Zein-Based Nanoparticles as Active Platforms for Sustainable Applications: Recent Advances and Perspectives
Abstract
1. Introduction
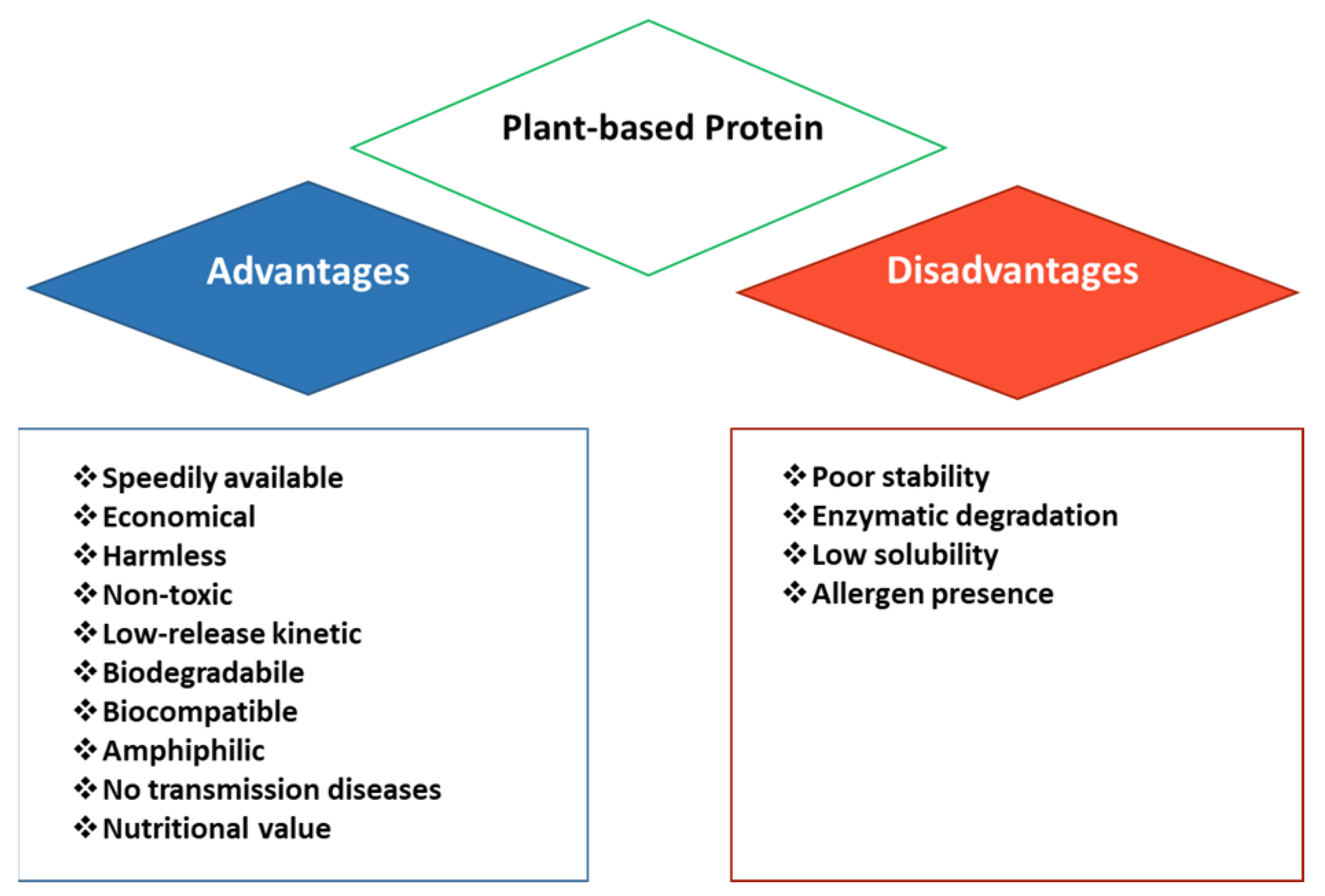
2. Zein: Structure, Properties, and Current Applications
3. Zein-Based Nanocarrier Production
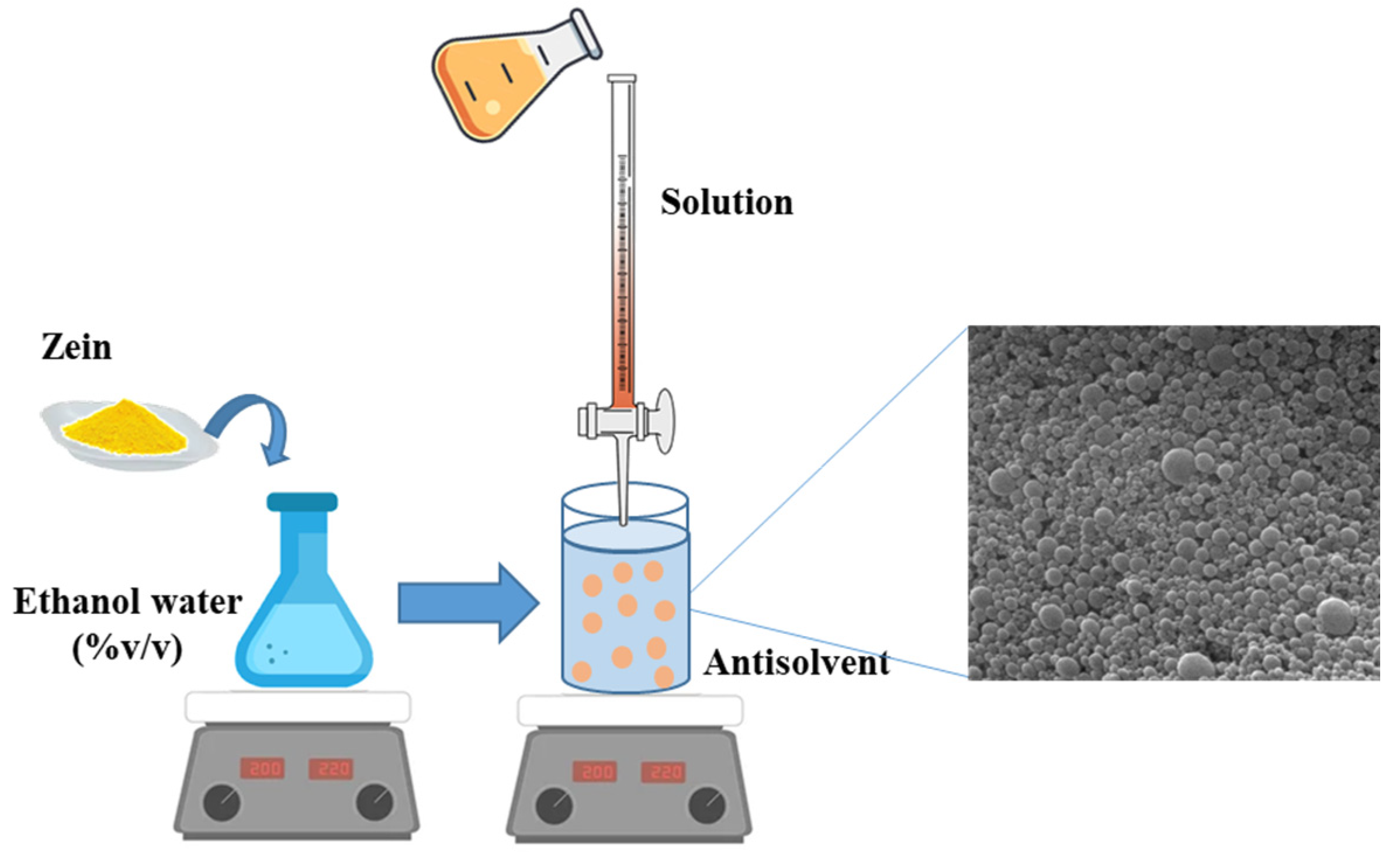
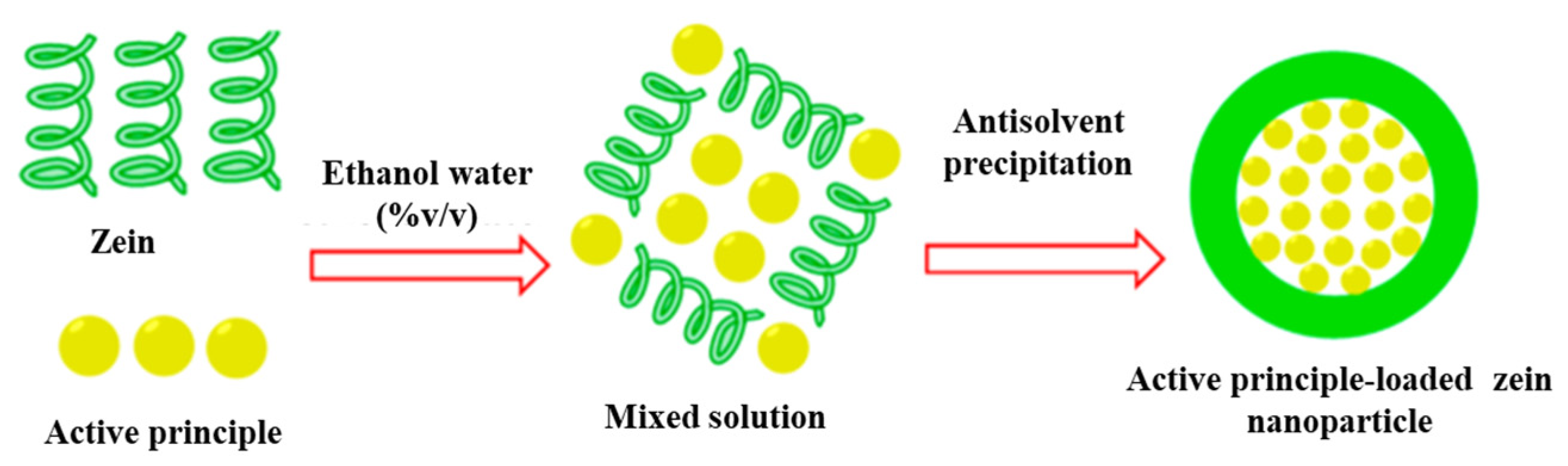
3.1. Liquid Antisolvent Process
3.2. Spray Drying
3.3. Supercritical Antisolvent Process
3.4. Coacervation
3.5. Emulsification Method
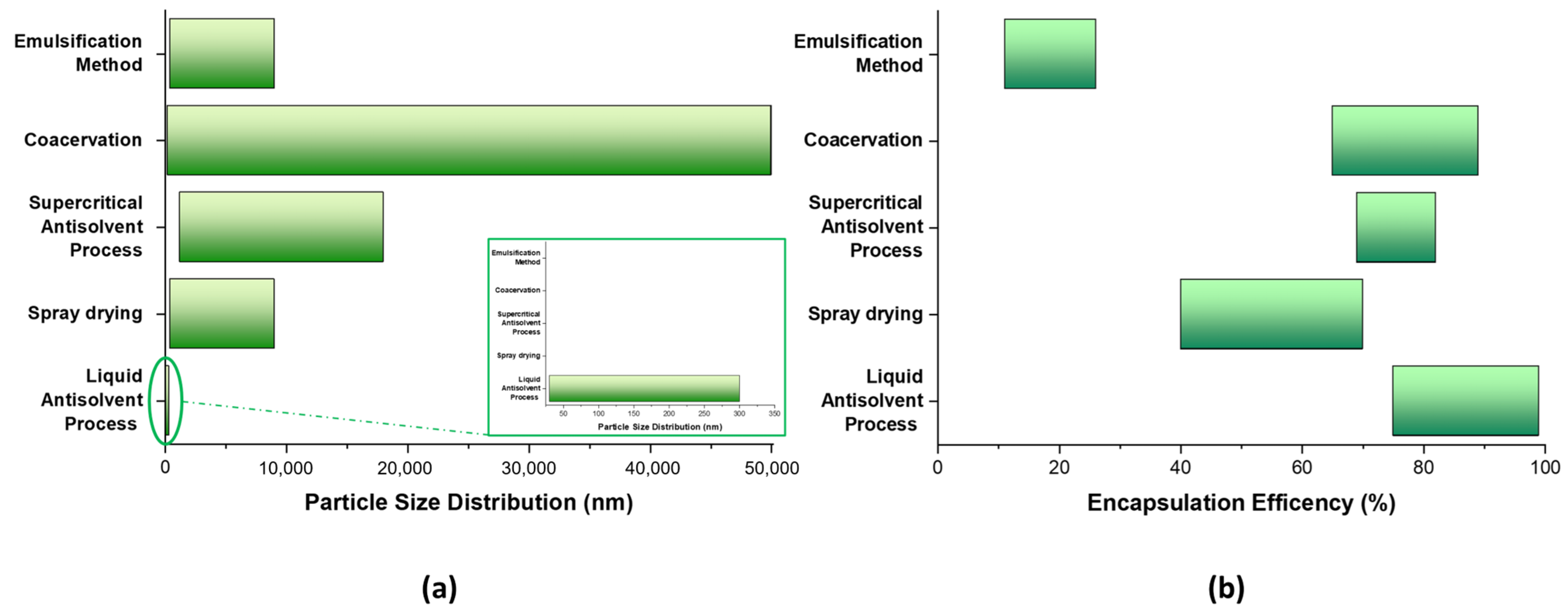
3.6. Comparison of the Zein-Based Nanocarrier Properties Obtained by Different Methods
4. Potential Application Fields of Zein Nanocarriers
4.1. Zein-Based Nanocarriers in Agriculture

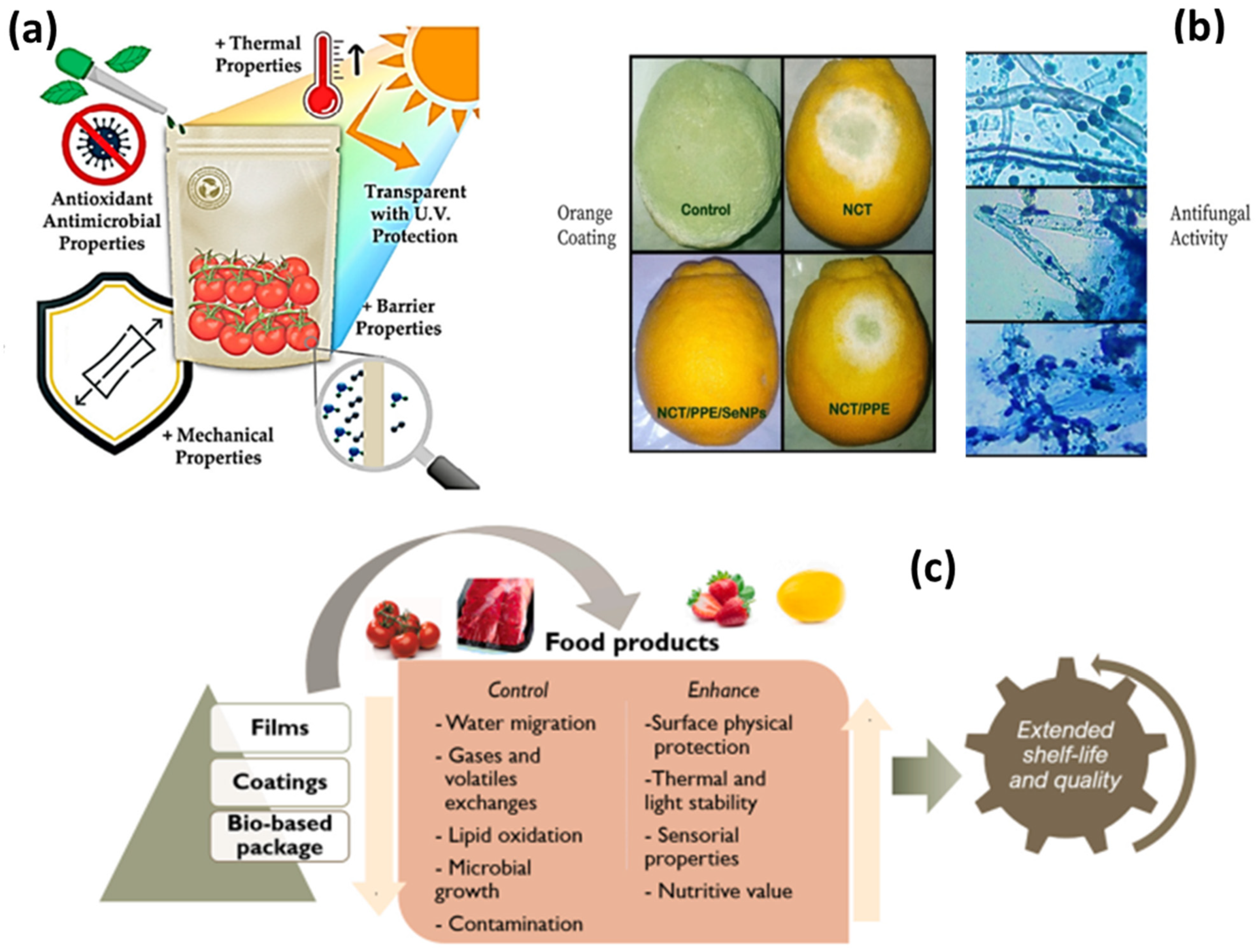
4.2. Zein-Based Nanocarriers in Food and Food Packaging
4.3. Zein-Based Nanocarriers in Cosmetics
4.4. Miscellaneous Applications
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-mediated drug delivery systems for anticancer agents: An overview and perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef]
- Olivieri, F.; Castaldo, R.; Cocca, M.; Gentile, G.; Lavorgna, M. Mesoporous silica nanoparticles as carriers of active agents for smart anticorrosive organic coatings: A critical review. Nanoscale 2021, 13, 9091–9111. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kang, P.M. Recent advances in nanocarrier-assisted therapeutics delivery systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Saif, A.; Anjum, L.; Faisal, Z.; Akram, N.; Shah, Y.A.; Irfan, R.; Saeed, F.; Afzaal, M.; Asif Shah, M. Recent advances in protein-based nanoparticles and their applications in the delivery of bioactive compounds. Int. J. Food Prop. 2023, 26, 2866–2880. [Google Scholar] [CrossRef]
- Mali, S.C.; Raj, S.; Trivedi, R. Nanotechnology a novel approach to enhance crop productivity. Biochem. Biophys. Rep. 2020, 24, 100821. [Google Scholar] [CrossRef]
- Han, M.; Liu, K.; Liu, X.; Rashid, M.T.; Zhang, H.; Wang, M. Research Progress of Protein-Based Bioactive Substance Nanoparticles. Foods 2023, 12, 2999. [Google Scholar] [CrossRef] [PubMed]
- Kučuk, N.; Primožič, M.; Knez, Ž.; Leitgeb, M. Sustainable Biodegradable Biopolymer-Based Nanoparticles for Healthcare Applications. Int. J. Mol. Sci. 2023, 24, 3188. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M. Nanomaterials: Types, properties, recent advances, and toxicity concerns. Curr. Opin. Environ. Sci. Health 2022, 25, 100319. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Rezigue, M.; Alsharedeh, R.H.; Obeid, M.A.; Mishra, V.; Serrano-Aroca, Á.; El-Tanani, M.; Tambuwala, M.M. Protein-based nanomaterials: A new tool for targeted drug delivery. Ther. Deliv. 2022, 13, 321–338. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q.; Zhang, Y. Biopolymer-based nanotechnology approaches to deliver bioactive compounds for food applications: A perspective on the past, present, and future. J. Agric. Food Chem. 2020, 68, 12993–13000. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.N.V.; Silva, C.U.A.; Siquenique, S.N.; Learmonth, D.A. Micro-and nanocarriers for encapsulation of biological plant protection agents: A systematic literature review. ACS Agric. Sci. Technol. 2022, 2, 838–857. [Google Scholar] [CrossRef]
- De Marco, I. Zein microparticles and nanoparticles as drug delivery systems. Polymers 2022, 14, 2172. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, L.; McClements, D.J.; Peng, X.; Xu, Z.; Meng, M.; Ji, H.; Qiu, C.; Long, J.; Jin, Z. Encapsulation of polyphenols in protein-based nanoparticles: Preparation, properties, and applications. Crit. Rev. Food Sci. Nutr. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale drug delivery systems: From medicine to agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Ojha, S.; Singh, D.; Sett, A.; Chetia, H.; Kabiraj, D.; Bora, U. Nanotechnology in crop protection. In Nanomaterials in Plants, Algae, and Microorganisms; Elsevier: Amsterdam, The Netherlands, 2018; pp. 345–391. [Google Scholar]
