Wide Response Range Photoelectrochemical UV Detector Based on Anodized TiO2-Nanotubes@Ti@quartz Structure
Abstract
1. Introduction
2. Materials and Methods
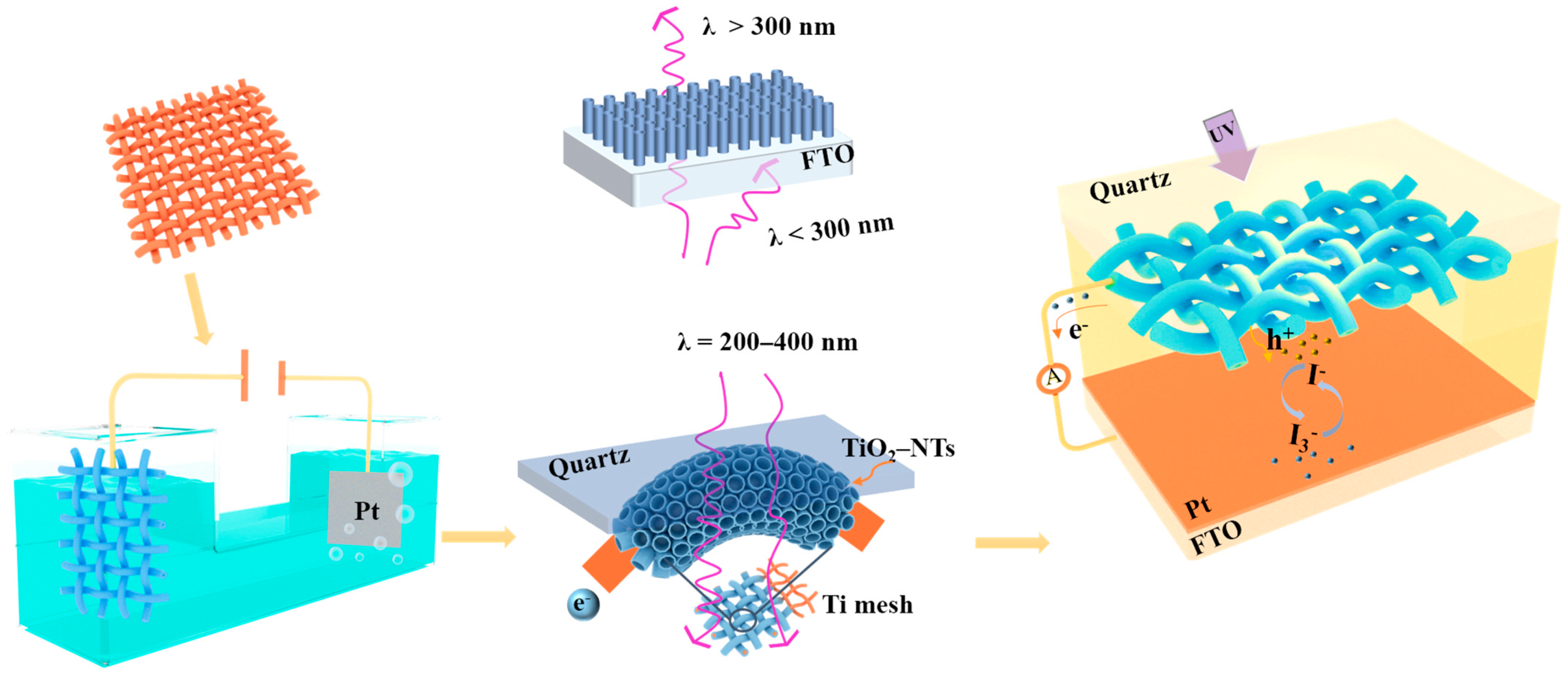
2.1. Preparation of Photoanodes
2.2. Encapsulation of PEC UV Detectors
2.3. Characterization
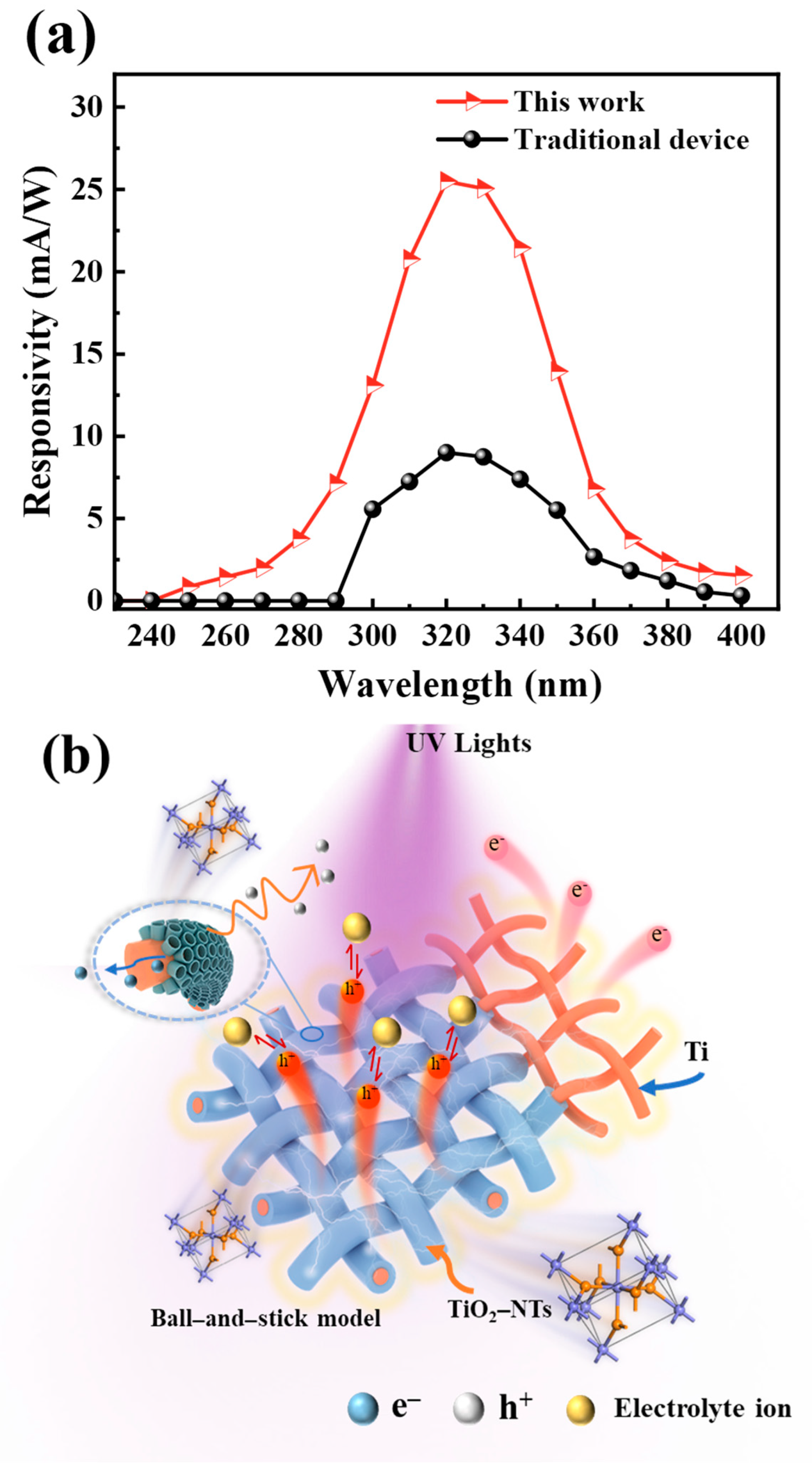
3. Results and Discussion
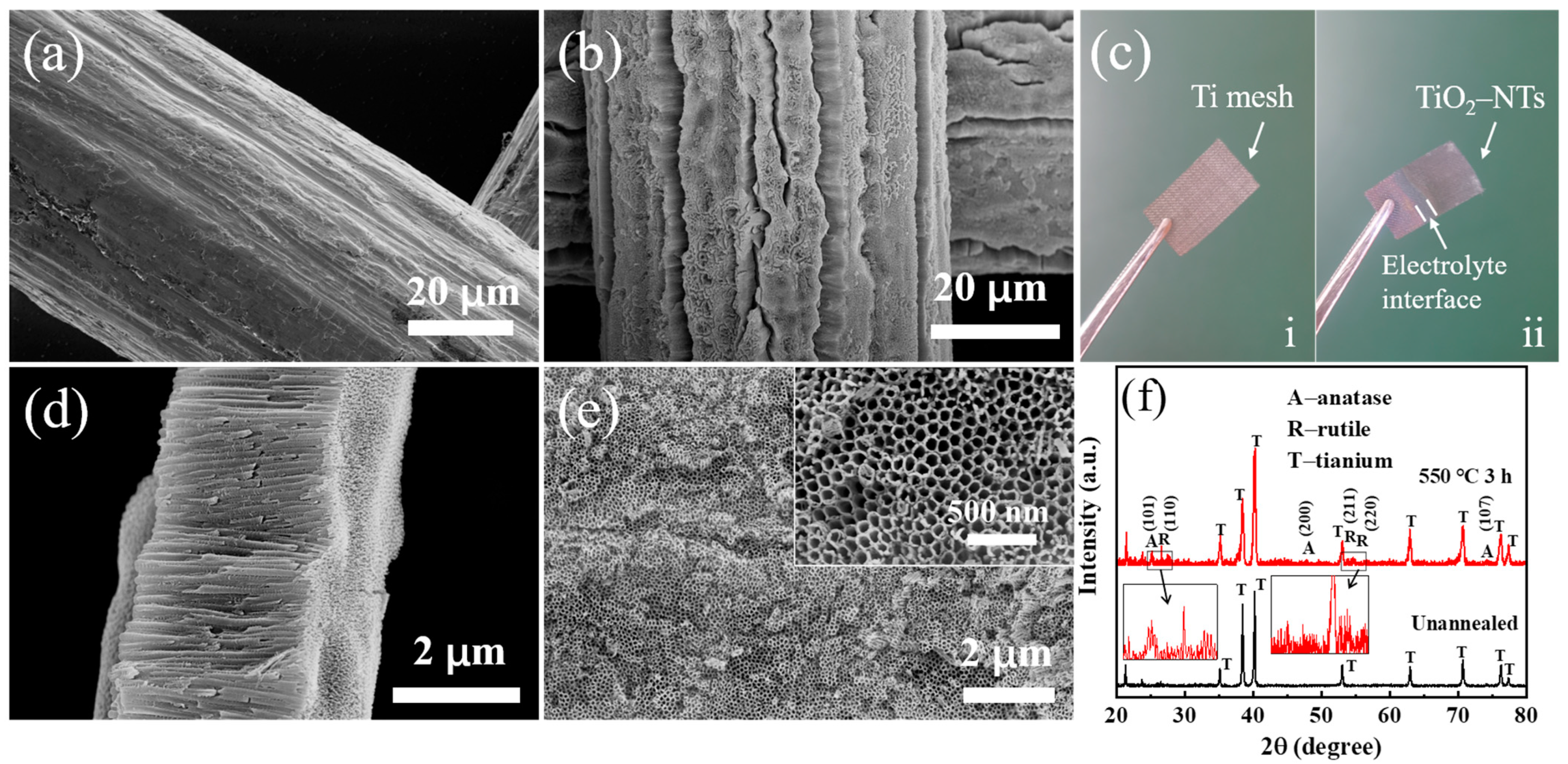
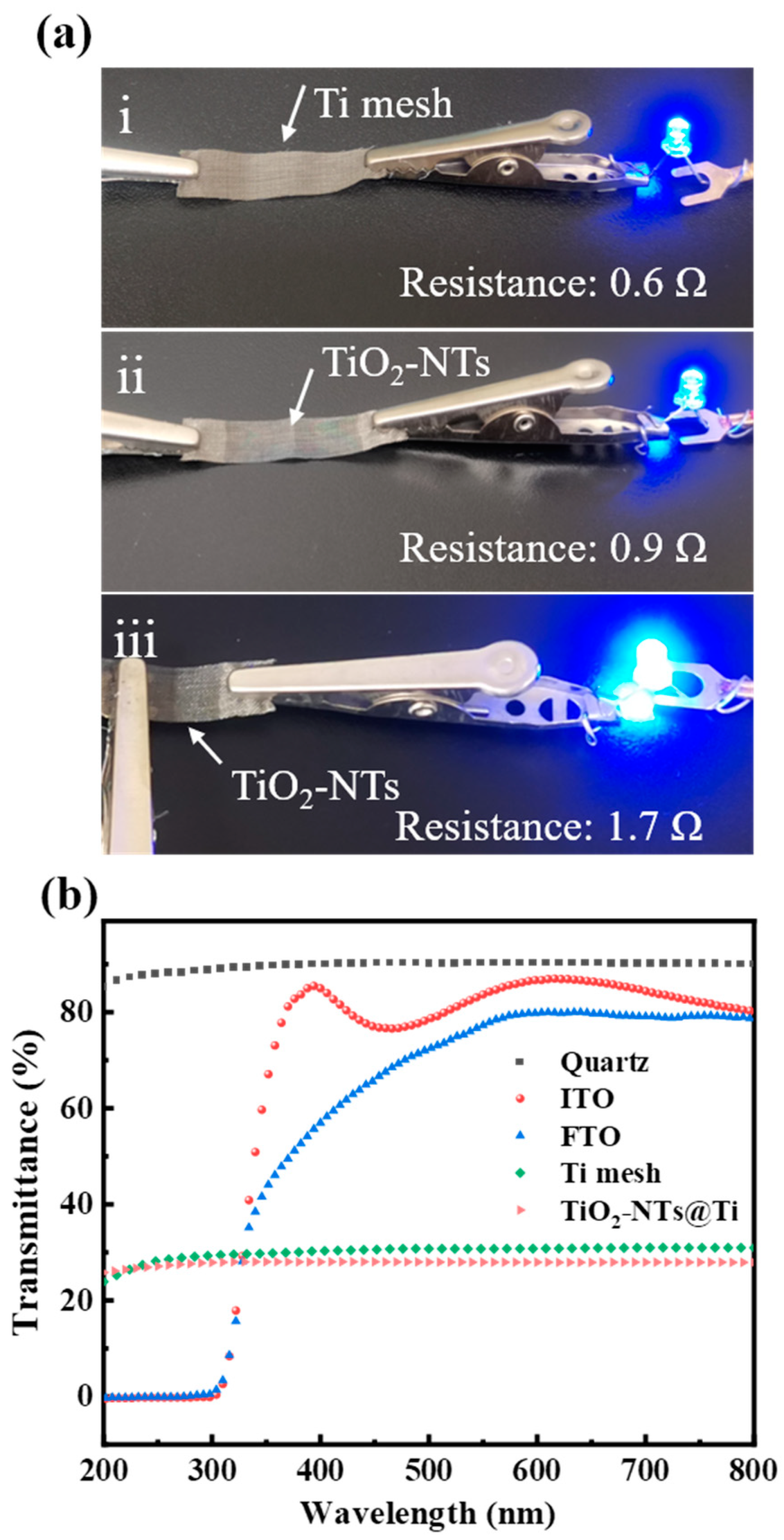
3.1. Characteristics of TiO2-NTs@Ti Mesh Photoanode
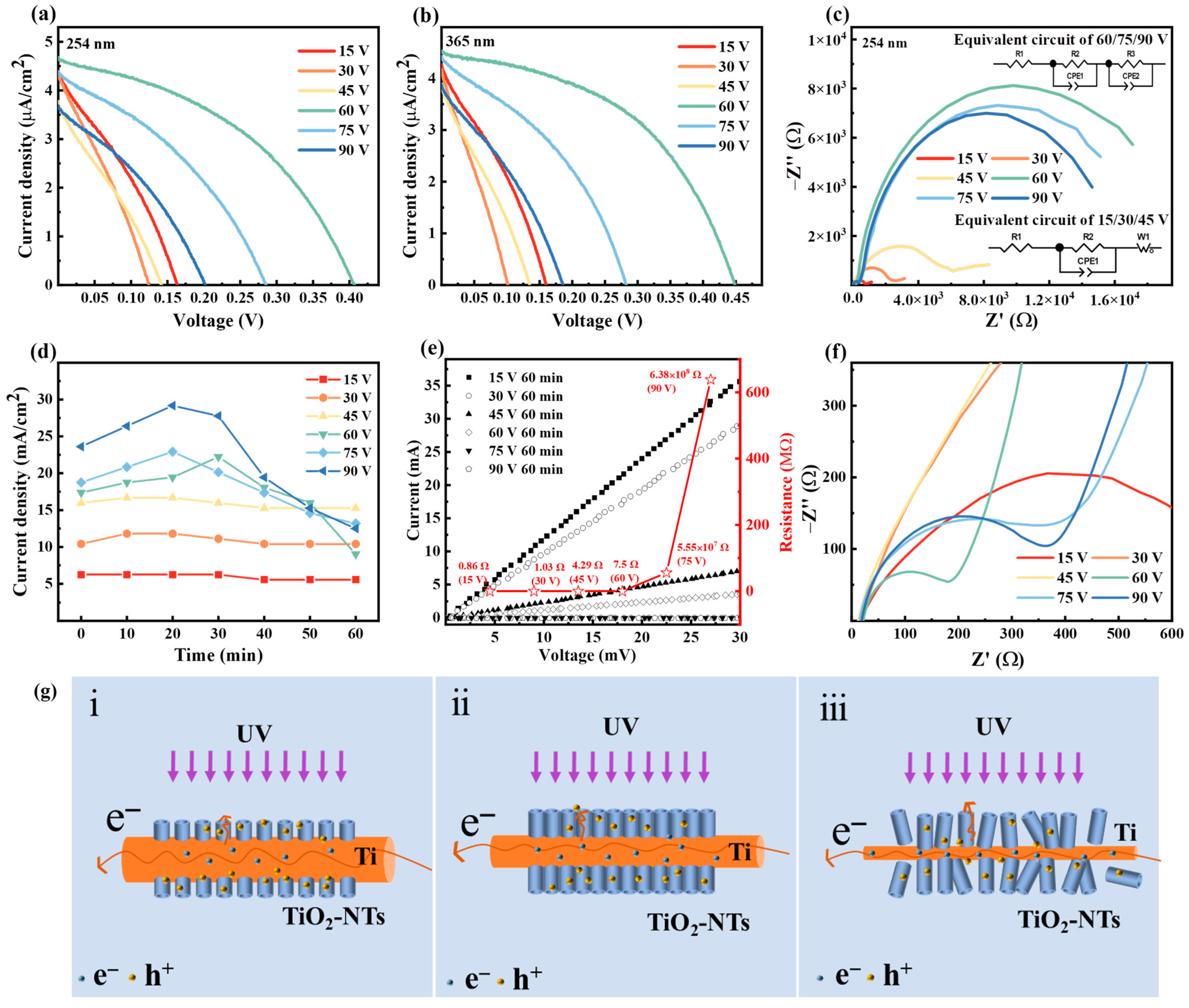
3.2. Performance of PEC UV Detectors
3.3. Extension of the Detection Range
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Sadler, B.M. Ultraviolet communications: Potential and state-of-the-art. IEEE Commun. Mag. 2008, 46, 67–73. [Google Scholar]
- Cai, S.; Zuo, C.; Zhang, J.; Liu, H.; Fang, X. A paper-based wearable photodetector for simultaneous UV intensity and dosage measurement. Adv. Funct. Mater. 2021, 31, 2100026. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y. Self-powered UV–visible photodetectors based on ZnO/Cu2O nanowire/electrolyte heterojunctions. J. Alloys Compd. 2016, 675, 325–330. [Google Scholar] [CrossRef]
- Yang, D.; Ma, D. Development of Organic Semiconductor Photodetectors: From Mechanism to Applications. Adv. Opt. Mater. 2019, 7, 1800522. [Google Scholar] [CrossRef]
