Up- and Down-Regulation of Enzyme Activity in Aggregates with Gold-Covered Magnetic Nanoparticles Triggered by Low-Frequency Magnetic Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GCMNPs Synthesis, Surface Modification, and Enzyme Conjugation
2.3. Enzymatic Activity
2.4. Sample Characterization
3. Results and Discussion
3.1. GCMNP Synthesis and Functionalization
3.2. Enzyme Conjugation to the GCMNPs’ Surfaces
| Sample | Mean Hydrodynamic Diameter, nm | Peak Positions, nm |
|---|---|---|
| GCMNP-LA | 52 ± 1 | 41 |
| GCMNP-PEG | 74 ± 1 | 68 |
| D1 | 147 ± 3 | 75, 93 |
| D2 | 158 ± 3 | 62, 108, 146, 329 |
| D3 | 104 ± 2 | 74, 302 |
| D4 | 159 ± 4 | 92, 171, 240 |
| D5 | 127 ± 2 | 72, 191 |
| M1 | 97 ± 2 | 62 |
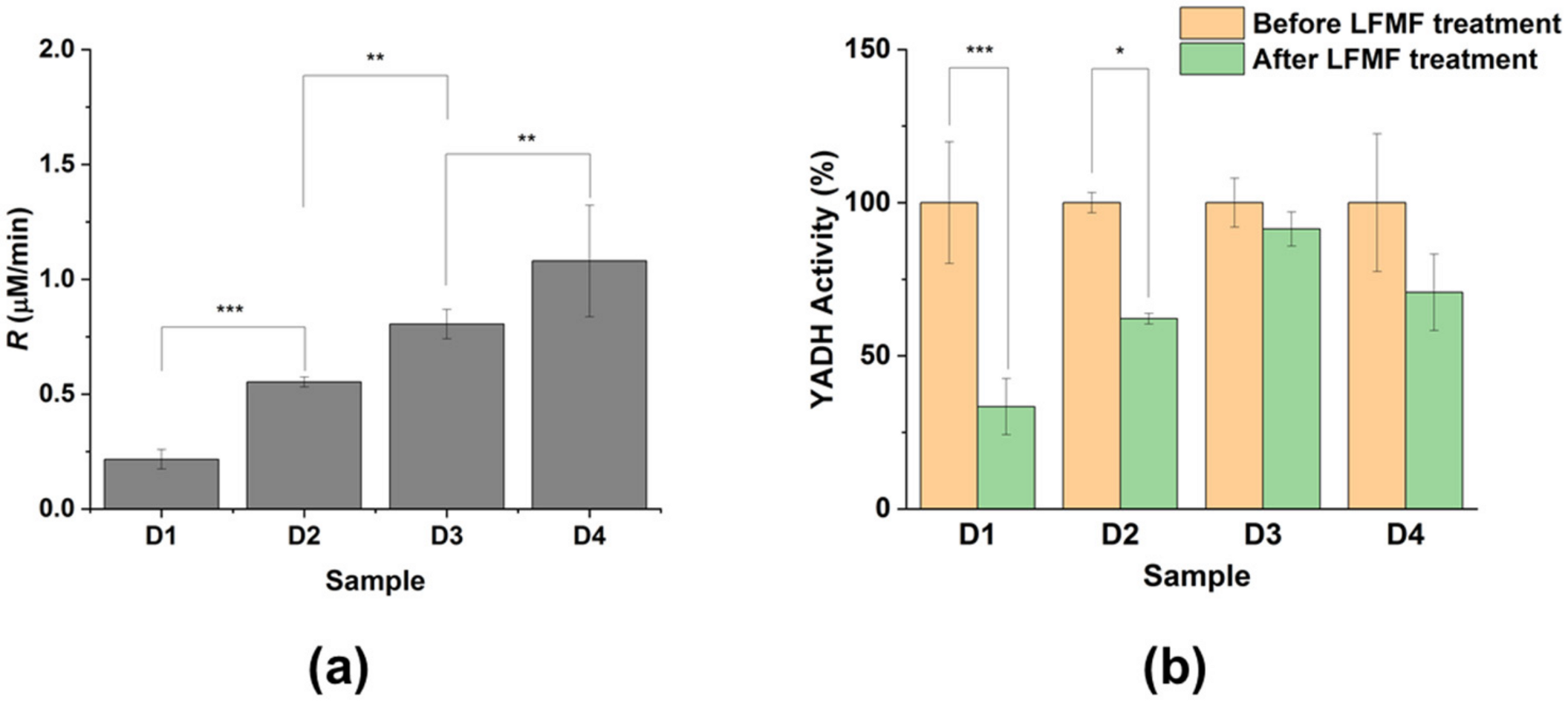
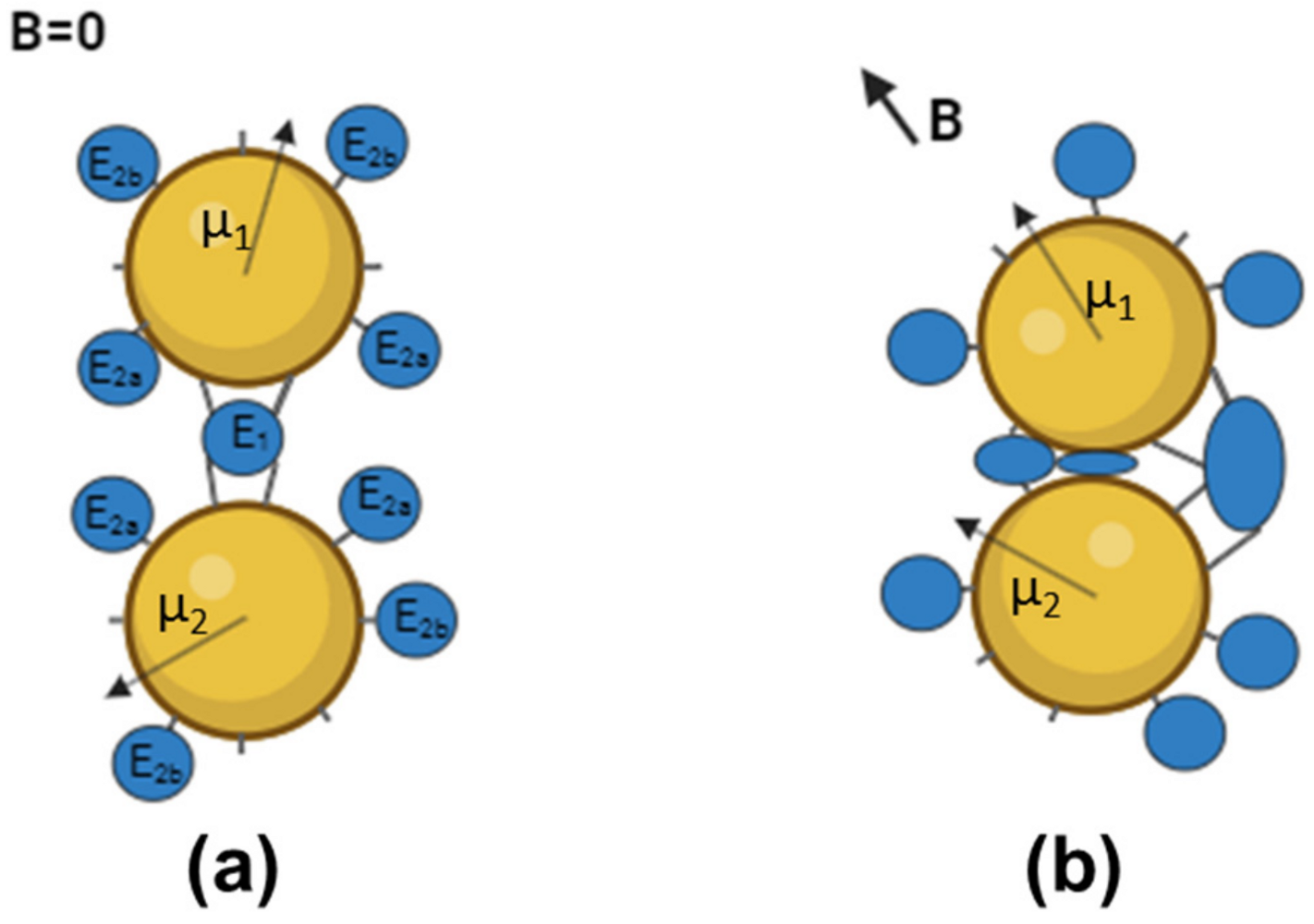
3.3. Down-Regulation of Enzymes in “Dimeric” Aggregates
- The optimization of the enzyme concentration during binding to the MNPs’ surface (at a constant MNPs concentration). An increase in enzyme concentration naturally leads to the enzyme’s increased activity in an aggregate. At the same time, an increase in enzyme concentration boosts non-covalent enzyme binding, which leads to a decrease in LFMF effectiveness.