- Pascoli, M.; De Lima, R.; Fraceto, L.F. Zein nanoparticles and strategies to improve colloidal stability: A mini-review. Front. Chem. 2018, 6, 6. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Xiang, H.; Meng, J.; Shao, W.; Zeng, D.; Ji, J.; Wang, P.; Zhou, X.; Qi, P.; Liu, L.; Yang, S. Plant protein-based self-assembling core–shell nanocarrier for effectively controlling plant viruses: Evidence for nanoparticle delivery behavior, plant growth promotion, and plant resistance induction. Chem. Eng. J. 2023, 464, 142432. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and zein-based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Wang, Y.; Padua, G.W. Formation of zein microphases in ethanol—Water. Langmuir 2010, 26, 12897–12901. [Google Scholar] [CrossRef]
- Momany, F.A.; Sessa, D.J.; Lawton, J.W.; Selling, G.W.; Hamaker, S.A.; Willett, J.L. Structural characterization of α-zein. J. Agric. Food Chem. 2006, 54, 543–547. [Google Scholar] [CrossRef]
- Li, C.; Song, R. The regulation of zein biosynthesis in maize endosperm. Theor. Appl. Genet. 2020, 133, 1443–1453. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, C.; Hu, Y.; Gao, M.; Luan, G. Zein as a structural protein in gluten-free systems: An overview. Food Sci. Hum. Wellness 2021, 10, 270–277. [Google Scholar] [CrossRef]
- Yan, X.; Li, M.; Xu, X.; Liu, X.; Liu, F. Zein-based nano-delivery systems for encapsulation and protection of hydrophobic bioactives: A review. Front. Nutr. 2022, 9, 999373. [Google Scholar] [CrossRef] [PubMed]
- Chengala, L.; Singh, N. Botanical pesticides—A major alternative to chemical pesticides: A review. Int. J. Life Sci. 2017, 5, 722–729. [Google Scholar]
- Guo, Y.; Liu, Z.; An, H.; Li, M.; Hu, J. Nano-structure and properties of maize zein studied by atomic force microscopy. J. Cereal Sci. 2005, 41, 277–281. [Google Scholar] [CrossRef]
- Luecha, J.; Hsiao, A.; Brodsky, S.; Liu, G.L.; Kokini, J.L. Green microfluidic devices made of corn proteins. Lab Chip 2011, 11, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Giteru, S.G.; Ali, M.A.; Oey, I. Recent progress in understanding fundamental interactions and applications of zein. Food Hydrocoll. 2021, 120, 106948. [Google Scholar] [CrossRef]
- Verdolotti, L.; Lavorgna, M.; Oliviero, M.; Sorrentino, A.; Iozzino, V.; Buonocore, G.; Iannace, S. Functional Zein–Siloxane Bio-Hybrids. ACS Sustain. Chem. Eng. 2014, 2, 254–263. [Google Scholar]
- Lawton, J.W. Zein: A history of processing and use. Cereal Chem. 2002, 79, 1–18. [Google Scholar] [CrossRef]
- Baloyi, J.T.; Taylor, J.; Taylor, J.R. Bioplastic film making properties of quality protein maize (QPM) zein. Cereal Chem. 2023, 100, 805–815. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Luecha, J.; Sozer, N.; Kokini, J.L. Synthesis and properties of corn zein/montmorillonite nanocomposite films. J. Mater. Sci. 2010, 45, 3529–3537. [Google Scholar] [CrossRef]
- Pooja, N.; Chakraborty, I.; Rahman, M.H.; Mazumder, N. An insight on sources and biodegradation of bioplastics: A review. 3 Biotech 2023, 13, 220. [Google Scholar] [CrossRef]
- Zucchelli, M.; Mazzon, G.; Bertolacci, L.; Carzino, R.; Zendri, E.; Athanassiou, A. Stone sustainable protection and preservation using a zein-based hydrophobic coating. Prog. Org. Coat. 2021, 159, 106434. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.; Yu, D.; Regenstein, J.M.; Dong, J.; Chen, W.; Xia, W. Chitosan/zein bilayer films with one-way water barrier characteristic: Physical, structural and thermal properties. Int. J. Biol. Macromol. 2022, 200, 378–387. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y. Physical and antimicrobial properties of zein and methyl cellulose composite films with plasticizers of oleic acid and polyethylene glycol. LWT 2021, 140, 110811. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Zhang, L.; Wang, X.; Li, L. Effects of plasticizer type and concentration on rheological, physico-mechanical and structural properties of chitosan/zein film. Int. J. Biol. Macromol. 2020, 143, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Wei, D.; Zhu, W.; Li, Z.; Jiang, Y. High-elongation zein films for flexible packaging by synergistic plasticization: Preparation, structure and properties. J. Cereal Sci. 2018, 79, 354–361. [Google Scholar] [CrossRef]