- Dastkhoon, M.; Ghaedi, M.; Asfaram, A.; Arabi, M.; Ostovan, A.; Goudarzi, A. Cu@ SnS/SnO2 nanoparticles as novel sorbent for dispersive micro solid phase extraction of atorvastatin in human plasma and urine samples by high-performance liquid chromatography with UV detection: Application of central composite design (CCD). Ultrason. Sonochem. 2017, 36, 42–49. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, W.; Yang, W.; He, J.H.; Fang, X. Recent Progress of Heterojunction Ultraviolet Photodetectors: Materials, Integrations, and Applications. Adv. Funct. Mater. 2020, 30, 1909909. [Google Scholar] [CrossRef]
- Guo, D.; Su, Y.; Shi, H.; Li, P.; Zhao, N.; Ye, J.; Wang, S.; Liu, A.; Chen, Z.; Li, C.; et al. Self-Powered Ultraviolet Photodetector with Superhigh Photoresponsivity (3.05 A/W) Based on the GaN/Sn:Ga2O3 pn Junction. ACS Nano 2018, 12, 12827–12835. [Google Scholar] [CrossRef]
- Razeghi, M.; Rogalski, A. Semiconductor ultraviolet detectors. J. Appl. Phys. 1996, 79, 7433–7473. [Google Scholar] [CrossRef]
- Omnès, F.; Monroy, E.; Muñoz, E.; Reverchon, J.-L. Wide bandgap UV photodetectors: A short review of devices and applications. Gallium Nitride Mater. Devices 2007, 6473, 111–125. [Google Scholar]
- Ouyang, W.; Chen, J.; Shi, Z.; Fang, X. Self-powered UV photodetectors based on ZnO nanomaterials. Appl. Phys. Rev. 2021, 8, 031315. [Google Scholar] [CrossRef]