- The optimization of a cross-linking agent’s concentration for the binding of an enzyme to MNPs’ surface (at a constant enzyme concentration). An increase in the EDC/S-NHS concentration naturally suppresses the enzyme activity in aggregates due to the possible modification of active site groups. At the same time, an increase in the EDC/S-NHS concentration can enhance LFMF effectiveness by promoting the formation of “dimeric” aggregates.
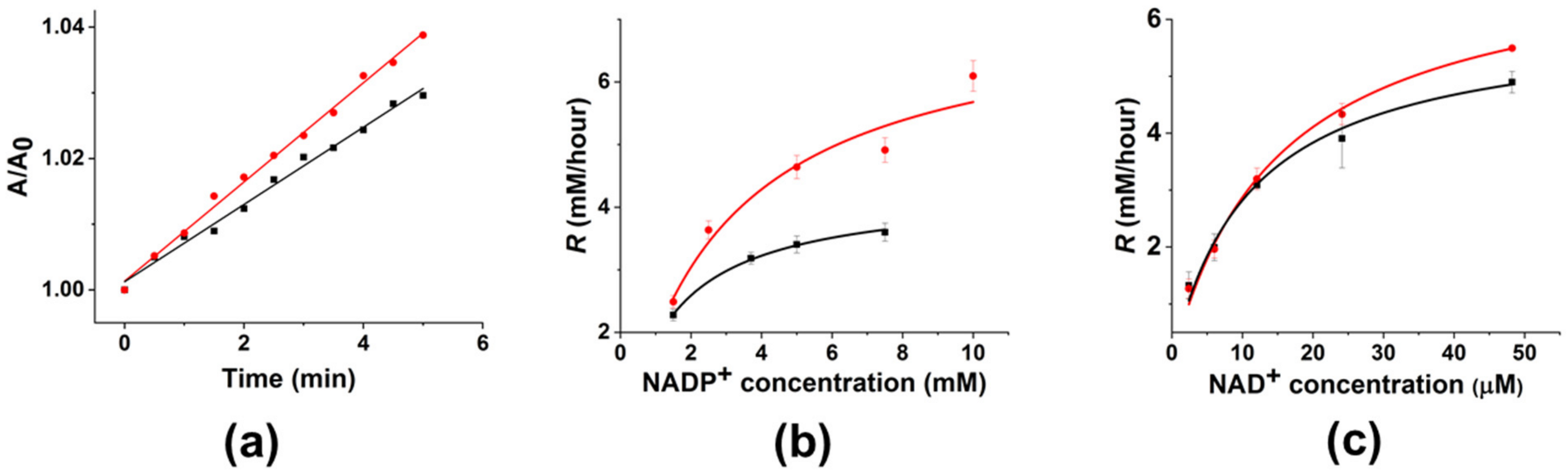
3.4. Up-Regulation of FDH in “Monomeric” Aggregates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cipolatti, E.P.; Costa, M.; Henriques, R.O.; Costa, J.C.; Machado, A.; Guimarães Freire, D.M.; Andrade, E.M. Enzymes in Green Chemistry: The State of the Art in Chemical Transformations. In Advances in Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–151. [Google Scholar]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Ang, E.L.; Zhao, H. Biocatalysis for the synthesis of pharmaceuticals and pharmaceutical intermediates. Bioorg. Med. Chem. 2018, 26, 1275–1284. [Google Scholar] [CrossRef]
- Okoro, G.; Husain, S.; Saukani, M.; Mutalik, C.; Yougbaré, S.; Hsiao, Y.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Photosynthetic Biohybrid Systems. ACS Mater. Lett. 2023, 5, 95–115. [Google Scholar] [CrossRef]
- Claaßen, C.; Gerlach, T.; Rother, D. Stimulus-Responsive Regulation of Enzyme Activity for One-Step and Multi-Step Syntheses. Adv. Synth. Catal. 2019, 361, 2387–2401. [Google Scholar] [CrossRef]
- Schmidt-Dannert, C.; Lopez-Gallego, F. A roadmap for biocatalysis—Functional and spatial orchestration of enzyme cascades. Microb. Biotechnol. 2016, 9, 601–609. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y. Temperature-Mediated Regulation of Enzymatic Activity. ChemCatChem 2016, 8, 2740–2747. [Google Scholar] [CrossRef]
- Klibanov, A.M.; Samokhin, G.P.; Martinek, K.; Berezin, I.V. Enzymatic mechanochemistry: A new approach to studying the mechanism of enzyme action. Biochim. Biophys. Acta 1976, 438, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Poloni, C.; Szymanski, W.; Feringa, B.L. Photo-controlled deactivation of immobilised lipase. Chem. Commun. 2014, 50, 12645–12648. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Schultz, C.; Kalivretenos, A.; Ghosh, B.; Broedel, S.E., Jr. Engineering a Hyper-catalytic Enzyme by Photoactivated Conformation Modulation. J. Phys. Chem. Lett. 2012, 3, 1142–1146. [Google Scholar] [CrossRef]