- Qu, L.; Chen, G.; Dong, S.; Huo, Y.; Yin, Z.; Li, S.; Chen, Y. Improved mechanical and antimicrobial properties of zein/chitosan films by adding highly dispersed nano-TiO2. Ind. Crops Prod. 2019, 130, 450–458. [Google Scholar] [CrossRef]
- Rouf, T.B.; Schmidt, G.; Kokini, J.L. Zein–Laponite nanocomposites with improved mechanical, thermal and barrier properties. J. Mater. Sci. 2018, 53, 7387–7402. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wang, B.-J.; Weng, Y.-M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT Food Sci. Technol. 2015, 63, 115–121. [Google Scholar] [CrossRef]
- Xue, F.; Zhao, M.; Liu, X.; Chu, R.; Qiao, Z.; Li, C.; Adhikari, B. Physicochemical properties of chitosan/zein/essential oil emulsion-based active films functionalized by polyphenols. Future Foods 2021, 3, 100033. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, X.; Wang, L.; Wang, H.; Hu, Z.; Ju, X.; Yuan, Y. A review of food preservation based on zein: The perspective from application types of coating and film. Food Chem. 2023, 424, 136403. [Google Scholar] [CrossRef]
- Tan, H.; Zhou, H.; Guo, T.; Li, J.; Zhang, C.; Wang, S.; Zhang, Y.; Ma, L. Zein structure and its hidden zearalenone: Effect of zein extraction methods. Food Chem. 2022, 374, 131563. [Google Scholar] [CrossRef] [PubMed]
- Pascoli, M.; de Albuquerque, F.P.; Calzavara, A.K.; Tinoco-Nunes, B.; Oliveira, W.H.C.; Gonçalves, K.C.; Polanczyk, R.A.; Vechia, J.F.D.; de Matos, S.T.S.; de Andrade, D.J. The potential of nanobiopesticide based on zein nanoparticles and neem oil for enhanced control of agricultural pests. J. Pest Sci. 2020, 93, 793–806. [Google Scholar] [CrossRef]
- Wu, Y.; Du, J.; Zhang, J.; Li, Y.; Gao, Z. pH Effect on the Structure, Rheology, and Electrospinning of Maize Zein. Foods 2023, 12, 1395. [Google Scholar] [CrossRef]
- Tivano, F.; Chiono, V. Zein as a renewable material for the preparation of green nanoparticles for drug delivery. Front. Biomater. Sci. 2023, 2, 1156403. [Google Scholar] [CrossRef]
- Podaralla, S.; Perumal, O. Influence of formulation factors on the preparation of zein nanoparticles. AAPS PharmSciTech 2012, 13, 919–927. [Google Scholar] [CrossRef]
- Takács, D.; Adžić, M.; Omerović, N.; Vraneš, M.; Katona, J.; Pavlović, M. Electrolyte-induced aggregation of zein protein nanoparticles in aqueous dispersions. J. Colloid Interface Sci. 2024, 656, 457–465. [Google Scholar] [CrossRef]
- Wang, L.; Hao, L.; Hou, H.; Wang, L.; Liu, S.; Tang, R.; Mao, J.; Zhou, Q.; Deng, Q.; Yan, L. Carboxymethylpachymaran-Coated Zein Nanoparticles for Oral Delivery of Curcumin: Formation, Characterization, Physicochemical Stability, and Controlled Release Properties. ACS Food Sci. Technol. 2023, 3, 170–181. [Google Scholar] [CrossRef]
- Da Rosa, C.G.; de Melo, A.P.Z.; Sganzerla, W.G.; Machado, M.H.; Nunes, M.R.; Maciel, M.V.d.O.B.; Bertoldi, F.C.; Barreto, P.L.M. Application in situ of zein nanocapsules loaded with Origanum vulgare Linneus and Thymus vulgaris as a preservative in bread. Food Hydrocoll. 2020, 99, 105339. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, Z.; Xue, C. Development of zein-based nutraceutical delivery systems: A systematic overview based on recent researches. Food Hydrocoll. 2023, 137, 108368. [Google Scholar] [CrossRef]
- Zada, M.H.; Rottenberg, Y.; Domb, A.J. Peptide loaded polymeric nanoparticles by non-aqueous nanoprecipitation. J. Colloid Interface Sci. 2022, 622, 904–913. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Joye, I.J.; McClements, D.J. Food-grade protein-based nanoparticles and microparticles for bioactive delivery: Fabrication, characterization, and utilization. Adv. Protein Chem. Struct. Biol. 2015, 98, 293–325. [Google Scholar]
