelectric Materials. Adv. Mater. 2012, 24, 6125–6135. [Google Scholar]
- Snyder, G.J.; Toberer, E.S. Complex Thermoelectric Materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Lee, K.H.; Mun, H.A.; Kim, H.S.; Hwang, S.W.; Roh, J.W.; Yang, D.J.; Shin, W.H.; Li, X.S.; Lee, Y.H.; et al. Dense Dislocation Arrays Embedded in Grain Boundaries for High-Performance Bulk Thermoelectrics. Science 2015, 348, 109–114. [Google Scholar] [CrossRef]
- Lee, J.K.; Oh, M.W.; Ryu, B.; Lee, J.E.; Kim, B.S.; Min, B.K.; Joo, S.J.; Lee, H.W.; Park, S.D. Enhanced Thermoelectric Properties of AgSbTe2 Obtained by Controlling Heterophases with Ce Doping. Sci. Rep. 2017, 7, 4496. [Google Scholar] [CrossRef]
- Zhao, L.D.; Wu, H.J.; Hao, S.Q.; Wu, C.I.; Zhou, X.Y.; Biswas, K.; He, J.Q.; Hogan, T.P.; Uher, C.; Wolverton, C.; et al. All-scale Hierarchical Thermoelectrics: MgTe in PbTe Facilitates Valence Band Convergence and Suppresses Bipolar Thermal Transport for High Performance. Energy Environ. Sci. 2013, 6, 3346–3355. [Google Scholar] [CrossRef]
- Kadel, K.; Kumari, L.; Li, W.Z.; Huang, J.Y.; Provencio, P.P. Synthesis and Thermoelectric Properties of Bi2Se3 Nanostructures. Nanoscale Res Lett 2011, 6, 57. [Google Scholar] [CrossRef]
- Sun, G.; Qin, X.; Li, D.; Zhang, J.; Ren, B.; Zou, T.; Xin, H.; Paschen, S.B.; Yan, X. Enhanced Thermoelectric Performance of n-type Bi2Se3 Doped with Cu. J. Alloys Compd. 2015, 639, 9–14. [Google Scholar] [CrossRef]
- Shikin, A.M.; Klimovskikh, I.I.; Eremeev, S.V.; Rybkina, A.A.; Rusinova, M.V.; Rybkin, A.G.; Zhizhin, E.V.; Sánchez-Barriga, J.; Varykhalov, A.; Rusinov, I.P.; et al. Electronic and Spin Structure of the Topological Insulator Bi2Te2.4Se0.6. Phys. Rev. B 2014, 89, 1–8. [Google Scholar] [CrossRef]
- Musah, J.-D.; Linlin, L.; Guo, C.; Novitskii, A.; Ilyas, A.O.; Serhiienko, I.; Khovaylo, V.; Roy, V.A.L.; Wu, C.-M.L. Enhanced Thermoelectric Performance of Bulk Bismuth Selenide: Synergistic Effect of Indium and Antimony co-doping. ACS Sustain. Chem. Eng. 2022, 10, 3862–3871. [Google Scholar]
- Lin, C.C.; Ginting, D.; Lydia, R.; Lee, M.H.; Rhyee, J.S. Thermoelectric Properties and Extremely Low Lattice Thermal Conductivity in p-type Bismuth Tellurides by Pb-doping and PbTe Precipitation. J. Alloys Compd. 2016, 671, 538–544. [Google Scholar]
- Theja, V.C.S.; Karthikeyan, V.; Musah, J.-D.; Wu, C.-M.L.; Roy, V.A.L. Thermoelectric Properties of Sulfide and Selenide-based Materials. In Sulfide and Selenide Based Materials for Emerging Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 293–328. [Google Scholar]
- Mohyedin, M.Z.; Taib, M.F.M.; Radzwan, A.; Mustaffa, M.; Shaari, A.; Hassan, O.H.; Ali, A.M.M.; Haq, B.U.; Yahya, M.Z.A. Enhanced Mechanism of Thermoelectric Performance of Bi2Se3 using Density Functional Theory. Mater. Renew. Sustain. Energy 2020, 9, 1–9. [Google Scholar]