- Schierling, B.; Noël, A.-J.; Wende, W.; Hien, L.T.; Volkov, E.; Kubareva, E.; Oretskaya, T.; Kokkinidis, M.; Römpp, A.; Spengler, B.; et al. Controlling the enzymatic activity of a restriction enzyme by light. Proc. Natl. Acad. Sci. USA 2010, 107, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.D.; Nakajima, Y.; Maeda, H.; Maruta, S. Photocontrol of kinesin ATPase activity using an azobenzene derivative. J. Biochem. 2007, 142, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Smith, K.A.; Hatton, T.A. Photocontrol of protein folding: The interaction of photosensitive surfactants with bovine serum albumin. Biochemistry 2005, 44, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Lee, C.T. Protein secondary structure controlled with light and photoresponsive surfactants. J. Phys. Chem. B 2006, 110, 16117–16123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, T.; Tian, X.; Gai, D. Effect of microwave irradiation on the structure of glucoamylase. Process Biochem. 2012, 47, 2323–2328. [Google Scholar] [CrossRef]
- Shinde, S.D.; Yadav, G.D. Insight into microwave-assisted lipase catalyzed synthesis of geranyl cinnamate: Optimization and kinetic modeling. Appl. Biochem. Biotechnol. 2015, 175, 2035–2049. [Google Scholar] [CrossRef] [PubMed]
- Welsch, N.; Wittemann, A.; Ballauff, M. Enhanced activity of enzymes immobilized in thermoresponsive core-shell microgels. J. Phys. Chem. B 2009, 113, 16039–16045. [Google Scholar] [CrossRef] [PubMed]
- Armenia, I.; Grazú Bonavia, M.V.; De Matteis, L.; Ivanchenko, P.; Martra, G.; Gornati, R.; de la Fuente, J.M.; Bernardini, G. Enzyme activation by alternating magnetic field: Importance of the bioconjugation methodology. J. Colloid Interface Sci. 2019, 537, 615–628. [Google Scholar] [CrossRef]
- Klyachko, N.L.; Sokolsky-Papkov, M.; Pothayee, N.; Efremova, M.V.; Gulin, D.A.; Kuznetsov, A.A.; Majouga, A.G.; Riffle, J.S.; Golovin, Y.I.; Kabanov, A.V.; et al. Changing the enzyme reaction rate in magnetic nanosuspensions by a non-heating magnetic field. Angew. Chem. Int. Ed. 2012, 51, 12016–12019. [Google Scholar] [CrossRef]
- Majouga, A.; Sokolsky-Papkov, M.; Kuznetsov, A.; Lebedev, D.; Efremova, M.; Beloglazkina, E.; Rudakovskaya, P.; Veselov, M.; Zyk, N.; Golovin, Y.; et al. Enzyme-functionalized gold-coated magnetite nanoparticles as novel hybrid nanomaterials: Synthesis, purification and control of enzyme function by low-frequency magnetic field. Colloids Surf. B 2015, 125, 104–109. [Google Scholar] [CrossRef]
- Efremova, M.V.; Veselov, M.M.; Barulin, A.V.; Gribanovsky, S.L.; Le-Deygen, I.M.; Uporov, I.V.; Kudryashova, E.V.; Sokolsky-Papkov, M.; Majouga, A.G.; Golovin, Y.I.; et al. In Situ Observation of Chymotrypsin Catalytic Activity Change Actuated by Nonheating Low-Frequency Magnetic Field. ACS Nano 2018, 12, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Veselov, M.M.; Uporov, I.V.; Efremova, M.V.; Le-Deygen, I.M.; Prusov, A.N.; Shchetinin, I.V.; Savchenko, A.G.; Golovin, Y.I.; Kabanov, A.V.; Klyachko, N.L. Modulation of α-Chymotrypsin Conjugated to Magnetic Nanoparticles by the Non-Heating Low-Frequency Magnetic Field: Molecular Dynamics, Reaction Kinetics, and Spectroscopy Analysis. ACS Omega 2022, 7, 20644–20655. [Google Scholar] [CrossRef] [PubMed]