- Zhang, R.; Han, Y.; Xie, W.; Liu, F.; Chen, S. Advances in protein-based nanocarriers of bioactive compounds: From microscopic molecular principles to macroscopical structural and functional attributes. J. Agric. Food Chem. 2022, 70, 6354–6367. [Google Scholar] [CrossRef]
- Yuan, Y.; Ma, M.; Xu, Y.; Wang, D. Surface coating of zein nanoparticles to improve the application of bioactive compounds: A review. Trends Food Sci. Technol. 2022, 120, 1–15. [Google Scholar] [CrossRef]
- Monteiro, R.A.; Camara, M.C.; de Oliveira, J.L.; Campos, E.V.R.; Carvalho, L.B.; de Freitas Proença, P.L.; Guilger-Casagrande, M.; Lima, R.; do Nascimento, J.; Gonçalves, K.C. Zein based-nanoparticles loaded botanical pesticides in pest control: An enzyme stimuli-responsive approach aiming sustainable agriculture. J. Hazard. Mater. 2021, 417, 126004. [Google Scholar] [CrossRef] [PubMed]
- Calliari, C.M.; Campardelli, R.; Pettinato, M.; Perego, P. Encapsulation of hibiscus sabdariffa extract into zein nanoparticles. Chem. Eng. Technol. 2020, 43, 2062–2072. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.; Germano-Costa, T.; Lima, R.; Vechia, J.F.D.; Soares, S.T.; de Andrade, D.J.; Gonçalves, K.C.; do Nascimento, J.; Polanczyk, R.A. Association of zein nanoparticles with botanical compounds for effective pest control systems. Pest Manag. Sci. 2019, 75, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, Y.; Ji, H.; Lin, Q.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A. Physicochemical stability, antioxidant activity, and antimicrobial activity of quercetin-loaded zein nanoparticles coated with dextrin-modified anionic polysaccharides. Food Chem. 2023, 415, 135736. [Google Scholar] [CrossRef]
- Ye, G.; Wu, T.; Li, Z.; Teng, M.; Ma, L.; Qin, M.; Zhao, P.; Fu, Q. Preparation and characterization of novel composite nanoparticles using zein and hyaluronic acid for efficient delivery of naringenin. Food Chem. 2023, 417, 135890. [Google Scholar] [CrossRef]
- Yang, Z.; McClements, D.J.; Peng, X.; Xu, Z.; Meng, M.; Chen, L.; Jin, Z. Fabrication of zein–carboxymethyl cellulose nanoparticles for co-delivery of quercetin and resveratrol. J. Food Eng. 2023, 341, 111322. [Google Scholar] [CrossRef]
- Ji, C.; Khan, M.A.; Chen, K.; Liang, L. Coating of DNA and DNA complexes on zein particles for the encapsulation and protection of kaempferol and α-tocopherol. J. Food Eng. 2023, 352, 111520. [Google Scholar] [CrossRef]
- Huang, X.; Li, T.; Li, S. Encapsulation of vitexin-rhamnoside based on zein/pectin nanoparticles improved its stability and bioavailability. Curr. Res. Food Sci. 2023, 6, 100419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, Y.; Hu, X.; Zeng, Z.; Hu, J.; Zeng, T.; Geng, F.; Wu, D. Nanoparticles loaded with phlorizin fabricate a fortified yogurt with antioxidant potential. Food Biosci. 2023, 54, 102849. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, Q.-F. Bioavailability enhancement of astilbin in rats through zein–caseinate nanoparticles. J. Agric. Food Chem. 2019, 67, 5746–5753. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Wang, D.; Tao, S.; Hu, X.; Wang, C.; Sun, Y.; Zhao, B.; Li, Y. Facile encapsulation of thymol within deamidated zein nanoparticles for enhanced stability and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 126940. [Google Scholar] [CrossRef]
- Makurunje, P.; Woodhouse, G.; Goddard, D.T.; Middleburgh, S.C. Self-contained dual-scale composite architectures in spray dried zirconium diboride. Ceram. Int. 2022, 48, 17529–17538. [Google Scholar] [CrossRef]
- Bhonsale, S.; Muñoz López, C.A.; Van Impe, J. Global Sensitivity Analysis of a Spray Drying Process. Processes 2019, 7, 562. [Google Scholar] [CrossRef]
- Eijkelboom, N.M.; van Boven, A.P.; Siemons, I.; Wilms, P.F.; Boom, R.M.; Kohlus, R.; Schutyser, M.A. Particle structure development during spray drying from a single droplet to pilot-scale perspective. J. Food Eng. 2023, 337, 111222. [Google Scholar] [CrossRef]