- Zhang, J.; Zhong, S.; Ke, S.H. Enhanced Thermoelectric Performance in Sb-Br codoped Bi2Se3 with Complex Electronic Structure and Chemical Bonding Softening. RSC Adv. 2022, 12, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Singh, D.J. Potential Thermoelectric Performance from Optimization of Holes-doped Bi2Se3. Phys. Rev. X 2011, 1, 021005. [Google Scholar]
- Uchiyama, T.; Goto, H.; Uesugi, E.; Takai, A.; Zhi, L.; Miura, A.; Hamao, S.; Eguchi, R.; Ota, H.; Sugimoto, K.; et al. Semiconductor-metal Transition in Bi2Se3 cause by Impurity Doping. Sci. Rep. 2023, 13, 1–10. [Google Scholar]
- Uesugi, E.; Uchiyama, T.; Goto, H.; Ota, H.; Ueno, T.; Fujiwara, H.; Terashima, K.; Yokoya, T.; Matsui, F.; Akimitsu, J.; et al. Fermi Level Tuning of Ag-doped Bi2Se3 Topological Insulator. Sci. Rep. 2019, 9, 3–10. [Google Scholar] [CrossRef]
- Musah, J.-D.; Guo, C.; Novitskii, A.; Serhiienko, I.; Adesina, A.E.; Khovaylo, V.; Wu, C.L.; Zapien, J.A.; Roy, V.A.L. Ultralow Thermal Conductivity in Dual-doped n-type Bi2Te3 Material for Enhanced Thermoelectric Properties. Adv. Electron Mater. 2021, 7, 2000910. [Google Scholar] [CrossRef]
- Gautam, S.; Aggarwal, V.; Singh, B.; Awana, V.P.S.; Ganesan, R.; Kushvaha, S.S. Signature of Weak-Antilocalization in Sputtered Topological Insulator Bi2Se3 Thin Films with Varying Thickness. Sci. Rep. 2022, 12, 9770. [Google Scholar]
- Iovu, M.S.; Kamitsos, E.I.; Varsamis, C.P.E.; Boolchand, P.; Popescu, M. Raman Spectra of AsxSe100-x and As40Se60 Glasses Doped with Metals. Chalcogenide Lett. 2005, 2, 21–25. [Google Scholar]
- Sheldrick, W.S. Zeitschrift Fur Anorg. Und Allg. Chemie 2012, 638, 2401–2424. [Google Scholar]
- Verma, A. Electronic and Optical Properties of Rare-earth Chalcogenides and Pnictides. African Rev. Phys. 2009, 3, 11–20. [Google Scholar]
- Zeemann, J.J. Crystal Structures, vol.2 by Rwg Wyckoff. Acta Crystallographica. 1965, 19, 490. [Google Scholar] [CrossRef]
- Hellman, O.; Broido, D.A. Phonon Thermal Transport in Bi2Te3 from First Principles. Phys. Rev. B. 2014, 90, 134309. [Google Scholar] [CrossRef]
- Wang, L.; Zhuang, L.; Xin, H.; Huang, Y.; Wang, D. Semi Quantitative Estimation of Ce3+/ Ce4+ ratio in YAG: Ce3+ Phosphor under Different Sintering Atmosphere. Open J. Inorg. Chem. 2015, 5, 12–18. [Google Scholar] [CrossRef]
- Surviliene, S.; Jasulaitiene, V.; Češuniene, A.; Lisowska-Oleksiak, A. The use of XPS for Study of the Surface Layers of Cr-Co Alloy Electrodeposited from Cr(III) Formate-urea Baths. Solid State Ion. 2008, 179, 222–227. [Google Scholar] [CrossRef]
- Meng, X.; Deng, J.; Zhu, J.; Bi, H.; Kan, E.; Wang, X. Cobalt Sulfide/Graphene Composite Hydrogel as Electrode for High Performance Pseudocapacitors. Sci. Rep. 2016, 6, 21717. [Google Scholar] [CrossRef]