- Wang, X.; Ding, K.; Huang, L.; Li, X.; Ye, L.; Luo, J.; Jiang, J.; Li, H.; Xiong, Y.; Ye, L.J.A.S.S. Enhancing the performance of Self-Powered Deep-Ultraviolet photoelectrochemical photodetectors by constructing α-Ga2O3@ a-Al2O3 Core-Shell nanorod arrays for Solar-Blind imaging. Appl. Surf. Sci. 2024, 648, 159022. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, H.; Li, H.; Jiang, Y.; Qu, L.; Wang, Y.; Gao, F.; Feng, W. Ultrathin In2O3 Nanosheets toward High Responsivity and Rejection Ratio Visible-Blind UV Photodetection. Small 2023, 19, 2205623. [Google Scholar] [CrossRef]
- Ni, S.; Guo, F.; Wang, D.; Liu, G.; Xu, Z.; Kong, L.; Wang, J.; Jiao, S.; Zhang, Y.; Yu, Q.; et al. Effect of MgO Surface Modification on the TiO2 Nanowires Electrode for Self-Powered UV Photodetectors. ACS Sustain. Chem. Eng. 2018, 6, 7265–7272. [Google Scholar] [CrossRef]
- Wei, K.; Li, B.; Gong, C.; Zhu, Z.; Zhang, Y.; Liu, M.; Ren, P.; Pan, X.; Wang, Y.; Zhou, J. Full near-ultraviolet response photoelectrochemical ultraviolet detector based on TiO2 nanocrystalline coated stainless steel mesh photoanode. Nanotechnology 2021, 32, 475503. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Ren, P.; Liu, M.; Gong, Z.; Zhang, M.; Zhao, H.; Zhang, Y.; Zhang, Y.; Liu, J. Expansion of the response range of photoelectrochemical UV detector using an ITO/Ag-nanowire/quartz UV-visible transparent conductive electrode. J. Mater. Chem. C 2022, 10, 4157–4165. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Wang, Y.; He, Y.; Pan, X.; Xie, E. An overview on emerging photoelectrochemical self-powered ultraviolet photodetectors. Nanoscale 2016, 8, 50–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, D.; Wu, Z.; Liu, Z. Free-standing TiO2 nanotube arrays made by anodic oxidation and ultrasonic splitting. Nanotechnology 2008, 19, 365708. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Moon, S.; Patil, S.S.; Lee, K. Visible photoresponse of TiO2 nanotubes in comparison to that of nanoparticles and anodic thin film. Catal. Today 2022, 403, 39–46. [Google Scholar] [CrossRef]
- Mohammadi, N.; Abrego-Martinez, J.C.; Mohamedi, M. Three-Dimensional Pinecone-like Binder-Free Pt-TiO2 Nanorods on Ti Mesh Structures: Synthesis, Characterization and Electroactivity towards Ethanol Oxidation. Molecules 2022, 27, 1921. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. Engl. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Guo, F.; Liu, J.; Yang, F. Kinetic analysis of the anodic growth of TiO2 nanotubes: Effects of voltage and temperature. J. Mater. Chem. C 2019, 7, 14098–14108. [Google Scholar] [CrossRef]
- Xiao, F.X. Construction of highly ordered ZnO-TiO2 nanotube arrays (ZnO/TNTs) heterostructure for photocatalytic application. ACS Appl. Mater. Interfaces 2012, 4, 7055–7063. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Z. Anodic formation of ordered TiO2 nanotube arrays: Effects of electrolyte temperature and anodization potential. J. Phys. Chem. C 2009, 113, 4026–4030. [Google Scholar] [CrossRef]
- Hou, X.; Wang, X.; Liu, B.; Wang, Q.; Wang, Z.; Chen, D.; Shen, G. SnO2@ TiO2 Heterojunction Nanostructures for Lithium-Ion Batteries and Self-Powered UV Photodetectors with Improved Performances. ChemElectroChem 2014, 1, 108–115. [Google Scholar] [CrossRef]
- Liu, Z.; Subramania, V.; Misra, M.J.T.J.O.P.C.C. Vertically oriented TiO2 nanotube arrays grown on Ti meshes for flexible dye-sensitized solar cells. J. Phys. Chem. C 2009, 113, 14028–14033. [Google Scholar] [CrossRef]
- Liao, J.; Lin, S.; Zhang, L.; Pan, N.; Cao, X.; Li, J. Photocatalytic degradation of methyl orange using a TiO2/Ti mesh electrode with 3D nanotube arrays. ACS Appl. Mater. Interfaces 2012, 4, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Rustomji, C.S.; Frandsen, C.J.; Jin, S.; Tauber, M.J. Dye-sensitized solar cell constructed with titanium mesh and 3-D array of TiO2 nanotubes. J. Phys. Chem. B 2010, 114, 14537–14543. [Google Scholar] [CrossRef]
- Kuang, D.; Brillet, J.; Chen, P.; Takata, M.; Uchida, S.; Miura, H.; Sumioka, K.; Zakeeruddin, S.M.; Grätzel, M.J.A. Application of highly ordered TiO2 nanotube arrays in flexible dye-sensitized solar cells. ACS Nano 2008, 2, 1113–1116. [Google Scholar] [CrossRef]