- Yang, D.-L.; Liu, R.-K.; Wei, Y.; Sun, Q.; Wang, J.-X. Micro-sized nanoaggregates: Spray-drying-assisted fabrication and applications. Particuology 2024, 85, 22–48. [Google Scholar] [CrossRef]
- Ordoubadi, M.; Wang, H.; Vehring, R. Mechanistic Formulation Design of Spray-Dried Powders. KONA Powder Part. J. 2023, 40, 149–171. [Google Scholar] [CrossRef]
- Spicher, M.T.; Schwaminger, S.P.; von der Haar-Leistl, D.; Fellenberg, E.; Berensmeier, S. Process development for pilot-scale spray drying of ultrasmall iron (oxyhydr) oxide nanoparticles. Powder Technol. 2023, 433, 119186. [Google Scholar] [CrossRef]
- Campión, R.; Gonzalez-Navarro, C.J.; López, A.L.M.; Martínez-Oharriz, M.C.; Matías, C.; Sáiz-Abajo, M.-J.; Collantes, M.; Peñuelas, I.; Irache, J.M. Zein-based nanospheres and nanocapsules for the encapsulation and oral delivery of quercetin. Int. J. Pharm. 2023, 643, 123216. [Google Scholar] [CrossRef]
- Coelho, S.C.; Laget, S.; Benaut, P.; Rocha, F.; Estevinho, B.N. A new approach to the production of zein microstructures with vitamin B12, by electrospinning and spray drying techniques. Powder Technol. 2021, 392, 47–57. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Leena, M.M.; Moses, J.; Anandharamakrishnan, C. Micro-and nano-encapsulation of β-carotene in zein protein: Size-dependent release and absorption behavior. Food Funct. 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Wong, J.J.L.; Wong, A.I.C.; Xu, Y.; Yuliarti, O. Zein as a water insoluble excipient for spray dry encapsulation of hydrophilic bioactives. J. Food Eng. 2020, 283, 110054. [Google Scholar] [CrossRef]
- Veneranda, M.; Hu, Q.; Wang, T.; Luo, Y.; Castro, K.; Madariaga, J.M. Formation and characterization of zein-caseinate-pectin complex nanoparticles for encapsulation of eugenol. LWT 2018, 89, 596–603. [Google Scholar] [CrossRef]
- Do Carmo, C.S.; Maia, C.; Poejo, J.; Lychko, I.; Gamito, P.; Nogueira, I.; Bronze, M.; Serra, A.T.; Duarte, C.M. Microencapsulation of a-tocopherol with zein and b-cyclodextrin using spray drying for colour stability and shelf-life improvement of fruit beverages. RSC Adv. 2017, 7, 32065. [Google Scholar] [CrossRef]
- Campardelli, R.; Adami, R.; Della Porta, G.; Reverchon, E. Nanoparticle precipitation by supercritical assisted injection in a liquid antisolvent. Chem. Eng. J. 2012, 192, 246–251. [Google Scholar] [CrossRef]
- Campardelli, R.; Oleandro, E.; Adami, R.; Reverchon, E. Polymethylmethacrylate (PMMA) sub-microparticles produced by Supercritical Assisted Injection in a Liquid Antisolvent. J. Supercrit. Fluids 2014, 92, 93–99. [Google Scholar] [CrossRef]
- Campardelli, R.; Oleandro, E.; Reverchon, E. Supercritical assisted injection in a liquid antisolvent for PLGA and PLA microparticle production. Powder Technol. 2016, 287, 12–19. [Google Scholar] [CrossRef]
- Palazzo, I.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Zein/luteolin microparticles formation using a supercritical fluids assisted technique. Powder Technol. 2019, 356, 899–908. [Google Scholar] [CrossRef]
- Liu, G.; An, D.; Li, J.; Deng, S. Zein-based nanoparticles: Preparation, characterization, and pharmaceutical application. Front. Pharmacol. 2023, 14, 1120251. [Google Scholar] [CrossRef]
- He, H.; Huang, Y.; Zhang, X.; Ouyang, Y.; Pan, P.; Lan, Y.; Zhong, Z.; Ping, L.; Lu, T.; Chen, Z. Supercritical fluid coating of flavonoids on excipients enhances drug release and antioxidant activity. Int. J. Pharm. 2023, 632, 122593. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Reverchon, E.; De Marco, I. Zein/diclofenac sodium coprecipitation at micrometric and nanometric range by supercritical antisolvent processing. J. CO2 Util. 2018, 27, 366–373. [Google Scholar] [CrossRef]
- Rosa, M.T.M.; Alvarez, V.H.; Albarelli, J.Q.; Santos, D.T.; Meireles, M.A.A.; Saldana, M.D. Supercritical anti-solvent process as an alternative technology for vitamin complex encapsulation using zein as wall material: Technical-economic evaluation. J. Supercrit. Fluids 2020, 159, 104499. [Google Scholar] [CrossRef]