- Feng, T.; Zhao, T.; Zhu, S.; Zhang, N.; Wei, Z.; Wang, K.; Li, L.; Wu, F.; Chen, R. Anion Doped Cobalt Selenide with Porous Architechture for High rate and Flexible Lithium-Sulfur Batteries. Small Methods 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Rowe, E.D.M.; Ph, D.; Sc, D.; Group, F. Handbook of Thermoelectrics: Macro to Nano; CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Kumar, A.S.O.; Shukla, V.; Srivastava, S.K. Role of Electronegativity on the Bulk Modulus, Magnetic Moment and Band gap of Co2MnAl based Heusler Alloys. J. Sci. Adv. Mater. Devices 2019, 4, 158–162. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Muthusamy, C. Role of Ionic Radii and Electronegativity of co-deopants (Co, Ni and Cr) on Properties of Cu Doped ZnO and Evaluation of In-vitro Cytotoxicity. Surf. Interfaces 2022, 30, 101968. [Google Scholar]
- Zhou, A.; Liu, L.S.; Zhai, P.C.; Zhao, W.Y.; Zhang, Q.J. Electronic Structures and Transport Properties of Single Filled CoSb3. J. Electron. Mater. 2010, 39, 1832–1836. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.H.; Back, S.Y.; Ahn, K.; Rhyee, J.S.; Park, S.D. Enhancement of Thermoelectric Properties in CuI-Doped Bi2Te2.7Se0.3 by Hot Deformation. J. Alloys Compd. 2018, 731, 531–536. [Google Scholar] [CrossRef]
- Pan, L.; Mitra, S.; Zhao, L.D.; Shen, Y.; Wang, Y.; Felser, C.; Berardan, D. The Role of Ionized Impurity Scattering on the Thermoelectric Performances of Rock Salt AgPbmSnSe2+m. Adv. Funct. Mater. 2016, 26, 5149–5157. [Google Scholar] [CrossRef]
- Ma, J.; Xin, X.Y.; Liu, H.Q.; Gu, Y.J.; Wang, Y.F. Origin of Improved Average Power Factor and Mechanical Properties of SnTe with High Dose Bi2Te3 Alloying. Ceram. Int. 2023, 49, 21916–21922. [Google Scholar] [CrossRef]
- Kulbachinskii, V.A.; Kytin, V.G.; Kudryashov, A.A.; Tarasov, P.M. Thermoelectric Properties of Bi2Te3, Sb2Te3 and Bi2Se3 Single Crystals with Magnetic Impurities. J. of solid State Chem. 2012, 193, 47–52. [Google Scholar] [CrossRef]
- Tang, Y.; Gibbs, Z.M.; Agapito, L.A.; Li, G.; Kim, H.S.; Nardelli, M.B.; Curtarolo, S.; Snyder, G.J. Convergence of Multi-valley Bands as the Electronic Origin of High Thermoelectric Performance in CoSb3 Skutterudites. Nat. Mater. 2015, 14, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Maitra, S.; Chatterjee, M.J.; Chattopadhyaya, M.; Kargupta, K.; Banerjee, D. Polypyrrole-bismuth Selenide (PPY-Bi2Se3) Composite: Thermoelectric Characterization and Effect of Nickel Doping. Synth. Met. 2022, 289, 117119. [Google Scholar] [CrossRef]
- Nolan, M.; Grigoleit, S.; Sayle, D.C.; Parker, S.C.; Watson, G.W. Density Funtional Theory Studies of the Structure and Electronic Structure of Pure and Defective low Index Surface of Ceria. Surface Science 2005, 576, 217–229. [Google Scholar] [CrossRef]
- Borah, D.; Baruah, M.K.; Saika, P.P.; Senapoty, K.K.; Barua, M.; Singha, R. Structural Characterization and Surface Environment of ZnO Nanoflowers. J. Mater. Environ. Sci. 2016, 7, 331–336. [Google Scholar]