- Varghese, O.K.; Gong, D.; Paulose, M.; Grimes, C.A.; Dickey, E.C. Crystallization and high-temperature structural stability of titanium oxide nanotube arrays. J. Mater. Res. 2011, 18, 156–165. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Aldalbahi, A.; Ahamad, T.; Alshehri, S.M.; Feng, P.X. Preparation of BiFeO3 and its photoelectric performance as photoanode of DSSC. Ceram. Int. 2021, 47, 27565–27570. [Google Scholar] [CrossRef]
- Zare, A.; Behaein, S.; Moradi, M.; Hosseini, Z. Application of a dual functional blocking layer for improvement of the responsivity in a self-powered UV photodetector based on TiO2 nanotubes. RSC Adv. 2022, 12, 9909–9916. [Google Scholar] [CrossRef] [PubMed]
- Nayak, J.; Prabakar, K.; Park, J.W.; Kim, H. Effect of synthesis temperature on structure, optical and photovoltaic properties of TiO2 nanorod thin films. Electrochim. Acta 2012, 65, 44–49. [Google Scholar] [CrossRef]
- Bisquert, J. Theory of the impedance of electron diffusion and recombination in a thin layer. J. Phys. Chem. B 2002, 106, 325–333. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Garcia-Belmonte, G.; Bisquert, J.; Zaban, A.; Salvador, P. Decoupling of transport, charge storage, and interfacial charge transfer in the nanocrystalline TiO2/electrolyte system by impedance methods. J. Phys. Chem. B 2002, 106, 334–339. [Google Scholar] [CrossRef]