- Kaushik, P.; Rawat, K.; Aswal, V.; Kohlbrecher, J.; Bohidar, H. Fluorescent complex coacervates of agar and in situ formed zein nanoparticles: Role of electrostatic forces. Carbohydr. Polym. 2019, 224, 115150. [Google Scholar] [CrossRef]
- Aloys, H.; Korma, S.A.; Alice, T.M.; Chantal, N.; Ali, A.H.; Abed, S.M.; Ildephonse, H. Microencapsulation by complex coacervation: Methods, techniques, benefits, and applications—A review. Am. J. Food Sci. Nutr. Res. 2016, 3, 188–192. [Google Scholar]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Li, X.; Erni, P.; Van Der Gucht, J.; De Vries, R. Encapsulation using plant proteins: Thermodynamics and kinetics of wetting for simple zein coacervates. ACS Appl. Mater. Interfaces 2020, 12, 15802–15809. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Su, C.; Li, X.; Wang, H.; Luo, M.; Chen, Z.; Zhang, B.; Zhu, J.; Yuan, Y. Preparation and characterization of bilayered microencapsulation for co-delivery Lactobacillus casei and polyphenols via Zein-chitosan complex coacervation. Food Hydrocoll. 2024, 148, 109410. [Google Scholar] [CrossRef]
- Farnad, N.; Farhadi, K. Simple and complex coacervation methods for the nanoencapsulation of Rosa damascena mill L. anthocyanin in zein/potato starch: A new approach to enhance antioxidant and thermal properties. J. Food Sci. 2023, 88, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.N.; Deore, S.L. Emulsion micro emulsion and nano emulsion: A review. Syst. Rev. Pharm. 2017, 8, 39. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Tavasoli, S.; Liu, Q.; Jafari, S.M. Development of Pickering emulsions stabilized by hybrid biopolymeric particles/nanoparticles for nutraceutical delivery. Food Hydrocoll. 2022, 124, 107280. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, S.; Zhao, C.; Liu, M.; Zhang, Z.; Xu, W.; Luo, D.; Shah, B.R. Stability, microstructural and rheological properties of Pickering emulsion stabilized by xanthan gum/lysozyme nanoparticles coupled with xanthan gum. Int. J. Biol. Macromol. 2020, 165, 2387–2394. [Google Scholar] [CrossRef]
- Souza, E.M.; Ferreira, M.R.; Soares, L.A. Pickering emulsions stabilized by zein particles and their complexes and possibilities of use in the food industry: A review. Food Hydrocoll. 2022, 131, 107781. [Google Scholar] [CrossRef]
- Xu, W.; Li, Z.; Li, H.; Sun, H.; Zheng, S.; Luo, D.; Li, Y.; Wang, Y.; Shah, B.R. Stabilization and microstructural network of pickering emulsion using different xanthan gum/lysozyme nanoparticle concentrations. LWT 2022, 160, 113298. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, M.; Mao, Z.; Xiao, J.; Huang, Q.; Lin, X.; Cao, Y. Modulation of interfacial phenolic antioxidant distribution in Pickering emulsions via interactions between zein nanoparticles and gallic acid. Int. J. Biol. Macromol. 2020, 152, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huan, Y.; Ren, P.; Li, J.; Wei, Z.; Xu, J.; Tang, Q. Zein/hyaluronic acid nanoparticle stabilized Pickering emulsion for astaxanthin encapsulation. Int. J. Biol. Macromol. 2024, 255, 127992. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; McClements, D.J.; He, X.; Xu, X.; Tan, F.; Yang, D.; Sun, Q.; Dai, L. Interfacial properties and structure of Pickering emulsions co-stabilized by different charge emulsifiers and zein nanoparticles. Food Hydrocoll. 2024, 146, 109285. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P. Pesticides in agriculture and environment: Impacts on human health. Contam. Agric. Environ. Health Risks Remediat. 2019, 1, 76–95. [Google Scholar]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1. [Google Scholar] [CrossRef]
- Chaza, C.; Sopheak, N.; Mariam, H.; David, D.; Baghdad, O.; Moomen, B. Assessment of pesticide contamination in Akkar groundwater, northern Lebanon. Environ. Sci. Pollut. Res. 2018, 25, 14302–14312. [Google Scholar] [CrossRef]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The future of food security and food safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef]