- Golovin, Y.I.; Gribanovsky, S.L.; Golovin, D.Y.; Klyachko, N.L.; Majouga, A.G.; Master, A.M.; Sokolsky, M.; Kabanov, A.V. Towards nanomedicines of the future: Remote magneto-mechanical actuation of nanomedicines by alternating magnetic fields. J. Control. Release 2015, 219, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Golovin, Y.I.; Klyachko, N.L.; Golovin, D.Y.; Efremova, M.V.; Samodurov, A.A.; Sokolski-Papkov, M.; Kabanov, A.V. A new approach to the control of biochemical reactions in a magnetic nanosuspension using a low-frequency magnetic field. Tech. Phys. Lett. 2013, 39, 240–243. [Google Scholar] [CrossRef]
- Alekseeva, A.A.; Kargov, I.S.; Kleimenov, S.Y.; Savin, S.S.; Tishkov, V.I. Additivity of the Stabilization Effect of Single Amino Acid Substitutions in Triple Mutants of Recombinant Formate Dehydrogenase from the Soybean Glycine max. Acta Naturae 2015, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Veskoukis, A.S.; Margaritelis, N.V.; Kyparos, A.; Paschalis, V.; Nikolaidis, M.G. Spectrophotometric assays for measuring redox biomarkers in blood and tissues: The NADPH network. Redox Rep. 2018, 23, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, F.; Zhou, H.; Su, H. The structural and bonding evolution in cysteine-gold cluster complexes. Phys. Chem. Chem. Phys. 2013, 15, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Mehn, D.; Caputo, F.; Rösslein, M.; Calzolai, L.; Saint-Antonin, F.; Courant, T.; Wick, P.; Gilliland, D. Larger or more? Nanoparticle characterisation methods for recognition of dimers. RSC Adv. 2017, 7, 27747–27754. [Google Scholar] [CrossRef]
- Sarcar, S.; Jain, T.K.; Maitra, A. Activity and stability of yeast alcohol dehydrogenase (YADH) entrapped in aerosol OT reverse micelles. Biotechnol. Bioeng. 1992, 39, 474–478. [Google Scholar] [CrossRef]
- Mesentsev, A.V.; Lamzin, V.S.; Tishkov, V.I.; Ustinnikova, T.B.; Popov, V.O. Effect of pH on kinetic parameters of NAD+-dependent formate dehydrogenase. Biochem. J. 1997, 321 Pt 2, 475–480. [Google Scholar] [CrossRef]
- Sadykhov, E.G.; Serov, A.E.; Voinova, N.S.; Uglanova, S.V.; Petrov, A.S.; Alekseeva, A.A.; Kleimenov, S.Y.; Popov, V.O.; Tishkov, V.I. A comparative study of the thermal stability of formate dehydrogenases from microorganisms and plants. Appl. Biochem. Microbiol. 2006, 42, 236–240. [Google Scholar] [CrossRef]
- Malito, E.; Alfieri, A.; Fraaije, M.W.; Mattevi, A. Crystal structure of a Baeyer-Villiger monooxygenase. Proc. Natl. Acad. Sci. USA 2004, 101, 13157–13162. [Google Scholar] [CrossRef]
- Qiu, L.; Gulotta, M.; Callender, R. Lactate dehydrogenase undergoes a substantial structural change to bind its substrate. Biophys. J. 2007, 93, 1677–1686. [Google Scholar] [CrossRef]