- Fu, A.; Chen, X.; Tong, L.; Wang, D.; Liu, L.; Ye, J. Remarkable Visible-Light Photocatalytic Activity Enhancement over Au/p-type TiO2 Promoted by Efficient Interfacial Charge Transfer. ACS Appl. Mater. Interfaces 2019, 11, 24154–24163. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, M.; Wu, W.; Wang, Z.; Liu, M.; Yang, T.; Renqianzhuoma. Wide Response Range Photoelectrochemical UV Detector Based on Anodized TiO2-Nanotubes@Ti@quartz Structure. Nanomaterials 2024, 14, 439. https://doi.org/10.3390/nano14050439
Wang Y, Zhang M, Wu W, Wang Z, Liu M, Yang T, Renqianzhuoma. Wide Response Range Photoelectrochemical UV Detector Based on Anodized TiO2-Nanotubes@Ti@quartz Structure. Nanomaterials. 2024; 14(5):439. https://doi.org/10.3390/nano14050439
Chicago/Turabian StyleWang, Youqing, Miaomiao Zhang, Wenxuan Wu, Ze Wang, Minghui Liu, Tiantian Yang, and Renqianzhuoma. 2024. "Wide Response Range Photoelectrochemical UV Detector Based on Anodized TiO2-Nanotubes@Ti@quartz Structure" Nanomaterials 14, no. 5: 439. https://doi.org/10.3390/nano14050439
APA StyleWang, Y., Zhang, M., Wu, W., Wang, Z., Liu, M., Yang, T., & Renqianzhuoma. (2024). Wide Response Range Photoelectrochemical UV Detector Based on Anodized TiO2-Nanotubes@Ti@quartz Structure. Nanomaterials, 14(5), 439. https://doi.org/10.3390/nano14050439