- Gupta, A.; Rayeen, F.; Mishra, R.; Tripathi, M.; Pathak, N. Nanotechnology Applications in Sustainable Agriculture: An Emerging Ecofriendly Approach. Plant Nano Biol. 2023, 4, 100033. [Google Scholar] [CrossRef]
- Machado, S.; Pereira, R.; Sousa, R.M.O. Nanobiopesticides: Are they the future of phytosanitary treatments in modern agriculture? Sci. Total Environ. 2023, 896, 166401. [Google Scholar] [CrossRef]
- Bramlett, M.; Plaetinck, G.; Maienfisch, P. RNA-based biocontrols—A new paradigm in crop protection. Engineering 2020, 6, 522–527. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.; Fraceto, L.F. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Yan, S.; Hu, Q.; Li, J.; Chao, Z.; Cai, C.; Yin, M.; Du, X.; Shen, J. A star polycation acts as a drug nanocarrier to improve the toxicity and persistence of botanical pesticides. ACS Sustain. Chem. Eng. 2019, 7, 17406–17413. [Google Scholar] [CrossRef]
- Prasad, A.; Astete, C.E.; Bodoki, A.E.; Windham, M.; Bodoki, E.; Sabliov, C.M. Zein nanoparticles uptake and translocation in hydroponically grown sugar cane plants. J. Agric. Food Chem. 2017, 66, 6544–6551. [Google Scholar] [CrossRef]
- Kacsó, T.; Neaga, I.O.; Erincz, A.; Astete, C.E.; Sabliov, C.M.; Oprean, R.; Bodoki, E. Perspectives in the design of zein-based polymeric delivery systems with programmed wear down for sustainable agricultural applications. Polym. Degrad. Stab. 2018, 155, 130–135. [Google Scholar] [CrossRef]
- Camara, M.C.; Monteiro, R.A.; Carvalho, L.B.A.; Oliveira, J.L.; Fraceto, L.F. Enzyme stimuli–responsive nanoparticles for bioinsecticides: An emerging approach for uses in crop protection. ACS Sustain. Chem. Eng. 2020, 9, 106–112. [Google Scholar] [CrossRef]
- De Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotechnology in packaging for food industry: Past, present, and future. Coatings 2023, 13, 1411. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Zhang, X.; Liu, J.; Gong, P.; Su, Z.; Fan, L.; Li, G. Emerging nanoparticles in food: Sources, application, and safety. J. Agric. Food Chem. 2023, 71, 3564–3582. [Google Scholar] [CrossRef] [PubMed]
- Chausali, N.; Saxena, J.; Prasad, R. Recent trends in nanotechnology applications of bio-based packaging. J. Agric. Food Res. 2022, 7, 100257. [Google Scholar] [CrossRef]
- Garavand, F.; Khodaei, D.; Mahmud, N.; Islam, J.; Khan, I.; Jafarzadeh, S.; Tahergorabi, R.; Cacciotti, I. Recent progress in using zein nanoparticles-loaded nanocomposites for food packaging applications. Crit. Rev. Food Sci. Nutr. 2022, 1–21. [Google Scholar] [CrossRef]
- Jaski, A.C.; Schmitz, F.; Horta, R.P.; Cadorin, L.; da Silva, B.J.G.; Andreaus, J.; Paes, M.C.D.; Riegel-Vidotti, I.C.; Zimmermann, L.M. Zein—A plant-based material of growing importance: New perspectives for innovative uses. Ind. Crops Prod. 2022, 186, 115250. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, X.; Chang, Y.; Li, D.; Sun, X.; Liu, X. Zein-derived peptides as nanocarriers to increase the water solubility and stability of lutein. Food Funct. 2018, 9, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, R.; Tang, H.; Li, J.-X. Interactions and characterization of Zein-BSA nanoparticles: Multi-spectral analysis and molecular simulations. J. Mol. Liq. 2023, 391, 123293. [Google Scholar] [CrossRef]
- Khezri, K.; Saeedi, M.; Dizaj, S.M. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Badhe, N.; Shitole, P.; Chaudhari, Y.; Matkar, S.; Jamdhade, P.; Gharat, T.; Doke, R. Nanoparticles in Cosmetics: The Safety and Hidden Risks. Biol. Forum Int. J. 2023, 15, 1156–1161. [Google Scholar]
- Salas, M.F.R.; Porras, P.C.; Vargas, M.J.C.; Molina, J.A.P.; Rojas, M.C.; Redondo, G.L.M. Nanotechnological Applications in Dermocosmetics. Eur. J. Pharm. Res. 2023, 3, 1–7. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
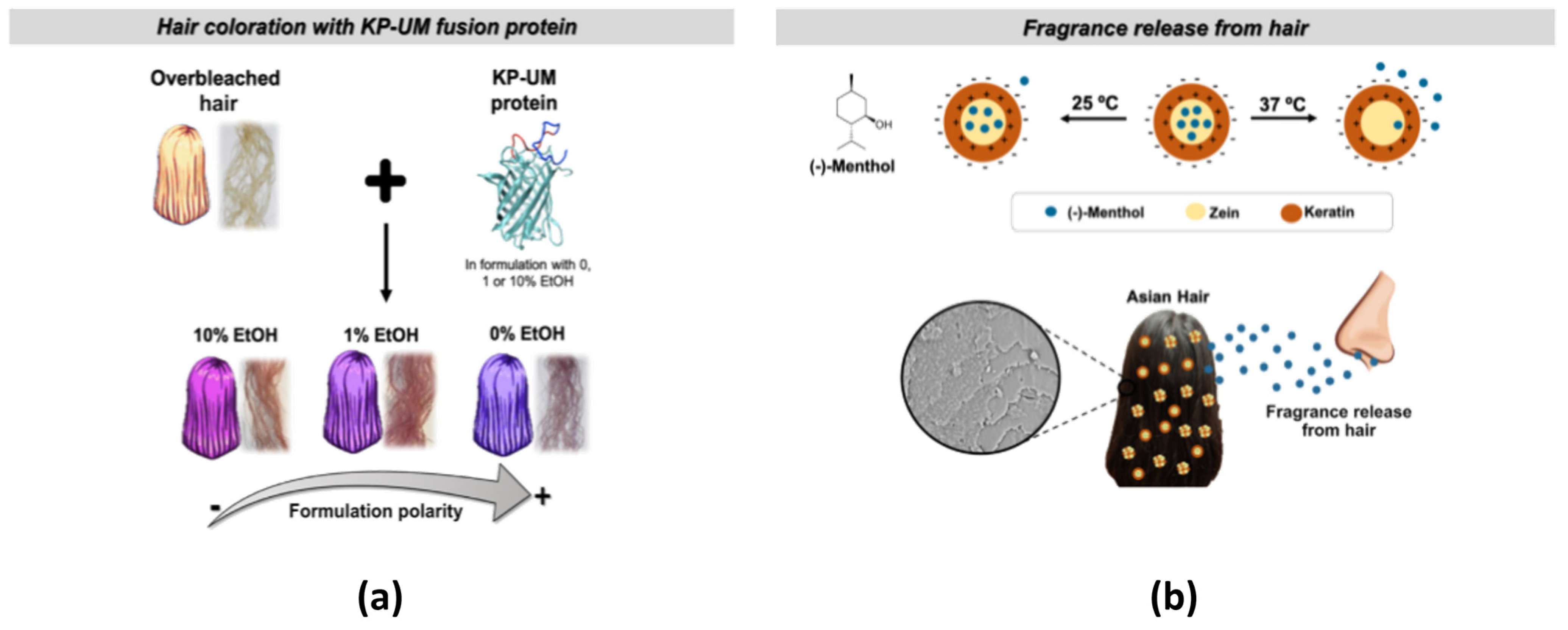