- Schirwitz, K.; Schmidt, A.; Lamzin, V.S. High-resolution structures of formate dehydrogenase from Candida boidinii. Protein Sci. 2007, 16, 1146–1156. [Google Scholar] [CrossRef]
- Pal, N.; Wu, M.; Lu, H.P. Probing conformational dynamics of an enzymatic active site by an in situ single fluorogenic probe under piconewton force manipulation. Proc. Natl. Acad. Sci. USA 2016, 113, 15006–15011. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; He, Y.; Lu, H.P. Interrogating the activities of conformational deformed enzyme by single-molecule fluorescence-magnetic tweezers microscopy. Proc. Natl. Acad. Sci. USA 2015, 112, 13904–13909. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; He, Y.; Lu, H.P. Manipulating and probing enzymatic conformational fluctuations and enzyme-substrate interactions by single-molecule FRET-magnetic tweezers microscopy. Phys. Chem. Chem. Phys. 2014, 16, 13052–13058. [Google Scholar] [CrossRef]
- Ganzhorn, A.J.; Green, D.W.; Hershey, A.D.; Gould, R.M.; Plapp, B.V. Kinetic characterization of yeast alcohol dehydrogenases. Amino acid residue 294 and substrate specificity. J. Biol. Chem. 1987, 262, 3754–3761. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, A.A.; Serenko, A.A.; Kargov, I.S.; Savin, S.S.; Kleymenov, S.Y.; Tishkov, V.I. Engineering catalytic properties and thermal stability of plant formate dehydrogenase by single-point mutations. Protein Eng. Des. Sel. 2012, 25, 781–788. [Google Scholar] [CrossRef]
- Vlasova, K.Y.; Vishwasrao, H.; Abakumov, M.A.; Golovin, D.Y.; Gribanovsky, S.L.; Zhigachev, A.O.; Poloznikov, A.; Majouga, A.G.; Golovin, Y.I.; Sokolsky-Papkov, M.; et al. Enzyme Release from Polyion Complex by Extremely Low Frequency Magnetic Field. Sci. Rep. 2020, 10, 4745. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselov, M.M.; Efremova, M.V.; Prusov, A.N.; Klyachko, N.L. Up- and Down-Regulation of Enzyme Activity in Aggregates with Gold-Covered Magnetic Nanoparticles Triggered by Low-Frequency Magnetic Field. Nanomaterials 2024, 14, 411. https://doi.org/10.3390/nano14050411
Veselov MM, Efremova MV, Prusov AN, Klyachko NL. Up- and Down-Regulation of Enzyme Activity in Aggregates with Gold-Covered Magnetic Nanoparticles Triggered by Low-Frequency Magnetic Field. Nanomaterials. 2024; 14(5):411. https://doi.org/10.3390/nano14050411
Chicago/Turabian StyleVeselov, Maxim M., Maria V. Efremova, Andrey N. Prusov, and Natalia L. Klyachko. 2024. "Up- and Down-Regulation of Enzyme Activity in Aggregates with Gold-Covered Magnetic Nanoparticles Triggered by Low-Frequency Magnetic Field" Nanomaterials 14, no. 5: 411. https://doi.org/10.3390/nano14050411
APA StyleVeselov, M. M., Efremova, M. V., Prusov, A. N., & Klyachko, N. L. (2024). Up- and Down-Regulation of Enzyme Activity in Aggregates with Gold-Covered Magnetic Nanoparticles Triggered by Low-Frequency Magnetic Field. Nanomaterials, 14(5), 411. https://doi.org/10.3390/nano14050411