- Kim, H.S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz Number with Seebeck Coefficient Measurement. APL Mater. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS Investigation of Cerium Oxides and Mixed Cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Paparazzo, E. On the Number, Binding Energies, and Mutual Intensities of Ce3d Peaks in the XPS Analysis of Cerium Oxide Systems: A Response to Murugan et al. Superlatt. Microstruct. 2015, 85, 321. Superlattices Microstruct. 2017, 105, 216–220. [Google Scholar] [CrossRef]
- Vijay, V.; Harish, S.; Archana, J.; Navaneethan, M. Synergistic Effect of Grain Boundaries and Phonon Engineering in Sb Substituted Bi2Se3 Nanostructures for Thermoelectric Applications. J. Colloid Interface Sci. 2022, 612, 97–110. [Google Scholar] [CrossRef]
- Hegde, G.S.; Prabhu, A.N.; Gao, Y.H.; Kuo, Y.K.; Reddy, V.R. Potential Thermoelectric Materials of Indium and Tellurium co-doped Bismuth Selenide Single Crystals Grown by Melt Growth Technique. J. Alloys Compd. 2021, 866, 158814. [Google Scholar] [CrossRef]
- Min, Y.; Roh, J.W.; Yang, H.; Park, M.; Kim, S.I.; Hwang, S.; Lee, S.M.; Lee, K.H.; Jeong, U. Surfactant-Free Scalable Synthesis of Bi2Te3 and Bi2Se3 Nanoflakes and Enhanced Thermoelectric Properties of their Nanocomposites. Adv. Mater. 2013, 25, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Dedi; Lee, P.C.; Wei, P.C.; Chen, Y.Y. Thermoelectric Characteristics of A Single-Crystalline Topological Insulator Bi2Se3 Nanowire. Nanomaterials 2021, 11, 819. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musah, J.-D.; Or, S.W.; Kong, L.; Roy, V.A.L.; Wu, C.-M.L. Eco-Friendly Cerium–Cobalt Counter-Doped Bi2Se3 Nanoparticulate Semiconductor: Synergistic Doping Effect for Enhanced Thermoelectric Generation. Nanomaterials 2023, 13, 2738. https://doi.org/10.3390/nano13202738
Musah J-D, Or SW, Kong L, Roy VAL, Wu C-ML. Eco-Friendly Cerium–Cobalt Counter-Doped Bi2Se3 Nanoparticulate Semiconductor: Synergistic Doping Effect for Enhanced Thermoelectric Generation. Nanomaterials. 2023; 13(20):2738. https://doi.org/10.3390/nano13202738
Chicago/Turabian StyleMusah, Jamal-Deen, Siu Wing Or, Lingyan Kong, Vellaisamy A. L. Roy, and Chi-Man Lawrence Wu. 2023. "Eco-Friendly Cerium–Cobalt Counter-Doped Bi2Se3 Nanoparticulate Semiconductor: Synergistic Doping Effect for Enhanced Thermoelectric Generation" Nanomaterials 13, no. 20: 2738. https://doi.org/10.3390/nano13202738
APA StyleMusah, J.-D., Or, S. W., Kong, L., Roy, V. A. L., & Wu, C.-M. L. (2023). Eco-Friendly Cerium–Cobalt Counter-Doped Bi2Se3 Nanoparticulate Semiconductor: Synergistic Doping Effect for Enhanced Thermoelectric Generation. Nanomaterials, 13(20), 2738. https://doi.org/10.3390/nano13202738