- Tinoco, A.; Gonçalves, F.; Costa, A.F.; Freitas, D.S.; Cavaco-Paulo, A.; Ribeiro, A. Keratin: Zein particles as vehicles for fragrance release on hair. Ind. Crops Prod. 2021, 159, 113067. [Google Scholar] [CrossRef]
- Tinoco, A.; Martins, M.; Cavaco-Paulo, A.; Ribeiro, A. Biotechnology of functional proteins and peptides for hair cosmetic formulations. Trends Biotechnol. 2022, 40, 591–605. [Google Scholar] [CrossRef]
- Ji, Z.; Yao, J.; Shao, Z.; Chen, X. Zein/chitosan composite nanospheres for controlled release of fragrances. Mater. Lett. 2023, 349, 134863. [Google Scholar] [CrossRef]
- Schmidt, G.; Christ, P.E.; Kertes, P.E.; Fisher, R.V.; Miles, L.J.; Wilker, J.J. Underwater Bonding with a Biobased Adhesive from Tannic Acid and Zein Protein. ACS Appl. Mater. Interfaces 2023, 15, 32863–32874. [Google Scholar] [CrossRef]
- Gonçalves, J.; Torres, N.; Silva, S.; Gonçalves, F.; Noro, J.; Cavaco-Paulo, A.; Ribeiro, A.; Silva, C. Zein impart hydrophobic and antimicrobial properties to cotton textiles. React. Funct. Polym. 2020, 154, 104664. [Google Scholar] [CrossRef]
- Tortorella, S.; Maturi, M.; Buratti, V.V.; Vozzolo, G.; Locatelli, E.; Sambri, L.; Franchini, M.C. Zein as a versatile biopolymer: Different shapes for different biomedical applications. RSC Adv. 2021, 11, 39004–39026. [Google Scholar] [CrossRef] [PubMed]
- Zaher, S.; Soliman, M.E.; Elsabahy, M.; Hathout, R.M. Protein nanoparticles as natural drugs carriers for cancer therapy. Adv. Tradit. Med. 2023, 23, 1035–1064. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Zein-based micro-and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131, 40696. [Google Scholar] [CrossRef]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef]



| Active Compound | Co-Carrier | Application | Properties and Encapsulation Highlights | Ref. |
|---|---|---|---|---|
| Geraniol + Eugenol Geraniol + Trans-Cinnamaldehyde | - | Agriculture |
| [62] |
| Limonene + Carvacrol | - | Agriculture |
| [60] |
| Neem oil | - | Agriculture |
| [48] |
| Quercetin | Carboxymethyl dextrin (CMD) | Food |
| [63] |
| Oregano Thyme | - | Food |
| [54] |
| Naringenin | Hyaluronic acid (HA) | Food |
| [64] |
| Quercetin Resveratrol | carboxymethyl cellulose (CMC) | Food |
| [65] |
| Kaempferol α-Tocopherol | DNA DNA-quercetin complex | Food |
| [66] |
| Vitexin-rhamnoside | Pectin | Food |
| [67] |
| Phlorizin (PHL) | Gum Arabic (GA) | Food |
| [68] |
| Astilbin | Caseinate | Agriculture, Food |
| [69] |
| Thymol | - | Cosmetic, Food |
| [70] |
| Active Compound | Co-Carrier | Application | Properties and Encapsulation Highlights | Ref. |
|---|---|---|---|---|
| Green Jelly Leaves (GJL) | Hydroxypropylmethyl cellulose (HPMC) | Food |
| [80] |
| Eugenol | pectin, sodium caseinate (NaCas) | Food Agriculture |
| [81] |
| a-Tocopherol (TOC) | cyclodextrin (CD) | Food Agriculture |
| [82] |
| vitamin B12 | Food |
| [78] | |
| β-carotene | Glycerol | Food |
| [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleandro, E.; Stanzione, M.; Buonocore, G.G.; Lavorgna, M. Zein-Based Nanoparticles as Active Platforms for Sustainable Applications: Recent Advances and Perspectives. Nanomaterials 2024, 14, 414. https://doi.org/10.3390/nano14050414
Oleandro E, Stanzione M, Buonocore GG, Lavorgna M. Zein-Based Nanoparticles as Active Platforms for Sustainable Applications: Recent Advances and Perspectives. Nanomaterials. 2024; 14(5):414. https://doi.org/10.3390/nano14050414
Chicago/Turabian StyleOleandro, Emilia, Mariamelia Stanzione, Giovanna Giuliana Buonocore, and Marino Lavorgna. 2024. "Zein-Based Nanoparticles as Active Platforms for Sustainable Applications: Recent Advances and Perspectives" Nanomaterials 14, no. 5: 414. https://doi.org/10.3390/nano14050414
APA StyleOleandro, E., Stanzione, M., Buonocore, G. G., & Lavorgna, M. (2024). Zein-Based Nanoparticles as Active Platforms for Sustainable Applications: Recent Advances and Perspectives. Nanomaterials, 14(5), 414. https://doi.org/10.3390/nano14050414







